Abstract
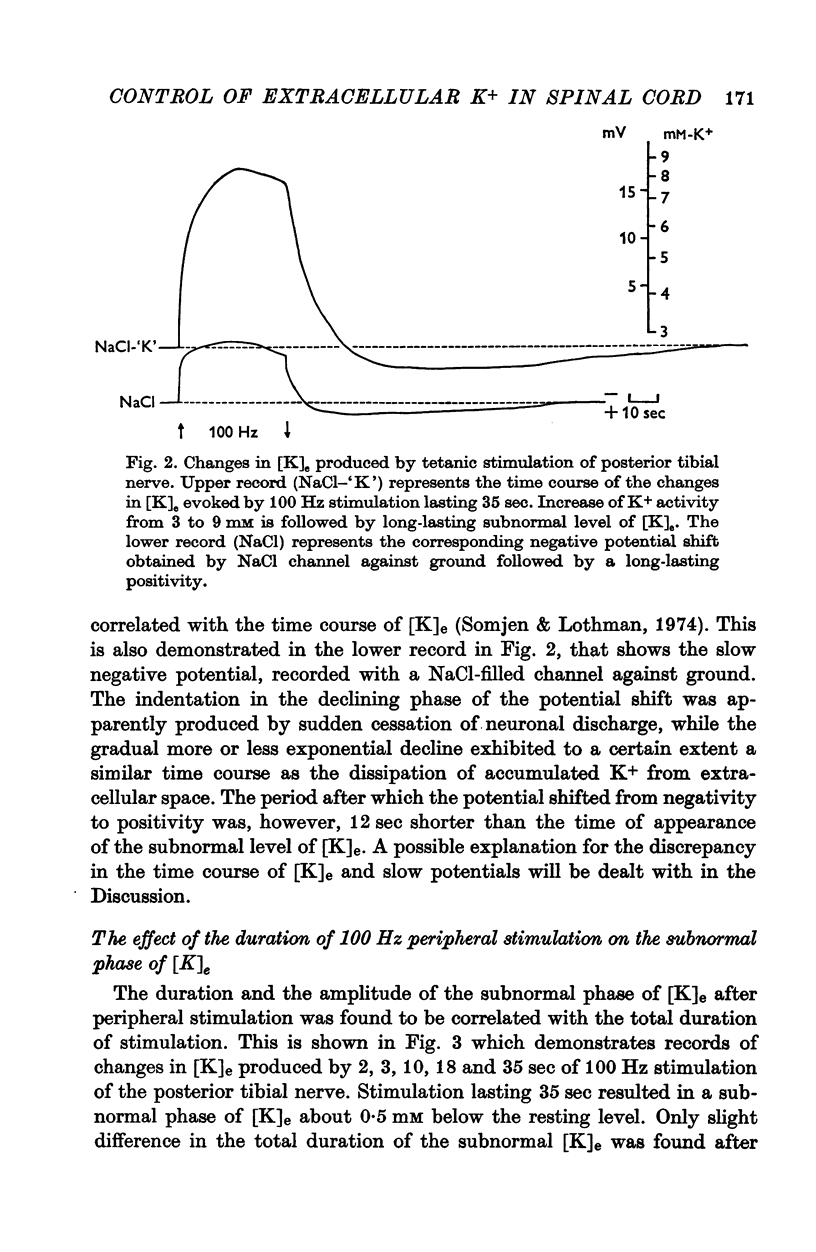
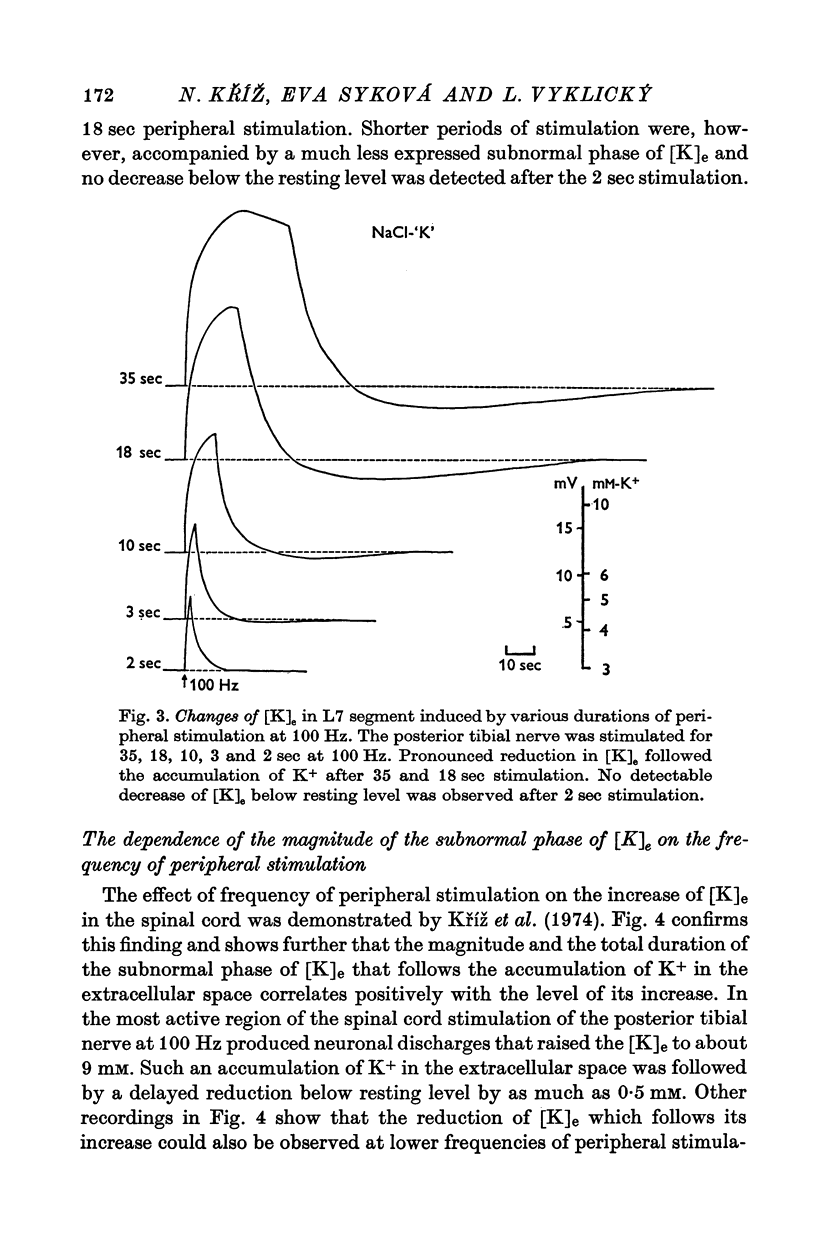
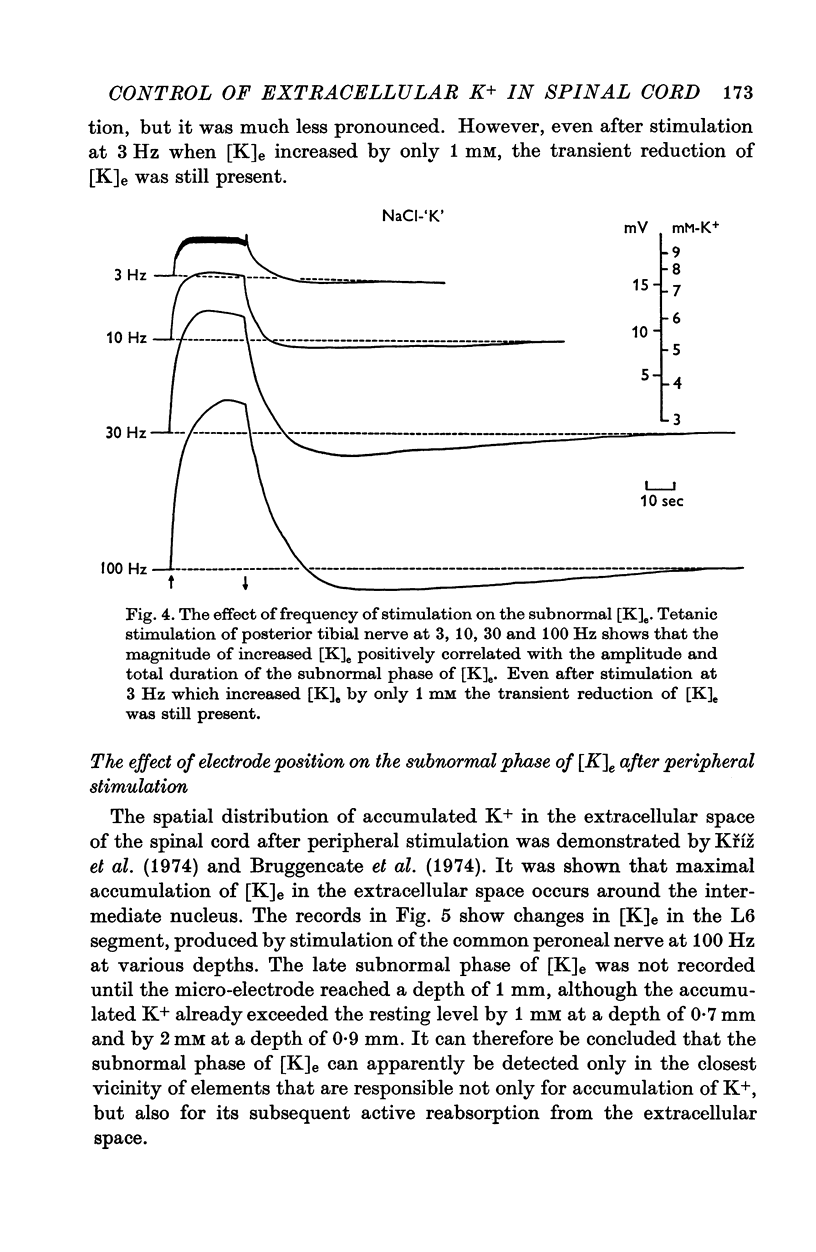
1. By means of K-specific double-barrelled micro-electrodes the time course of changes in K+ concentration in the extracellular space of the lumbar spinal cord was examined after peripheral tetanic stimulation and after a single volley in a mixed peripheral nerve in non-anaesthetized, intercollicularly decerebrated and spinalized cats. 2. Tetanic stimulation (100 Hz) which increases the [K]e from 3 to 9 mM is followed by a phase of reduced [K]e during which [K]e decreases by 0.5 mM below resting level, lasting 1-2 minutes before returning to its original resting level. Evidence is presented that this subnormal phase of [K]e reflects active processes redistributing accumulated K+ from extracellular space. 3. The subnormal phase of [K]e can be registered only when the microelectrode is located in very close vicinity of discharging neurones and is not primarily dependent on the absolute level of increased [K]e. This can be considered as evidence that the neurones and not the glial cells are responsible for active reabsorption of K+ from the extracellular space. 4. Increased E1K]e is reflected in focally recorded potentials as a negativity and decreased [K]e as a positivity. The latency of focally recorded positivity is, however, shorter than the latency of reduced [K]e. This makes it likely that the positivity reflects not only passive hyperpolarization of glial elements, but also an active, electrogenic ion transport across neuronal membrane. 5. The shortest latency of increased [K]e induced by a single volley in a mixed peripheral nerve was found to be 9 msec; the peak, representing 0.5 mM, was attained after 40 msec and the total duration was 200 msec. A theoretical consideration is put forward that the time course of transient increase in [K]e is consistent with the suggestion that K+ which accumulates in the spinal cord after neuronal discharge is responsible for primary afferent depolarization. 6. Evidence is presented that increased [K]e, induced by a long lasting peripheral stimulation, is accompanied by decreased efficacy of impulse transmission.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHARD C. G. The cord dorsum potentials in relation to peripheral source of afferent stimulation. Cold Spring Harb Symp Quant Biol. 1952;17:221–232. doi: 10.1101/sqb.1952.017.01.021. [DOI] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. The interpretation of potential changes in the spinal cord. J Physiol. 1938 Apr 14;92(3):276–321. doi: 10.1113/jphysiol.1938.sp003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans J., Burke R., Fedina L., Lundberg A. The effect of dopa on the spinal cord. 8. Presynaptic and "remote" inhibition of transmission from Ia afferents to alpha motoneurones. Acta Physiol Scand. 1974 Mar;90(3):618–639. doi: 10.1111/j.1748-1716.1974.tb05627.x. [DOI] [PubMed] [Google Scholar]
- Grossman R. G., Rosman L. J. Intracellular potentials of inexcitable cells in epileptogenic cortex undergoing fibrillary gliosis after a local injury. Brain Res. 1971 May 7;28(2):181–201. doi: 10.1016/0006-8993(71)90654-8. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E. Extracellular K + activity and slow potential changes in spinal cord and medulla. Can J Physiol Pharmacol. 1972 Dec;50(12):1214–1217. doi: 10.1139/y72-177. [DOI] [PubMed] [Google Scholar]
- Kríz N., Syková E., Ujec E., Vyklický L. Changes of extracellular potassium concentration induced by neuronal activity in the sinal cord of the cat. J Physiol. 1974 Apr;238(1):1–15. doi: 10.1113/jphysiol.1974.sp010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Rapid changes of potassium concentration at the outer surface of exposed single neurons during membrane current flow. J Gen Physiol. 1973 Mar;61(3):385–399. doi: 10.1085/jgp.61.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Goldring S. Slow hyperpolarization in cells presumed to be glia in cerebral cortex of cat. J Neurophysiol. 1973 Sep;36(5):879–892. doi: 10.1152/jn.1973.36.5.879. [DOI] [PubMed] [Google Scholar]
- Somjen G. G., Lothman E. W. Potassium, sustained focal potential shifts, and dorsal root potentials of the mammalian spinal cord. Brain Res. 1974 Mar 29;69(1):153–157. doi: 10.1016/0006-8993(74)90382-5. [DOI] [PubMed] [Google Scholar]
- Sypert G. W., Ward A. A., Jr Unidentified neuroglia potentials during propagated seizures in neocortex. Exp Neurol. 1971 Nov;33(2):239–255. doi: 10.1016/0014-4886(71)90018-5. [DOI] [PubMed] [Google Scholar]
- Ujec E., Beránek R. Differential high-impedance DC amplifier with negative input capacity. Physiol Bohemoslov. 1967;16(1):89–96. [PubMed] [Google Scholar]
- Vyklicky L., Sykova E., Kriz N., Ujec E. Post-stimulation changes of extracellular potassium concentration in the spinal cord of the rat. Brain Res. 1972 Oct 27;45(2):608–611. doi: 10.1016/0006-8993(72)90492-1. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Syková E., Kríz N. Slow potentials induced by changes of extracellular potassium in the spinal cord of the cat. Brain Res. 1975 Apr 4;87(1):77–80. doi: 10.1016/0006-8993(75)90782-9. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Kritz N., Bures J. Potassium-selective microelectrodes used for measuring the extracellular brain potassium during spreading depression and anoxic depolarization in rats. Brain Res. 1972 Apr 14;39(1):255–259. doi: 10.1016/0006-8993(72)90802-5. [DOI] [PubMed] [Google Scholar]
- ten Bruggencate G., Lux H. D., Liebl L. Possible relationships between extracellular potassium activity and presynaptic inhibition in the spinal cord of the cat. Pflugers Arch. 1974;349(4):301–317. doi: 10.1007/BF00588416. [DOI] [PubMed] [Google Scholar]


