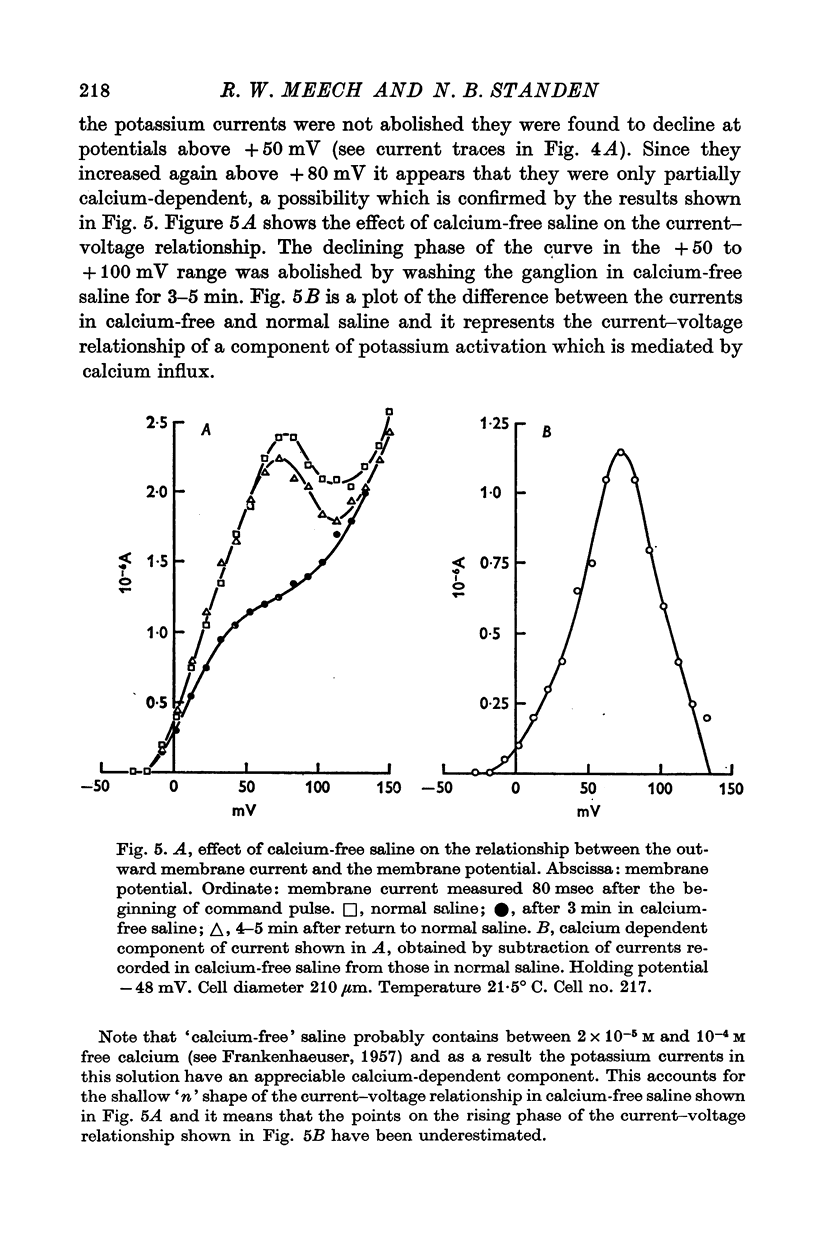
Abstract
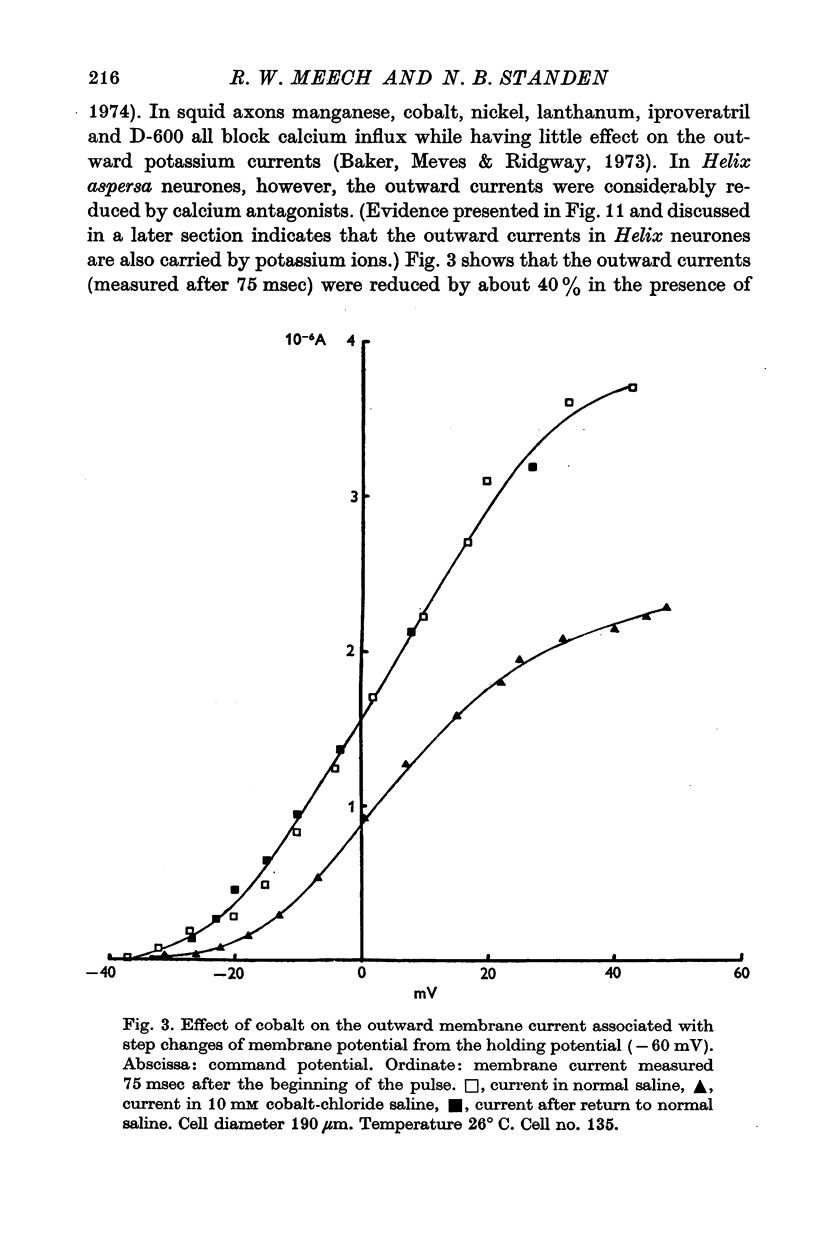
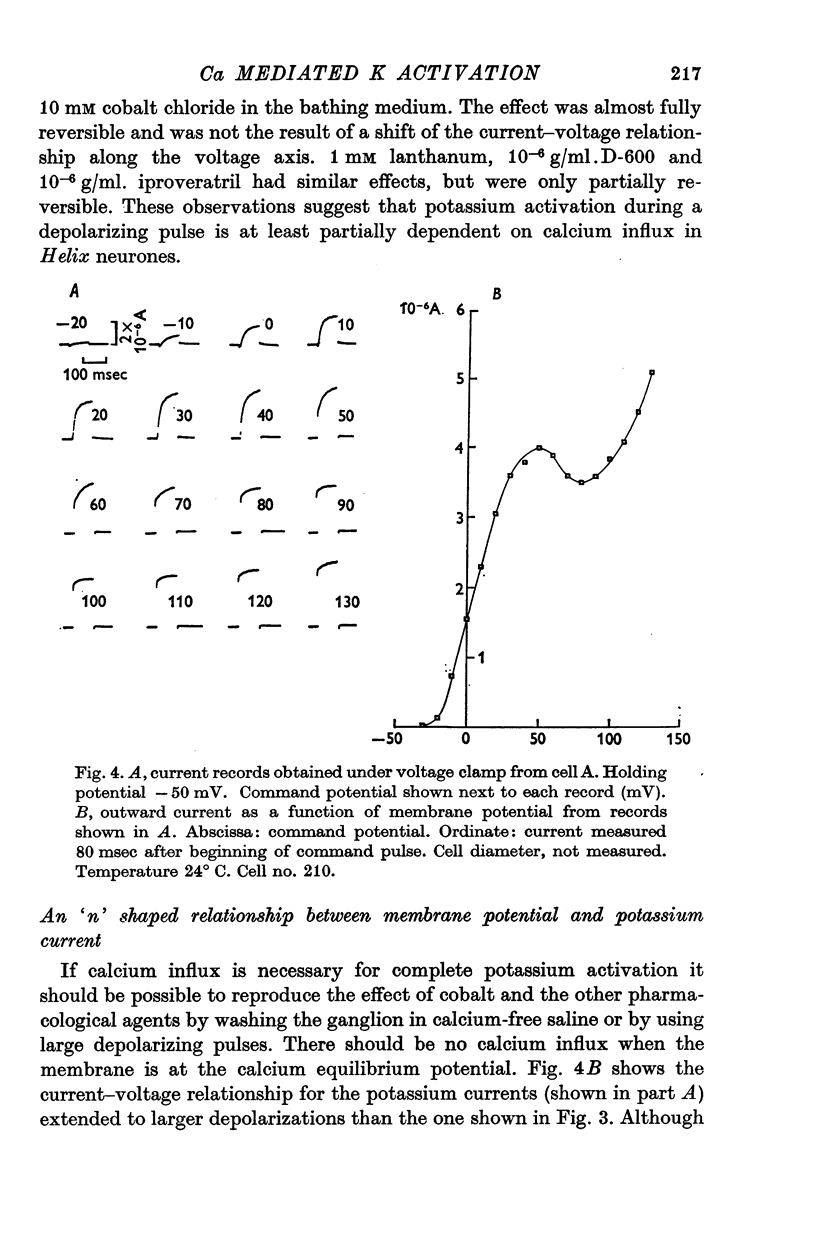
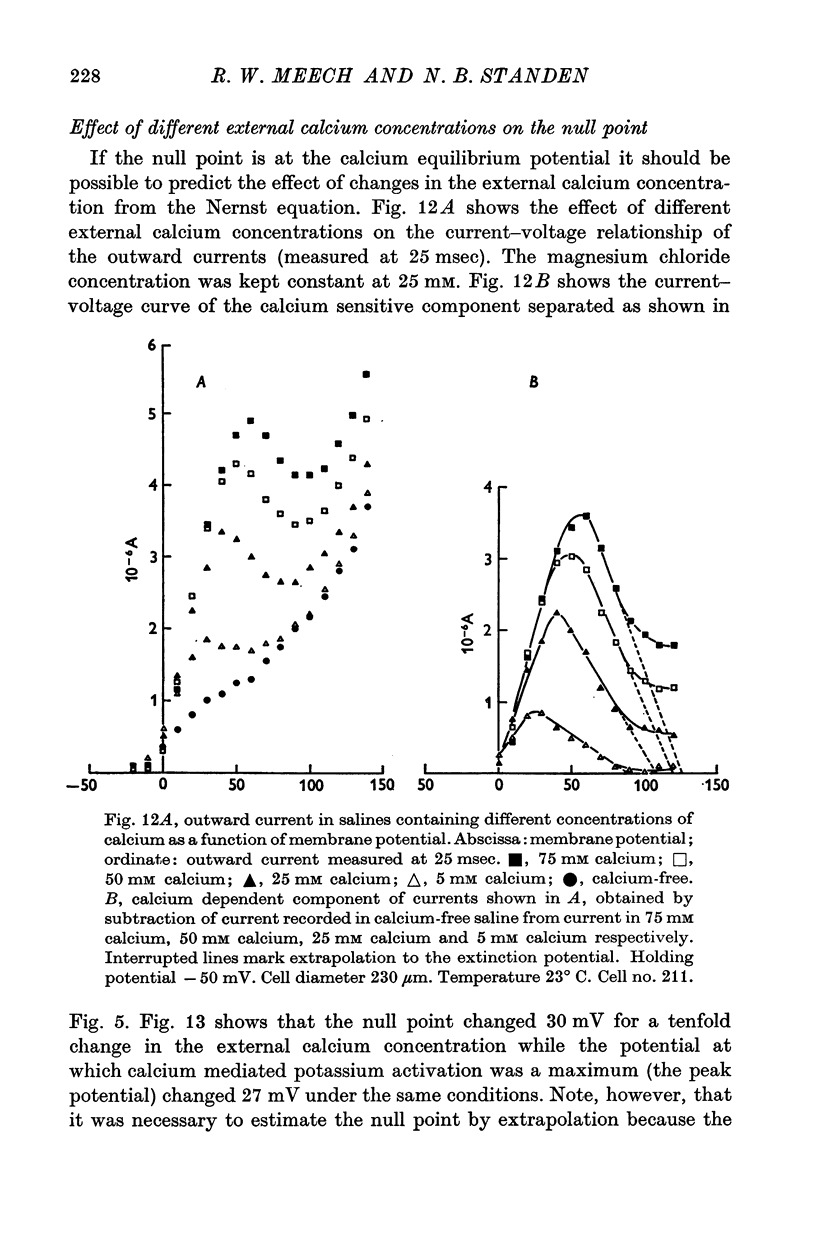
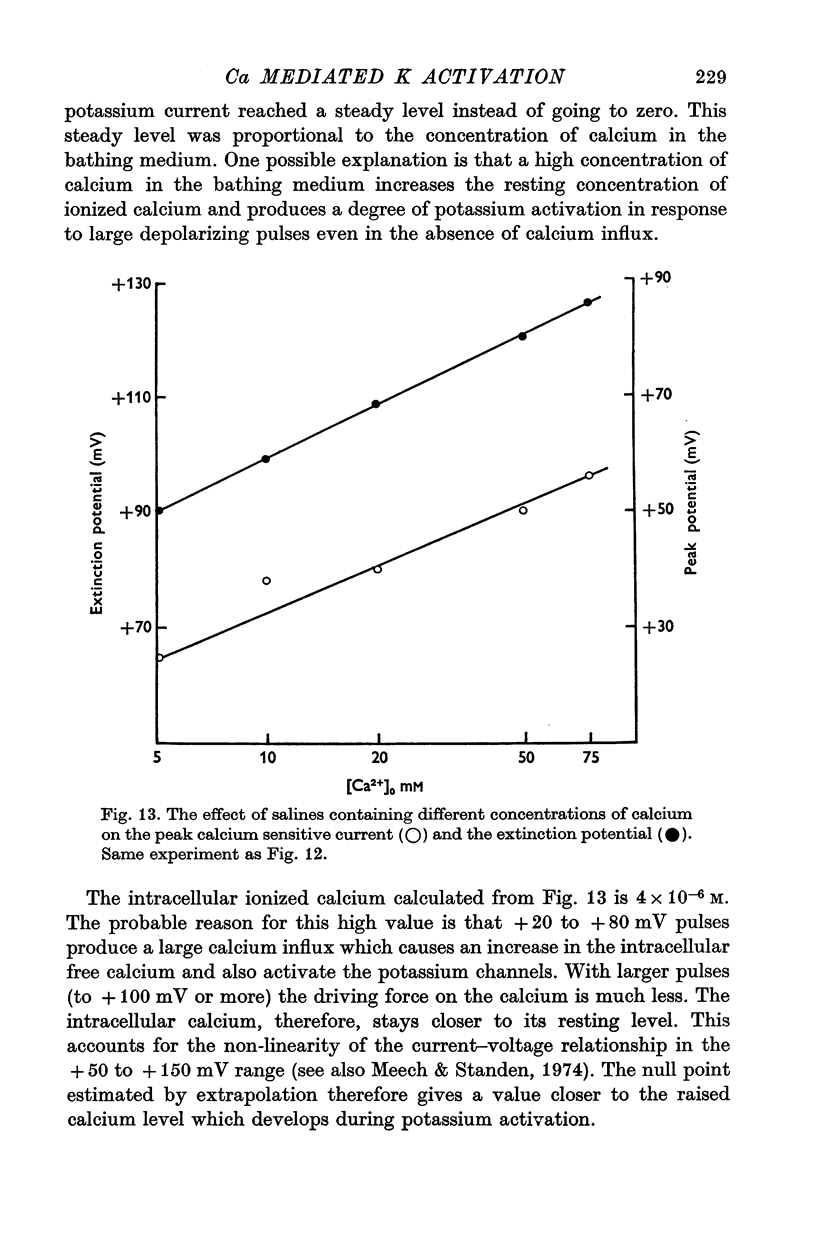
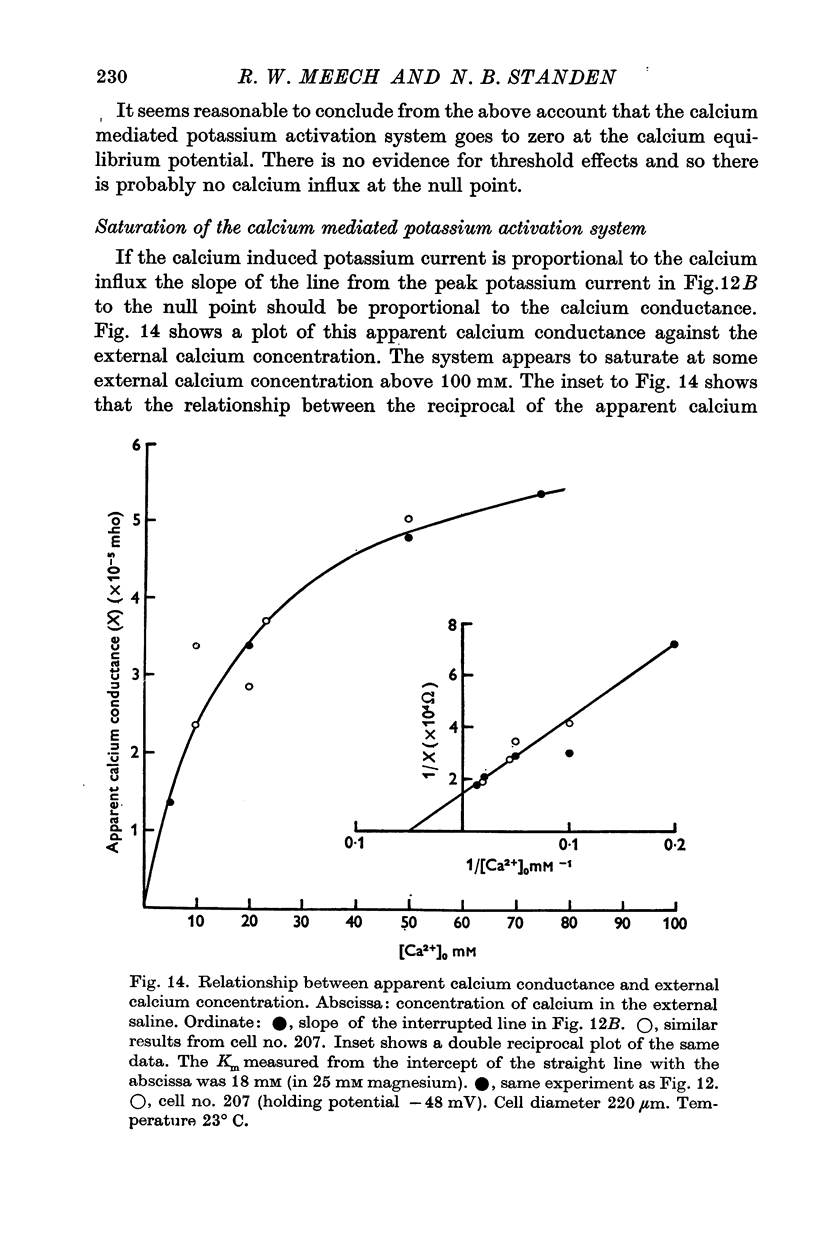
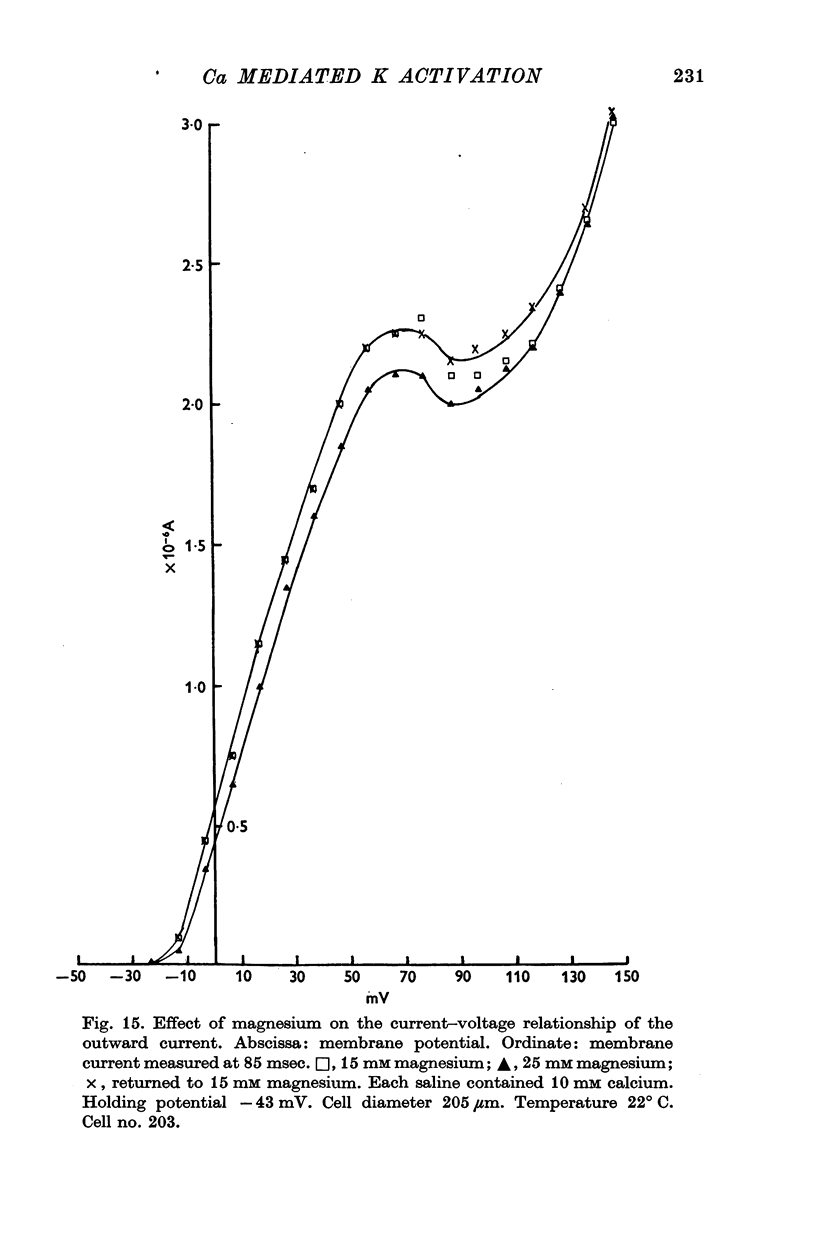
1. Helix aspersa neurones under voltage clamp generate prolonged outward currents (potassium currents) in response to depolarizing command pulses. 2. The potassium currents recorded from cell A were reversibly reduced 25-50% by 10 mM cobalt ions in the bathing medium; 1 mM lanthanum, 10(-6) g/ml. D-600 and 10(-6) g/ml. iproveratril had similar effects but were only partially reversible. 3. The relationship between the potassium currents and the membrane potential had an "n" shape in normal saline. In calcium-free saline (containing 25 mM magnesium) the potassium currents were reduced and the "n" shape was abolished. The effect of calcium-free saline was readily reversible. 4. The voltage-dependence of the calcium-sensitive potassium currents was similar to that of the "late" calcium channel in squid axons (Baker, Hodgkin & Ridgway, 1971). 5. When cell A was depolarents were made up of two exponentially declining components. The slower of the two components was reduced in calcium-free saline. 6. When cell A was depolarized by 150 mV for 10 msec and then repolarized the "tail" currents were made up of a single rapidly declining component. The reversal potential of this component changed by 58 mV for a tenfold change in the external potassium concentration as predicted by the Nernst equation. 7. The reversal potential of "tail" currents having both components was less sensitive to changes in the external potassium concentration. 8. Tetraethylammonium (TEA) ions blocked both calcium dependent and voltage sensitive potassium currents. Each receptor was found to bind a single molecule of TEA. The dissociaton constant was about 10 mM in each case. 9. The intracellular concentration of ionized calcium was estimated from the potential at which there was no apparent calcium influx (the null point). It was between 3 x 10(-8) M and 8 x 10(-8) M with 10(-2) M calcium in the bathing medium. 10. The null point changed 30 mV for a tenfold change in the external calcium concentration as predicted by the Nernst equation. 11. It is concluded that depolarization of Helix neurones activates two typesof potassium channel. One channel is voltage dependent and highly selective for potassium. Activation of the other channel is dependent on the influx (or injection, see Meech, 1972, 1974a) of calcium. This calcium mediated potassium activation system saturates at high external calcium concentrations and is inhibited by external magnesium ions.
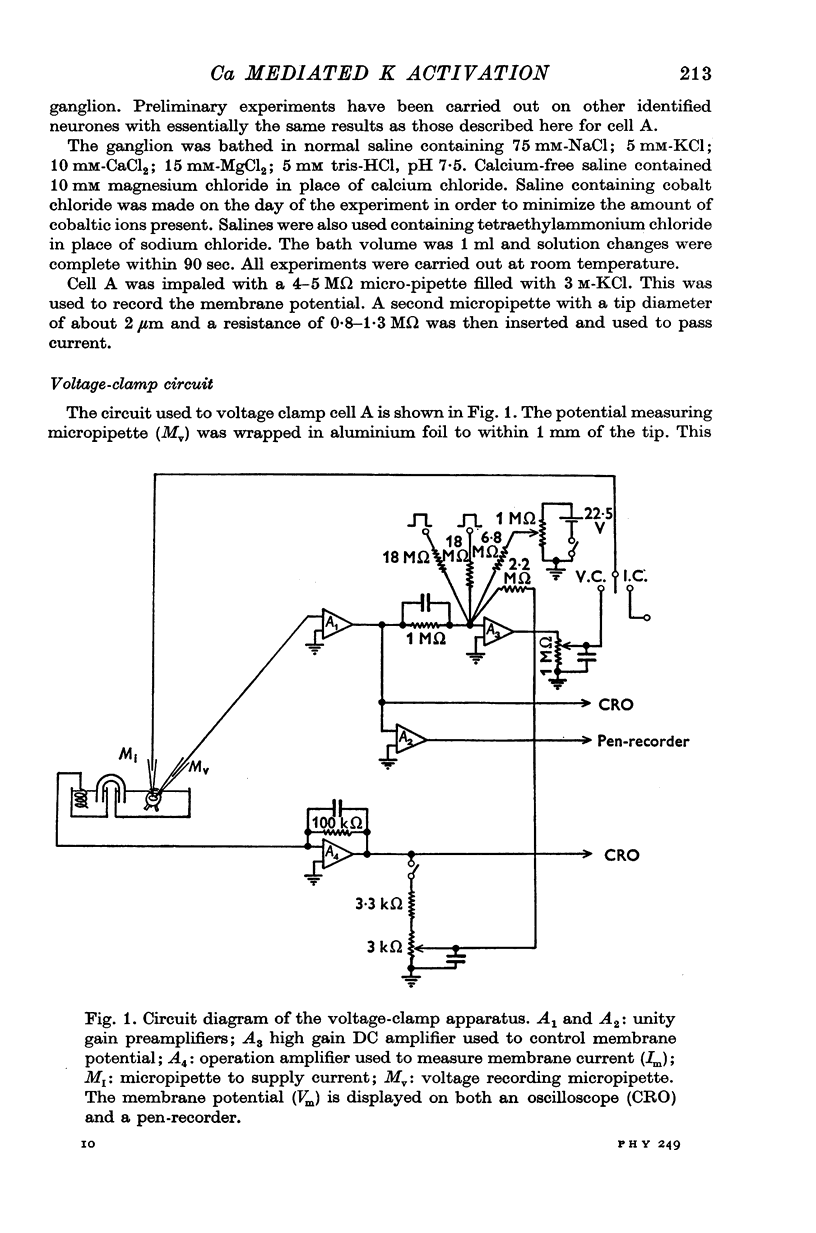
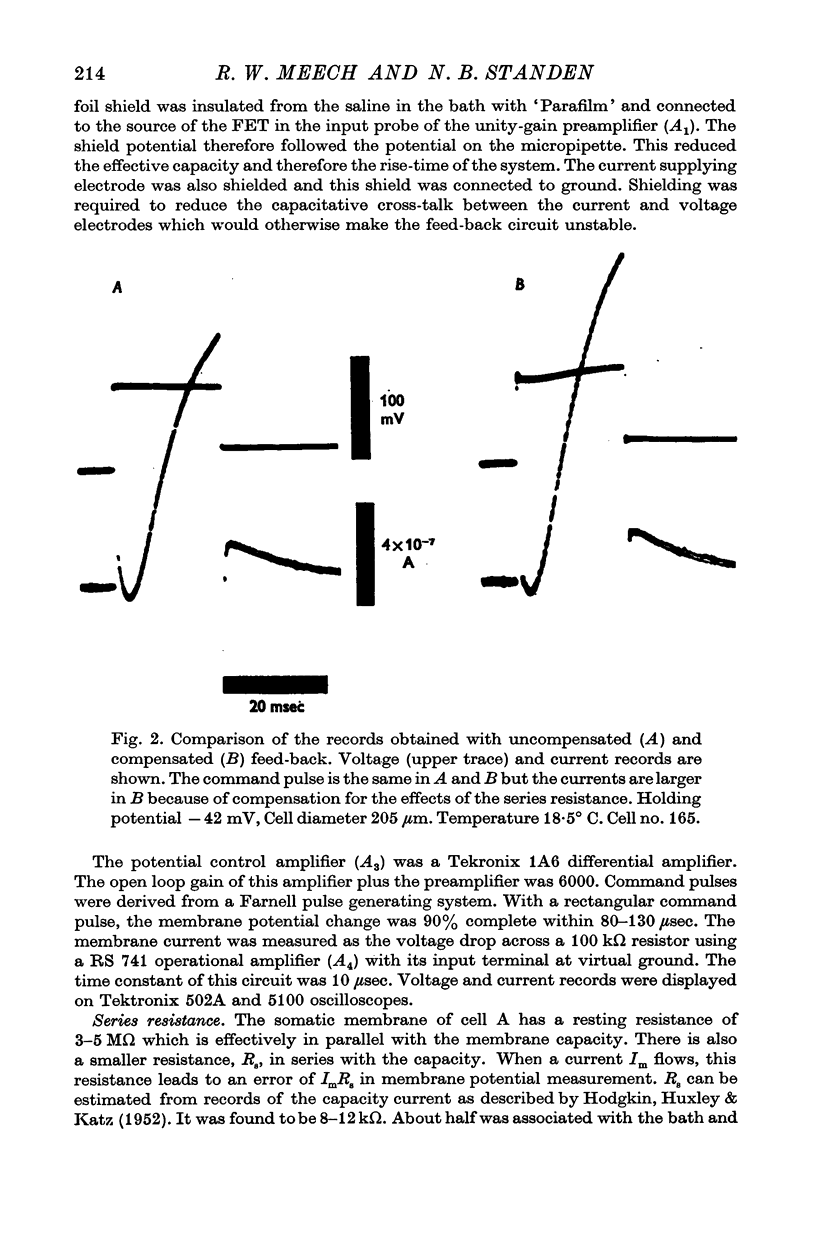
Full text
PDF


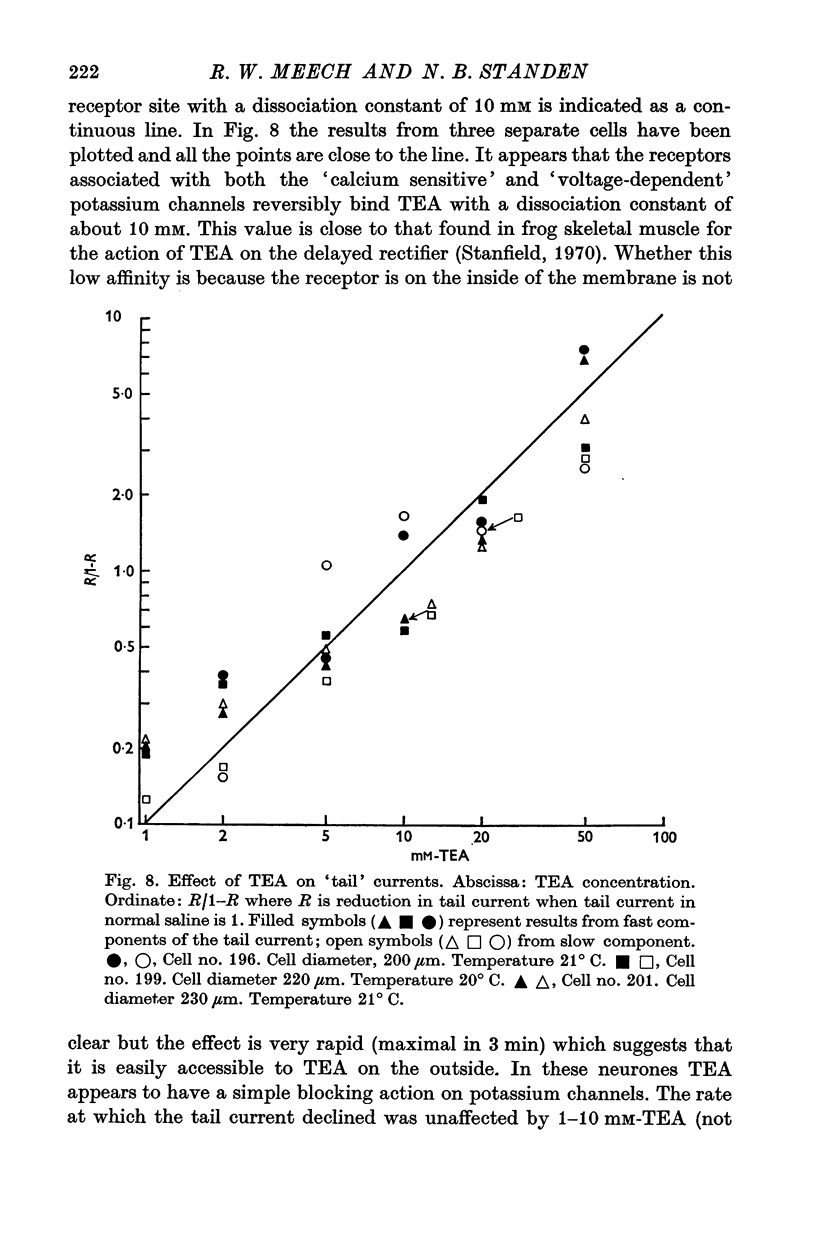
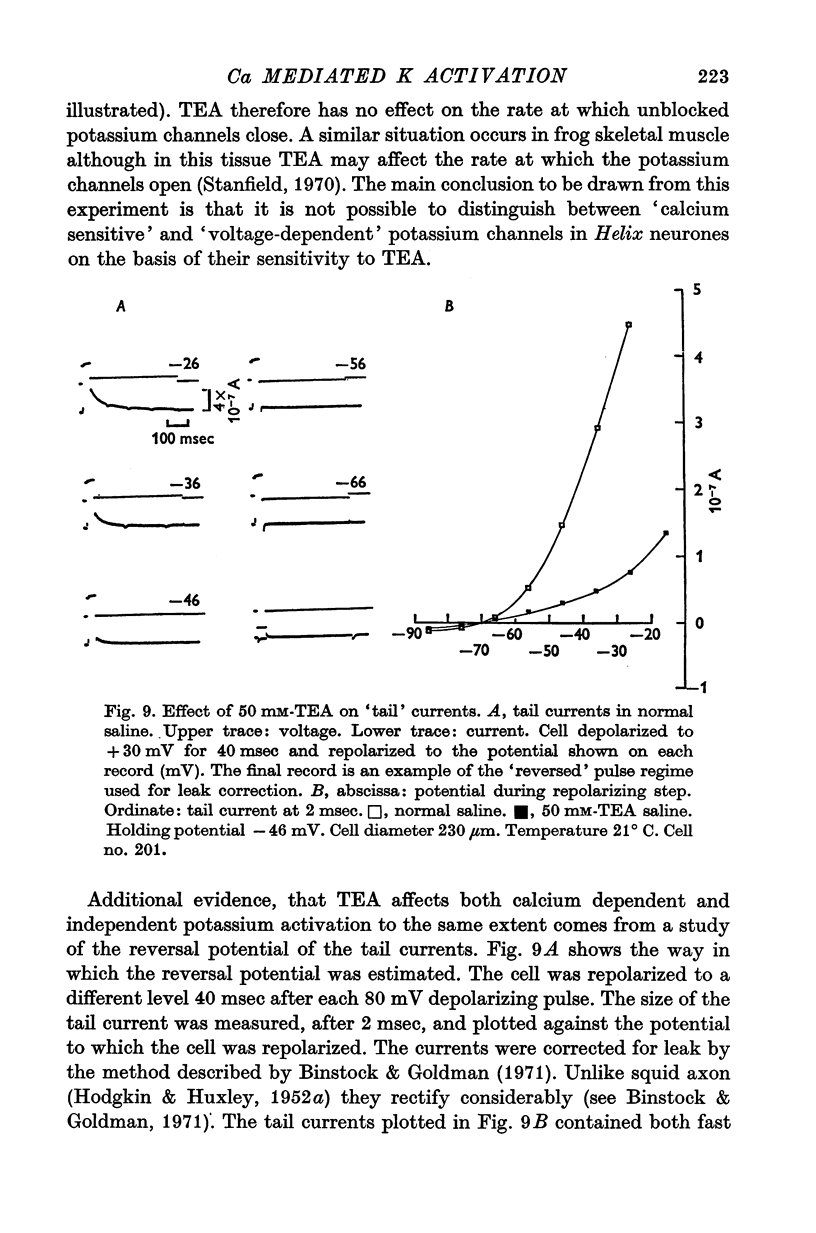
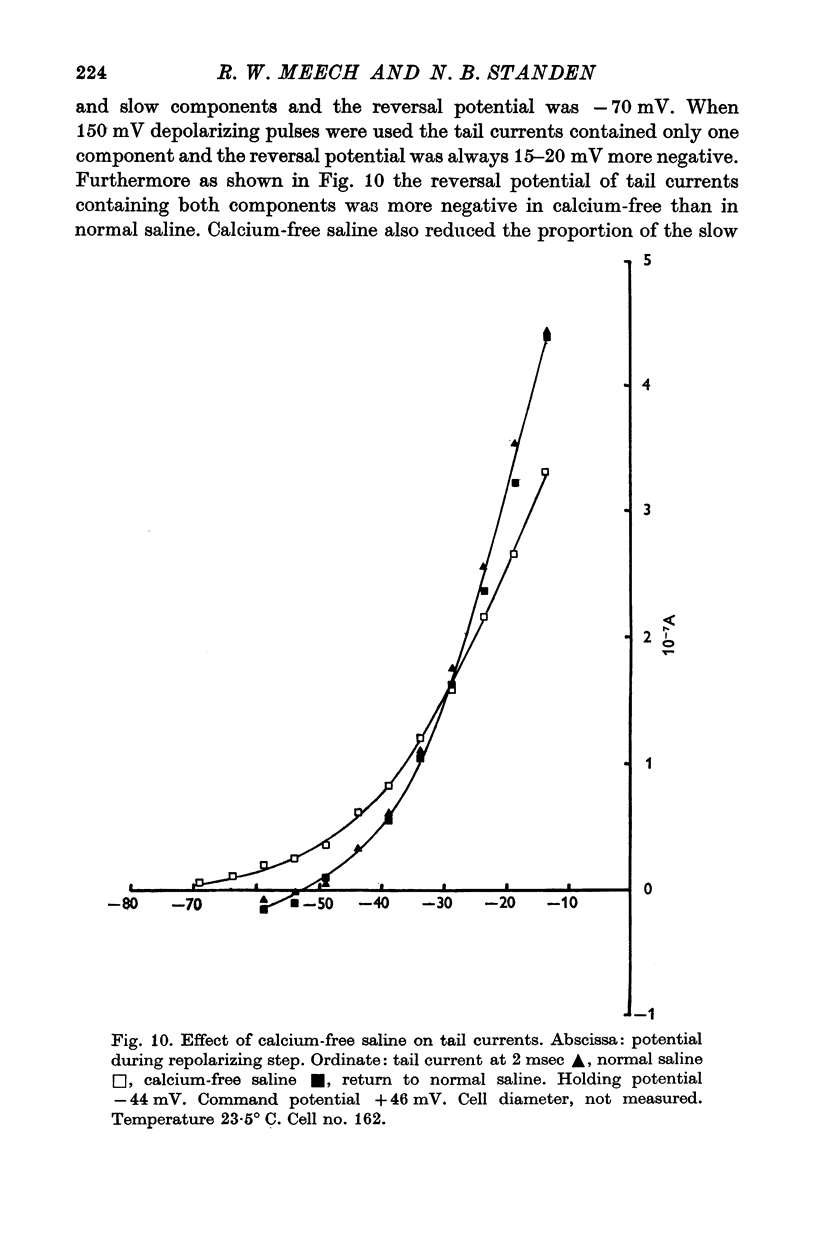
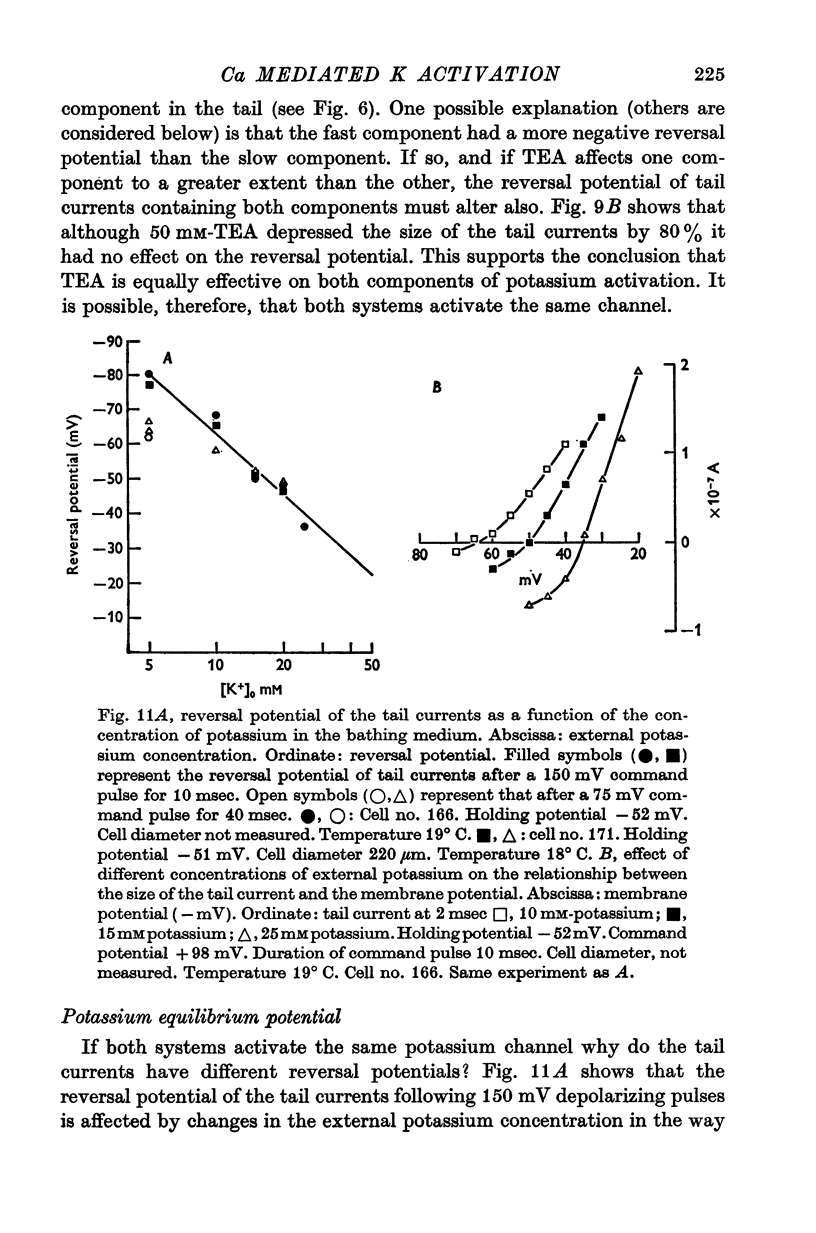










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
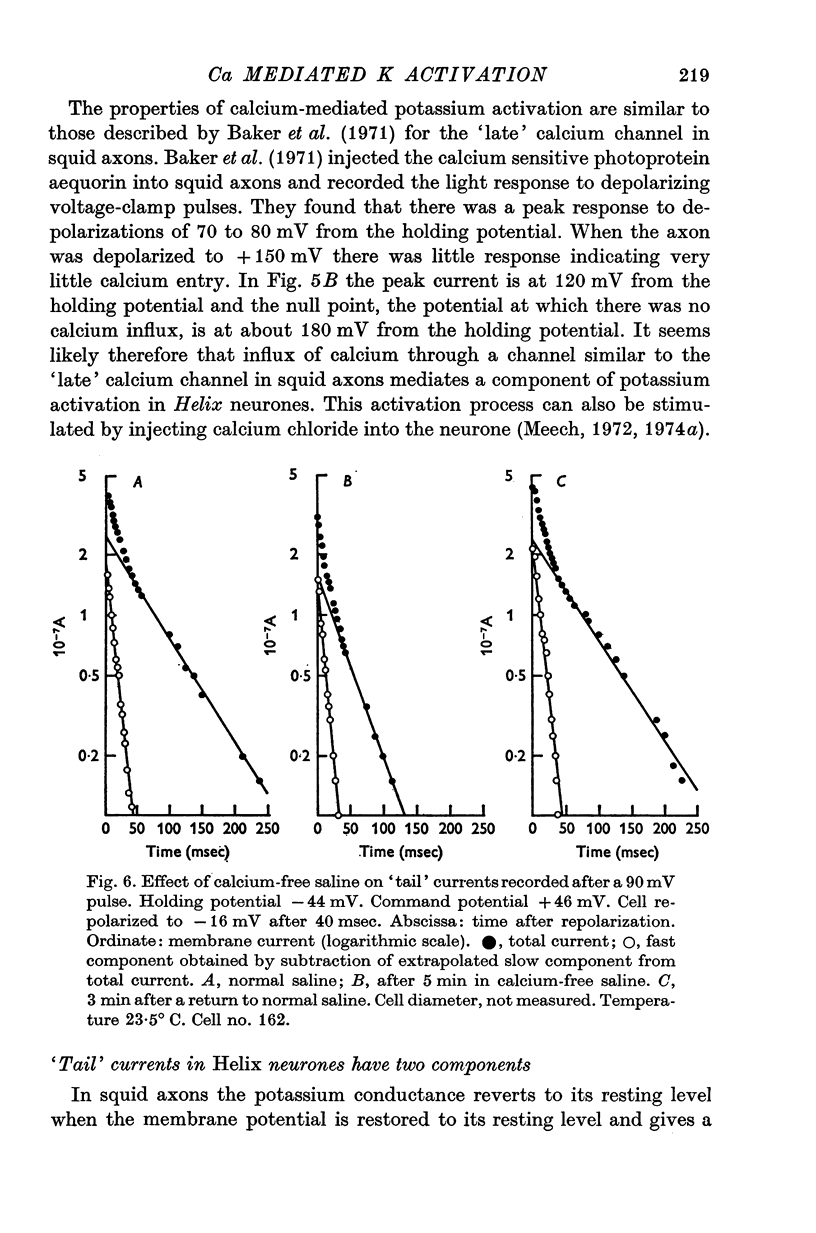
- BENNETT M. V., CRAIN S. M., GRUNDFEST H. Electrophysiology of supramedullary neurons in Spheroides maculatus. III. Organization of the supramedullary neurons. J Gen Physiol. 1959 Sep;43:221–250. doi: 10.1085/jgp.43.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
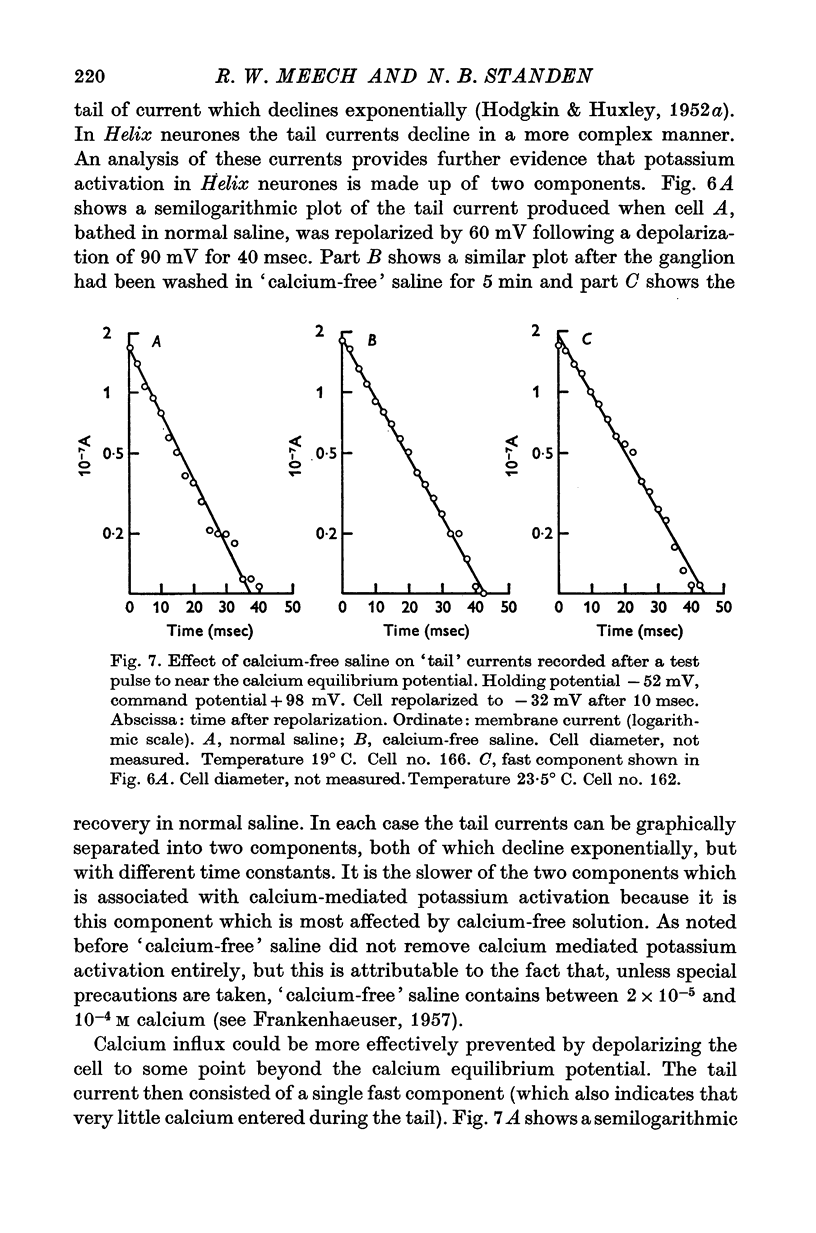
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L., Goldman L. Rectification in instantaneous potassium current-voltage relations in Myxicola giant axons. J Physiol. 1971 Sep;217(3):517–531. doi: 10.1113/jphysiol.1971.sp009583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundfest H. Comparative electrobiology of excitable membranes. Adv Comp Physiol Biochem. 1966;2:1–116. doi: 10.1016/b978-0-12-395511-1.50006-8. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., SAITO N. Membrane potential change and membrane current in supramedullary nerve cell of puffer. J Neurophysiol. 1959 Mar;22(2):204–221. doi: 10.1152/jn.1959.22.2.204. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., SAITO N. Voltage-current relations in nerve cell membrane of Onchidium verruculatum. J Physiol. 1959 Oct;148:161–179. doi: 10.1113/jphysiol.1959.sp006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S., Satow Y. Sodium- and calcium-dependent spike potentials in the secretory neuron soma of the X-organ of the crayfish. J Gen Physiol. 1971 Feb;57(2):216–236. doi: 10.1085/jgp.57.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge D., Miller J. Different spike mechanisms in axon and soma of molluscan neurone. Nature. 1974 Nov 8;252(5479):155–156. doi: 10.1038/252155a0. [DOI] [PubMed] [Google Scholar]
- Kado R. T. Aplysia giant cell: soma-axon voltage clamp current differences. Science. 1973 Nov 23;182(4114):843–845. doi: 10.1126/science.182.4114.843. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Magura I. S. Calcium ions as inward current carriers in mollusc neurones. Comp Biochem Physiol. 1970 Aug 15;35(4):857–866. doi: 10.1016/0010-406x(70)90080-0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Lisiewicz A. Injections of calcium ions into spinal motoneurones. J Physiol. 1972 Sep;225(2):363–390. doi: 10.1113/jphysiol.1972.sp009945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K. Influence of ionic environment on the relationship between pre- and postsynaptic potentials. J Neurobiol. 1970;1(4):435–437. doi: 10.1002/neu.480010407. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Calcium-mediated potassium activation in Helix neurones. J Physiol. 1974 Mar;237(2):43P–44P. [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kusano K. Behavior of delayed current under voltage clamp in the supramedullary neurons of puffer. J Gen Physiol. 1966 Mar;49(4):613–628. doi: 10.1085/jgp.49.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B. Properties of a calcium channel in snail neurones. Nature. 1974 Jul 26;250(464):340–342. doi: 10.1038/250340a0. [DOI] [PubMed] [Google Scholar]
- Wald F. Ionic differences between somatic and axonal action potentials in snail giant neurones. J Physiol. 1972 Jan;220(2):267–281. doi: 10.1113/jphysiol.1972.sp009706. [DOI] [PMC free article] [PubMed] [Google Scholar]


