Abstract
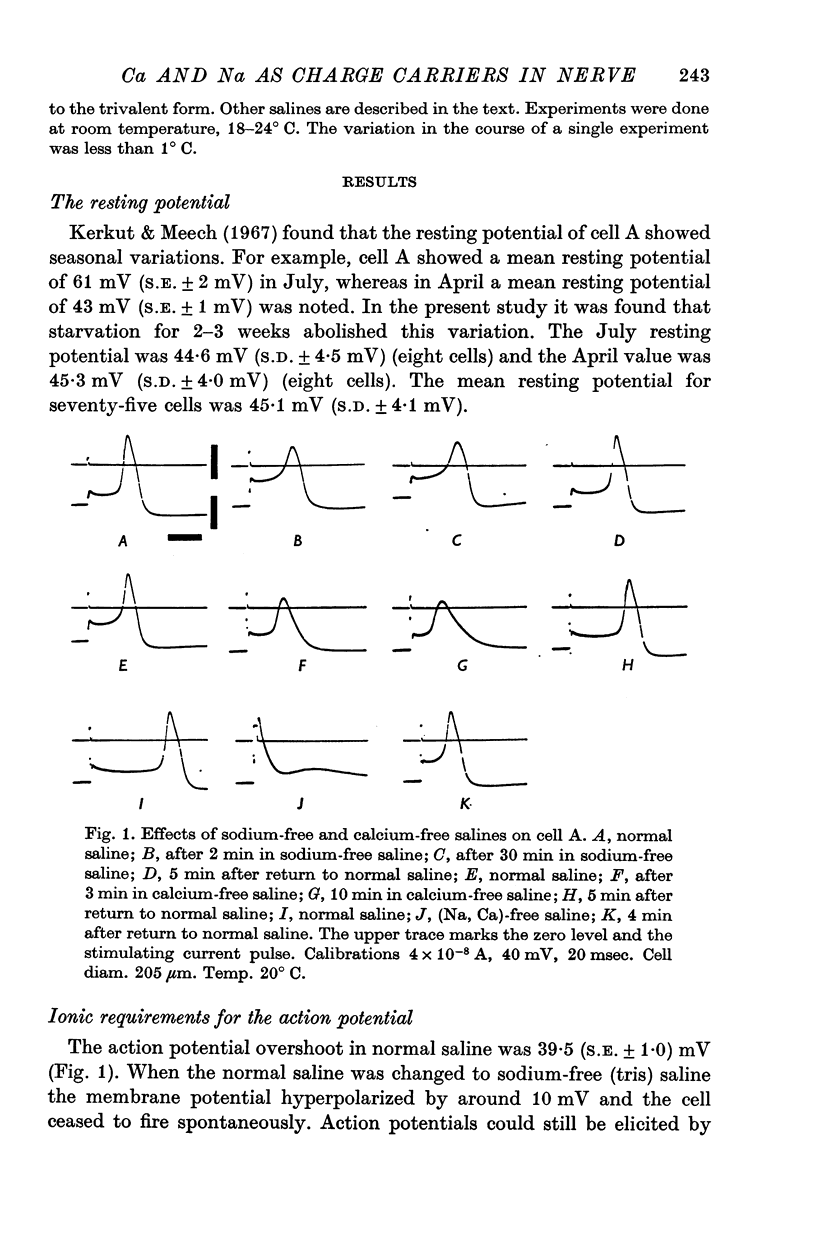
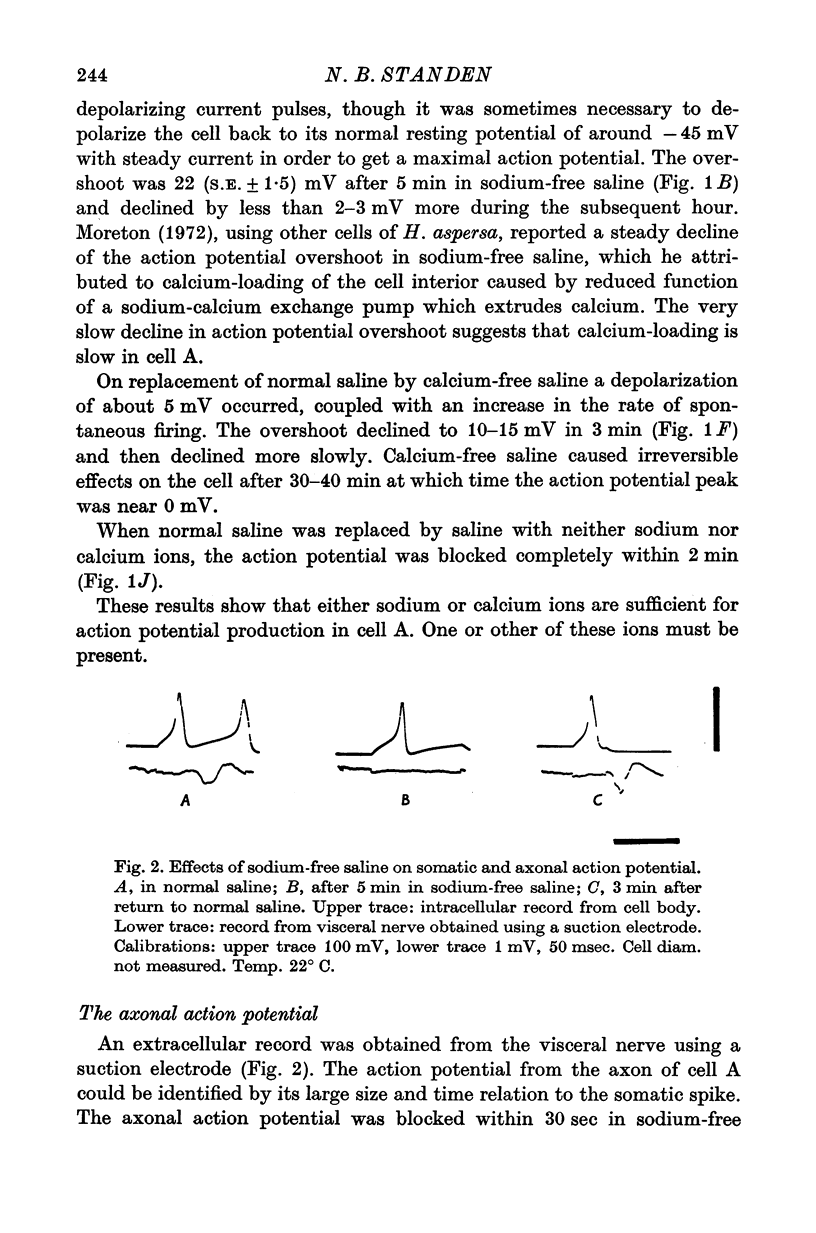
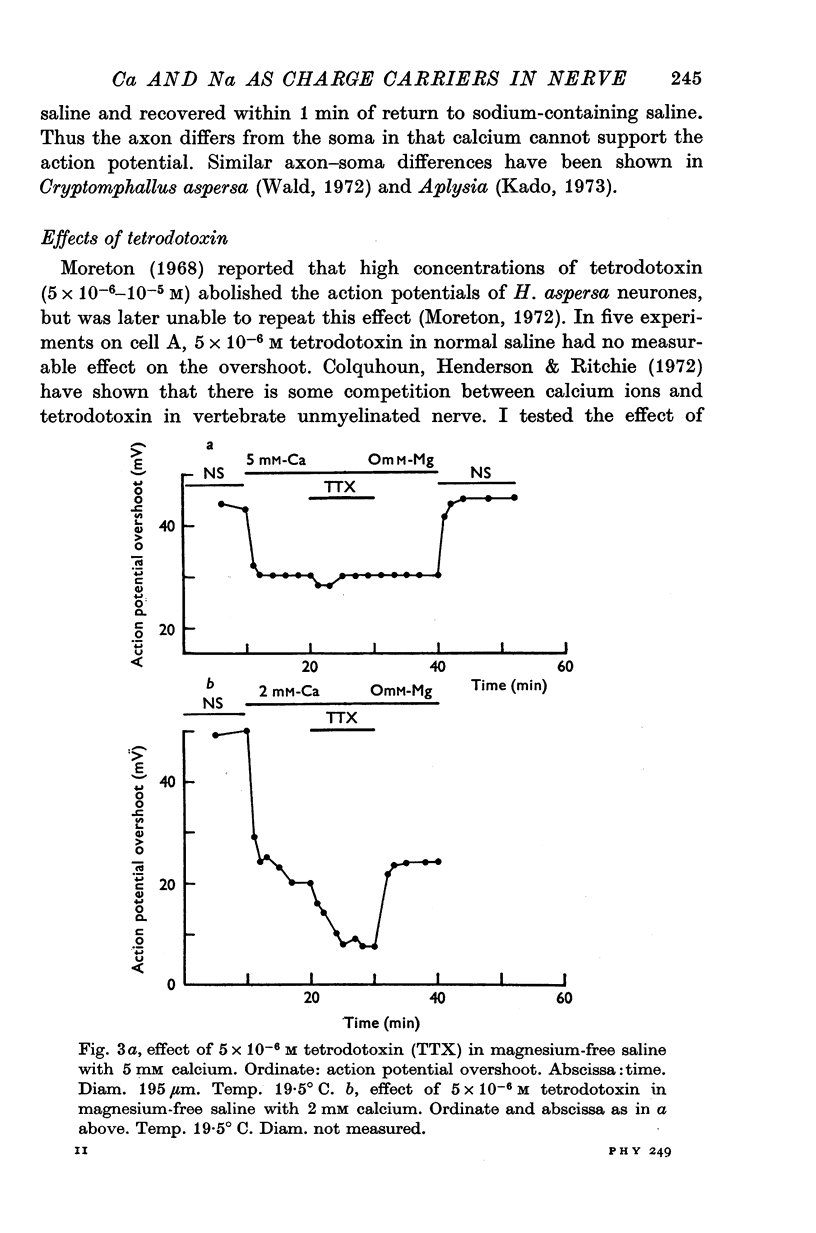
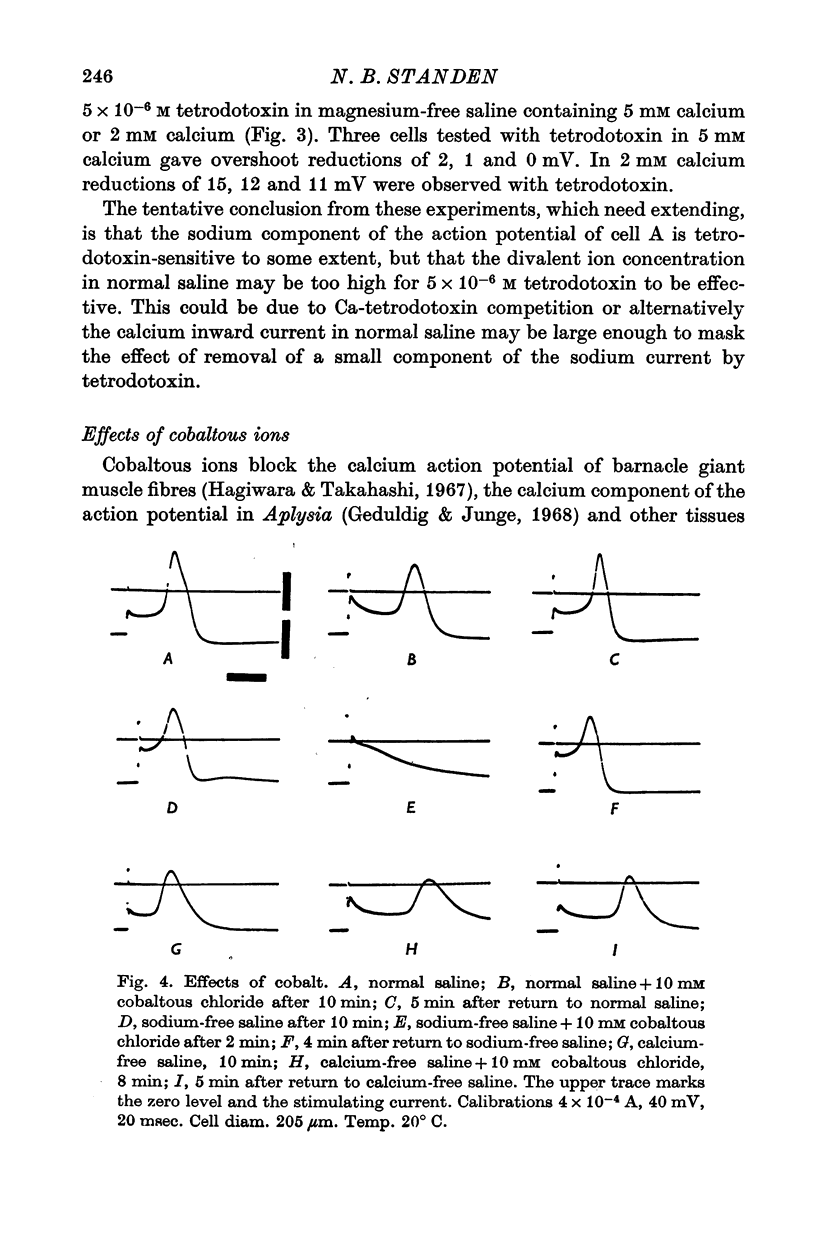
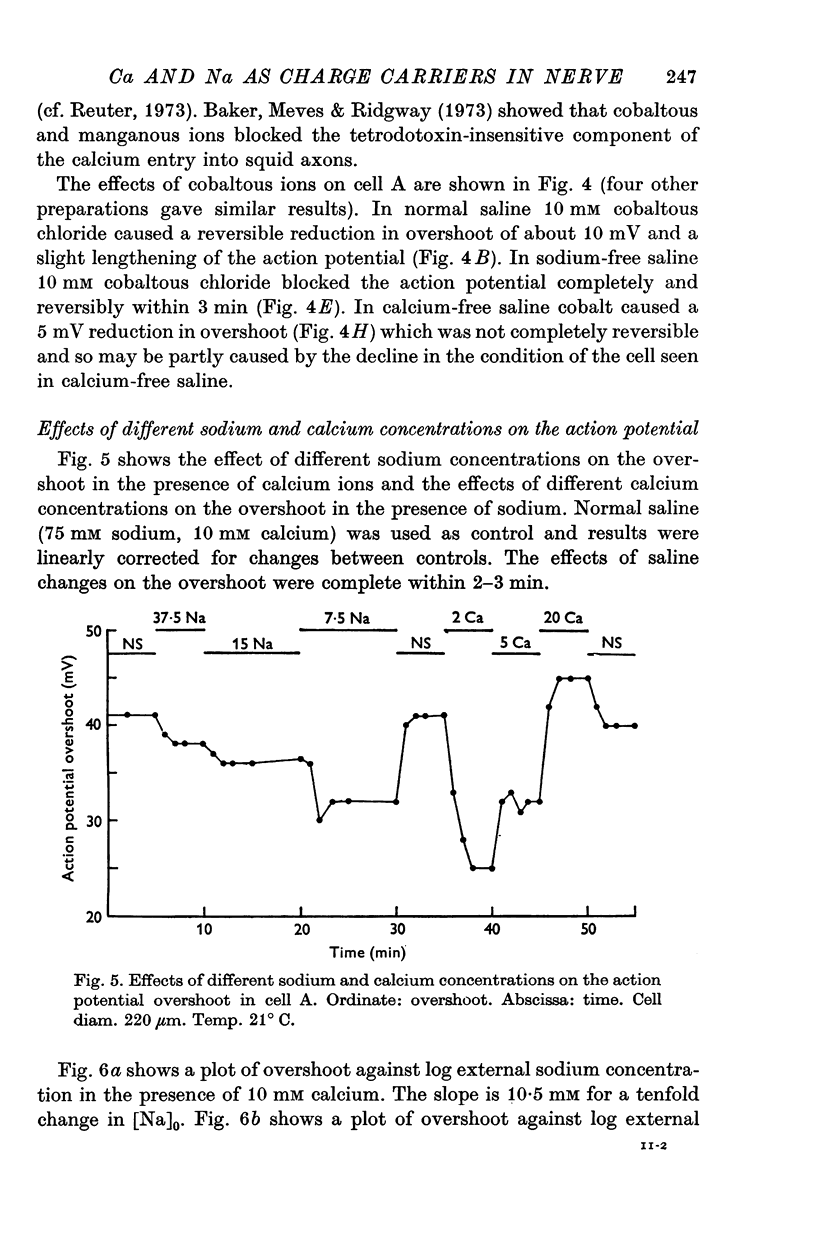
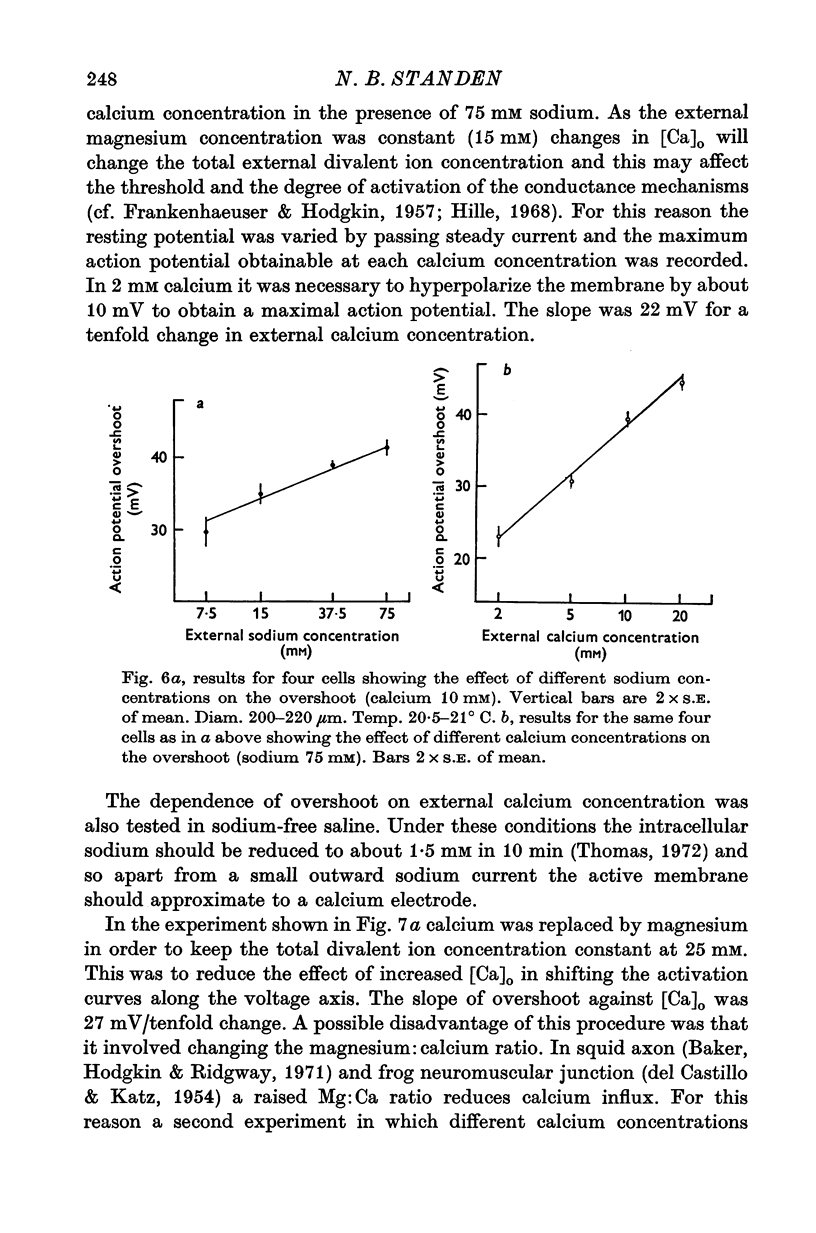
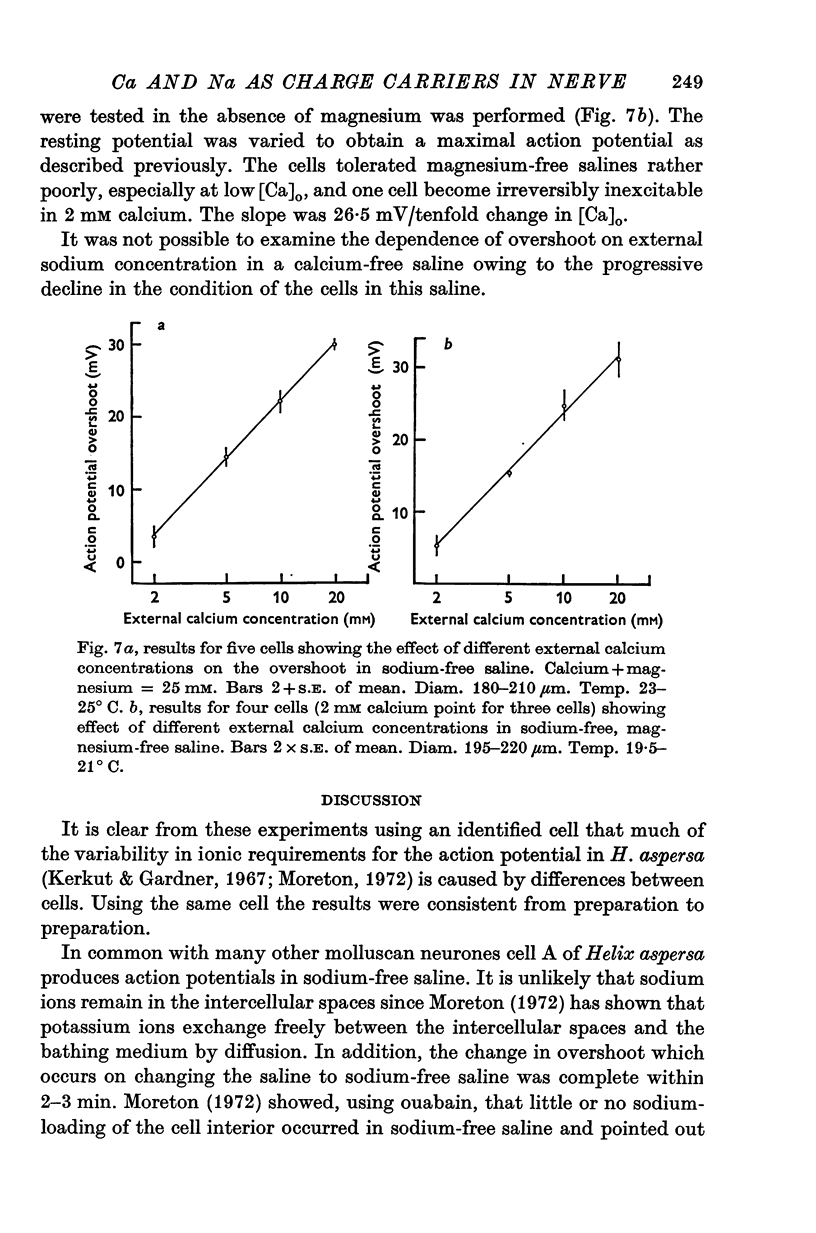
1. The soma of cell A in Helix aspersa produced action potentials in sodium-free or calcium-free saline, but not in saline with neither sodium nor calcium. 2. The axon had a sodium-dependent action potential. 3. Tetrodotoxin (5 x 10(-6) M) had no effect on the overshoot except at low external divalent ion concentrations. 4. The action potential in sodium-free saline was blocked by cobalt. 5. The slope of action potential overshoot against sodium concentration in the presence of 10 mM calcium was 10.5 mV/tenfold change. That of overshoot against calcium concentration in the presence of 75 mM sodium was 22 mV/tenfold change. 6. In sodium-free saline the slope of overshoot versus calcium concentration was 27 mV/tenfold change. 7. It is concluded that calcium is an important charge carrier in the action potential of cell A.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Gruener R. Voltage clamp of the Aplysia giant neurone: early sodium and calcium currents. J Physiol. 1970 Nov;211(1):217–244. doi: 10.1113/jphysiol.1970.sp009276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., CHICHIBU S., NAKA K. I. THE EFFECTS OF VARIOUS IONS ON RESTING AND SPIKE POTENTIALS OF BARNACLE MUSCLE FIBERS. J Gen Physiol. 1964 Sep;48:163–179. doi: 10.1085/jgp.48.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado R. T. Aplysia giant cell: soma-axon voltage clamp current differences. Science. 1973 Nov 23;182(4114):843–845. doi: 10.1126/science.182.4114.843. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Meech R. W. The effect of ions on the membrane potential of snail neurones. Comp Biochem Physiol. 1967 Feb;20(2):411–429. doi: 10.1016/0010-406x(67)90257-5. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. The ionic requirements for the production of action potentials in helix pomatia neurones. Pflugers Arch. 1968;304(3):215–241. doi: 10.1007/BF00592126. [DOI] [PubMed] [Google Scholar]
- Moreton R. B. Electrophysiology and ionic movements in the central nervous system of the snail, Helix aspersa. J Exp Biol. 1972 Oct;57(2):513–541. doi: 10.1242/jeb.57.2.513. [DOI] [PubMed] [Google Scholar]
- Moreton R. B. Ionic mechanism of the action potentials of giant neurones of Helix aspersa. Nature. 1968 Jul 6;219(5149):70–71. doi: 10.1038/219070a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Strom-Spannungsbeziehungen von Purkinje-Fasern bei verschiedenen extracellulären Calcium-Konzentrationen und unter Adrenalineinwirkung. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(4):357–367. [PubMed] [Google Scholar]
- Sattelle D. B. Electrophysiology of the giant nerve cell bodies of Limnaea stagnalis (L.) (Gastropoda: Pulmonata). J Exp Biol. 1974 Jun;60(3):653–671. doi: 10.1242/jeb.60.3.653. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald F. Ionic differences between somatic and axonal action potentials in snail giant neurones. J Physiol. 1972 Jan;220(2):267–281. doi: 10.1113/jphysiol.1972.sp009706. [DOI] [PMC free article] [PubMed] [Google Scholar]


