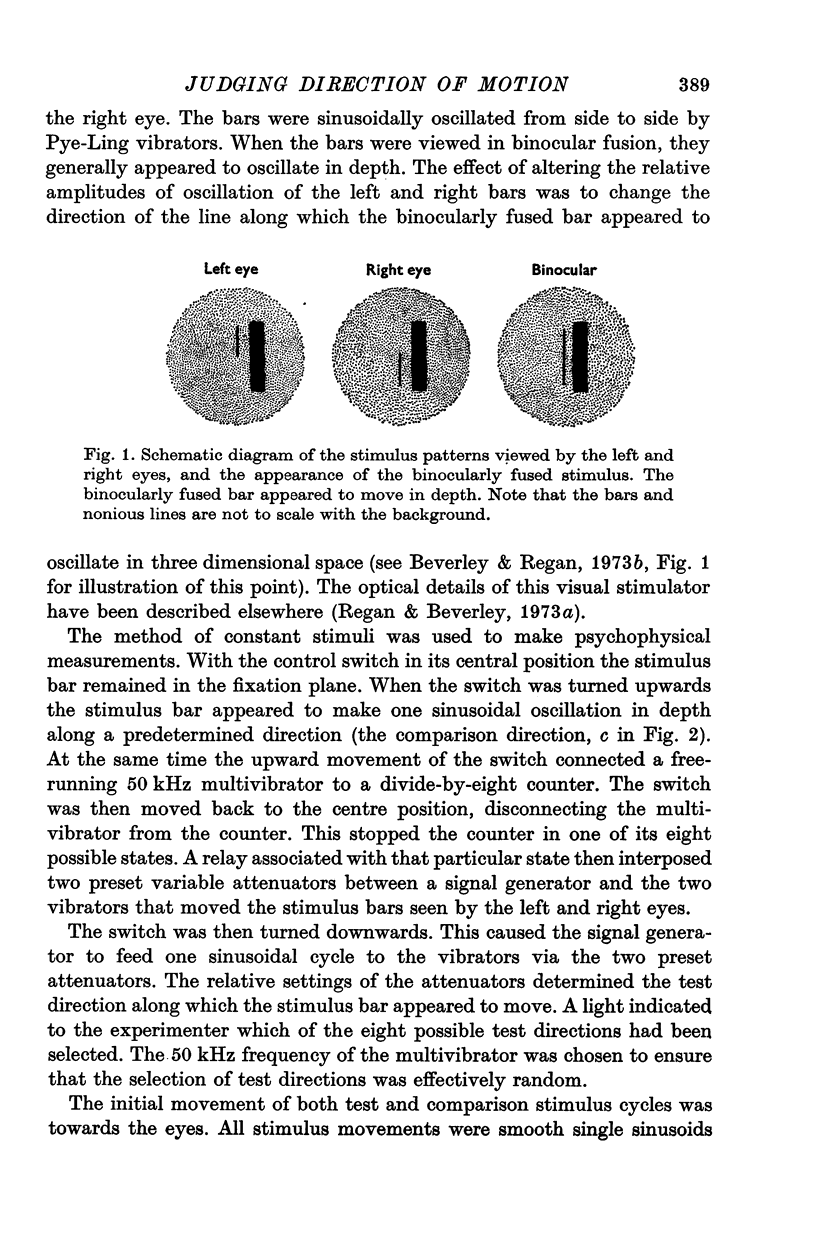
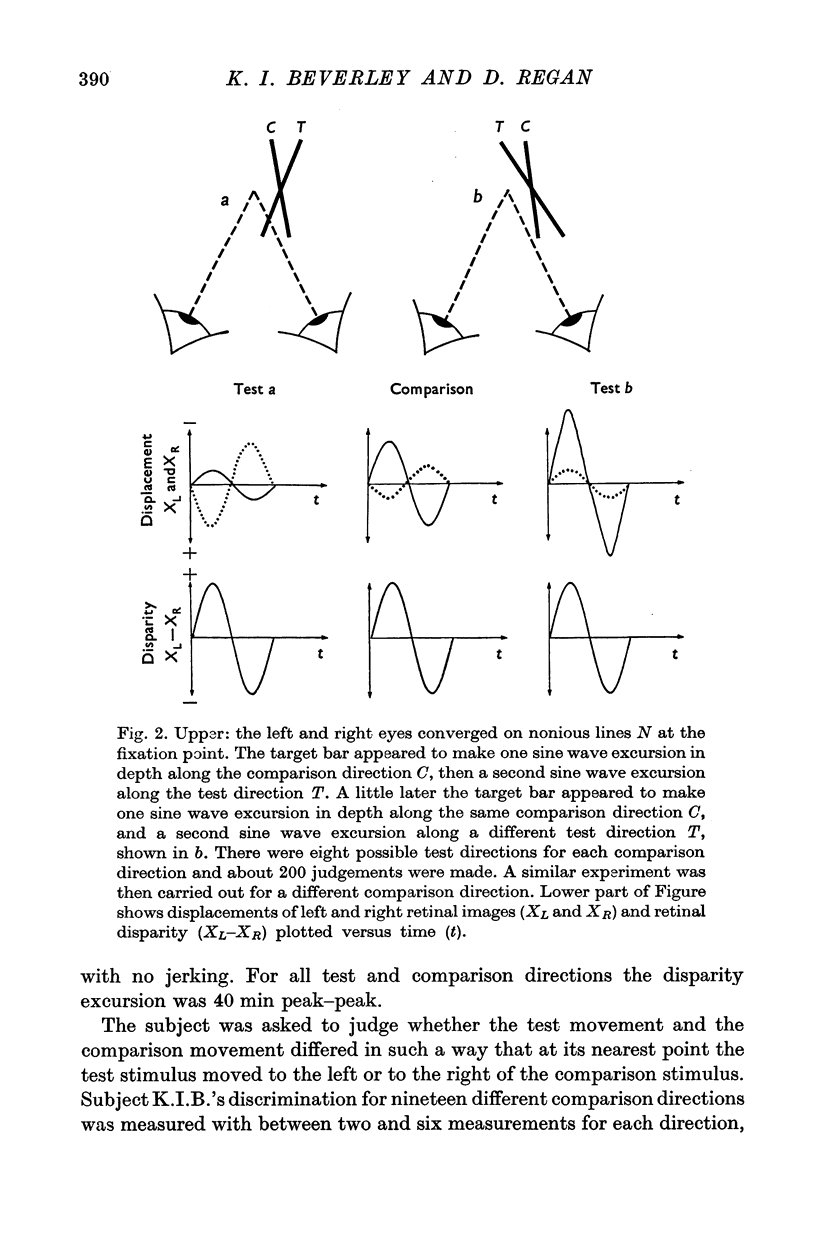
Abstract
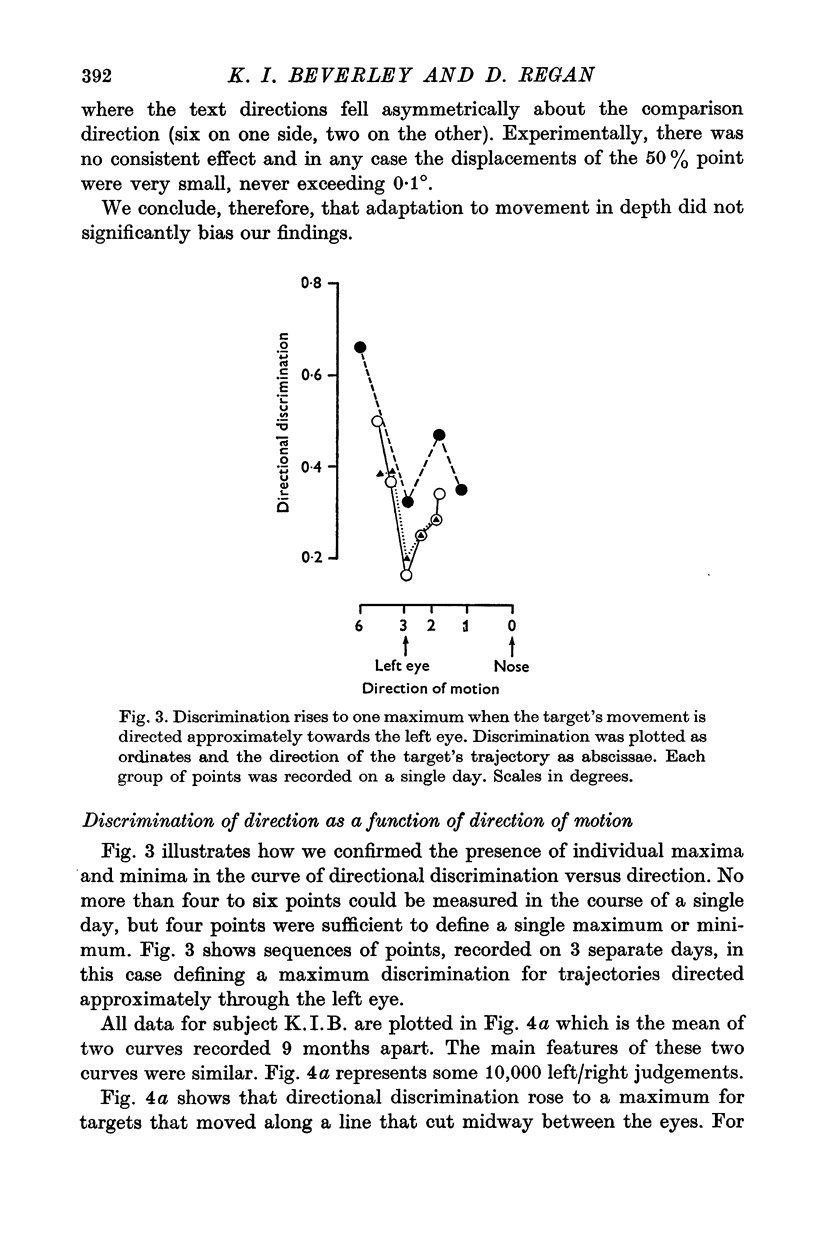
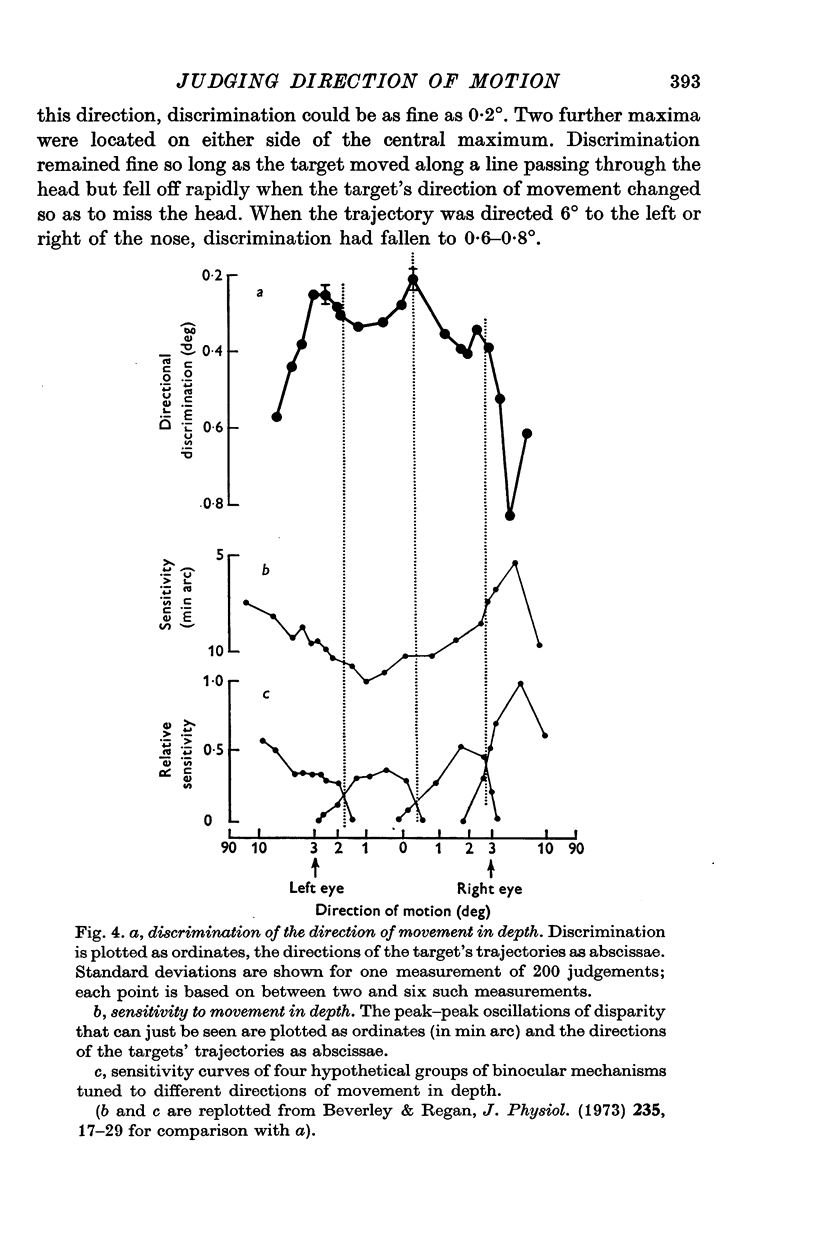
1. Binocular discrimination of the direction of a target's motion in depth was measured in terms of the smallest angular difference in direction that could be detected with a probability 50% better than chance. Directional discrimination was measured for targets moving along 16 different trajectories directed to the left and right of the nose. 2. The relative velocities of the retinal images in the left and right eyes gave a sensitive cue to the direction of the target's motion in depth. 3. The direction of motion was bets discriminated when the target moved along a line directed close to the nose. A change in direction of only 0.2 degrees from this direction of motion could be detected. Discrimination showed two other maxima, one on each side of the central maximum. Discrimination fell to about 0.6-0.8 degrees when the target's direction was changed by only 6 degrees to either side of the nose. 4. The curve of sensitivity to movement in depth had a generally inverse shape to the directional discrimination curve: sensitivity was minimal for trajectories directed near the nose and increased for trajectories directed so as to miss the head. 5. The directional discrimination curve can be related to the sensitivity curves of the four postulated neural mechanisms tuned to different directions of motion in depth; there are three discrimination maxima and, correspondingly, three trajectories for which the slopes of adjacent sensitivity curves differ maximally. This suggests that binocular psychophysical judgements of the direction along which a target moves in depth are to some extent mediated by neural mechanisms that compare (e.g. subtract) the outputs of directionally tuned movement detectors. One function of such neural comparators might be to enhance psychophysical sensitivity to the direction along which a target moves in depth, and thus to provide a physiological basis for precisely judging whether or not an object will hit the head. 6. We suggest that the neural basis for judging the direction of moving objects has an analogy in colour vision where opponent-colour mechanisms enhance sensitivity to wave-length differences in such a way that wave-lengths are more easily discriminated in those parts of the spectrum where the slopes of the pigment action spectra differ maximally.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley K. I., Regan D. Evidence for the existence of neural mechanisms selectively sensitive to the direction of movement in space. J Physiol. 1973 Nov;235(1):17–29. doi: 10.1113/jphysiol.1973.sp010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley K. I., Regan D. Temporal integration of disparity information in stereoscopic perception. Exp Brain Res. 1974 Jan 31;19(2):228–232. doi: 10.1007/BF00238537. [DOI] [PubMed] [Google Scholar]
- Beverley K. I., Regan D. Visual sensitivity to disparity pulses: evidence for directional selectivity. Vision Res. 1974 May;14(5):357–361. doi: 10.1016/0042-6989(74)90095-9. [DOI] [PubMed] [Google Scholar]
- Bragg V. C., Collins F. G. Audiometer modification and pulse-tone technique for pure-tone threshold determination. Aerosp Med. 1969 Jan;40(1):9–11. [PubMed] [Google Scholar]
- HURVICH L. M., JAMESON D. Some quantitative aspects of an opponent-colors theory. II. Brightness, saturation, and hue in normal and dichromatic vision. J Opt Soc Am. 1955 Aug;45(8):602–616. doi: 10.1364/josa.45.000602. [DOI] [PubMed] [Google Scholar]
- Lewis C. E., Jr, Blakeley W. R., Swaroop R., Masters R. L., McMurty T. C. Landing performance by low-time private pilots after the sudden loss of binocular vision--cyclops II. Aerosp Med. 1973 Nov;44(11):1241–1245. [PubMed] [Google Scholar]
- Regan D., Beverley K. I. Disparity detectors in human depth perception: evidence for directional selectivity. Science. 1973 Aug 31;181(4102):877–879. doi: 10.1126/science.181.4102.877. [DOI] [PubMed] [Google Scholar]
- Regan D., Beverley K. I. Some dynamic features of depth perception. Vision Res. 1973 Dec;13(12):2369–2379. doi: 10.1016/0042-6989(73)90236-8. [DOI] [PubMed] [Google Scholar]
- Regan D., Beverley K. I. The dissociation of sideways movements from movements in depth: psychophysics. Vision Res. 1973 Dec;13(12):2403–2415. doi: 10.1016/0042-6989(73)90238-1. [DOI] [PubMed] [Google Scholar]
- Vos J. J., Walraven P. L. An analytical description of the line element in the zone-fluctuation model of colour vision. II. The derivation of the line element. Vision Res. 1972 Aug;12(8):1345–1365. doi: 10.1016/0042-6989(72)90182-4. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Cells responding to changing image size and disparity in the cortex of the rhesus monkey. J Physiol. 1974 Nov;242(3):827–841. doi: 10.1113/jphysiol.1974.sp010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S. M. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol. 1974 Feb;236(3):549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]


