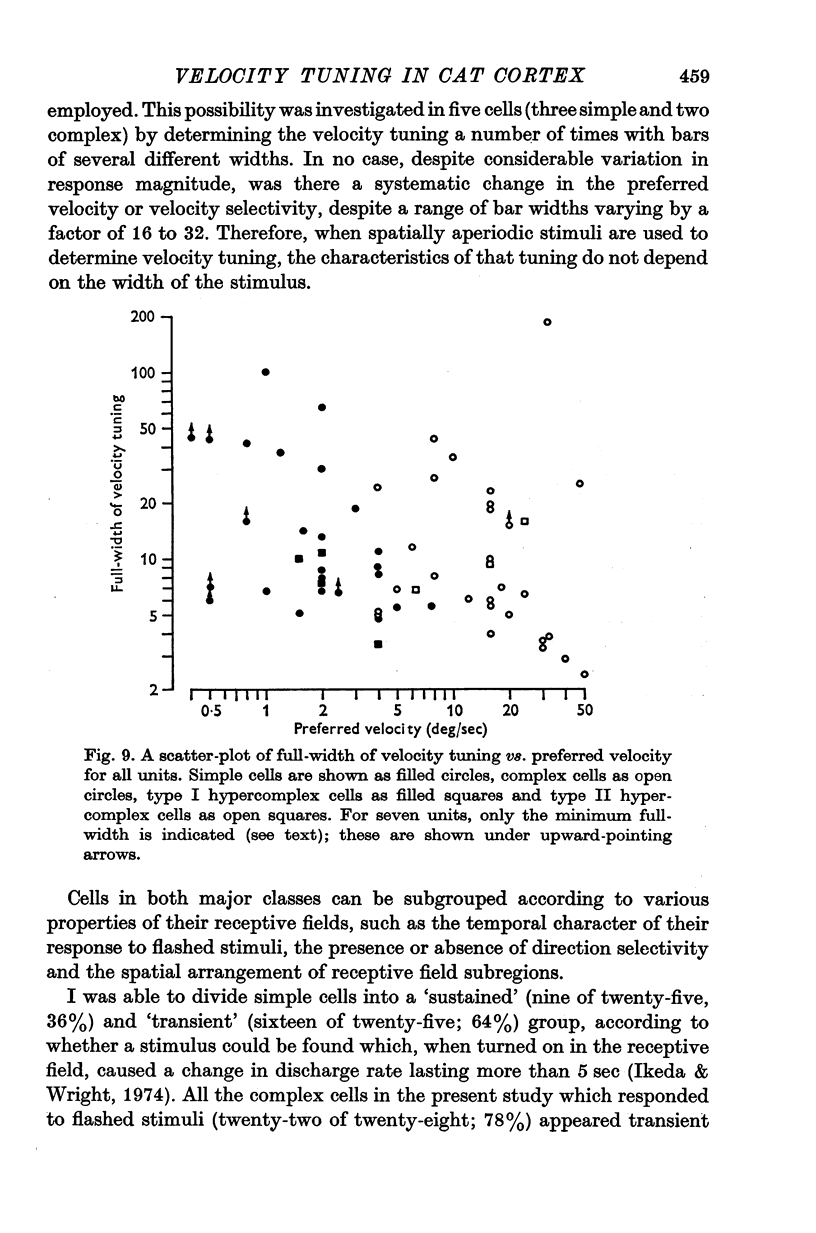
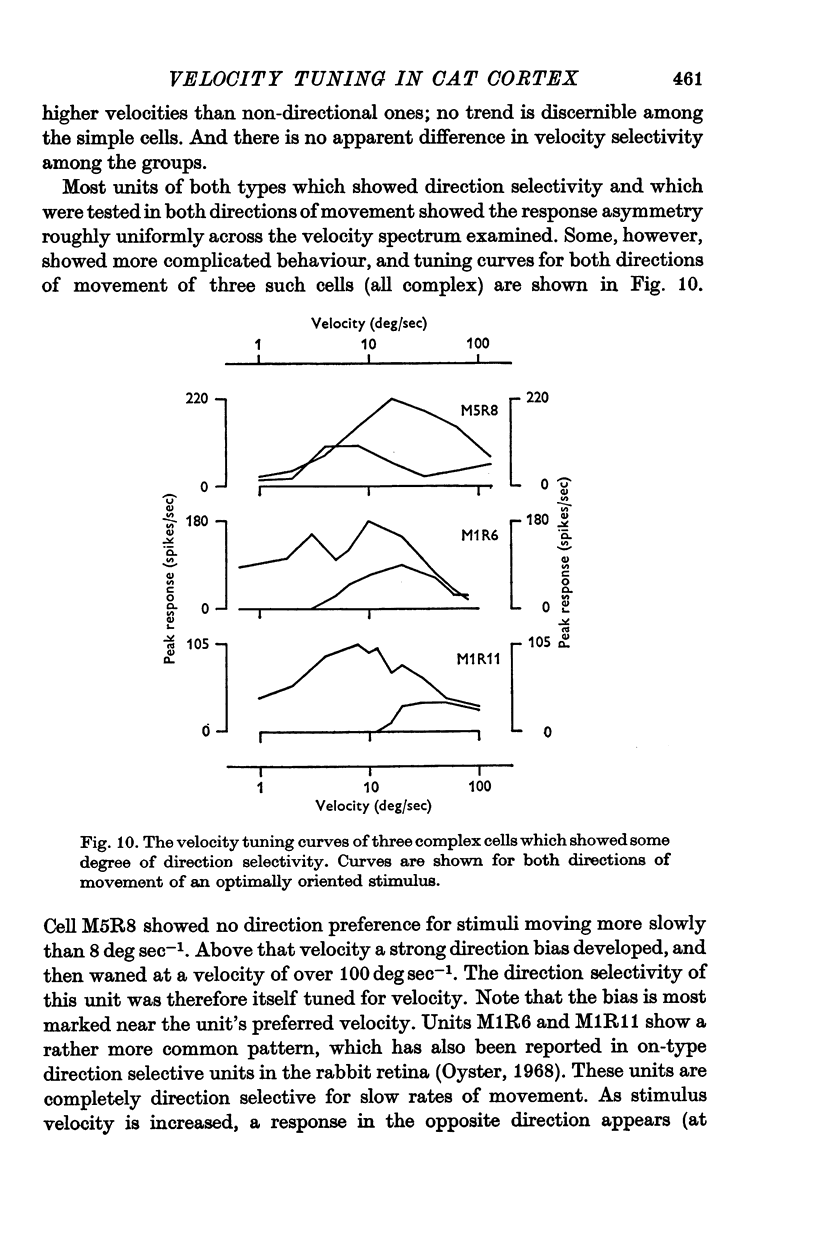
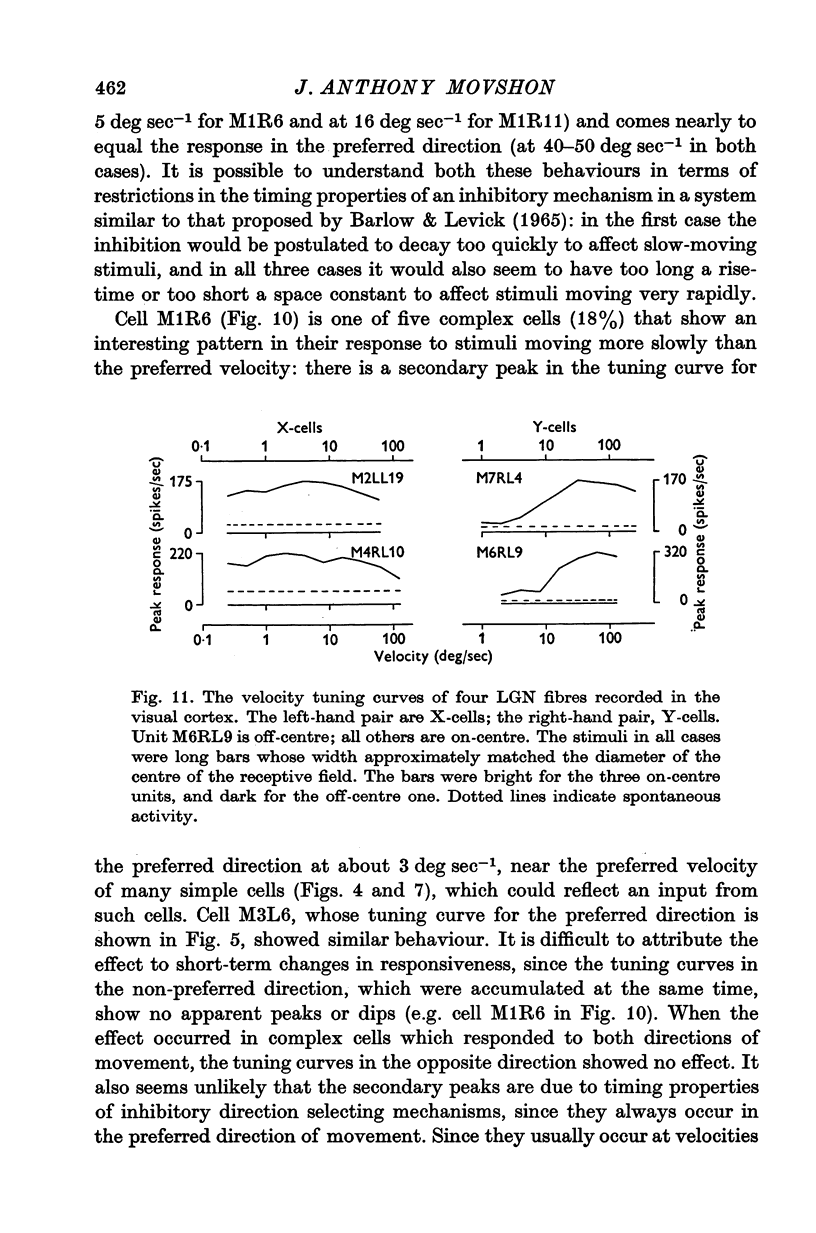
Abstract
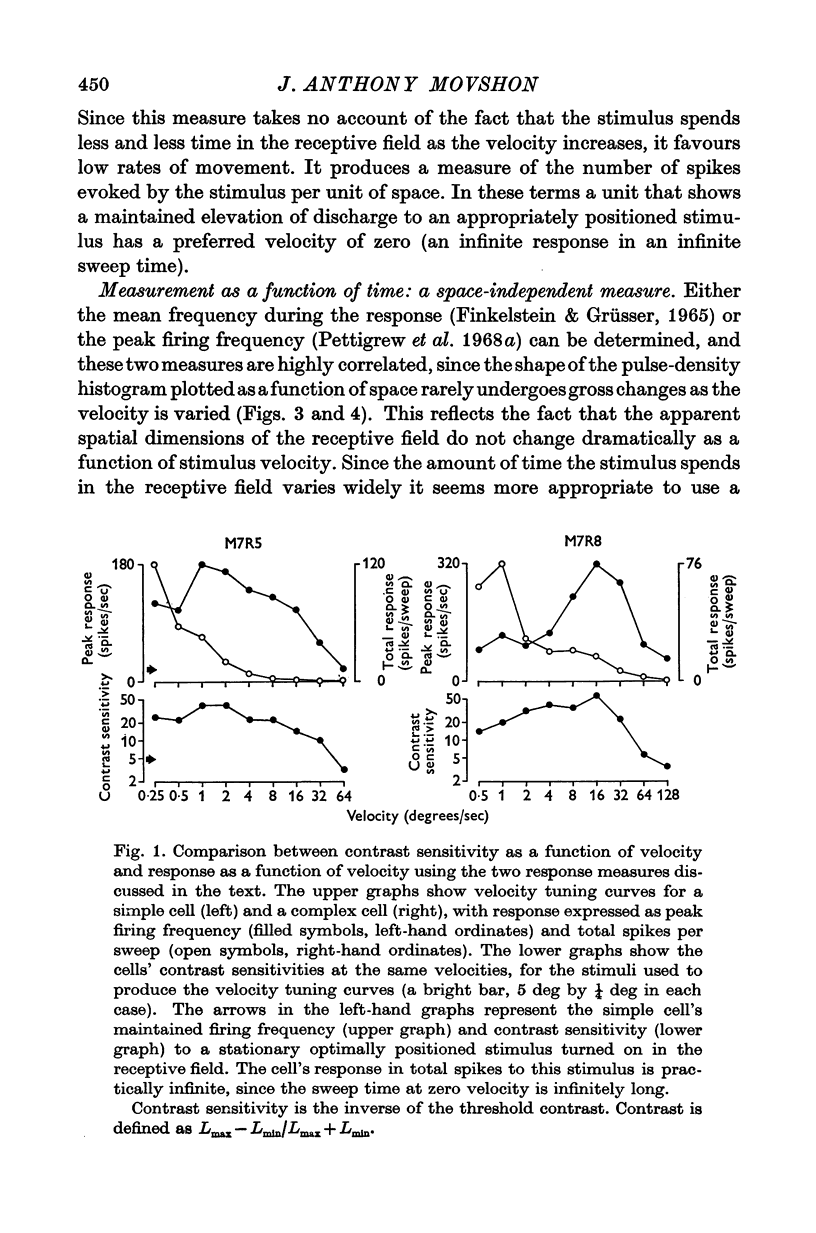
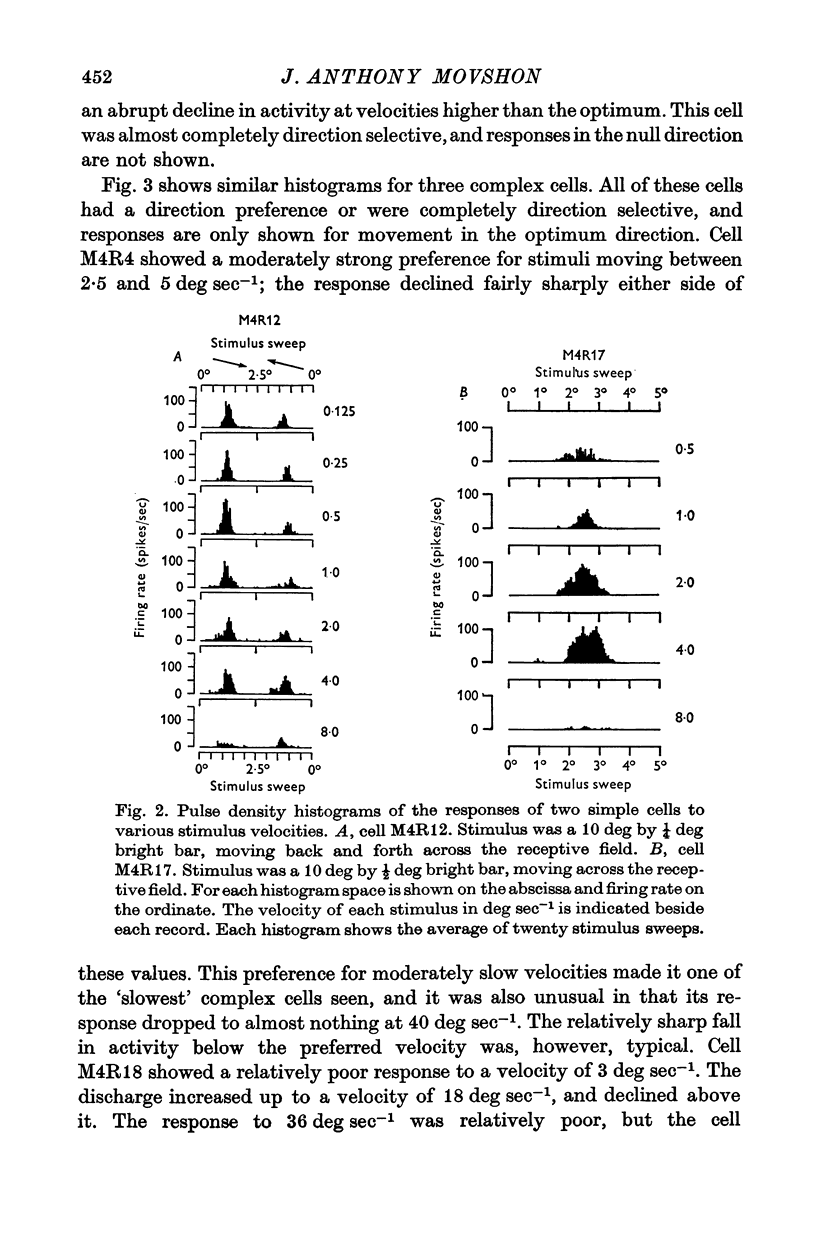
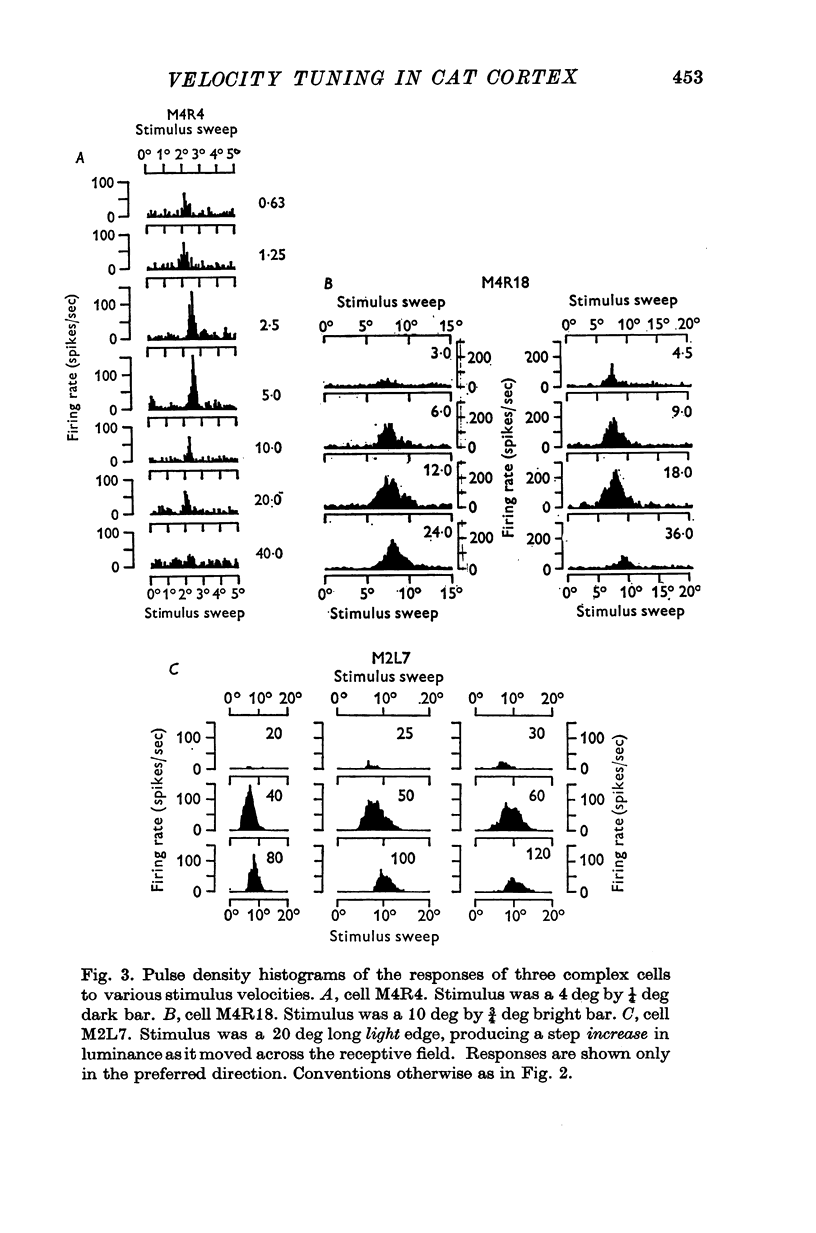
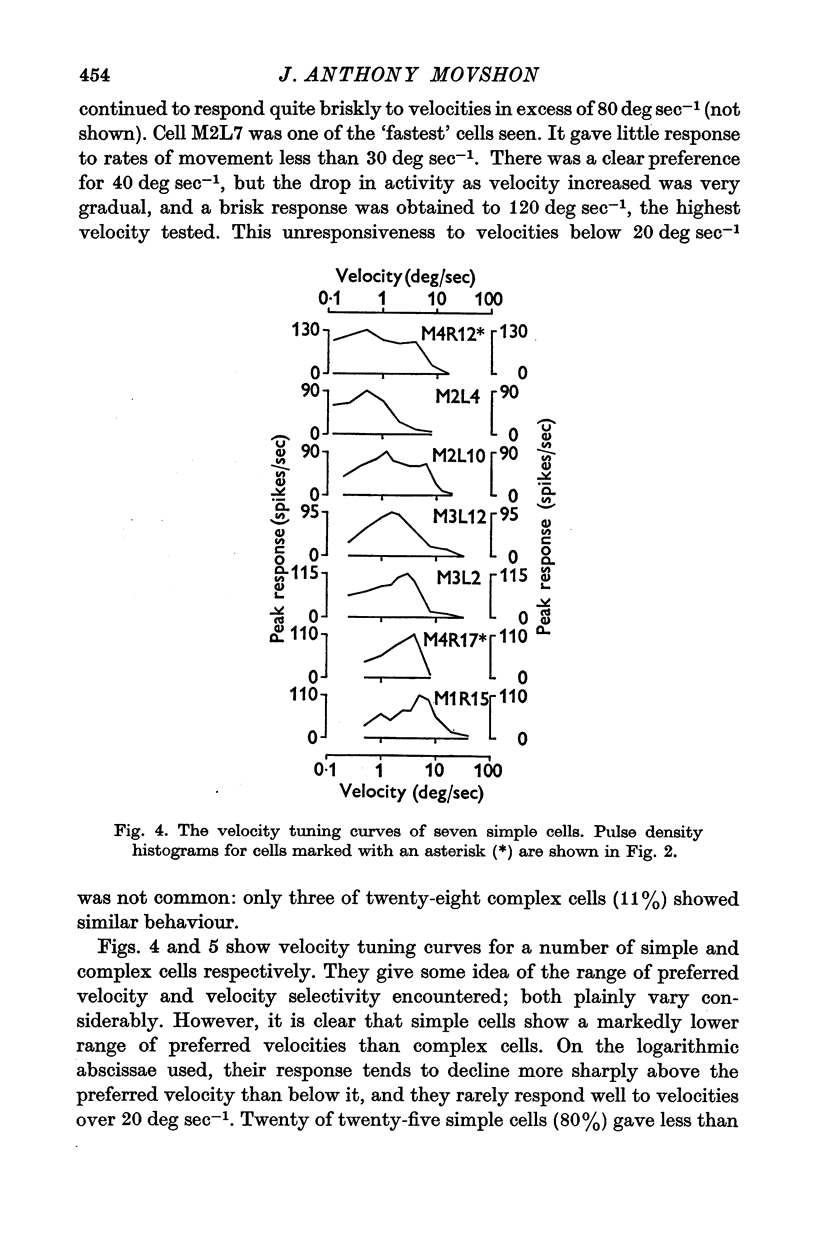
1. The activity of single units was recorded from the striate cortex (area 17) of anaesthetized, paralysed cats. Responses to stimuli moving at different velocities were examined. 2. Peak evoked firing frequency, rather than fotal evoked spikes, is used throughout as a measure of response. The former mea-ure gives curves of response vs. velocity that correlate well with curves of contrast sensitivity vs. velocity, wheras the latter does not. 3. Cortical receptive fields were classified according to the criteria of Hubel & Wiesel. Simple cells were found to prefer lower velocities (mean 2-2 deg sec-1) than complex cells( mean 18-8 deg sec-1). The response of simple cells to stimuli moving faster than 20 deg sec-1 is generally poor; complex cells usually discharge briskly to these speeds. 4. Cells classified as hypercomplex by the end-inhibition criterion were further chara-terized as type I or type II, according to the suggestion of Dreher (1972). Type I units are indistinguishable from simple cells in their velocity tuning, and type II units equally clearly resemble complex cells. These results are therefor consistent with Dreher's sbudivision. 5. Teh selectivity of cells for velocity is variable but can be quite marked. The average selectivities of simple and complex cells are not significantly different. There is an inverse correlation between preferred velocity and the sharpness of velocity selectivity for simple cells; no trend is apparent for other cell types. 6. No clear correlation is observed between the velocity preferances of units and their degree of direction selectivity, or receptive field arrangement. Simple cells with 'sustainef' temporal responses to flashed stimuli tend to prefer slower rates of movement than 'transient' ones, and to be less selective for velocity. 7. The results for different cortical cell-types are compared with the velocity tuning of X- and Y-cells in the lateral geniculate nucleus.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Tobin E. A. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15(4):439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Cleland B. G., Cooper G. F., Enroth-Cugell C. The angular selectivity of visual cortical cells to moving gratings. J Physiol. 1968 Sep;198(1):237–250. doi: 10.1113/jphysiol.1968.sp008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Cooper G. F., Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969 Jul;203(1):223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Optical and retinal factors affecting visual resolution. J Physiol. 1965 Dec;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Kuhnt U., Benevento L. A. An intracellular analysis of visual cortical neurones to moving stimuli: response in a co-operative neuronal network. Exp Brain Res. 1974;21(3):251–274. doi: 10.1007/BF00235746. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O., Ito M. Functional synaptic organization of primary visual cortex neurones in the cat. Exp Brain Res. 1968;6(4):324–352. doi: 10.1007/BF00233183. [DOI] [PubMed] [Google Scholar]
- Dreher B. Hypercomplex cells in the cat's striate cortex. Invest Ophthalmol. 1972 May;11(5):355–356. [PubMed] [Google Scholar]
- Dreher B., Sanderson K. J. Receptive field analysis: responses to moving visual contours by single lateral geniculate neurones in the cat. J Physiol. 1973 Oct;234(1):95–118. doi: 10.1113/jphysiol.1973.sp010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein D., Grüsser O. J. Frog retina: detection of movement. Science. 1965 Nov 19;150(3699):1050–1051. doi: 10.1126/science.150.3699.1050. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J., Sherman S. M. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972 Jul;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Evidence for "sustained" and "transient" neurones in the cat's visual cortex. Vision Res. 1974 Jan;14(1):133–136. doi: 10.1016/0042-6989(74)90128-x. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M., Fiore L. Post-synaptic inhibitory components of the responses to moving stimuli in area 17. Brain Res. 1974 Nov 8;80(1):122–126. doi: 10.1016/0006-8993(74)90728-8. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Van Essen D. C. Cell structure and function in the visual cortex of the cat. J Physiol. 1974 May;238(3):515–547. doi: 10.1113/jphysiol.1974.sp010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A. Proceedings: Velocity preferences of simple and complex cells in the cat's striate cortex. J Physiol. 1974 Oct;242(2):121P–123P. [PubMed] [Google Scholar]
- Movshon J. A., Tolhurst D. J. Proceedings: On the response linearity of neurones in cat visual cortex. J Physiol. 1975 Jul;249(1):56P–57P. [PubMed] [Google Scholar]
- Oyster C. W. The analysis of image motion by the rabbit retina. J Physiol. 1968 Dec;199(3):613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Binocular interaction on single units in cat striate cortex: simultaneous stimulation by single moving slit with receptive fields in correspondence. Exp Brain Res. 1968;6(4):391–410. doi: 10.1007/BF00233186. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Rose D., Blakemore C. An analysis of orientation selectivity in the cat's visual cortex. Exp Brain Res. 1974 Apr 30;20(1):1–17. doi: 10.1007/BF00239014. [DOI] [PubMed] [Google Scholar]
- Rose D. Proceedings: The hypercomplex cell classification in the cat's striate cortex. J Physiol. 1974 Oct;242(2):123P–125P. [PubMed] [Google Scholar]
- Singer W., Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res. 1973 Jan 30;49(2):291–307. doi: 10.1016/0006-8993(73)90424-1. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Singer W., Pöppel E., Creutzfeldt O. Inhibitory interaction in the cat's lateral geniculate nucleus. Exp Brain Res. 1972;14(2):210–226. doi: 10.1007/BF00234800. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Stone J. Morphology and physiology of the geniculocortical synapse in the cat: the question of parallel input to the striate cortex. Invest Ophthalmol. 1972 May;11(5):338–346. [PubMed] [Google Scholar]
- Tolhurst D. J., Sharpe C. R., Hart G. The analysis of the drift rate of moving sinusoidal gratings. Vision Res. 1973 Dec;13(12):2545–2555. doi: 10.1016/0042-6989(73)90251-4. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Konishi M., Creutzfeldt O. D. Postsynaptic potentials in the cat's visual cortex following electrical stimulation of afferent pathways. Exp Brain Res. 1966;1(3):272–283. doi: 10.1007/BF00234347. [DOI] [PubMed] [Google Scholar]


