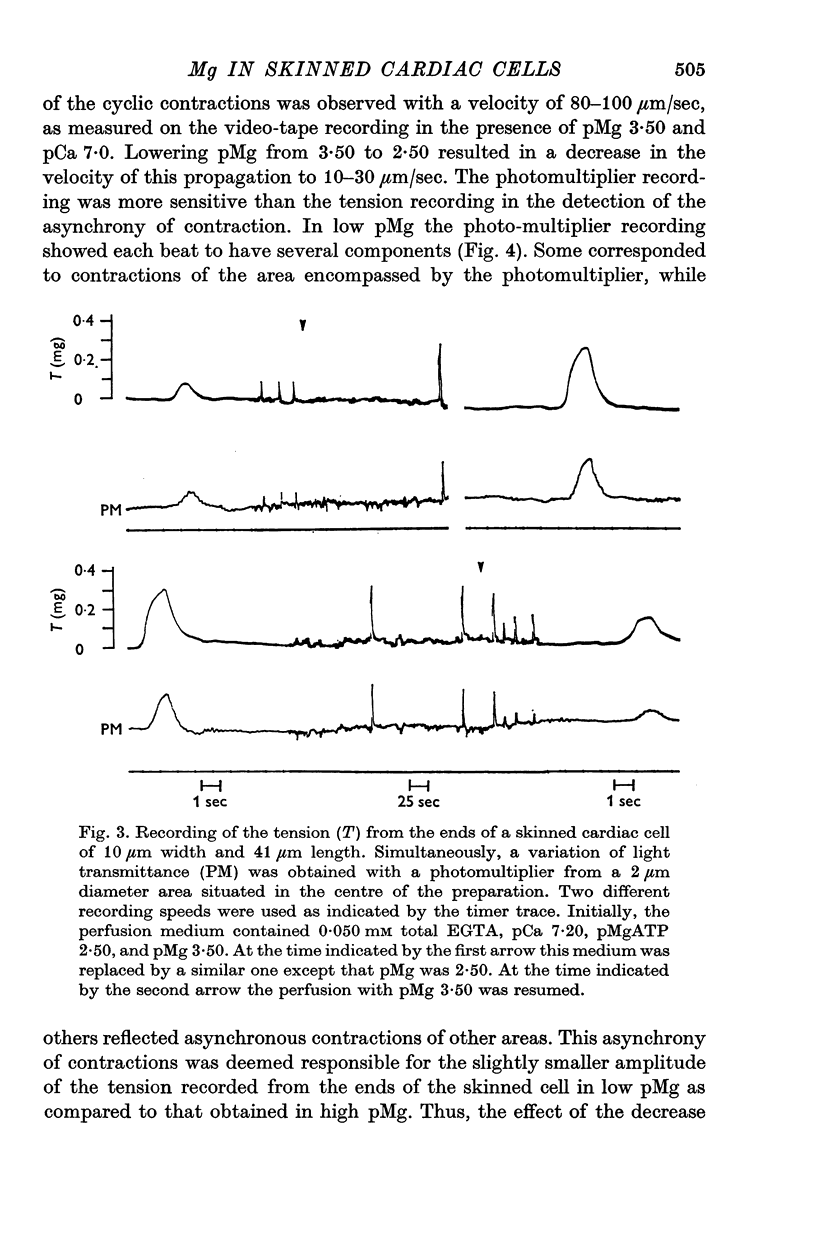
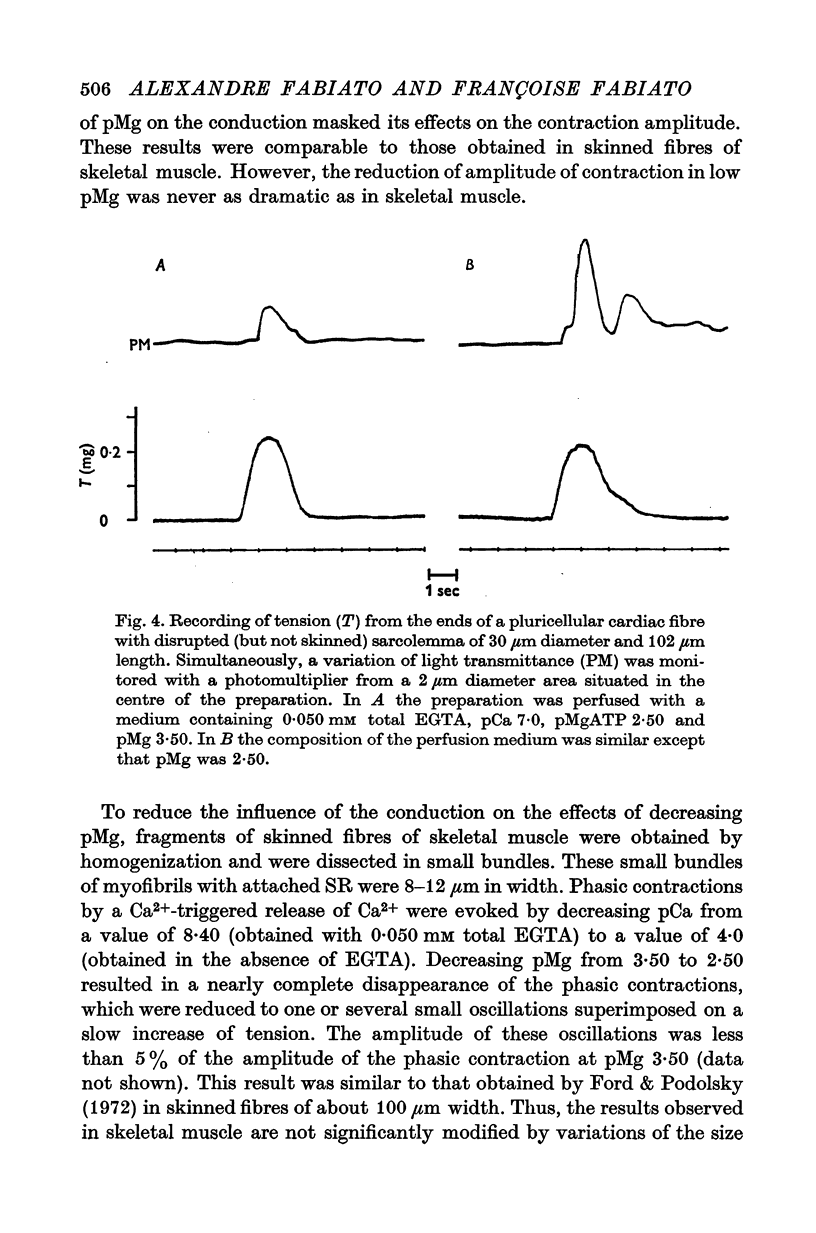
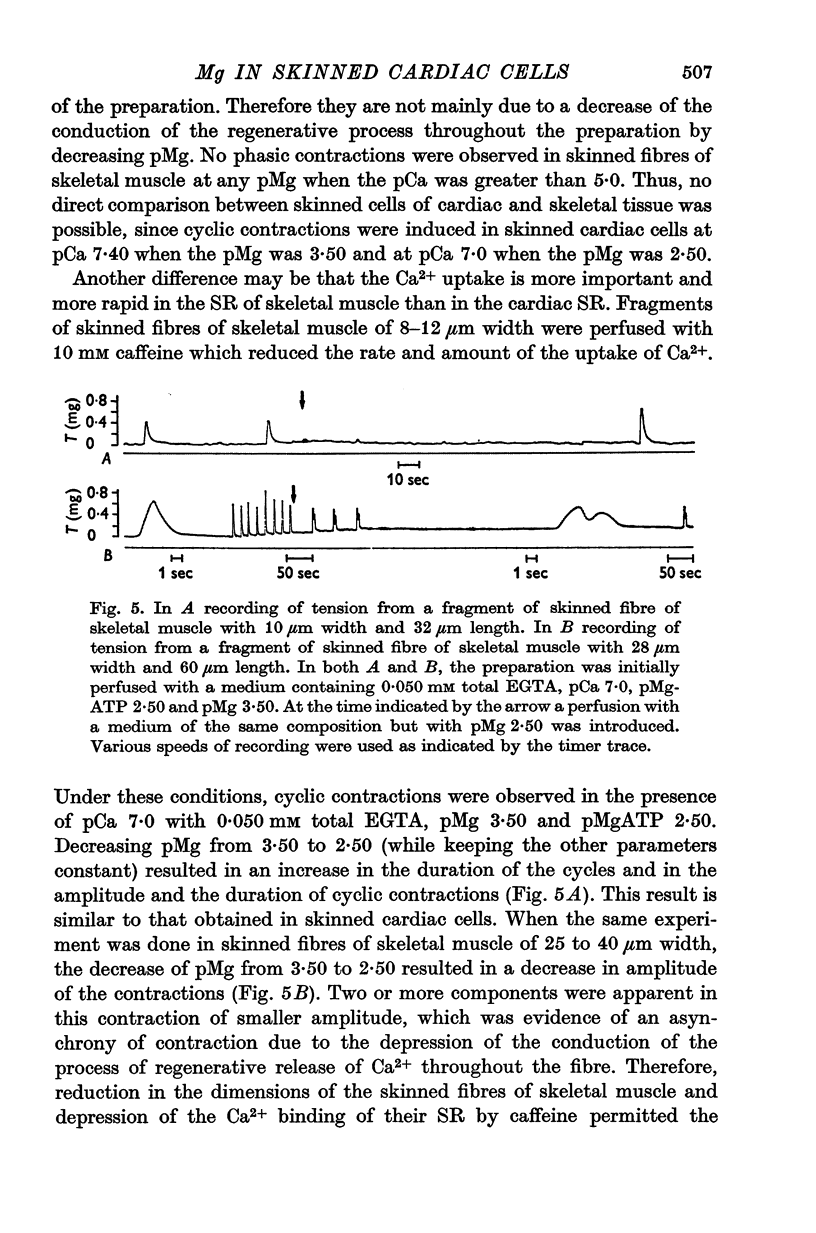
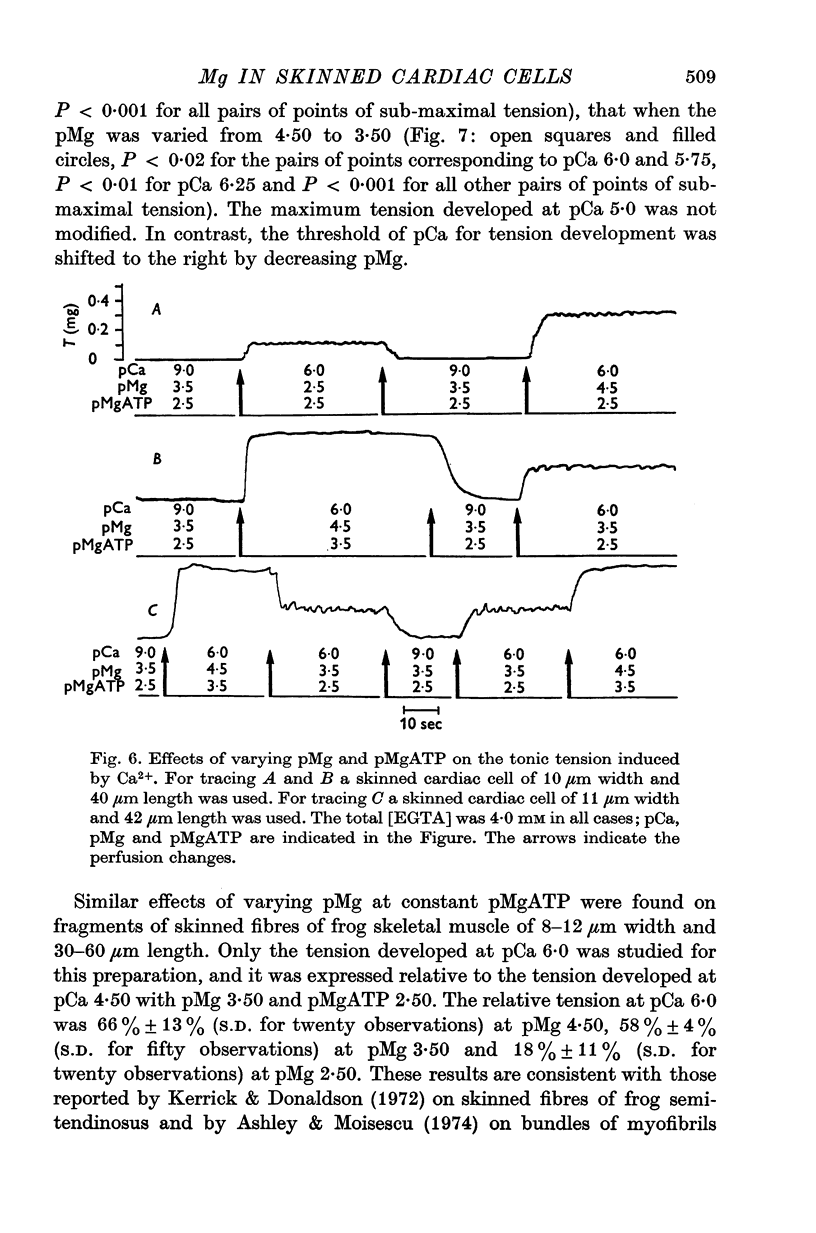
Abstract
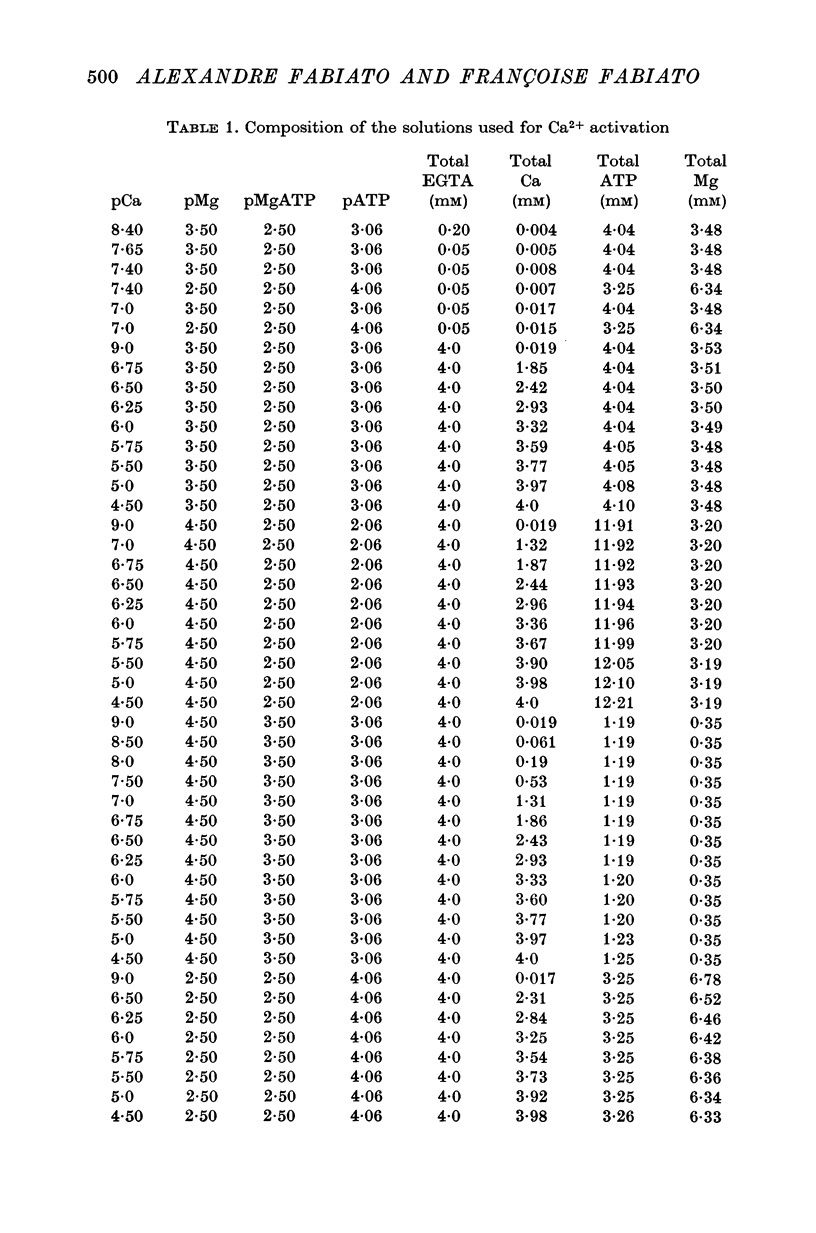
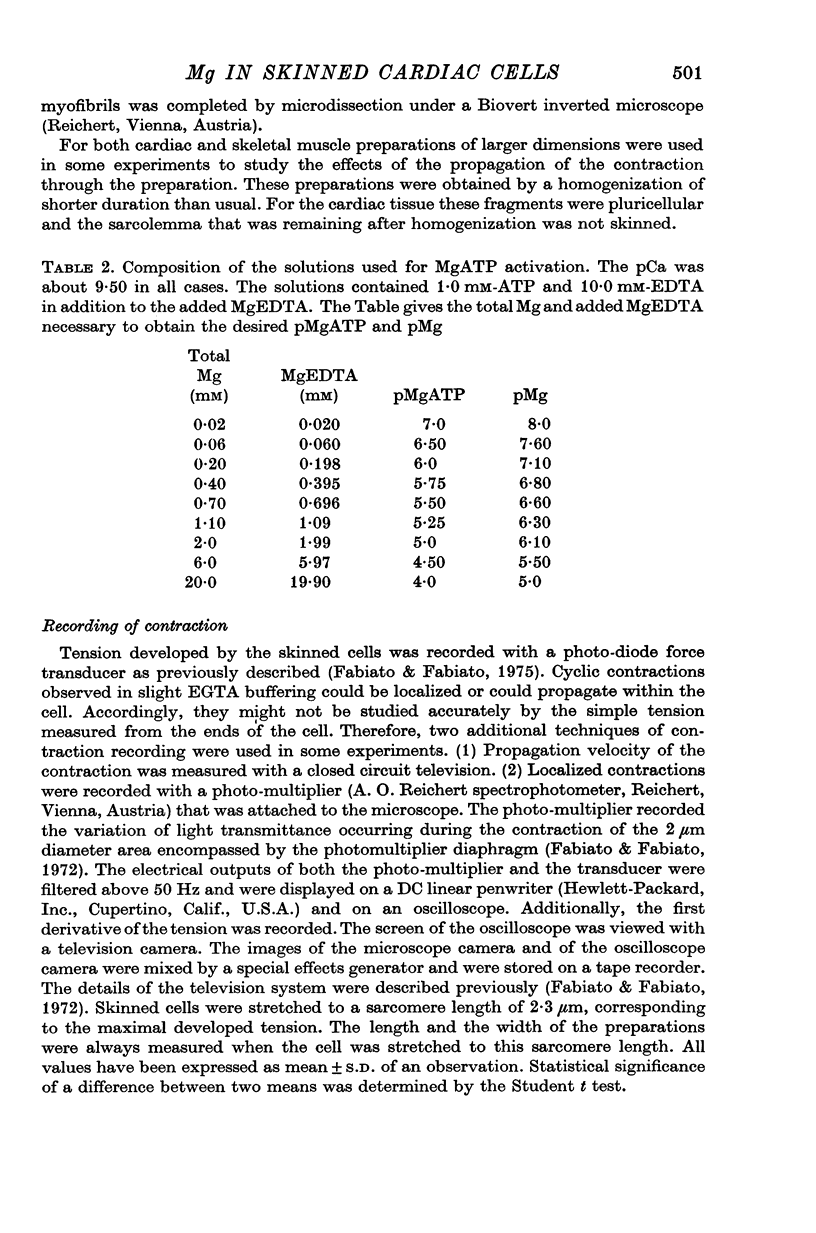
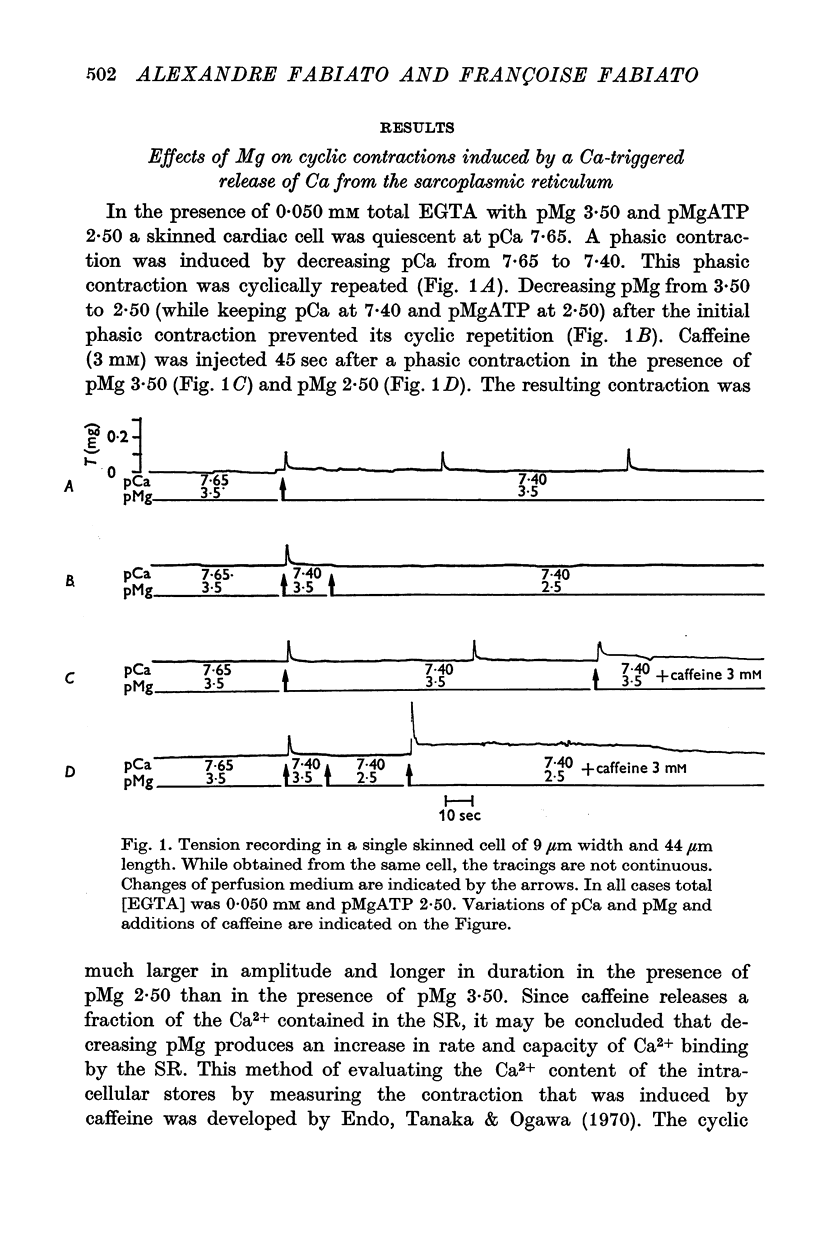
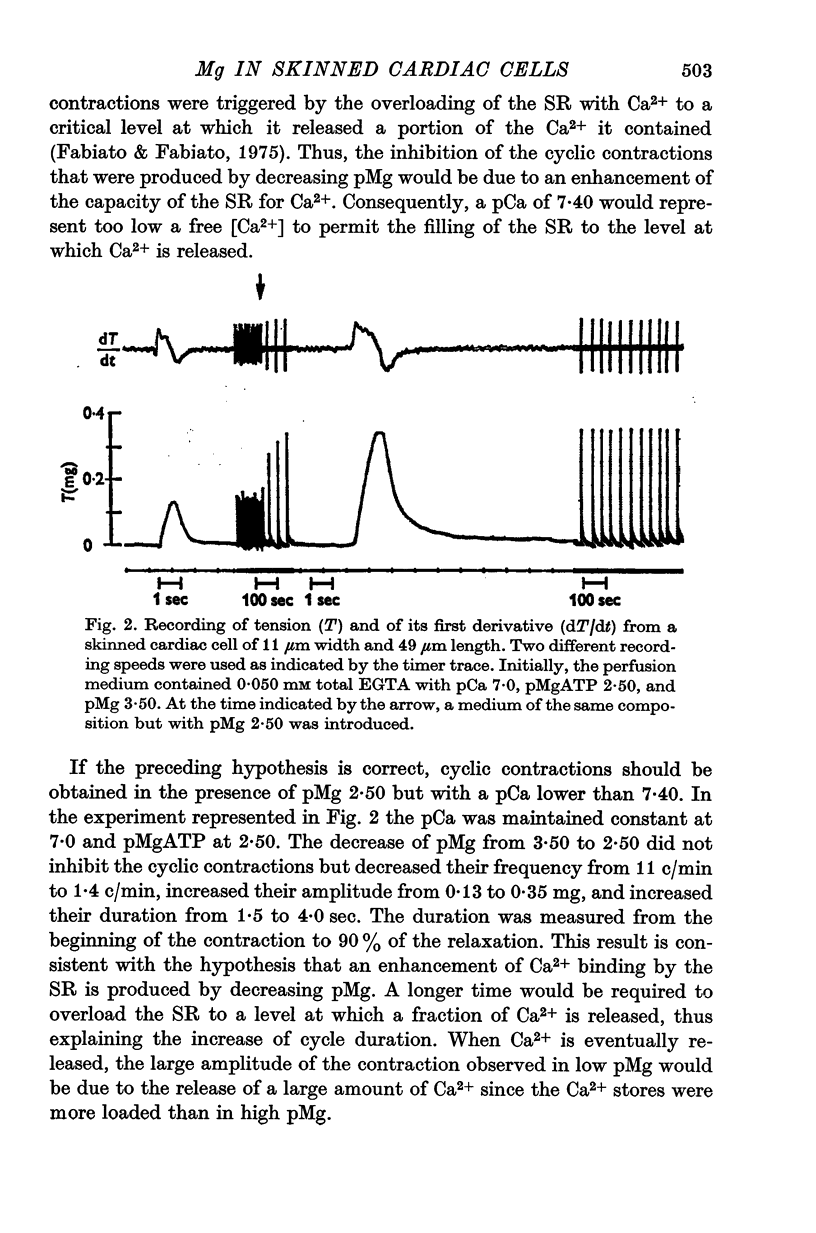
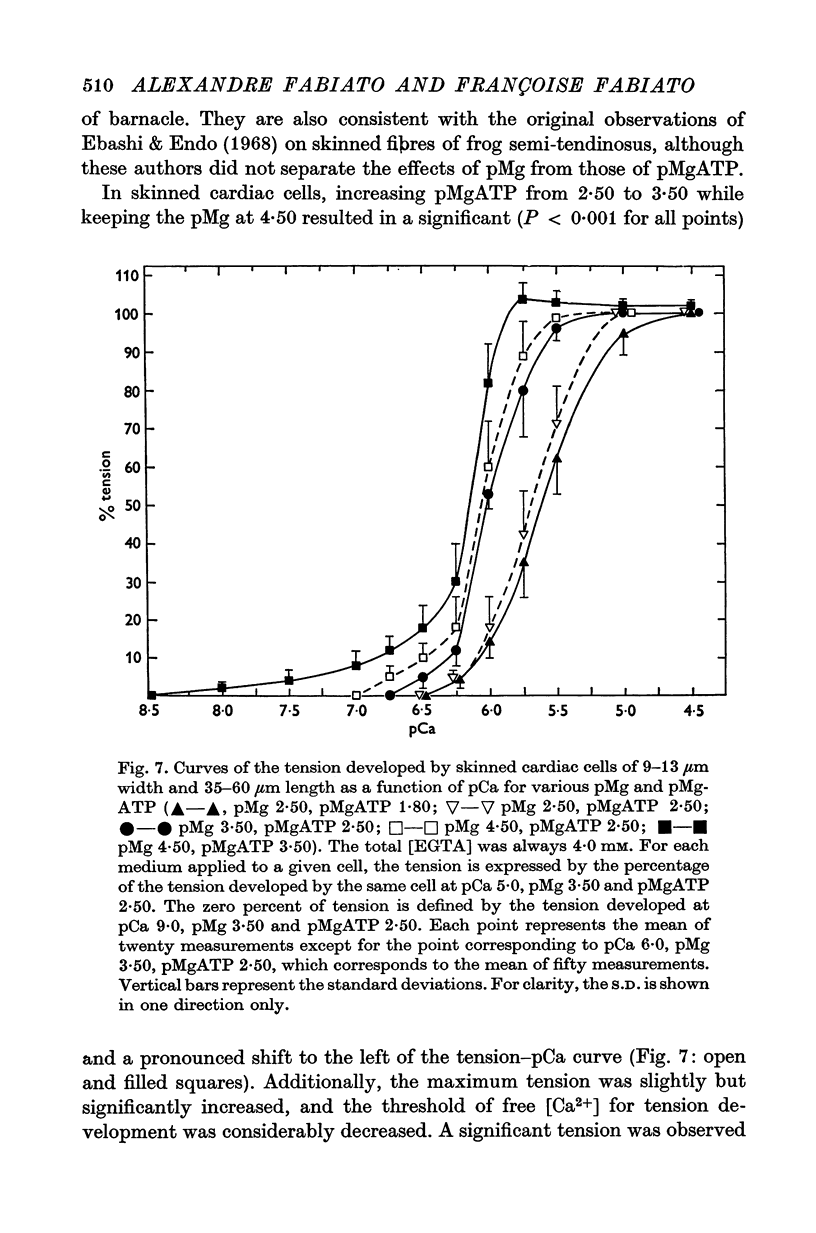
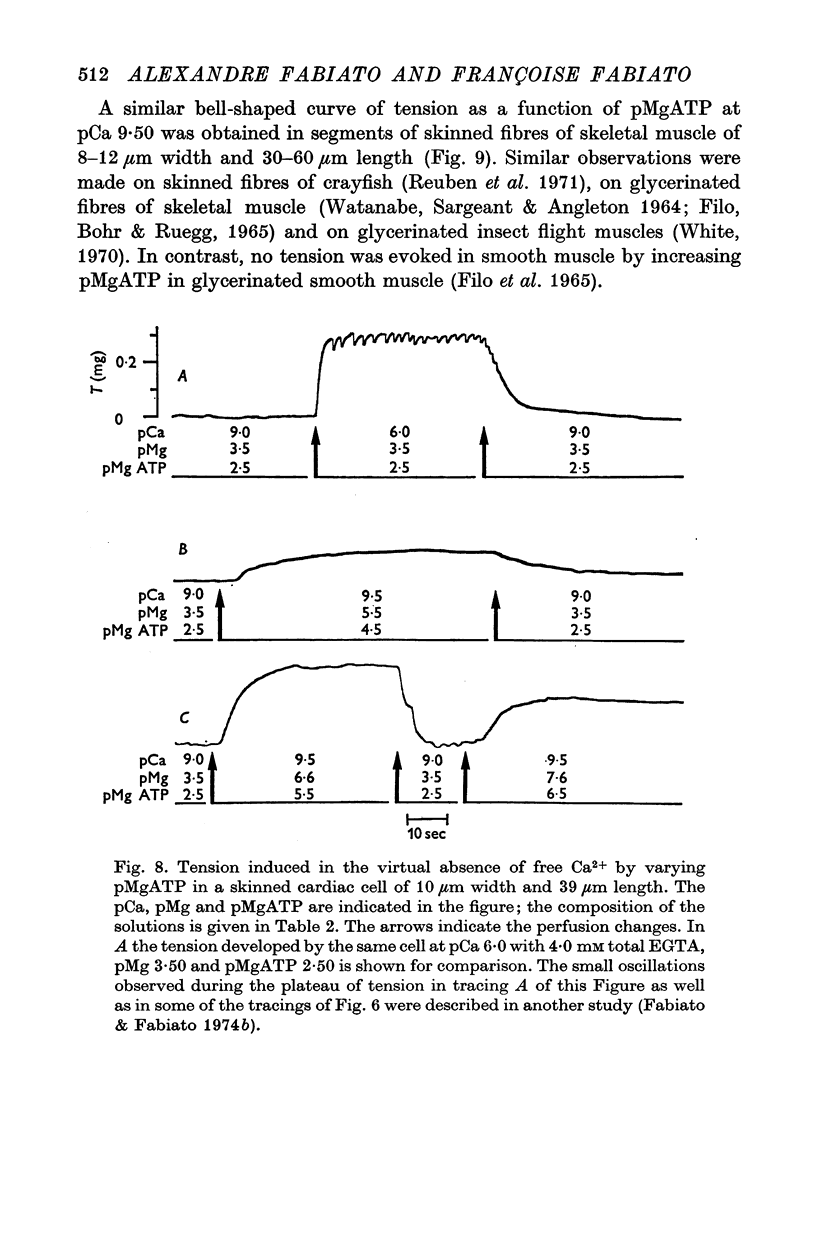
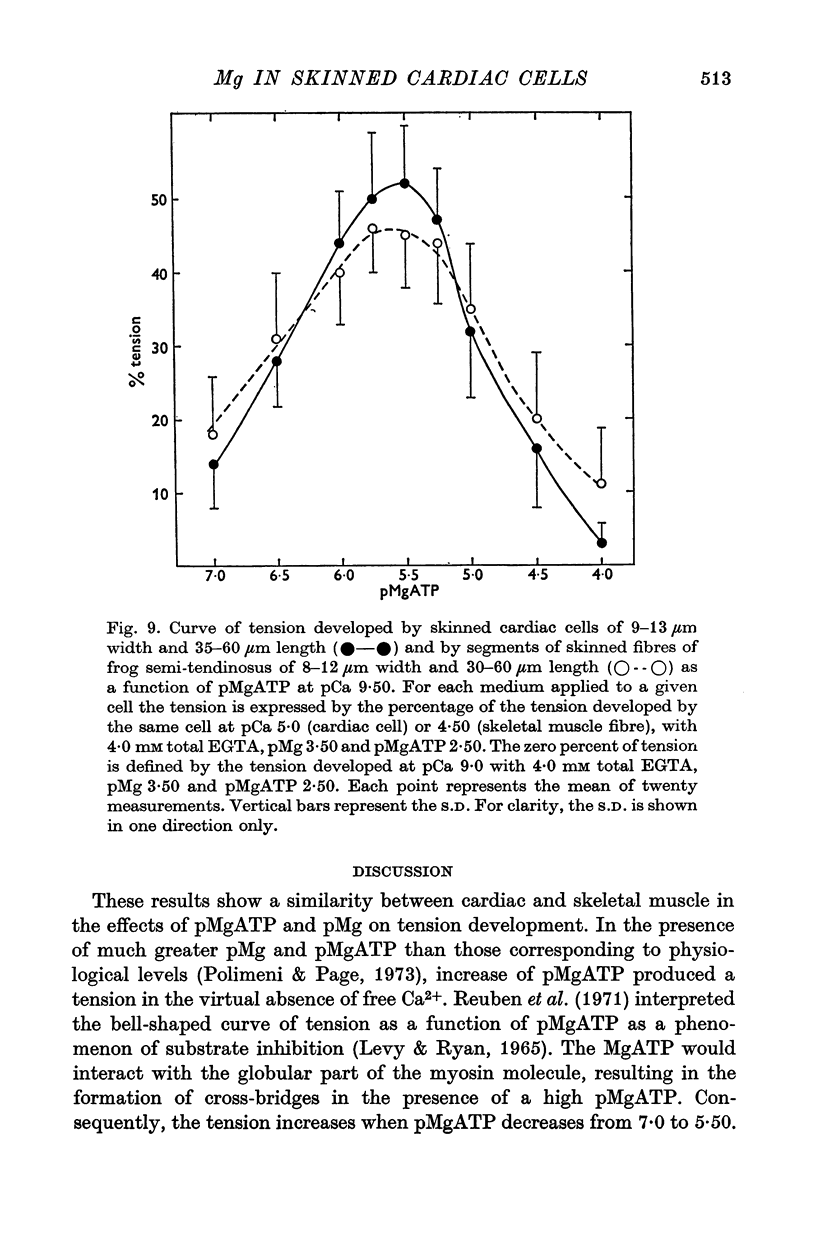
1. In the presence of a slight buffering of the free [Ca2+] with 0.050 mM total EGTA cyclic contractions were induced by a Ca2+-triggered release of Ca2+ on skinned (sarcolemma-free) segments of single cardiac cells from rat ventricle. The threshold of the free [Ca2+] trigger was elevated when the free [Mg2+] was increased. 2. At a suprathreshold free [Ca2+] increasing the free [Mg2+] resulted in a decrease in frequency and in an increase in amplitude of the phasic contractions. Addition of caffeine at a specified interval after a cyclic contraction produced a larger contraction when free [Mg2+] was higher. It was concluded that an increase of free [Mg2+] increased the capacity and the rate of binding for Ca2+ by the sarcoplasmic reticulum (SR). 3. Small skinned fibres of skeletal muscle which were perfused with 10 mM caffeine yielded results similar to those obtained in skinned cardiac cells. It was concluded that the mechanism of action of free Mg2+ was similar in both preparations, but that the SR of skeletal muscle had a higher capacity and rate of binding for Ca2+ than the cardiac SR. 4. With a strong buffering of the free [Ca2+] with 4-0 mM total EGTA, a smaller tonic tension was developed for a given pCa in the presence of a higher free [Mg2+]. This result was nearly identical in skinned cells from cardiac and skeletal muscle tissue. 5. A decrease of the [MgATP2-] produced a tension in the skinned cardiac cells that were perfused in Ca2+ free media. The maximum tension was observed for [MgATP2-] 10(-5-50)M as in skinned fibres of skeletal muscle. A further decrease of [MgATP2-] resulted in a decrease of tension.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Moisescu D. G. Proceedings: The influence of Mg2+ concentration and of pH upon the relationship between steady-state isometric tension and Ca2+ concentration in isolated bundles of barnacle myofibrils. J Physiol. 1974 Jun;239(2):112P–114P. [PubMed] [Google Scholar]
- Brandt P. W., Reuben J. P., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. II. Role of calcium. J Gen Physiol. 1972 Mar;59(3):305–317. doi: 10.1085/jgp.59.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Carvalho A. P., Leo B. Effects of ATP on the interaction of Ca++, Mg++, and K+ with fragmented sarcoplasmic reticulum isolated from rabbit skeletal muscle. J Gen Physiol. 1967 May;50(5):1327–1352. doi: 10.1085/jgp.50.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Lipmann F. ADENOSINE TRIPHOSPHATE-LINKED CONCENTRATION OF CALCIUM IONS IN A PARTICULATE FRACTION OF RABBIT MUSCLE. J Cell Biol. 1962 Sep 1;14(3):389–400. doi: 10.1083/jcb.14.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Entman M. L., Snow T. R., Freed D., Schwartz A. Analysis of calcium binding and release by canine cardiac relaxing system (sarcoplasmic reticulum). The use of specific inhibitors to construct a two-component model for calcium binding and transport. J Biol Chem. 1973 Nov 25;248(22):7762–7772. [PubMed] [Google Scholar]
- FILO R. S., BOHR D. F., RUEGG J. C. GLYCERINATED SKELETAL AND SMOOTH MUSCLE: CALCIUM AND MAGNESIUM DEPENDENCE. Science. 1965 Mar 26;147(3665):1581–1583. doi: 10.1126/science.147.3665.1581. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972 Sep;31(3):293–307. doi: 10.1161/01.res.31.3.293. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Intracellular calcium movements in skinned muscle fibres. J Physiol. 1972 May;223(1):21–33. doi: 10.1113/jphysiol.1972.sp009831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F., Reddy Y., Briggs F. N. The interaction of cations with the calcium-binding site of troponin. Biochim Biophys Acta. 1970 Nov 17;221(2):407–409. doi: 10.1016/0005-2795(70)90290-4. [DOI] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan D. J. Modification of sarcoplasmic reticulum adenosine triphosphatase by adenosine triphosphate magnesium. Arch Biochem Biophys. 1974 May;162(1):6–11. doi: 10.1016/0003-9861(74)90098-8. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Donaldson S. K. The effects of Mg 2+ on submaximum Ca 2+ -activated tension in skinned fibers of frog skeletal muscle. Biochim Biophys Acta. 1972 Jul 12;275(1):117–122. doi: 10.1016/0005-2728(72)90030-8. [DOI] [PubMed] [Google Scholar]
- LEVY H. M., RYAN E. M. EVIDENCE THAT CALCIUM ACTIVATES THE CONTRACTION OF ACTOMYOSIN BY OVERCOMING SUBSTRATE INHIBITION. Nature. 1965 Feb 13;205:703–705. doi: 10.1038/205703b0. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Page E., Polimeni P. I. Magnesium exchange in rat ventricle. J Physiol. 1972 Jul;224(1):121–139. doi: 10.1113/jphysiol.1972.sp009884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni P. I., Page E. Magnesium in heart muscle. Circ Res. 1973 Oct 5;33(4):367–374. doi: 10.1161/01.res.33.4.367. [DOI] [PubMed] [Google Scholar]
- Reuben J. P., Brandt P. W., Berman M., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol. 1971 Apr;57(4):385–407. doi: 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro R. J., Briggs F. N. Estimating the functional capabilities of sarcoplasmic reticulum in cardiac muscle. Calcium binding. Circ Res. 1974 Apr;34(4):531–540. doi: 10.1161/01.res.34.4.531. [DOI] [PubMed] [Google Scholar]
- Veloso D., Guynn R. W., Oskarsson M., Veech R. L. The concentrations of free and bound magnesium in rat tissues. Relative constancy of free Mg 2+ concentrations. J Biol Chem. 1973 Jul 10;248(13):4811–4819. [PubMed] [Google Scholar]
- WATANABE S., SARGEANT T., ANGLETON M. ROLE OF MAGNESIUM IN CONTRACTION OF GLYCERINATED MUSCLE FIBERS. Am J Physiol. 1964 Oct;207:800–808. doi: 10.1152/ajplegacy.1964.207.4.800. [DOI] [PubMed] [Google Scholar]
- Watchorn E., McCance R. A. Subacute magnesium deficiency in rats. Biochem J. 1937 Aug;31(8):1379–1390. doi: 10.1042/bj0311379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Rigor contraction and the effect of various phosphate compounds on glycerinated insect flight and vertebrate muscle. J Physiol. 1970 Jul;208(3):583–605. doi: 10.1113/jphysiol.1970.sp009138. [DOI] [PMC free article] [PubMed] [Google Scholar]


