Abstract
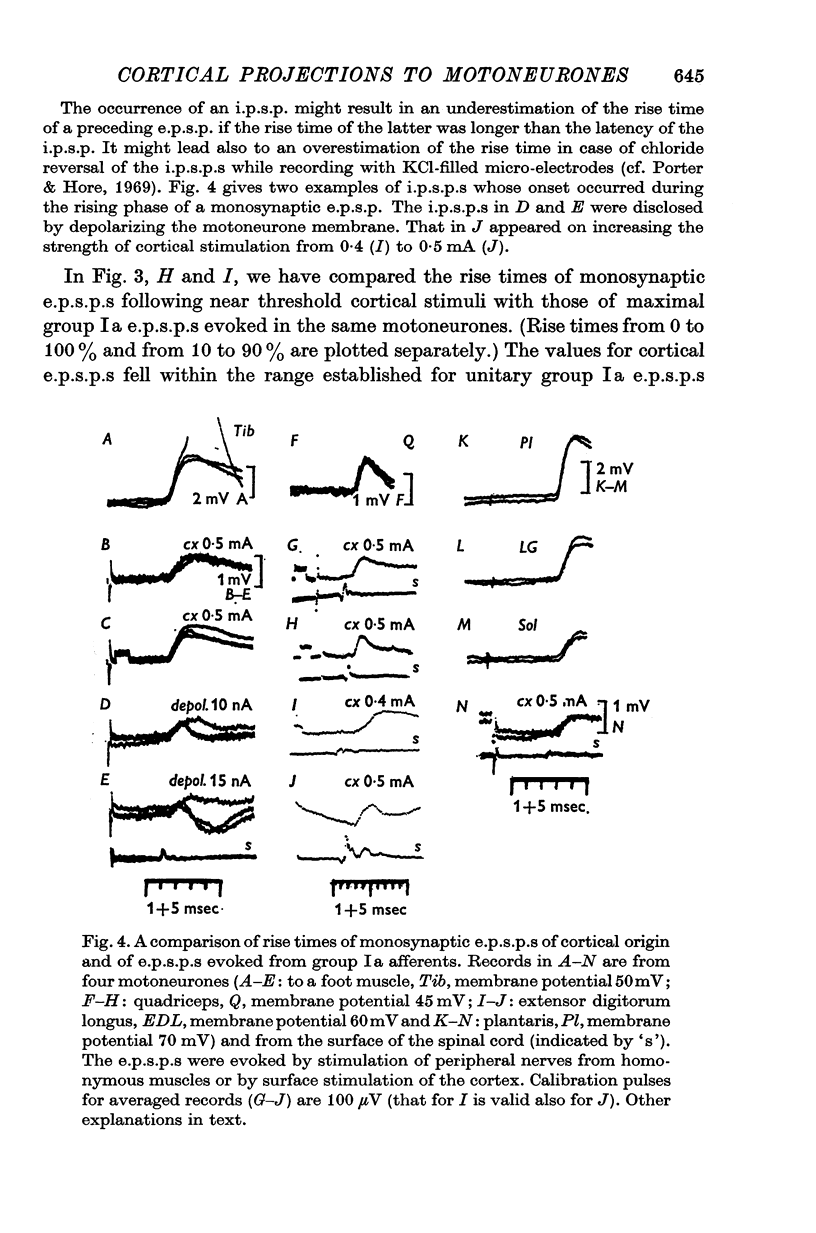
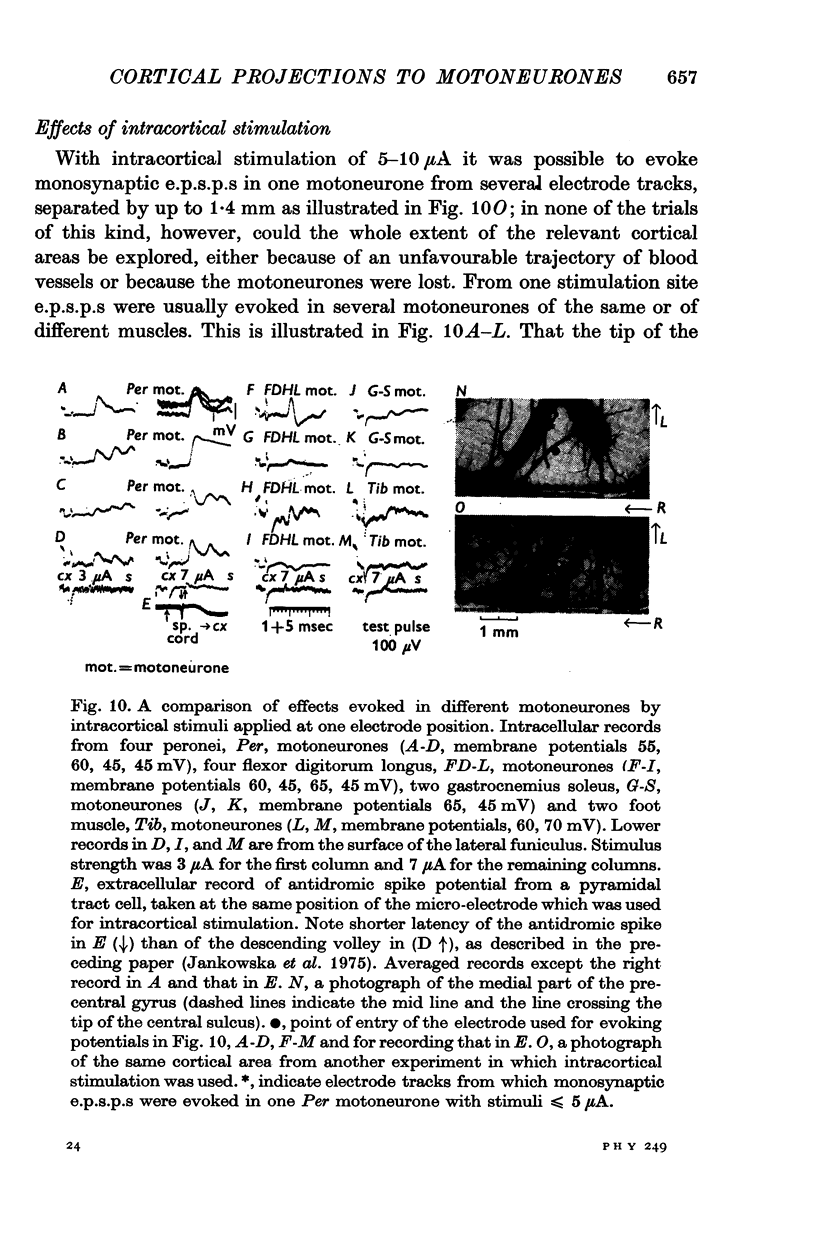
1. We have investigated the spatial organization of monosynaptic corticospinal projections to hind-limb motoneurones, using near threshold stimulation of the surface of the precentral gyrus to activate pyramidal tract (PT) cells and intracellular recording from motoneurones to detect the resulting e.p.s.p.s. 2. Monosynaptic e.p.s.p.s. of cortical origin were seen in all motoneurone species investigated, those of distal as well as of proximal hind-limb muscles. The proportion of motoneurones in which the e.s.p.s. were evoked and the amplitudes of the latter indicated a more extensive cortical projection to motor nuclei for distal than for proximal muscles, as previously found for forelimb motoneurones. 3. Cortical areas from which monosynaptic e.p.s.p.s. were evoked in individual motoneurones were remarkably large, most often between 3 and 7 mm2. Several motoneurones appeared to have two or three separate areas within the hind-limb division of the motor cortex. 4. Areas of location of pyramidal tract cells projecting to various motoneurones innervating one muscle were usually not identical. They overlapped often only partially or did not overlap at all. 5. Areas of location of pyramidal tract cells projecting to motor nuclei for different muscles often showed an extensive overlap. When it occurred, various motoneurones of a given motor nucleus had common cortical projection areas with motoneurones of other motor nuclei, either to synergistic or to antagonistic muscles. Our results give further evidence for overlapping of areas of cortical projections to motoneurones and speak against a mosaic-like organization of pyramidal tract cells projecting to different motor nuclei. 6. The rise times of cortically evoked e.p.s.p.s. indicate that the corticospinal tract fibres terminate on motoneurones at approximately similar distances from the soma as group Ia afferents. The small amplitudes of the majority of e.p.s.p.s. evoked by near threshold cortical stimulation therefore suggest that unitary e.p.s.p.s of cortical origin are small and that the density of pyramidal tract cells projecting to individual motoneurones is usually low, even in the centrum of projection areas. 7. Effects of intracortical stimulation depended on the stimulus strength. With currents of 2-3 muA, e.p.s.p.s were usually evoked in one motoneurone species or in close synergists. With currents of 5-10 muA, largest e.p.s.p.s a number of other motoneurones. Latencies of descending volleys in the lumbar corticospinal tract indicated that intracortical stimuli activated pyramidal tract cells indirectly; the effects of these stimuli could thus not be used to indicate the location of pyramidal tract cells responsible for them.
Full text
PDF




























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Hagan P. J., Phillips C. G., Powell T. P. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon's hand. Proc R Soc Lond B Biol Sci. 1975 Jan 21;188(1090):31–36. doi: 10.1098/rspb.1975.0002. [DOI] [PubMed] [Google Scholar]
- Asanuma H. Cerebral cortical control of movement. Physiologist. 1973 May;16(2):143–166. [PubMed] [Google Scholar]
- Asanuma H., Fernandez J., Scheibel M. E., Scheibel A. B. Characteristics of projections from the nucleus ventralis lateralis to the motor cortex in the cats: an anatomical and physiological study. Exp Brain Res. 1974;20(4):315–330. doi: 10.1007/BF00237378. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Rosén I. Spread of mono- and polysynaptic connections within cat's motor cortex. Exp Brain Res. 1973 Mar 19;16(5):507–520. doi: 10.1007/BF00234477. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Rosén I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res. 1972;14(3):243–256. doi: 10.1007/BF00816161. [DOI] [PubMed] [Google Scholar]
- Brooks V. B., Jasper H. H., Patton H. D., Purpura D. P., Brookhart J. M. Symposium on cerebral and cerebellar motor control. Brain Res. 1970 Feb 3;17(3):539–552. doi: 10.1016/0006-8993(70)90266-0. [DOI] [PubMed] [Google Scholar]
- Brooks V. B., Stoney S. D., Jr Motor mechanisms: the role of the pyramidal system in motor control. Annu Rev Physiol. 1971;33:337–392. doi: 10.1146/annurev.ph.33.030171.002005. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERN J. E., LANDGREN S., PHILLIPS C. G., PORTER R. Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon's motor cortex. J Physiol. 1962 Apr;161:73–90. doi: 10.1113/jphysiol.1962.sp006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Tanaka R. Disynaptic corticospinal effects in forelimb motoneurones in the cat. Brain Res. 1974 Jul 26;75(2):312–315. doi: 10.1016/0006-8993(74)90752-5. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975 Aug;249(3):617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Tanaka R. Neuronal mechanism of the disynaptic inhibition evoked in primate spinal motoneurones from the corticospinal tract. Brain Res. 1974 Jul 19;75(1):163–166. doi: 10.1016/0006-8993(74)90778-1. [DOI] [PubMed] [Google Scholar]
- LANDGREN S., PHILLIPS C. G., PORTER R. Cortical fields of origin of the monosynaptic pyramidal pathways to some alpha motoneurones of the baboon's hand and forearm. J Physiol. 1962 Apr;161:112–125. doi: 10.1113/jphysiol.1962.sp006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDGREN S., PHILLIPS C. G., PORTER R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurones of the baboon's hand and forearm. J Physiol. 1962 Apr;161:91–111. doi: 10.1113/jphysiol.1962.sp006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A., VOORHOEVE P. Effects from the pyramidal tract on spinal reflex arcs. Acta Physiol Scand. 1962 Nov-Dec;56:201–219. doi: 10.1111/j.1748-1716.1962.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the spinal cord. UCLA Forum Med Sci. 1969;11:231–265. [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- PATTON H. D., AMASSIAN V. E. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954 Jul;17(4):345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G. Cortical motor threshold and the thresholds and distribution of excited Betz cells in the cat. Q J Exp Physiol Cogn Med Sci. 1956 Jan;41(1):70–84. doi: 10.1113/expphysiol.1956.sp001164. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., PORTER R. THE PYRAMIDAL PROJECTION TO MOTONEURONES OF SOME MUSCLE GROUPS OF THE BABOON'S FORELIMB. Prog Brain Res. 1964;12:222–245. doi: 10.1016/s0079-6123(08)60625-1. [DOI] [PubMed] [Google Scholar]
- PRESTON J. B., WHITLOCK D. G. A comparison of motor cortex effects on slow and fast muscle innervations in the monkey. Exp Neurol. 1963 Apr;7:327–341. doi: 10.1016/0014-4886(63)90079-7. [DOI] [PubMed] [Google Scholar]
- PRESTON J. B., WHITLOCK D. G. Intracellular potentials recorded from motoneurons following precentral gyrus stimulation in primate. J Neurophysiol. 1961 Jan;24:91–100. doi: 10.1152/jn.1961.24.1.91. [DOI] [PubMed] [Google Scholar]
- Phillips C. G. Proceedings: Hughlings Jackson Lecture. Cortical localization and "sensori motor processes" at the "middle level" in primates. Proc R Soc Med. 1973 Oct;66(10):987–1002. [PMC free article] [PubMed] [Google Scholar]
- Phillips C. G. The Ferrier lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Porter R., Hore J. Time course of minimal corticomotoneuronal excitatory postsynaptic potentials in lumbar motoneurons of the monkey. J Neurophysiol. 1969 May;32(3):443–451. doi: 10.1152/jn.1969.32.3.443. [DOI] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Smith T. G., Nelson P. G., Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967 Sep;30(5):1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Rispal-Padel L., Massion J., Grangetto A. Relations between the ventrolateral thalamic nucleus and motor cortex and their possible role in the central organization of motor control. Brain Res. 1973 Sep 28;60(1):1–20. doi: 10.1016/0006-8993(73)90847-0. [DOI] [PubMed] [Google Scholar]
- Strick P. L. Cortical projections of the feline thalamic nucleus ventralis lateralis. Brain Res. 1970 May 20;20(1):130–134. doi: 10.1016/0006-8993(70)90162-9. [DOI] [PubMed] [Google Scholar]
- Strick P. L., Sterling P. Synaptic termination of afferents from the ventrolateral nucleus of the thalamus in the cat motor cortex. A light and electron microscopy study. J Comp Neurol. 1974 Jan 1;153(1):77–106. doi: 10.1002/cne.901530107. [DOI] [PubMed] [Google Scholar]
- Tamarova Z. A., Shapobalov A. I., Karamian O. A., Kurchavyi G. G. Kortiko-piramidnye i kortiko-ekstrapiramidnye sinapticheskie vliianiia na poiasnichnye motoneirony obez'iany. Neirofiziologiia. 1972 Nov-Dec;4(6):587–596. [PubMed] [Google Scholar]
- UEMURA K., PRESTON J. B. COMPARISON OF MOTOR CORTEX INFLUENCES UPON VARIOUS HIND-LIMB MOTONEURONS IN PYRAMIDAL CATS AND PRIMATES. J Neurophysiol. 1965 Mar;28:398–412. doi: 10.1152/jn.1965.28.2.398. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M. The pyramidal tract: recent investigations on its morphology and function. Ergeb Physiol. 1969;61:72–136. doi: 10.1007/BFb0111447. [DOI] [PubMed] [Google Scholar]
- von Bonin G., Mehler W. R. On columnar arrangement of nerve cells in cerebral cortex. Brain Res. 1971 Mar 19;27(1):1–9. doi: 10.1016/0006-8993(71)90367-2. [DOI] [PubMed] [Google Scholar]




