Abstract
Objective
To determine the incidence of epilepsy in a general practice population and its variation with socioeconomic deprivation.
Design
Prospective surveillance for new cases over an 18 or 24 month period.
Participants
All patients on practice registers categorised for deprivation with the Carstairs score of their postcode.
Setting
20 general practices in London and south east England.
Main outcome measure
Confirmed diagnosis of epilepsy.
Results
190 new cases of epilepsy were identified during 369 283 person years of observation (crude incidence 51.5 (95% confidence interval 44.4 to 59.3) per 100 000 per year). The incidence was 190 (138 to 262) per 100 000 in children aged 0-4 years, 30.8 (21.3 to 44.6) in those aged 45-64 years, and 58.7 (42.5 to 81.0) in those aged ⩾65 years. There was no apparent difference in incidence between males and females. The incidence showed a strong association with socioeconomic deprivation, the age and sex adjusted incidence in the most deprived fifth of the study population being 2.33 (1.46 to 3.72) times that in the least deprived fifth (P=0.001 for trend across fifths). Adjustment for area (London v outside London) weakened the association with deprivation (rate ratio 1.62 (0.91 to 2.88), P=0.12 for trend).
Conclusions
The incidence of epilepsy seems to increase with socioeconomic deprivation, though the association may be confounded by other factors.
What is already known on this topic
Epilepsy is associated with a wide range of markers of social and economic disadvantage
A small number of epidemiological studies have confirmed this association but have not established the direction of causality
What this study adds
The incidence of epilepsy, adjusted for age and sex, in the most deprived fifth of the study population was 2.3 times that in the least deprived fifth
Socioeconomic deprivation is an important risk factor for the development of epilepsy, though the results may partly reflect differences in incidence within and outside London
Introduction
Epilepsy is associated with a wide range of markers of social and economic disadvantage, including poor academic achievement, unemployment, underemployment, and low income.1–4 Because of this association it is often assumed that people who are socially and economically deprived are more likely to develop epilepsy. This hypothesis is supported to some extent by the observation that the incidence of epilepsy is higher in developing countries than in developed countries.5
A few epidemiological studies have confirmed an association between the prevalence of epilepsy and markers of social disadvantage.6 Prevalence studies, however, cannot establish the direction of causality, and the employment problems and social disadvantage experienced by people with epilepsy may cause downward social “drift.”7 The association between socioeconomic factors and incident epilepsy is poorly understood8 and to date has not been examined within the general community with methods that prospectively ascertain cases.
The NHS and the World Health Organization aim to reduce inequalities in health.9,10 This can be achieved by concentrating resources on conditions that affect socially and economically deprived people. Understanding of the role that deprivation has in epilepsy gives insight into its aetiology and management. We determined the incidence of epilepsy in an unselected community based population and its variation with socioeconomic deprivation.
Methods
Over an 18 or 24 month period we prospectively ascertained all incident cases of epilepsy in an unselected community population served by eight general practices in London (86 989 person years) and 12 practices outside London (282 294 person years). For these practices we were sole providers of secondary care for seizure disorders.
We advertised the linkage scheme to all general practices within the region. Practices that were willing to cooperate were selected if their patient details were stored on a computerised database. The follow up time differed because of researcher funding.
The practices outside London were all in south east England. Epilepsy was defined as the occurrence of one or more unprovoked seizure. We excluded provoked seizures, acute symptomatic seizures, and febrile convulsions.
We used a range of methods to identify cases of epilepsy, including a fast track clinic and active surveillance. An audit that involved a systematic search of all individual primary care records was performed at the end of the study period. This was 24 months (1 June 1995 to 31 May 1997) in 12 practices and 18 months (1 January 1995 to 30 June 1997) in the eight remaining practices. The methods by which cases were ascertained have been fully described previously.11
For each patient we collected clinical and demographic data, including postcode. Data were anonymised before analysis. The postcodes were used to assign to each individual a Carstairs score of social deprivation for the enumeration district in which he or she lived. An enumeration district on average contains 140 households and is the smallest area for which census data are available. The Carstairs score is an index of deprivation based on four variables available from the 1991 census: overcrowding, social class of head of household, car ownership, and unemployment.12 The distribution of the scores was banded into fifths, with the lowest fifth denoting the least deprived and the highest fifth the most deprived.
Statistical analysis
We calculated incidence rates of epilepsy by five year age group and by sex using person time at risk (18 or 24 months, depending on area). We based the multivariate analysis on random effects Poisson regression with the Huber-White estimator of variance and specified practice level clustering to allow for similarity of rates within practices. We grouped Carstairs scores into fifths for analysis. Reported P values represent tests for linear trend applied to the grouped data.
Results
We identified 268 new cases of seizures during 369 283 person years of observation (see table A on bmj.com). We excluded 78 cases of provoked or acute seizures and febrile convulsions. The 190 remaining cases were included in the analysis, giving a crude incidence rate of 51.5 (95% confidence interval 44.4 to 59.3) per 100 000 per year.
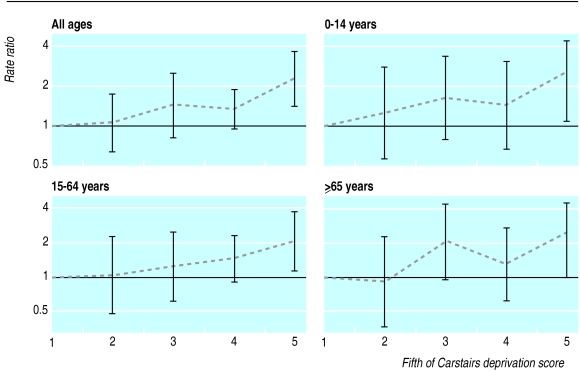
We found a strong relation between incidence of epilepsy and age. The incidence was highest between 0 and 4 years and lowest between 45 and 64 years (table 1). Males and females had similar incidence of epilepsy. We observed a steep rise in incidence with socioeconomic deprivation (tables 1 and 2, figure). The incidence, adjusted for age and sex, in the most deprived fifth of the study population was 2.33 (1.46 to 3.72) times that in the least deprived fifth (P=0.001 for trend). There were similar socioeconomic gradients in the 0-14, 15-64, and ⩾65 age groups (figure).
Table 1.
Number of patients diagnosed with epilepsy and rate per 100 000 population (95% confidence interval)*
| Epilepsy cases
|
Person years of follow up
|
Rate/100 000 population (95% confidence interval)
|
|
|---|---|---|---|
| Age group (years): | |||
| 0-4 | 37 | 19 487 | 190.0 (138.0 to 262.0) |
| 5-14 | 28 | 37 128 | 75.4 (52.1 to 109) |
| 15-44 | 60 | 158 663 | 37.8 (29.4 to 48.7) |
| 45-64 | 28 | 90 909 | 30.8 (21.3 to 44.6) |
| ⩾65 | 37 | 63 087 | 58.7 (42.5 to 81.0) |
| Sex: | |||
| Male | 96 | 182 040 | 52.7 (43.2 to 64.4) |
| Female | 94 | 187 240 | 50.2 (41.0 to 61.5) |
| Area: | |||
| London | 74 | 86 989 | 85.1 (67.7 to 107) |
| Outside London | 116 | 282 294 | 41.1 (34.3 to 49.3) |
| Fifths of Carstairs score: | |||
| 1 (least deprived) | 27 | 79 257 | 34.1 (23.4 to 49.7) |
| 2 | 28 | 76 115 | 36.8 (25.4 to 53.3) |
| 3 | 40 | 73 620 | 54.3 (39.9 to 74.1) |
| 4 | 36 | 68 198 | 52.8 (38.1 to 73.2) |
| 5 (most deprived) | 54 | 61 604 | 87.7 (67.1 to 114) |
Totals differ in some cases because of missing data, accounted for in the statistical analysis.
Table 2.
Rate ratios* for fifths of Carstairs deprivation score and other explanatory factors
| Model 1 (unadjusted)
|
Model 2†
|
Model 3†
|
||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI)
|
P value for trend
|
Odds ratio (95% CI)
|
P value for trend
|
Odds ratio (95% CI)
|
P value for trend
|
|||
| Fifth of Carstairs score: | ||||||||
| 1 (least deprived) | 1 | 1 | 1 | |||||
| 2 | 1.07 (0.67 to 1.69) | 1.05 (0.66 to 1.70) | 1.04 (0.65 to 1.68) | |||||
| 3 | 1.50 (0.88 to 2.56) | <0.001 | 1.45 (0.84 to 2.51) | 0.001 | 1.42 (0.81 to 2.50) | 0.12 | ||
| 4 | 1.41 (1.02 to 1.94) | 1.38 (0.97 to 1.96) | 1.16 (0.76 to 1.77) | |||||
| 5 (most deprived) | 2.35 (1.53 to 3.60) | 2.33 (1.46 to 3.72) | 1.62 (0.91 to 2.88) | |||||
| Age group‡: | ||||||||
| 0-4 | 1 | 1 | ||||||
| 5-14 | 0.41 (0.21 to 0.79) | 0.41 (0.21 to 0.79) | ||||||
| 15-44 | 0.20 (0.11 to 0.39) | 0.02 | 0.20 (0.11 to 0.38) | 0.02 | ||||
| 45-64 | 0.19 (0.10 to 0.36) | 0.19 (0.10 to 0.36) | ||||||
| ⩾65 | 0.37 (0.18 to 0.78) | 0.37 (0.18 to 0.76) | ||||||
| Sex: | ||||||||
| Female | 1 | 1 | ||||||
| Male | 1.05 (0.84 to 1.32) | 0.67 | 1.05 (0.84 to 1.31) | 0.68 | ||||
| Area: | ||||||||
| London | 1 | |||||||
| Outside London | 0.59 (0.37 to 0.95) | 0.03 | ||||||
All rate ratios based on models with practice level random effects and robust standard errors.
Model 2 adjusted for age and sex; model 3 adjusted for age, sex, and area.
For clarity results are for five age bands but models were constructed with five year age groups. Results shown for deprivation and other variables in models 2 and 3 are adjusted with this finer age stratification.
Populations served by London practices, however, were more deprived on average than those outside London. When we considered mean Carstairs scores of the nine most deprived practice populations, eight were based in London and the overlap in deprivation scores of individuals in London and non-London practices was small (see table A on bmj.com). When we made an additional adjustment for area, the association between epilepsy incidence and deprivation fifth was weakened and was not significant at the 5% level (table 2).
Discussion
This prospective study based on an unselected population represents the first to examine socioeconomic status as a risk factor for the development of epilepsy. The overall incidence rates obtained are comparable with those from previous epidemiological studies of incidence in the United Kingdom.5
Our main observation was the relation between the incidence of epilepsy and Carstairs deprivation score. However, interpretation of this apparent association is complicated by the fact that the main contrasts in deprivation were those between the populations in London and outside London. Indeed, in the multivariable analyses, when we made additional adjustment for area (London versus outside London) the strong gradient with deprivation was somewhat weakened—though the broad pattern remained—and the association was no longer significant (P=0.12 for trend). The question then is whether the observed deprivation gradient represents a “cause and effect” association or whether it is a spurious (confounded) association generated by a London versus outside London difference in some factor that affects incidence or case ascertainment, or both.
Alternative interpretations of results
There are three possible explanations. Firstly, patients may have had different access to epilepsy services or diagnostic facilities in the two areas. We think this is unlikely to have had an appreciable effect because of our dedicated surveillance and reporting methods that included a general practice-hospital linkage scheme, with standardised access to diagnostic facilities and epilepsy services. Also an audit of all patient records in participating practices found no evidence of any systematic difference in case reporting between practices.11 Thus, the procedures for reporting and referral should have minimised the possibility of variation in case identification.
Secondly, there may be differences in other demographic factors such as ethnicity. Ethnicity has been identified as a determinant of incidence in several US community based epilepsy studies, with epilepsy being more common among Afro-Americans than the white population.8,14,15 In the United Kingdom, a retrospective study found epilepsy to be less prevalent among people of south Asian origin,16 although this may be because of lower reporting among this group or a lower prevalence due to selective immigration.
In our study, the proportion of people of Afro-Caribbean, African, or Asian descent was relatively small and varied little between practices, though records of ethnic background were not available for individual patients. Overall, the proportion of non-white patients was no greater than 10% in any of the general practices, and it is therefore unlikely that confounding by ethnicity could account for the strong deprivation gradient we observed.
Thirdly, practices in and outside London may have differed in the accuracy and completeness of their patient registers. Because of high population mobility within inner city areas, general practices in London may be more susceptible to “list inflation”—that is, to have more people on their lists than they should because patients who move from the practice area are not removed from registry lists. Where this occurs the population at risk would be overestimated and hence the incidence of epilepsy would be underestimated. Again we believe the magnitude of this problem was small in this study because all participating practices had good computerised systems and were obliged by the health authority to update their patient lists regularly. Moreover, the likely direction of bias would almost certainly act to diminish any association with socioeconomic gradient as the incidence rates would be underestimated in the more deprived practices within the London area.
Thus, we consider that these explanations are unlikely to account for the deprivation gradient we observed, and we conclude that the evidence is in favour of poor socioeconomic status being a risk factor for the development of epilepsy. This is a pattern similar to that seen for a range of other conditions such as coronary artery disease and many cancers, whose incidences show strong gradients with socioeconomic class.13
Possible mechanisms
The pathophysiological mechanisms by which low socioeconomic status might increase risk of epilepsy are not clear. But several other risk factors such as incidence of birth defects, trauma, infection, and poor nutrition are known to be more common among socioeconomically deprived populations. These would certainly provide a plausible reason for a higher incidence of epilepsy in more disadvantaged groups. Genetic factors may also have a role. The children of parents with epilepsy are more likely to develop seizures, and when one parent is affected the probability of a child developing epilepsy before the age of 20 years is raised from 1% in the general population to 6%.17 The genetic basis for many epilepsies is increasingly being recognised, although the relation between genetic factors and social disadvantage is likely to be complex. Although children of parents with epilepsy may be socially disadvantaged because of their parent's condition, genes associated with epilepsy may also be important in determining educational achievement and other aspects of medical health.
Supplementary Material
Figure.
Age and sex adjusted rate ratios by fifth of Carstairs deprivation score
Acknowledgments
We thank the general practitioners in the participating practices who allowed us access to their surgeries and assisted in the identification of patients with epilepsy. We also thank Dr Ben Armstrong for advice on statistical methods.
Footnotes
Funding: Wellcome Trust, Brain Research Trust, the University College Hospital NHS Trust, National Society for Epilepsy. PW is funded by a Public Health Career Scientist Award.
Competing interests: None declared.
An extra table of details of the general practices can be found on bmj.com
References
- 1.Rodin E. Vocational and educational problems with epileptic patients. Epilepsia. 1972;13:149–160. doi: 10.1111/j.1528-1157.1972.tb04562.x. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby A, Buck D, Baker G, McNamee P, Graham JS, Chadwick D. Uptake and costs of care for epilepsy: findings from a UK regional study. Epilepsia. 1998;39:776–786. doi: 10.1111/j.1528-1157.1998.tb01164.x. [DOI] [PubMed] [Google Scholar]
- 3.Lisle JR, Waldron HA. Employees with epilepsy in the NHS. BMJ. 1986;292:305–306. doi: 10.1136/bmj.292.6516.305-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart YM, Shorvon SD. The nature of epilepsy in the general population. I. Characteristics of patients receiving medication for epilepsy. Epilepsy Res. 1995;21:43–49. doi: 10.1016/0920-1211(95)00007-w. [DOI] [PubMed] [Google Scholar]
- 5.Sander JW, Shorvon SD. Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry. 1996;61:433–443. doi: 10.1136/jnnp.61.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan CLI, Ahmed Z, Kerr MP. Social deprivation and prevalence of epilepsy and associated health usage. J Neurol Neurosurg Psychiatry. 2000;69:13–17. doi: 10.1136/jnnp.69.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacoby A, Baker GA, Steen N, Potts P, Chadwick DW. The clinical course of epilepsy and its psychosocial correlates: findings from a UK community study. Epilepsia. 1996;37:148–161. doi: 10.1111/j.1528-1157.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 8.Shamansky SL, Glaser GH. Socioeconomic characteristics of childhood seizure disorders in the New Haven area: an epidemiologic study. Epilepsia. 1979;20:457–474. doi: 10.1111/j.1528-1157.1979.tb04828.x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Targets for all. Copenhagen: World Health Organization; 1985. [Google Scholar]
- 10.Department of Health. The new NHS: modern and dependable. London, Stationery Office. 1997. [Google Scholar]
- 11.MacDonald BK, Cockerell OC, Sander JW, Shorvon S. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain. 2000;123:665–676. doi: 10.1093/brain/123.4.665. [DOI] [PubMed] [Google Scholar]
- 12.Carstairs V, Morris R. Deprivation and health in Scotland. Aberdeen: Aberdeen University Press; 1991. [Google Scholar]
- 13.Acheson D, Barker D, Chambers J, Graham H, Marmot M, Whitehead M. Independent inquiry into inequalities in health: report. London: Stationery Office; 1998. pp. 1–164. [Google Scholar]
- 14.Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile convulsions. N Engl J Med. 1976;295:1029–1033. doi: 10.1056/NEJM197611042951901. [DOI] [PubMed] [Google Scholar]
- 15.Haerer AF, Andreson DW. Prevalence and clinical features of epilepsy in a biracial United States population. Epilepsia. 1986;27:66–75. doi: 10.1111/j.1528-1157.1986.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 16.Wright J, Pickard N, Hakin N. A population-based study of prevalence, clinical characteristics and effect of ethnicity in epilepsy. Seizure. 2000;9:309–313. doi: 10.1053/seiz.2000.0422. [DOI] [PubMed] [Google Scholar]
- 17.Annegers JF, Annegers JF, Risch N, Hauser WA, Susser M. Relations of genetic and environmental factors in the etiology of epilepsy. Ann Neurol. 2001;39:442–449. doi: 10.1002/ana.410390406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.