Abstract
Objective
To compare the effects and side effects of low dosage tricyclic antidepressants with placebo and with standard dosage tricyclics in acute phase treatment of depression.
Design
Systematic review of randomised trials comparing low dosage tricyclics (⩽100 mg/day) with placebo or with standard dosage tricyclics in adults with depression.
Main outcome measures
Relative risk of response in depression (random effects model), according to the original authors' definition but usually defined as 50% or greater reduction in severity of depression. Relative risks of overall dropouts and dropouts due to side effects.
Results
35 studies (2013 participants) compared low dosage tricyclics with placebo, and six studies (551 participants) compared low dosage tricyclics with standard dosage tricyclics. Low dosage tricyclics, mostly between 75 and 100 mg/day, were 1.65 (95% confidence interval 1.36 to 2.0) and 1.47 (1.12 to 1.94) times more likely than placebo to bring about response at 4 weeks and 6-8 weeks, respectively. Standard dosage tricyclics failed, however, to bring about more response but produced more dropouts due to side effects than low dosage tricyclics.
Conclusions
Treatment of depression in adults with low dose tricyclics is justified. However, more rigorous studies are needed to definitively establish the relative benefits and harms of various dosages.
What is already known on this topic
Tricyclics are still prescribed as often as selective serotonin reuptake inhibitors and other newer antidepressants worldwide
Experts have often claimed that clinicians prescribe tricyclics at less than adequate dosages
What this study adds
Tricyclics at dosages below the recommended range are more effective than placebo
They may or may not be as effective as standard dosage tricyclics but result in fewer dropouts due to side effects
The minimum effective dosage and ranges for antidepressants has not been established—a simple set of numbers that every practising doctor and patient would want to know
Introduction
Despite the growing popularity of selective serotonin reuptake inhibitors and other newer antidepressants, tricyclic andtidepressants are still extensively prescribed worldwide. In the United Kingdom between 1991 and 1996, there was a 460% increase in prescriptions for selective serotonin reuptake inhibitors, but there was also a 40% increase in prescriptions for tricyclics for patients starting treatment, with these new patients still outnumbering those taking selective serotonin reuptake inhibitors by 56%.1 In the United States between 1990 and 1995 antidepressant use increased by 73% mainly because of patients being prescribed selective serotonin reuptake inhibitors, but even today tricyclics are prescribed as often as selective serotonin reuptake inhibitors.2,3 Other countries show similar trends.4
Evidence for the recommended dosage of tricyclics is poor.5,6 Many of the existing guidelines recommend dosages greater than 100 mg/day or 125 mg/day, but there is a lack of convincing evidence that lower dosages are not effective.7,8 This uncertainty casts doubt on the widely held view that depression is undertreated both in primary care and in psychiatric settings.9,10 It also questions whether selective serotonin reuptake inhibitors should be preferred over tricyclics when controlled trials failed to find differences in effectiveness between the two, because it is easier to achieve “adequate” dosage with selective serotonin reuptake inhibitors.11
Methods
Inclusion criteria
We included randomised trials comparing low dosage tricyclics with placebo or with standard dosages of the same tricyclic in the acute phase treatment of adults with depression. Low dosage was defined as 100 mg/day or less of imipramine, amitriptyline, clomipramine, desipramine, doxepin, dothiepin, trimipramine, or lofepramine. We excluded nortriptyline because the standard dosage is debatable. Standard dosage was defined as greater than 100 mg/day. Our trial was to last at least four weeks.
Our primary outcome was the effect of treatment on depression, according to the original authors' definition but usually defined as 50% or greater reduction in severity of depression. The severity of symptoms was measured by either observer rating (preferred) or self report.
Identification of trials
We electronically searched the Cochrane Collaboration depression, anxiety, and neurosis controlled trials register up to November 2000 for any trials in which tricyclics were given. This database incorporates results of group searches of Medline (1966 onwards), Embase (1980 onwards), CINAHL (1982 onwards), PsycINFO (1974 onwards), PSYNDEX (1977 onwards), and LILACS (1982-99). We also hand searched the major psychiatric and medical journals. Two reviewers (HM and TAF) then manually examined the potential papers to see if they were randomised trials comparing low dosage tricyclics with placebo or with standard dosage for any form of depression. All potential identified papers were then checked according to the strict eligibility criteria by two independent reviewers (TAF and CB).
To identify further reports TAF checked the references of this preliminary list of selected studies along with references of other relevant review papers. To identify more recent reports HM subjected nine of the most representative studies to SciSearch. TAF contacted authors of major papers and other experts in the specialty.
Quality assessment and data extraction
TAF and CB assessed the methodological quality of the selected studies. The criteria for quality assessment were based on the recommendations of the Cochrane Collaboration Handbook and focused on concealment of allocation and double blinding.12 HM and TAF independently extracted data from the original reports using data extraction forms. Disagreements between the two reviewers were resolved by consensus.
Statistical analysis
Data were entered twice into Review Manager (version 4.1) using the duplicate data entry facility. For dichotomous outcomes, we calculated relative risks and their 95% confidence intervals with a random effects model because they may be more generalisable and more easily interpreted than those obtained with fixed effects models, odds ratios, or risk differences.13,14 We assessed heterogeneity between studies with the Q statistic and by visual inspection of the results. For continuous outcomes, we calculated standardised weighted mean differences with a random effects model.
We first performed per protocol analysis according to the values reported by the original authors. When data on dropouts were included by the last observation carried forward method, we analysed them according to the primary studies. We also performed a worst case scenario intention to treat analysis whereby dropouts were considered non-responders in the active treatment group but as responders in the placebo group. This extreme scenario was to guard against favouring active drugs that could be the more harmful.
We performed a funnel plot analysis to check for publication bias. To examine the robustness of the findings we performed two sensitivity analyses, by limiting the included studies to those using operational diagnostic criteria for major depression and to those in which the dosage was 75 mg/day or less.
Subgroup analyses should be performed and interpreted with caution because multiple analyses lead to false positive conclusions. We did, however, perform two subgroup analyses, where possible: for older people (age 65 or more) separately, because these people may be more vulnerable to side effects associated with tricylics and a decreased dosage is often recommended for them; and for psychiatric patients and primary care patients separately, because it is sometimes believed that results obtained from either of these settings may not be straightforwardly applicable to the other setting.
Results
Study inclusion and characteristics
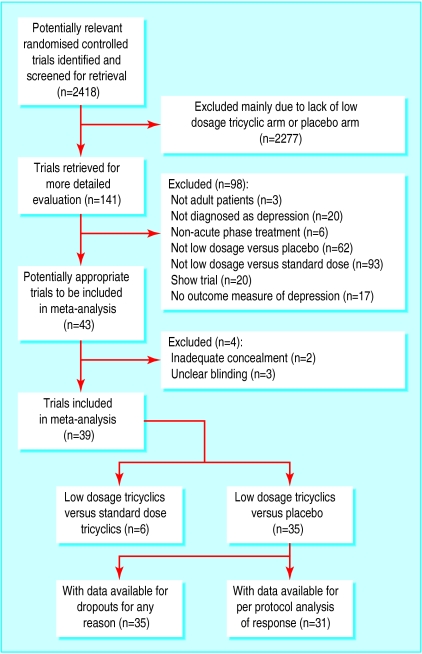
Of the 2418 citations originally identified in our electronic search, 141 were potentially relevant and were assessed for strict eligibility and quality. The inter-rater reliability of the two reviewers for this first stage of study selection was good (agreement 97%, κ=0.61). After the reference search, SciSearch, and personal contacts, we ultimately agreed on 35 studies (2013 participants) that compared low dosage tricyclics with placebo, and six studies (551 participants) that compared low dosage tricyclics with standard dosage tricyclics. Two of these had three arms of standard dosage tricylics, low dosage tricylics, and placebo (fig 1). The inter-rater reliability for this second stage of assessment for eligibility and validity was excellent, with weighted kappas between 0.58 and 0.86. The inter-rater reliability of the two validity criteria was also satisfactory, with weighted kappas of 0.58 and 0.79, respectively.
Figure 1.
Process of inclusion of studies for review and analysis
Sixteen studies used amitriptyline as active drugs and 13 used imipramine. The remaining randomised controlled trials studied clomipramine (3 trials), doxepine (3), dothiepin (2), trimipramine (2), and lofepramine (1). Five studies focused on depression in people aged 65 or more. Ten studies were conducted in primary care and 12 studies in psychiatric settings. Six studies dealt with depression seen in patients with comorbid physical conditions such as migraine or rheumatoid arthritis. All of the included studies were randomised controlled trials with both patients and doctors blinded (table; the complete list of included studies is also available in the Cochrane Library). However, only four studies reported enough details on their randomisation procedure.
Low dosage tricyclics versus placebo
Effectiveness
Low dosage tricyclics, on average between 75 and 100 mg/day, were 65% (36% to 100%; random effects model), 47% (12% to 94%), and 114% (41% to 226%) more likely than placebo to bring about response at 4 weeks, 6-8 weeks, and 3-12 months, respectively. On average 45% of patients taking low dosage tricyclics responded at 4 weeks, 59% at 6-8 weeks, and 53% at 3-12 months. Heterogeneity was noted only for the outcome at 6-8 weeks (fig 2).
Figure 2.
Low dosage tricyclics versus placebo for all depression: per protocol relative risk of response
This advantage of low dosage tricyclics was not maintained when we undertook the strict intention to treat analyses based on the worst case scenario. Effectiveness was, however, corroborated by secondary analyses based on continuous measures. People taking low dosage tricyclics had scores for severity of depression that were 0.29 (0 to 0.59; random effects model), 0.59 (0.30 to 0.87), 0.59 (0.20 to 0.99), and 0.89 (0.10 to 1.68) standard deviations lower than those taking placebo at 2 weeks, 4 weeks, 6-8 weeks, and 3-12 months, respectively. Heterogeneity was noted for all these time periods (fig 3).
Figure 3.
Low dosage tricyclics versus placebo for all depression: per protocol standardised weighted mean difference in depressive severity
Acceptability
No difference was found in total number of dropouts between low dosage tricyclics and placebo groups (relative risk 1.08, 0.93 to 1.26). Overall, 439 of 1840 (24%) enrolled participants dropped out by the end of the trial. People taking low dosage tricyclics, however, were 111% (35% to 228%) more likely than those taking placebo to drop out due to side effects. People taking low dosage tricylics were also 63% (36% to 95%) more likely to experience at least one side effect.
Funnel plot analysis and sensitivity analyses
The funnel plot showed some publication bias because the five smallest studies reported large relative risks in favour of low dosage tricyclics. These studies mainly dated from the 1960s and ‘70s and involved patients recruited outside a clinical setting. When we omitted these studies the plot was no longer asymmetrical and the relative risk decreased only slightly from 1.65 (1.36 to 2.00) to 1.58 (1.31 to 1.90) at 4 weeks, and from 1.47 (1.12 to 1.94) to 1.28 (1.05 to 1.55) at 6-8 weeks. The outcome at 6-8 weeks was no longer heterogeneous. The pooled standardised mean difference for the continuous outcome changed to –0.31 (–0.47 to –0.15) at 4 weeks and –0.32 (–0.49 to –0.15) at 6-8 weeks; these results were also no longer heterogeneous.
When we limited the included studies to those that used operational diagnostic criteria for depression, the results were essentially identical. When we limited the included studies to patients taking less than 75 mg/day of tricyclics they were still more likely to show response than those taking placebo at 4 weeks (relative risk 1.63, 1.29 to 2.07). The corresponding standardised mean difference in measurements of depression was –0.44 (–0.72 to –0.17). Patients receiving this minimal dosage were still more likely to drop out due to side effects (relative risk 2.17, 1.05 to 4.50) or to experience at least one side effect (relative risk 2.18, 1.28 to 3.73) than those taking placebo.
Subgroup analyses
Based on these sensitivity analyses, we did not need to restrict ourselves to studies employing operational diagnostic criteria or to those that administered strictly low dosage tricyclics to arrive at conclusions generalisable to present day patients. The following subgroup analyses therefore deal with studies treating any depression.
Old people—Only five studies explicitly dealt with depression in people aged 65 or more (n=265). Due to lack of power, the meta-analysis of these five studies produced only the following significant findings. The people taking low dosage tricyclics were more likely to show response at 6-8 weeks than those taking placebo, but they were also more likely to experience at least one side effect (relative risk 1.52, 1.09 to 2.11 and 1.26, 1.10 to 1.45, respectively). The point estimates of the obtained relative risks and standardised mean differences were in accordance with the overall results.
Primary care settings—Five studies recruited patients with depression in primary care settings; five further studies included patients with various physical conditions such as migraine, low back pain, and rheumatoid arthritis, presumably in primary care settings.
Overall there were 558 participants. Although there were no significant findings in the random effects estimates of our primary outcome, the resulting 95% confidence intervals were compatible with the overall findings. For example, at 4 weeks participants taking low dosage tricyclics were 28% (–2% to 68%; random effects model) more likely to show response than those taking placebo; at 6-8 weeks, participants taking low dosage tricyclics were 23% (–3% to 55%) more likely to do so. The continuous outcomes were again supportive of the overall conclusions because the standardised mean difference was –0.29 (–0.58 to 0.01) at 4 weeks and –0.41 (–0.62 to –0.19) at 6-8 weeks.
Psychiatric settings—We also performed a meta-analysis on only such studies that made clear that they were conducted with patients seen in psychiatric settings and not comorbid with other major psychiatric disorders such as eating disorders or Alzheimer’s disease. Twelve studies (n=912) were available; two with inpatients and the others with outpatients.
Among the psychiatric patients, the relative risk for showing response with low dosage tricyclic rather than with placebo was 2.40 (1.11 to 5.16; random effects model) at 2 weeks, 1.89 (1.24 to 2.87) at 4 weeks, 1.66 (0.87 to 3.15) at 6-8 weeks, and 2.06 (1.34 to 3.17) at 6 months. The standardised mean difference also supported the effectiveness of low dosage tricyclics in psychiatric patients with depression. Exclusion of the outliers lessened heterogeneity associated with some of the results but did not substantially affect the relative risks or standardised mean differences. The tricyclic was more likely to cause dropouts due to side effects or at least one side effect (relative risk 3.80, 1.63 to 8.86 and 1.43, 1.19 to 1.73, respectively) than placebo.
Low dosage tricyclics versus standard dosage tricyclics
Effectiveness
Standard dosage tricyclics were not significantly more effective at achieving response than low dosage tricyclics at 1-8 weeks (fig 4): relative risk 0.89 (0.74 to 1.07) at 4 weeks and 1.11 (0.76 to 1.61) at 6-8 weeks. In terms of the standardised mean difference, standard dosage tricyclics outperformed the low dosage tricyclics at 4 weeks only (standardised weighted mean difference 0.29, 0.08 to 0.50).
Figure 4.
Low dosage tricyclics versus standard dosage tricyclics for all depression: per protocol relative risk of response
Acceptability
Overall there was no difference in the acceptability of the treatments when measured by leaving study early for any reason (relative risk 0.95, 0.75 to 1.20). Low dosage regimens, however, were 55% (24% to 73%) less likely than standard dosage regimens to cause dropouts due to side effects.
Discussion
Low dosage tricyclic antidepressants between 75 and 100 mg/day and possibly below this range brings about more reduction in depression at 4-8 weeks of treatment and beyond, as well as more dropouts due to side effects and more people with at least one side effect than placebo in both primary care and psychiatric settings. Given the average event rates for controls in the included studies, the number needed to treat to bring about response in depression was between 4 and 6 at 1-6 months of treatment, and the number needed to harm to produce one dropout due to side effects was around 24. Standard dosage tricyclics, however, may or may not be able to bring about more reduction in depression than low dosage tricyclics, although they cause more dropouts due to side effects than placebo (number needed to harm around 11).
Reaching definitive conclusions from these data, however, is not straightforward. The strength of our conclusions is compromised by several factors. Firstly, the quality of the included studies was not ideal. The success of blinding was not ascertained in any. Many studies did not employ operational diagnostic criteria and interview schedules to diagnose depression. Some studies used ad hoc outcome measures of unknown reliability and validity. Although the dropout rates were not high overall, as our worst case scenario intention to treat analyses showed, they were large enough to hamper drawing definitive conclusions. The dropout is always a problem but here it is even more prominent because, in the case of low dosage tricyclics, there is a trade-off between response and dropouts. If dropouts are not dealt with appropriately, the higher dosage always wins because it increases response at the expense of dropouts. Secondly, the quality of reporting in the included studies was not ideal. We are uncertain whether the random allocation was adequately concealed in most of the studies. Some studies failed to report standard deviations for their outcome measures. Thirdly, and perhaps due to the above factors, we noted heterogeneity for some of the pooled results. A few studies were extreme outliers, all in favour of the low dosage regimen. Lastly, most of the included studies lasted up to eight weeks only.
We evaluated the seriousness of these shortcomings with several sensitivity analyses. Omitting the positive small studies removed heterogeneity of the pooled analyses and yet showed little changes in relative risks and standardised mean differences. Limiting the studies to those that employed modern operational diagnostic criteria or those that used strictly low dosage regimens did not materially affect the pooled estimates of effect sizes.
These sensitivity analyses greatly strengthen the inferences that in the treatment of depression tricyclics at dosages lower than the usually recommended range are more effective than placebo but possibly a little bit less effective than standard dosage tricyclics although with fewer side effects. The evidence suggests that academicians have been on weak ground in criticising clinicians' use of low dose tricyclics. Every trial protocol should include strategies for ensuring follow up of all the participants even if they stop the prescribed drug, because it is the only way to adhere to the intention to treat principle and to produce results permitting strong inferences about treatment effects. Only then can the relative benefits and harms of various dosages be definitively established.
Table.
Details of included studies
| Study
|
Methods (allocation, blindness, and duration)
|
Participants (diagnosis, age group, patient status)
|
Interventions
|
Outcomes (depression severity and response)
|
|---|---|---|---|---|
| Ahmedw1 | Random, “double blind,” 12 weeks | “Internal heat” (75% were potentially depressed), mainly adult, outpatients | Imipramine 50 mg/d, placebo, or benzoctamin | Ad hoc depression severity scale |
| Blashkiw2 | Random with adequate concealment, “double blind,” 4 weeks | Ad hoc operational criteria for depression, mainly adult (mean 38), outpatients at general practice | Amitriptyline 150 mg/d or 75 mg/d or placebo | Hamilton rating scale for depression-17; ⩾50% reduction in score calculated from mean and SD |
| Brickw3 | Random, “double blind,” 7 weeks | Mild to severe (except very severe) depression or anxiety according to the Minnesota multiphasic personality inventory and Taylor, mainly adult (mean 34-36), inmates | Amitriptyline 30 mg/d, placebo, or amitriptyline with emylcamate | Minnesota multiphasic personality inventory—depression scale; excellent or good response according to patients' subjective evaluation |
| Burchw4 | Random, “double blind,” 4-6 weeks | Primary depressive illness according to Feighner criteria, mainly adult (range 18-65), inpatients | Amitriptyline 40 mg/d (28-70 mg/d) or 158 mg/d (55-280 mg/d) | Montogomery-Asberg depression rating scale; ⩽9 |
| Burchw5 | Random, “double blind,” 4-6 weeks | Primary depressive illness according to Feighner criteria, mainly old (range ⩽65), inpatients | Amitriptyline 57.5 mg/d (20-125 mg/d) or 144 mg/d (100-190 mg/d) | Montogomery-Asberg depression rating scale; <9 |
| Couchw6 | Random, “double blind,” 4 weeks | Migraine with Hamilton rating scale for depression-18 ⩾14, at least mildly depressed, mainly adult (range 15-60), outpatients at headache clinic | Amitriptyline 94 mg/d (50-100 mg/d) or placebo | HRSD-18; becoming “non-depressed” |
| Diamondw7 | Random, “double blind,” 4 weeks | Chronic tension headache with “depression” or “anxiety and depression,” mainly adult (range 20-60) | Amitriptyline <60 mg/d or placebo | 4 point scale; excellent or good according to physician's global evaluation |
| GramW8 | Random, “double blind,” 6 weeks | DSM-III-R major depression, mainly adult (range 18-70), outpatients and inpatients | Clomipramine 25 mg/d, 50 mg/d, 75 mg/d, 125 mg/d, or 200 mg/d | Hamilton rating scale for depression-17; ⩽7 |
| Fryerw9 | Random, “double blind,” 4 weeks | ⩾70 of T score for depression subscale of Minnesota multiphasic personality inventory, mainly adult, inpatients | Imipramine 100 mg/d or placebo | Minnesota multiphasic personality inventory depression subscale |
| Goldbergw10 w11 | Random, “double blind,” 4 weeks | “Anxiety neurosis” (usually mixed anxiety and depression), mainly adult (range 19-59), outpatients | Doxepin 78.4 mg/d (25-150 mg/d) or placebo | Noticeable to moderate change on overall global improvement |
| Goldbergw12 | Random, “double blind,” 4 weeks | “Psychoneurotic (mixed anxiety depressive), mainly adult (range 19-60), outpatients | Doxepin 94.6 mg/d (50-100 mg/d) or placebo | Noticeable to moderate change on overall global improvement |
| Goldbergw13 | Random, “double blind,” 6 weeks | Neurotic depression according to New York University criteria | Amitriptyline 91.5 mg/d (75-200 mg/d), placebo, or trazodone | Hamilton rating scale for depression-21; ⩾50% reduction in score |
| Hollandaw14 | Random, “double blind,” 4 weeks | Endogenous or involutional or reactive depression according to traditional criteria, mainly adult (range 17-58), status not specified | Doxepin 60 mg/d or placebo | Noticeable to moderate overall improvement |
| Hormazabalw15 | Random, “double blind,” 4 weeks | Depressive psychosis, depressive neurosis, reactive depression, and others, mainly adult (mean 44-42), mainly outpatients | Amitriptyline 86.4 (SD=21) mg/d, placebo, or cianopramine | Hamilton rating scale for depression-21; noticeable to moderate improvement on global evaluation |
| Houstonw16 | Random, “double blind,” 52 weeks | ⩾7 on Leeds scale, mainly adult (range 20-46), outpatients at general practice | Amitriptyline (slow release) 50 mg/d or placebo | Leeds scale D score; ⩾50% reduction in Leeds scale calculated from mean and SD |
| Jacobsonw17 | Random, “double blind,” 4 weeks | Depression according to Feighner criteria, unknown, unknown | Amitriptyline 75-100 mg/d, placebo, or amitriptyline with chlordiazepoxide | Hamilton rating scale for depression-24; ⩾50% reduction in score calculated from mean and SD |
| Jenkinsw18 | Random, “double blind,” 4 weeks | Low back pain with Becks depression inventory score ⩾14, mainly adult (range 18-49), unknown | Imipramine 75 mg/d or placebo | Beck depression inventory; ⩾50% reduction in score |
| Kerrw19 | Random, “double blind,” 4 weeks | “Anxiety and depression associated with menopause,” mainly adult (range 44-57), unknown | Amitriptyline 55 mg/d (30-100 mg/d) or placebo | One item assessment in 5 grades; “marked improvement or complete relief of symptoms” |
| Laederach-Hofmannw20 | Random, “double blind,” 8 weeks | Obese binge eaters not being anorexic or bulimic according to DSM-IV (baseline Hamilton rating scale for depression-21 scores were 21 to 23 on average), mainly adult (range 20-60), outpatients | Imipramine 75 mg/d or placebo | Hamilton rating scale for depression-21, modified; ⩾50% reduction in score calculated from mean and SD |
| Lecrubierw21 w22 | Random, “double blind,” 6 months | DSM-III-R dysthymia (40%), dysthymia with major depression (40%), and major depression in partial remission (20%); mainly adult (range 18-73, mean 43); outpatients | Imipramine 100 mg/d, placebo, or amisulpiride | Montgomery-Asberg depression rating scale; much improved or much improved on clinical global impression |
| Macfarlanew23 | Random, “double blind,” 12 weeks | Rheumatoid arthritis with score of ⩾50 on Zung self rating depression scale, mainly adult (range 18-73), outpatients | Trimipramine 25-75 mg/d or placebo | Zung self rating depression scale; ⩾50% reduction in score calculated from mean and SD |
| Morakinyow24 | Random, “double blind,” 6 weeks | “Depression” according to traditional diagnosis, mainly adult (range 20-50), unknown | Amitriptyline 75 mg/d, placebo, or amitriptyline with chlordiazepoxide | “Improved” according to overall response in three grades of “improved,” “doubtful,” and “not improved” |
| Murphyw25 w26 | Random, “double blind,” 6 weeks | “Depression” according to traditional diagnosis, mainly adult (range 18-70), outpatients at general practice | Imipramine 100 mg/d, placebo, or mianserin | Ad hoc physician scale; ⩾50% reduction in score calculated from mean and SD |
| Nandiw27 | Random with adequate allocation concealment, “double blind,” 4 weeks | “Depression” according to traditional diagnosis, mainly adult, community residents | Imipramine 97.4 mg/d, placebo, or natural process | Hamilton rating scale for depression; ⩾50% reduction in score calculated from mean and SD |
| Petracaw28 | Random, “double blind,” 6 weeks | DSM-III-R dysthymia or major depression, mainly old (mean 72), unknown | Clomipramine 100 mg/d or placebo | Hamilton rating scale for depression-17; ⩾50 reduction in score calculated from mean and SD |
| Philippw29 | Random, “double blind,” 8 weeks | ICD-10 moderate depressive episode (patients with mild or severe condition excluded), mainly adult (range 18-65), outpatients at general practice | Imipramine 100 mg/d, placebo or hypericum extract | Hamilton rating scale for depression-17; much or very much improved on clinical global impression |
| Rampellow30 | Random, “double blind,” 6 weeks | DSM-III-R major depression or bipolar depression, anxious, mainly adult (range 18-62), outpatients | Amitriptyline 100 mg/d, placebo, or amineptine | Hamilton rating scale for depression; ⩾50% reduction in score calculated from mean and SD |
| Reiflerw31 w32 | Random, “double blind,” 8 weeks | Alzheimer's disease with major depression (DSM-III), mainly old (mean 72), outpatients | Imipramine 83 mg/d or placebo | Hamilton rating scale for depression-17; ⩾50% reduction in score calculated from mean and SD |
| Rickelsw33 w34 | Random, “double blind,” 4 weeks | “Mildly to moderately depressed” (reactive neurotic depression, mixed anxiety depressive reaction), mainly adult (mean 43-45), outpatients | Amitriptyline 100 mg/d or placebo | Physician depression scale |
| Rickelsw35 | Random, “double blind,” 4 weeks | “Symptoms of depression and anxiety,” mainly adult, volunteers | Amitriptyline 70 mg/d (50-100 mg/d) or placebo | Physician depression scale; moderate to noticeable global improvement |
| Robertsonw36 | Random, “double blind,” 6 weeks | Epilepsy with research diagnostic criteria major depression, mainly adult (range 18-70), unknown | Amitriptyline 75 mg/d or placebo | Hamilton rating scale for depression-21; ⩾50% reduction in score calculated from mean and SD |
| Rouillonw37 | Random, “double blind,” 8 weeks | DSM-III-R residual depression, mainly adult (range 18-65), outpatients | Clomipramine 97.5 mg/d (75-150 mg/d) or placebo | Montgomery-Asberg depression rating scale; <10 |
| Schweizerw38 w39 | Random, “double blind,” 8 weeks | DSM-III-R unipolar major depression, comorbid with various chronic physical conditions, mainly old (range 65-89, mean 72), outpatients | Imipramine 89 mg/d (25-150 mg/d) or placebo | Hamilton rating scale for depression-17; ⩾50% reduction in score |
| Simpsonw40 | Random, “double blind,” 6 weeks | Research diagnostic criteria endogenous major depression, mainly adult (range 22-60), outpatients | Trimipramine 75 mg/d or 150 mg/d | Hamilton rating scale for depression-21; noticeable to moderate improvement on clinical global impression |
| Tanw41 w42 | Random, “double blind,” 4 weeks | “Depression” without dementia or major physical illness, mainly old (>65, mean 80), inpatients | Lofepramine 70 mg/d or placebo | Montgomery-Asberg depression rating scale; ⩾50% reduction in score calculated from mean and SD |
| Tetreaultw43 | Random, “double blind,” 6 weeks | Kiloh and Garside's “neurotic reactive depression,” not specified, inpatients | Imipramine 50-100 mg/d or placebo | Wechsler scale; ⩾50% reduction in score calculated from mean and SD |
| Thompsonw44 | Random, “double blind,” 4 weeks | Doctor's usual diagnosis of “depression” (73% were research diagnostic criteria definite or probable major depression), not specified, outpatients at general practice | Dothiepin 75 mg/d or placebo | Hamilton rating scale for depression-17; ⩾50% reduction in score calculated from mean and SD |
| Tyrerw45 w46 | Random, “double blind,” 6 weeks | DSM-III dysthymic disorder, mainly adult (range 17-76), outpatients at general practice | Dothiepin 51.3 mg/d (25-150 mg/d) or placebo | Montgomery-Asberg depression rating scale |
| Weissmanw47 | Random, “double blind,” 6 weeks | DSM-III moderate to severe major depression, with minor chronic physical conditions, mainly old (range 60-85), outpatients | Imipramine 97.5 mg/d (25-225 mg/d) or placebo | Hamilton rating scale for depression-23; ⩾50% reduction in score calculated from mean and SD |
| WHO (Cali)w48 | Random, “double blind,” 4 weeks | ICD-9 manic-depressive illness or neurotic depression, mainly adult (range 18-65), outpatients (70%) and inpatients (30%) | Amitriptyline 37.5-75 mg/d or 75-150 mg/d | Hamilton rating scale for depression-17; ⩾50% reduction in score calculated from mean and SD |
| WHO (Lucknow)w49 | Random, “double blind,” 4 weeks | ICD-9 manic depressive illness or neurotic depression, mainly adult (range 18-65), outpatients (20%) and inpatients (80%) | Imipramine 37.5-75 mg/d or 75-150 mg/d | Hamilton rating scale for depression-17; ⩾50% reduction in score calculated from mean and SD |
| WHO (Nagasaki)w50 | Random, “double blind,” 4 weeks | ICD-9 manic depressive illness or neurotic depression, mainly adult (range 18-65), outpatients (85%) and inpatients (15%) | Amitriptyline 37.5-75 mg/d or 75-150 mg/d | Hamilton rating scale for depression-17; ⩾50% reduction in score calculated from mean and SD |
| WHO (Nashville)w51 | Random, “double blind,” 4 weeks | ICD-9 manic depressive illness or neurotic depression, mainly adult (range 18-65), outpatients | Imipramine 37.5-75 mg/d or 75-150 mg/d | Hamilton rating scale for depression-17; ⩾50% reduction in score calculated from mean and SD |
w1 Ahmed MH, Onyemelukwe GC, Onyewoto II. A double blind controlled clinical trial of benzoctamine (Tacitin) and imipramine (Tofranil) in the treatment of “internal heat” and its associated symptoms. East African Medical Journal 1988;65(4):230-7.
w2 Blashki TG, Mowbry R, Davies B. Controlled trial of amitriptyline in general practice. British Medical Journal 1971;1:133-8.
w3 Brick H, Doub WH- Jr, Perdue WC. Effects of amitriptyline on depressive and anxiety states in penitentiary inmates. Diseases of the Nervous System 1962;23:572-8.
w4 Burch JE, Ahmed O, Hullin RP, Mindham RH. Antidepressive effect of amitriptyline treatment with plasma drug levels controlled within three different ranges. Psychopharmacology 1988;94(2):197-205.
w5 Burch JE, Ahmed O, Hullin RP, Mindham RH. Antidepressive effect of amitriptyline treatment with plasma drug levels controlled within three different ranges. Psychopharmacology 1988;94(2):197-205.
w6 Couch JR, Hassanein RS. Amitriptyline in migraine prophylaxis. Archives of Neurology 1979;36(11):695-9.
w7 Diamond S, Baltes BJ. Chronic tension headache - treated with amitriptyline - a double-blind study. Headache 1971;11(3):110-6.
w8 Gram LF, Kragh-Sorensen P, Bech P, Bolwig TG, Vestergaard P, Larsen JK. Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clinical Pharmacology and Therapeutics 1999;66(2):152-65.
w9 Fryer DG, Timberlake WD. A trial of imipramine (Tofranil) in depressed patients with chronic physical disease. Journal of Chronic Diseases 1963;16:173-8.
w10 Goldberg HL, Finnery RJ. The use of doxepin in the treatment of symptoms of anxiety neurosis and accompanying depression: a collaborative controlled study. American Journal of Psychiatry 1972;129(1):74-7.
w11 Goldberg HL, Finnerty RJ, Cole JO. Doxepin: is a single daily dose enough? American Journal of Psychiatry 1974;131(9):1027-9.
w12 Goldberg HL, Finnerty RJ. Trazodone in the treatment of neurotic depression. Journal of Clinical Psychiatry 1980;41(12Pt1):430-4.
w13 Goldberg HL, Finnerty RJ, Cole JO. Doxepin: is a single daily dose enough? American Journal of Psychiatry 1974;131(9):1027-9.
w14 Hollanda Junior L, Silva CN, da, Lira BS, Ferreira SC. Double-blind clinical study with a new psychotropic agent: doxepin versus placebo. Hospital Rio Journal 1970;77(3):799-803.
w15 Hormazabal L, Omer LM, Ismail S. Cianopramine and amitriptyline in the treatment of depressed patients: a placebo-controlled study. Psychopharmacology 1985;86(1-2):205-8.
w16 Houston J, Berg I, Butler A, McGuire R. Amitriptyline for depressed women with young children in general practice. British Journal of Psychiatry 1983;142:103-4.
w17 Jacobson AF. Doctor-patient concordance in a placebo-controlled trial of limbitrol versus its components proceedings. Psychopharmacology Bulletin 1978;14(3):61-3.
w18 Jenkins DG, Ebbutt AF, Evans CD. Tofranil in the treatment of low back pain. Journal of International Medical Research 1976;4:28-40.
w19 Kerr MM. Amitriptyline in emotional states at the menopause. New Zealand Medical Journal 1970;72(461):243-5.
w20 Laederach-Hofmann K, Graf C, Horber F, Lippuner K, Lederer S, Michel R, Schneider M. Imipramine and diet counseling with psychological support in the treatment of obese binge eaters: a randomized, placebo-controlled double-blind study. International Journal of Eating Disorders 1999;26(3):231-44.
w21 Boyer P, Lecrubier Y. Atypical antipsychotic drugs in dysthymia: placebo controlled studies of amisulpride versus imipramine, versus amineptine. European Psychiatry: the Journal of the Association of European Psychiatrists 1996;11(suppl 3):S135-40.
w22 Lecrubier Y, Boyer P, Turjanski S, Rein W. Amisulpride versus imipramine and placebo in dysthymia and major depression Amisulpride Study Group. Journal of Affective Disorders 1997;43(2):95-103.
w23 Macfarlane JG, Jalali S, Grace EM. Trimipramine in rheumatoid arthritis: a randomized double-blind trial in relieving pain and joint tenderness. Current Medical Research and Opinion 1986;10:89-93.
w24 Morakinyo VO. Amytriptyline and chlordiazepoxide (Limbitrol) in depressive states in Nigeriansa double-blind study. African Journal of Medical Sciences 1970;1(4):409-14.
w25 Murphy JE, Donald JF, Molla AL. Mianserin in the treatment of depression in general-practice. Practitioner 1976;217(1297):135-8.
w26 Murphy JE. Mianserin in the treatment of depressive illness and anxiety states in general-practice. British Journal of Clinical Pharmacology 1978;5(suppl 1):81S-5S.
w27 Nandi DN, Ajmany S, Ganguli H, Banerjee G, Boral GC, Ghosh A, Sarkar S. A clinical evaluation of depressives found in a rural survey in India. British Journal of Psychiatry 1976;128:523-7.
w28 Petracca G, Teson A, Chemerinski E, Leiguarda R, Starkstein SE. A double-blind placebo-controlled study of clomipramine in depressed patients with Alzheimer's disease. Journal of Neuropsychiatry and Clinical Neurosciences 1996;8(3):270-5.
w29 Philipp M, Kohnen R, Hiller K-O. Hypericum extract versus imipramine or placebo in patients with moderate depression: randomised multicentre study of treatment for eight weeks. British Medical Journal 1999;319:1534-8.
w30 Rampello L, Nicoletti G, Raffaele R, Drago F. Comparative effects of amitriptyline and amineptine in patients affected by anxious depression. Neuropsychobiology 1995;31(3):130-4.
w31 Reifler BV, Teri L, Raskind M, Veith R, Barnes R, White E, McLean P. Double-blind trial of imipramine in Alzheimer's disease patients with and without depression. American Journal of Psychiatry 1989;146(1):45-9.
w32 Teri L, Reifler BV, Veith RC, Barnes R, White E, McLean P, Raskind M. Imipramine in the treatment of depressed Alzheimer's patients: impact on cognition. Journal of Gerontology 1991;46(6):P372-7.
w33 Rickels K, Gordon PE, Jenkins BW, Perloff M, Sachs T, Stepansky W. Drug treatment in depressive illness. Diseases of the Nervous System 1970;31(1):30-42.
w34 Rickels K, Hesbacher P, Downing RW. Differential drug effects in neurotic depression. Diseases of the Nervous System 1970;31(7):468-75.
w35 Rickels K, Csanalosi I, Chung HR, Case WG, Pereira Ogan A, Downing RW. Amitriptyline in anxious-depressed outpatients: a controlled study. American Journal of Psychiatry 1974;131(1):25-30.
w36 Robertson MM, Trimble MR. The treatment of depression in patients with epilepsy A double-blind trial. Journal of Affective Disorders 1985;9(2):127-36.
w37 Rouillon F, Markabi S, Febvre N, Phillips R, Vaillant J. Controlled study of treatment of residual depression by clomipramine versus placebo. Encephale 1994;20(2):139-45.
w38 Schweizer E, Rickels K, Hassman H. A double-blind, placebo-controlled comparison of imipramine and buspirone in the treatment of major depression in the elderly in the community. Psychopharmacology Bulletin 1994:639.
w39 Schweizer E, Rickels K, Hassman H, Garcia Espana F. Buspirone and imipramine for the treatment of major depression in the elderly. Journal of Clinical Psychiatry 1998;59(4):175-83.
w40 Simpson GM, Pi EH, Gross L, Baron D, November M. Plasma levels and therapeutic response with trimipramine treatment of endogenous depression. Journal of Clinical Psychiatry 1988;49(3):113-6.
w41 Tan RS, Barlow RJ, Abel C, Reddy S, Palmer AJ, Fletcher AE, Nicholl CG, Pitt BM, Bulpitt CJ. The effect of low dose lofepramine in depressed elderly patients in general medical wards. British Journal of Clinical Pharmacology 1994;37(4):321-4.
w42 Tan RS. Lowering antidepressant dosages in the elderly. Clinical Gerontologist 1995;16(1):67-70.
w43 Tetreault L, Doucet P, Blanchet A, Bordeleau JM. Comparative evaluation of the antidepressive properties of opipramol, imipramine and placebo in neurotic depression. L'union Médicale du Canada 1966;95(5):546-53.
w44 Thompson C, Thompson CM. The prescribing of antidepressants in general practice II: a placebo-controlled trial of low-dose dothiepin. Human Psychopharmacology 1989;4:191-204.
w45 Tyrer P, Seivewright N, Murphy S, Ferguson B, Kingdon D, Barczak P, Brothwell J, Darling C, Gregory S, Johnson AL. The Nottingham study of neurotic disorder: comparison of drug and psychological treatments. Lancet 1988;2:235-40.
w46 Tyrer P, Seivewright N, Ferguson B, Murphy S, Darling C, Brothwell J, Kingdon D, Johnson AL. The Nottingham Study of Neurotic Disorder: relationship between personality status and symptoms. Psychological Medicine 1990;20(2):423-31.
w47 Weissman MM, Prusoff B, Sholomskas AJ, Greenwald S. A double-blind clinical trial of alprazolam, imipramine, or placebo in the depressed elderly. Journal of Clinical Psychopharmacology 1992;12(3):175-82.
w48 Dose effects of antidepressant medication in different populations A World Health Organization collaborative study. Journal of Affective Disorders 1986;(suppl 2):1-67.
w49 Dose effects of antidepressant medication in different populations A World Health Organization collaborative study. Journal of Affective Disorders 1986;(suppl 2):1-67.
w50 Dose effects of antidepressant medication in different populations A World Health Organization collaborative study. Journal of Affective Disorders 1986;(suppl 2):1-67.
w51 Dose effects of antidepressant medication in different populations A World Health Organization collaborative study. Journal of Affective Disorders 1986;(suppl 2):1-67.
Acknowledgments
This systematic review was conducted within the framework of the Cochrane Collaboration Depression, Anxiety, and Neurosis Group. We thank Gordon Guyatt and David Streiner for their helpful comments on earlier drafts.
Footnotes
Funding: St Luke's Life Science Institute, Tokyo, Japan provided 700 000 yen (£3659; $5696; €5835).
Competing interests: TAF has received fees for speaking from several pharmaceutical companies, some of which manufacture various types of antidepressants including paroxetine, fluroxamine, milnacipran.
References
- 1.Lawrenson RA, Tyrer F, Newson RB, Farmer RD. The treatment of depression in UK general practice: selective serotonin reuptake inhibitors and tricyclic antidepressants compared. J Affect Disord. 2000;59:149–157. doi: 10.1016/s0165-0327(99)00147-0. [DOI] [PubMed] [Google Scholar]
- 2.Pincus HA, Tanielian TL, Marcus SC, Olfson M, Zarin DA, Thompson J, et al. Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA. 1998;279:526–531. doi: 10.1001/jama.279.7.526. [DOI] [PubMed] [Google Scholar]
- 3.Sleath BL, Rubin RH, Huston SA. Antidepressant prescribing to Hispanic and non-Hispanic white patients in primary care. Ann Pharmacother. 2001;35(4):419–423. doi: 10.1345/aph.10245. [DOI] [PubMed] [Google Scholar]
- 4.Roberts E, Norris P. Growth and change in the prescribing of anti-depressants in New Zealand: 1993-1997. N Z Med J. 2001;114:25–27. [PubMed] [Google Scholar]
- 5.Depression Guideline Panel. Clinical practice guideline: depression in primary care 2: treatment of major depression. Rockville, MD: US Department of Heath and Human Services, Agency for Health Care Policy and Research; 1993. . [AHCPR Publication 93-0551.] [Google Scholar]
- 6.Anderson IM, Nutt DJ, Deakin JF. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. British Association for Psychopharmacology. J Psychopharmacol. 2000;14:3–20. doi: 10.1177/026988110001400101. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (Revision) Am J Psychiatry. 2000;157(Apr suppl):1–45. [PubMed] [Google Scholar]
- 8.Paykel ES, Priest RG. Recognition and management of depression in general practice: consensus statement. BMJ. 1992;305:1198–1202. doi: 10.1136/bmj.305.6863.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschfeld RM, Keller MB, Panico S, Arons BS, Barlow D, Davidoff F, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277:333–340. [PubMed] [Google Scholar]
- 10.Furukawa TA, Kitamura T, Takahashi K. Treatment received by depressed patients in Japan and its determinants: naturalistic observation from a multi-center collaborative follow-up study. J Affect Disord. 2000;60:173–179. doi: 10.1016/s0165-0327(99)00175-5. [DOI] [PubMed] [Google Scholar]
- 11.Donoghue J, Taylor DM. Suboptimal use of antidepressants in the treatment of depression. CNS Drugs. 2000;13:365–383. [Google Scholar]
- 12.Mulrow CD, Oxman AD. Cochrane library. Issue 4. Oxford: Update Software; 1997. Cochrane collaboration handbook [updated 1 Mar 1997] [Google Scholar]
- 13.Furukawa TA, Guyatt GH, Grifitth LE. Can we individualize the Number Needed to Treat (NNT)? An empirical study of summary effect measures in meta-analyses. Int J Epidemiol. 2002;31:72–76. doi: 10.1093/ije/31.1.72. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Charette LJ, McPeek B, Mosteller F. Reporting on methods in clinical trials. N Engl J Med. 1982;306:1332–1337. doi: 10.1056/NEJM198206033062204. [DOI] [PubMed] [Google Scholar]