Abstract
Circumnutation and winding in plants are universal growth movements that allow plants to survive despite their sessile nature. However, the detailed molecular mechanisms controlling these phenomena remain unclear. We previously found that a gravitropic mutant of Japanese morning glory (Pharbitis nil or Ipomoea nil), Shidare-asagao (weeping), is defective not only in circumnutation but also in the winding response. This phenotype is similar to that of the Arabidopsis SCARECROW (SCR) mutant. We therefore investigated whether morning glory SCR (PnSCR) is involved in the weeping phenotype. We found that one amino acid was inserted into the highly conserved VHIID motif in weeping-type PnSCR; this mutation caused abnormal endodermal differentiation. We introduced either the mutant or WT PnSCR into Arabidopsis scr mutants for complementation tests. PnSCR of the WT, but not of weeping, rescued the shoot gravitropism and circumnutation of scr. These results show that both the abnormal gravitropism and the circumnutation defect in weeping are attributable to a loss of PnSCR function. Thus, our data show that gravisensing endodermal cells are indispensable for shoot circumnutation and the winding response and that PnSCR is responsible for the abnormal phenotypes of weeping.
Keywords: Arabidopsis, gravitropic mutant, gravity sensing, morning glory, SCARECROW
The stationary nature of plants distinguishes them from other organisms. Because of this unique nature, higher plants have evolved various mechanisms for responding to environmental cues, enabling them to use limited resources or escape from environmental stresses. One of the most important mechanisms that plants have acquired is the ability to sense gravity and use it as a basis for governing their growth orientation, a process known as gravitropism. Using gravitropism, plants can effectively take up water and nutrients from the soil or effectively absorb light energy from the atmosphere by expanding either roots or leaves, respectively. In addition to gravitropism, gravity affects how plants build their bodies, anchor themselves, and elevate their apical meristems to higher positions. For example, plants synthesize tough cell walls to withstand gravitational forces (1, 2), and cucurbitaceous plants develop a peg that functions to pull the seed coat out only on the gravistimulated side of the region between the root and hypocotyl (3, 4). The graviresponse also participates in the regulation of apical dominance (5). Intense studies of these forms of gravimorphogenesis led to many discoveries of the molecular mechanisms of gravitropism (6, 7). However, the mechanisms for other types of gravimorphogenesis remain unclear, despite their importance.
Plant organs display helical growth movements known as circumnutation (8-11). These movements help plant organs find suitable environmental cues. The amplitude, period, and shape of the circumnutation depend on the plant species, the plant organs involved, and the developmental stage of growth. Circumnutation interacts with other types of movements, such as tropisms (12, 13). Although the mechanism of circumnutation is unclear, Johnsson et al. (10) proposed a two-oscillator model to explain the phenomenon. In this model, circumnutational movement involves a gravitropic reaction that acts as an externally driven feedback oscillator, together with an endogenous or intrinsic oscillator that sends a rhythmic signal into the feedback system. The endogenous oscillator has been modeled as a growth wave traveling around the elongating organs that could be coupled with the oscillation of growth substances such as auxin and calcium (8, 10). There has been no direct evidence yet for the involvement of the graviresponse as an external oscillator in circumnutation, and it is rather controversial because the hypocotyls of space-flown sunflowers showed circumnutation in microgravity, although the period and amplitude of the movement were smaller (14). Recently, Yoshihara and Iino (15) reported that in rice coleoptiles circumnutation might be independent of gravitropism, but its mechanism might include gravity perception.
On the other hand, climbing plants grasp a support by various means; for example, in some plants such as morning glory their stems wind along the support for growing upward (12). Generally, relatively more substantial circumnutation can be observed in the vines and shoots of climbing plants (e.g., morning glory) that need to be anchored for support than in the shoots of nonclimbing plants (8, 9). It is therefore thought that circumnutation provides the motive power for the winding response of climbing plants, but there is no direct evidence for the causal relationship between circumnutation and winding response. Thus, the detailed mechanisms explaining the relationships among graviresponse, circumnutation, and winding response are still obscure.
To gain insight into this issue, we have used a gravitropic mutant of Japanese morning glory (Pharbitis nil or Ipomoea nil), Shidareasagao (weeping). The weeping shoots display agravitropism whereas the roots are gravitropically normal. Since its discovery in 1953, the morning glory cultivar weeping has been commonly cultivated as an ornamental plant in Japan, and horticulturalists have long been interested in the gene responsible for the abnormal phenotype. We previously found that the shoots of weeping are defective not only in circumnutation but also in the winding response (16). Thus, this mutant could be a model plant for studying the mechanisms of gravity-influenced morphogenesis, including circumnutation and the winding response.
To understand the molecular mechanisms underlying circumnutation, the winding response, and their relationship to the graviresponse, we looked for a mutated gene responsible for the phenotype of the weeping morning glory. Recently, we found that weeping lacks the proper endodermis required for gravisensing; this phenotype is very similar to the Arabidopsis agravitropic mutant sgr1/scr (16, 17). Moreover, we showed a possible role of the endodermis-mediated graviresponse in circumnutation by analyzing several Arabidopsis agravitropic mutants, including scr (16). Our observations suggested that the abnormal phenotypes of weeping were caused by defective differentiation of the endodermis. Here, we show that the abnormal phenotypes of weeping are attributable to a mutation in the P. nil SCARECROW (PnSCR) gene. The endodermis fails to develop in plants with mutant-type PnSCR (PnSCRm). By carrying out complementation experiments with the Arabidopsis scr mutant, we demonstrated that gravisensing endodermal cells are required for shoot circumnutation and winding movements in morning glory.
Materials and Methods
Plant Materials and Growth Conditions. Seeds of the weeping morning glory (P. nil or I. nill cv. Shidare-asagao, weeping) were propagated in the laboratory at Tohoku University, and seeds of cultivar Violet that is commonly used as a WT were purchased from Marutane Seed Co., Kyoto. The morning glory seeds were soaked in sulfuric acid for 60 min and then washed overnight with running tap water. The imbibed seeds were sown in vinyl pots (10 cm in diameter) filled with commercial soil composite, and seedlings were grown in a greenhouse as described by Hatakeda et al. (16).
We used two strains of Arabidopsis thaliana, the WT ecotype Columbia and the agravitropic mutant sgr1-1/scr-3, for complementation experiments. Agravitropic mutants of Arabidopsis, sgr2 and zig/sgr4, were also used for observation of their circumnutation. Arabidopsis seeds were germinated and seedlings were grown as described by Hatakeda et al. (16).
RNA Isolation and Cloning of Full-Length PnSCR cDNA. Total RNA was extracted from shoot apices of WT plants by using TRI reagent (Sigma-Aldrich) according to the manufacturer's instructions. A cDNA of WT morning glory SCR was amplified by PCR in the following way. The respective sequences of the upstream and downstream degenerate oligonucleotide primers, 5′-TTCCACATTCTTGCTTCT(C/A)G(C/A)CCTGG-3′ and 5′-TGTA(C/A)CCGTC(C/G)GAGGGGAACAT(T/G)CC-3′, were based on conserved amino acid regions of the SCR proteins of various plants. First-strand cDNA synthesis and amplification were performed with a One-Step RT-PCR Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Amplified cDNA fragments were then cloned into the pGEM-T Easy vector (Promega) according to the manufacturer's instructions. Based on the sequence information for the PnSCR cDNA, a full-length cDNA was obtained with a GeneRacer Kit (Invitrogen) according to the manufacturer's instructions. The full-length cDNA fragment from WT and weeping morning glory were amplified by PCR and cloned into pQE31 (Qiagen). DNA sequences were determined by using an ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, CA) following standard procedures.
Full-length PnSCR genomic DNA fragments from WT and weeping morning glory were amplified by PCR and cloned into a binary vector for subsequent plant transformation.
F2 Linkage Analysis. For F2 linkage analysis, weeping was crossed with Violet, and the resulting F1 plants were self-pollinated to generate F2 plants. Using the F2 population, linkage between agravitropism and mutation in PnSCR was verified with a cleaved amplified polymorphic sequence marker assay. Darkgrown F2 seedlings were first distinguished by their gravitropic responses. Then, genomic DNA was extracted from each plant, and the DNA fragment including the mutated region was amplified by PCR. The amplified PCR fragment was further digested by Sau3AI to discriminate between the WT and weeping PnSCR. We analyzed 25 individual F2 seedlings displaying either the gravitropic or agravitropic phenotype.
Plant Transformation. The PnSCR genomic DNA fragments from the WT and weeping cultivars were cloned into a pBI101 vector in which the GUS gene had been replaced with the Arabidopsis SCR (AtSCR) promoter (pAtSCR) (kindly donated by Philip N. Benfey, Duke University, Durham, NC). The PnSCR genomic DNA fragments were inserted downstream of the pAtSCR. These constructs were introduced into Agrobacterium tumefaciens strain GV3101 and transformed into the sgr1-1/scr-3 mutant by the floral dip method (18). T1 plants were selected by resistance to kanamycin. The presence of the transgene in these plants was tested by PCR.
Gravitropism Assay. Inflorescence stems, intact plants, or apical stems ≈4 cm in length excised from the young primary inflorescence were used to examine the gravitropic responses. The 4-cm-long stem included shoot apices but excluded all lateral organs. The excised inflorescence stems were preincubated in a vertical position under white f luorescent lamps (40-50 μmol·s-1·m-2) at 23°C and then placed in a horizontal position in the dark at 23°C. The curvature of the stem was measured as the angle formed between the growing direction of the apex and the horizontal baseline. At least eight individuals of each genotype were examined. To examine the gravitropic response of intact inflorescence stems, plastic pots with plants were placed in a horizontal position in the dark at 23°C.
Microscopic Observation of Inflorescence Stem Tissues. For histological analysis, stem segments were cut from primary inflorescence stems that grew upright after bolting. The segments were fixed in 10% (vol/vol) formaldehyde, 5% (vol/vol) acetic acid, and 50% (vol/vol) ethanol in 0.2-ml tubes under vacuum, with maintenance of the growth orientation of the stems. After fixation, the samples were dehydrated by a series of ethanol washes and embedded in Technovit 7100 (Heraeus Kulzer, Wehrheim, Germany) according to the manufacturer's instructions. Sections (5 μm thick) were prepared by using a rotary microtome, stained with 0.05% toluidine blue, and observed under an Olympus BX50F microscope (Olympus Optical, Tokyo).
Analysis of Nutational Movement. To observe the nutational movements of Arabidopsis shoots, potted plants were placed in a dark box when their inflorescence stems were ≈9-10 cm long. Photographs of apical shoots were taken with a digital camera (CAMEDIA E-10, Olympus) at 5-min intervals for at least 6 h. To avoid the occurrence of phototropic curvature of the inflorescence stems, plants were illuminated from above by the programmed flash photography. The amplitude of movement was measured from the images by using image analysis software (macscope 2.56, Mitani, Fukui, Japan). This image analysis also allowed the movement of shoot tips to be tracked by plotting the positions on x and y axes. 2D movements of shoot tips and the time required for shoot tips to turn around once were defined as amplitude and period, respectively. Movements of at least three individual Arabidopsis plants of each genotype were recorded and measured.
Results
Characterization of the Morning Glory SCR Gene. Fig. 1 shows the phenotypes of typical plants of the gravitropic mutant of morning glory weeping and the WT morning glory, Violet. The WT morning glory displays gravitropism and a winding response, whereas weeping does not display either phenomenon. Recently, we demonstrated that weeping lacks the normal endodermal cell layer responsible for the gravitropic response and has a defect in circumnutation (16). As Arabidopsis SCR has been shown to be necessary for proper endodermal development, we examined whether the abnormal phenotypes of weeping are caused by either the gene expression or loss of function of the morning glory homologue of SCR (PnSCR). We first used a PCR-based strategy to isolate a cDNA of the SCR-homologous gene from the WT morning glory, Violet (see Materials and Methods). Sequence analysis revealed that an ORF of the SCR-homologous gene has a coding capacity for 783 aa (Fig. 6, which is published as supporting information on the PNAS web site). The ORF encodes a protein with 58% sequence identity to the A. thaliana and Pisum sativum SCR proteins, and 61% sequence identity to the Zea mays SCR protein (Fig. 6). We therefore designated this SCR-homologous gene PnSCR (GenBank accession no. AB200391). We carried out a phylogenetic analysis of SCR family members from plants (Fig. 7, which is published as supporting information on the PNAS web site). PnSCR and SCR proteins from other plant species (P. sativum SCR, AtSCR, Z. mays SCR, and Oryza sativa SCR) form a clade, suggesting that PnSCR isolated by us encodes an ortholog of SCR. The SCR protein is a member of family of plant-specific transcriptional regulators (19), called GRAS, based on the locus designations of three genes for GIBBERELLIC ACID-INSENSITIVE (GAI), REPRESSOR OF ga1-3 (RGA), and SCR (19). The characteristic C-terminal sequence motifs of this protein family, the VHIID domain, two-Leu-heptad repeats, and SAW domain that is characterized by three pairs of absolutely conserved C-terminal residues (R-E, W-G, and W-W) (19), were identified in PnSCR (Fig. 6).
Fig. 1.
Growth of the morning glory cultivars Violet and weeping. (A) A typical plant of the WT morning glory cultivar Violet was grown for 2.5 weeks. (B) A typical plant of cultivar Shidare-asagao (weeping) was grown until its shoot displayed weeping growth. Plants were grown at 25°C under continuous white light. The diameter of the pot is 10 cm. Arrow g indicates the direction of gravitational force.
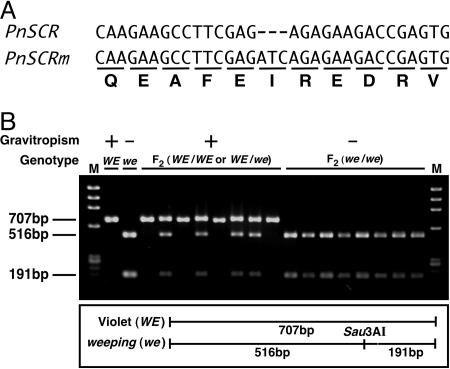
To examine the contribution of weeping-type PnSCR to the weeping phenotypes, we also isolated a cDNA of PnSCR from weeping. Sequence analysis of weeping PnSCR revealed that this gene had one amino acid inserted between amino acids 523 and 524 in the deduced peptide sequence; this insertion occurred in the VHIID motif that is highly conserved among the GRAS family proteins (Fig. 2A). The inserted amino acid was predicted to be an isoleucine.
Fig. 2.
Comparison of the VHIID motif of Violet-type PnSCR and weeping-type PnSCR and F2 linkage analysis between agravitropism and the PnSCR mutation. (A) A part of the VHIID motif of Violet-type PnSCR (PnSCR) and weeping-type PnSCR (PnSCRm). (B) Linkage between agravitropism and the mutation. Dark-grown F2 seedlings were distinguished by their gravitropic responses. Then, PnSCR was amplified by PCR from genomic DNA isolated from each F2 seedling. The PCR products were digested with Sau3AI and analyzed by agarose gel electrophoresis. M, molecular weight marker (φX174/HaeIII); WE, Violet; we, weeping. Data from eight F2 individuals of each phenotype are shown.
To investigate whether this mutation is linked to the agravitropism of weeping, we carried out a linkage analysis of F2 generations using a cleaved amplified polymorphic sequence marker so that the three-bases insertion made the cleavage site of Sau3AI. As shown in Fig. 2B, all F2 generations showing agravitropism were homozygous for the weeping-type PnSCR.
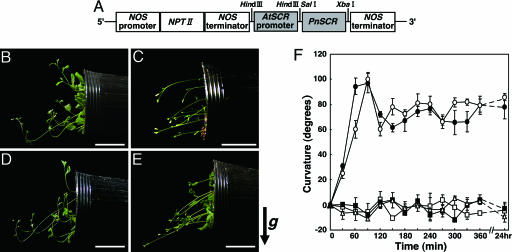
Complementation Tests for PnSCR Using scr Mutant of Arabidopsis. To verify that the PnSCR mutation causes the loss of PnSCR function, we introduced the PnSCRm or the WT PnSCR into the Arabidopsis sgr1-1/scr-3 mutant for complementation tests. The sgr1-1/scr-3 mutant lacks shoot gravitropism because of the abnormal differentiation of its endodermis. We used the pAtSCR to drive the introduced genes (Fig. 3A). It has been shown that the AtSCR gene is expressed specifically in the endodermal cell layer of the root, the hypocotyl, and the inflorescence stem (20, 21).
Fig. 3.
Shoot gravitropism of WT, scr, and transformant Arabidopsis. (A) Construct of pBI101ΔGUS::pAtSCR::PnSCR used for complementation tests. (B) Gravitropism of WT Arabidopsis. (C) Gravitropism of sgr1-1/scr-3 with pBI101ΔGUS::pAtSCR. (D) Complementation of sgr1-1/scr-3 gravitropism with pAtSCR::PnSCR.(E) Complementation test with pAtSCR::PnSCRm. WT and transgenic plants were placed in a horizontal position at 23°C for 6 h in the dark. Arrow g indicates the direction of gravitational force. (Scale bars: 2 cm.) (F) Time courses for gravitropic responses of the excised inflorescence stems of WT, sgr1-1/scr-3, and transgenic plants: WT (○), scr/PnSCR (•), scr/PnSCRm (□), scr/Vector (▪), sgr1-1/scr-3 (▵). The excised inflorescence stems were gravistimulated by being placed in a horizontal position at 23°C in the dark. Data represent means ± SE.
Inflorescence stems of the WT plants responded to gravity and began to bend upward within 30 min, and the curvature reached 90° within 90 min (Fig. 3 B and F). In contrast, both the parental scr-3 and scr-3/Vector (negative controls) did not respond gravitropically even after 24 h (Fig. 3 C and F). Transformation of scr-3 plants with pAtSCR::PnSCR restored the shoot gravitropism, providing gravitropic kinetics similar to those of the WT (Fig. 3 D and F). Thus, the WT PnSCR protein fully complemented the scr-3 phenotypes. In contrast, transformation of scr-3 plants with pAtSCR::PnSCRm did not rescue the shoot gravitropism, and the plants did not respond at all to gravity even after 24 h (Fig. 3 E and F). These results strongly suggest that the abnormal gravitropism in weeping is attributable to the loss-of-function mutation of PnSCR.
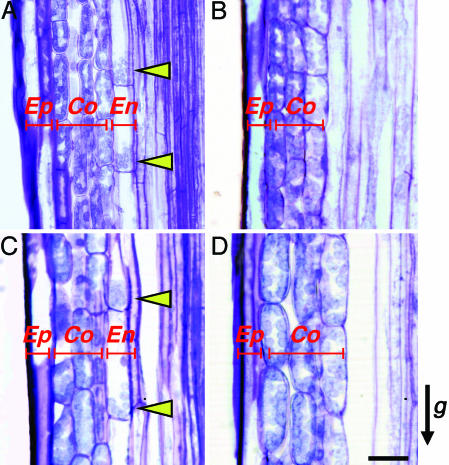
As described above, AtSCR plays an important role in the differentiation of the endodermis. If PnSCR also acts on endodermal development in the morning glory, it would be expected that pAtSCR::PnSCR could rescue the endodermal development of scr, whereas pAtSCR::PnSCRm could not rescue it. To test this hypothesis, we examined the inflorescence stem tissues of transgenic plants. In the WT shoots, one epidermal cell layer, usually three cortex layers, and one endodermal cell layer were arranged in a concentric manner from the outer side of the stem inward to its core. The endodermal cells were almost uniform in size and shape and contained amyloplasts sediment in the direction of gravity (Fig. 4A). In shoots of both scr-3 and scr-3/Vector, the cell layer containing sediment amyloplasts was not found (ref. 17 and Fig. 4B). Inflorescence stems of scr-3/pAtSCR::PnSCR plants also contained one layer of epidermis, usually three layers of cortex, and one layer of endodermis with amyloplast sedimentation (Fig. 4C), and thus were very similar to those of the WT. In contrast, we found no cell layer containing sediment amyloplasts in scr-3/pAtSCR::PnSCRm inflorescence stems (Fig. 4D). These observations confirm our hypothesis that PnSCR is necessary for the differentiation of endodermis and graviresponses in morning glory.
Fig. 4.
Histological analysis of WT and transformant Arabidopsis stained with toluidine blue. Longitudinal sections of inflorescence stems of WT (A), scr/Vector (B), scr/pAtSCR::PnSCR (C), and scr/pAtSCR::PnSCRm (D) Arabidopsis. Arrowheads indicate the sediment amyloplasts. Arrow g indicates the direction of gravitational force. Ep, epidermis; Co, cortex; En, endodermis. (Scale bar: 20 μm.)
Function of PnSCR for Shoot Circumnutation. To test whether the mutation of PnSCR causes abnormal shoot circumnutation, we analyzed the circumnutation of WT, scr, and transgenic Arabidopsis plants.
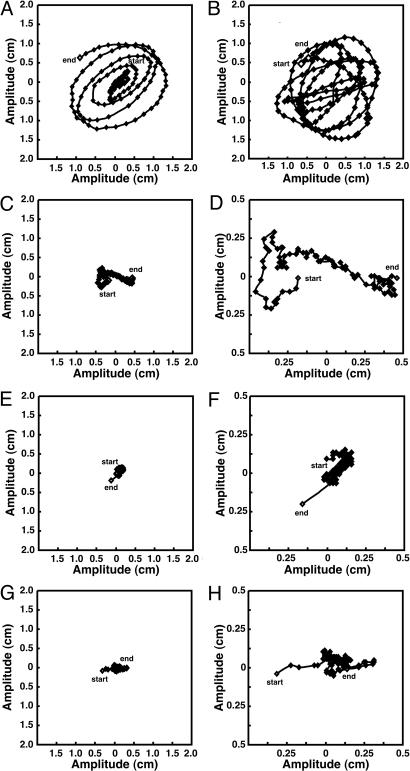
In WT inflorescences, the amplitude and period (see Materials and Methods) of circumnutation were ≈20 mm and 120 min, respectively. The apical bud movement of the WT, as observed from above, was circular or elliptical (Fig. 5A). In contrast, the scr-3 mutant and scr-3/Vector inflorescences exhibited very small movements that were not circular (Fig. 5 E-H). In scr-3/pAtSCR::PnSCR inflorescences, the circumnutation was restored to that of the WT (Fig. 5B). In contrast, inflorescences of scr-3/pAtSCR::PnSCRm plants continued to show severely reduced circumnutation (Fig. 5 C and D).
Fig. 5.
The circumnutation of inflorescence stems of WT, scr, and transformant Arabidopsis. Potted plants were placed in a dark box when their inflorescence lengths were ≈9-10 cm. Shoot movement was observed from above. (A) WT. (B) scr/pAtSCR::PnSCR. (C) scr/pAtSCR::PnSCRm. (E) sgr1-1/scr-3. (G) scr/Vector. D, F, and H show magnified images of C, E, and G, respectively. The start and the end of the movement are indicated by ⋄.
Growth and Development of Transgenic Plants. Because the scr-3 mutant displays a dwarf phenotype, it has been suggested that SCR is involved in the determination of plant size (22). To investigate whether PnSCR is involved in the determination of plant sizes and how PnSCRm affects plant sizes, we analyzed the phenotypes of transgenic Arabidopsis plants.
In scr-3/pAtSCR::PnSCR plants, the length and width of the inflorescence stem and the lengths of rosette leaves were identical to those of the WT. In contrast, scr-3/pAtSCR::PnSCRm plants showed a middle phenotype between WT and scr-3 (Table 1). Thus, PnSCRm only partially brought about dwarf phenotype, which differed from that of other scr mutants. Therefore, the weeping mutation appeared to be a novel type of mutation among those of scr mutants reported.
Table 1. Phenotype of inflorescence stems and rosette leaves of WT, transformants, and scr Arabidopsis.
| Phenotype
|
|||||
|---|---|---|---|---|---|
| Length/width | WT | PnSCR | PnSCRm | Vector | sgr1-1/scr-3 |
| Length of inflorescence stems, cm* | 36.8 ± 0.9 | 37.2 ± 1.5 | 27.5 ± 1.0 | 16.1 ± 1.4 | 15.1 ± 1.4 |
| Width of inflorescence stems, mm† | 0.98 ± 1.26 | 0.99 ± 0.03 | 0.67 ± 0.01 | 0.50 ± 0.04 | 0.46 ± 0.03 |
| Length of rosette leaves, mm‡ | 31.5 ± 1.3 | 31.9 ± 2.2 | 20.7 ± 1.1 | 8.3 ± 0.4 | 7.9 ± 0.3 |
Data represent means ± SE.
Lengths of the primary inflorescence stems of about 5-week-old plants were measured
Widths of the thickest point on the first internode of the primary inflorescence stems were measured
Lengths of the longest rosette leaves were measured
Discussion
Circumnutation and winding movements are universal features of growing plants and improve survival in these stationary organisms. However, the detailed molecular mechanisms controlling these phenomena have remained unclear, despite their importance. Here, we identified PnSCR as a gene regulating circumnutation and the winding response in a typical climbing plant, the morning glory. A sequence analysis and functional complementation experiments with PnSCR revealed that insertion of a single amino acid into the VHIID motif caused a loss of PnSCR function, resulting in abnormal development of the endodermis that is required for gravisensing in the shoots of dicotyledonous plants. This loss of gravisensing led to abnormal circumnutation in weeping, which in turn abolished the winding response. In addition to identifying PnSCR as the mutated gene responsible for abnormal phenotypes of weeping, these results suggest that circumnutation and winding movements are gravity-dependent morphogenetic phenomena in plants.
The Arabidopsis sgr1/scr and sgr7/shr mutants both lack normally differentiated endodermis (17, 20, 23, 24). In Arabidopsis, SCR and SHORT-ROOT (SHR) control ground tissue patterning during root and shoot development (17, 25, 26). The SCR gene is expressed in the initial daughter cell before its asymmetric division (20, 21). SHR is necessary for the maintenance of SCR expression, indicating that SHR is upstream of SCR (24). Thus, these two loci cooperatively play a key role in endodermis development. In scr-3/pAtSCR::PnSCR plants, endodermis development in inflorescence stems was restored to that of the WT. In addition, the hypocotyls of scr-3/pAtSCR::PnSCR plants normally differentiated endodermis, and thus were very similar to those of the WT (data not shown). The phylogenetic analysis of SCR family members revealed that PnSCR fell within a clade including SCR proteins from other plant species. These observations confirm that PnSCR is orthologous to SCR and that the abnormal differentiation of endodermis in weeping is caused by the mutation of PnSCR.Ofthe four scr-mutant alleles of Arabidopsis that have been isolated, all of the gene products are truncated by frameshift mutation or T-DNA insertion (17, 20). The in-frame mutation in the VHIID motif of PnSCR indicates that weeping mutation is a novel type of mutation among that of scr mutants. We do not know how this 3-bp insertion occurred in the PnSCR at present, but we speculate that it is a footprint generated by an excision of a DNA transposon in the Tpn1 family belonging to the CACTA superfamily. In fact, the Tpn1-related elements are thought to act as major spontaneous mutagens for the generation of various floricultural traits in the Japanese morning glory and are shown to generate 3-bp insertions (27, 28).
SCR and SHR belong to the GRAS family of proteins, which regulate diverse aspects of plant development (19, 29). All GRAS proteins share a conserved C-terminal GRAS domain, but their N termini are more divergent. The GRAS domain contains several conserved motifs, including two-Leu-heptad repeats (LHR I, II), VHIID, PFYRE, and SAW (19). The LHR I-VHIID-LHRII region may function as a DNA-binding domain, analogous to the basic leucine zipper protein-DNA interaction (30), with the LHRs mediating protein-protein interactions and the VHIID motif mediating protein-DNA interactions. Recently, Muangprom et al. (31) reported that conserved amino acid sequences near the VHIID motif play an important role in plant size determination; a novel Q-to-R mutation near the VHIID motif in the REPRESSOR OF ga1-3 (RGA) homologue of Brassica rapa results in a gibberellin-insensitive dwarf. Moreover, the same mutation in Arabidopsis RGA also confers a dwarf phenotype in transgenic Arabidopsis. The rice SCR is involved in asymmetric cell division in the cortex/endodermis progenitor cell and in the process of stomata and ligule formation in leaves (32), suggesting that SCR is broadly involved in asymmetric division in various biological events, including the determination of plant size. Surprisingly, the weeping-type PnSCR conferred a semidwarf phenotype to the transgenic scr mutant of Arabidopsis, supporting a role for the conserved VHIID motif of GRAS proteins in size determination in plants.
In the present study, we demonstrated that proper development of endodermis also plays an important role in shoot nutational movement in morning glory. However, it remains obscure whether the endodermis-mediated gravisensing is solely indispensable for circumnutation. To solve this issue, we analyzed the shoot circumnutation of two agravitropic mutants of Arabidopsis, sgr2 and zig/sgr4, which have endodermal cell layers with abnormal amyloplast sedimentation (33, 34). We found that inflorescence stems of these mutants were defective in nutational movement (Fig. 8, which is published as supporting information on the PNAS web site). In addition, we previously reported that circumnutation in Arabidopsis mutant, pgm, known to show a reduced gravitropism caused by the loss of starch granules, was smaller than that of the WT (16). Taken together, our data provide evidence that gravisensing and circumnutation are linked. On the other hand, Brown and Chapman (14) reported that sunflower hypocotyls showed circumnutation in microgravity. This observation appears to conflict with our conclusion. However, it was also noted that the magnitude of circumnutation of sunflower hypocotyls was smaller under microgravity conditions than that on the ground. In this spaceflight experiment, the seeds were germinated on Earth and thus the seedlings had sensed gravity before and during the launch. Thus, an oscillatory movement of the plant organs might be established on the ground, which continued for a time in orbit. To answer the question whether circumnutation occurs in the gravity-free condition of space or not, seeds should be germinated in microgravity and nutational movements of the seedlings should be compared with the controls in space but not with those on the ground.
Because the vine shoot of weeping completely lacks the ability to wind around a support, PnSCR might also be required for the winding response of the vine shoot in morning glory, a typical climbing plant. In classical observations, Darwin and Darwin (12) proposed that both the thigmomorphogenetic response caused by the vine touching a support and shoot circumnutation were important for climbing plants to wind around a support. We have found that weeping expresses normal thigmomorphogenesis (data not shown). Thus, it seems that shoot circumnutation plays a key role in the winding phenomenon and that touch response does not play a large role in the winding phenomenon. Alternatively, climbing plants may have a specialized touch-response in the shoot apex because of circumnutation. We demonstrated that the gravisensing cell or endodermis-mediated graviresponse is indispensable for circumnutation and the winding response in morning glory. However, how gravity regulates these phenomena is still unclear. To answer this question, factors responsible for these phenomena and other functions downstream of gravisensing should be identified. The identification of PnSCR as the gene responsible for gravitropism and winding in climbing plants has provided a molecular basis for elucidating the detailed mechanism of the relationship of the gravisensing/graviresponse to circumnutation and/or winding movement(s).
Supplementary Material
Acknowledgments
We thank Mr. Shozo Haga and Mr. Masatoshi Matsunoki for the seeds of the weeping morning glory, Dr. Philip N. Benfey for the plasmid containing the SCR promoter, and Dr. Atsushi Higashitani (Tohoku University) for helpful discussion. This work was financially supported by Grant-in-Aid for Scientific Research (B) 16380166 from the Japan Society for the Promotion of Science; Grant-in-Aid for Scientific Research on Priority Areas 17051003 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.T.); Grant-in-Aid for Young Scientists (B) 15770039 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y.M.); and Japan Society for the Promotion of Science Research Fellowships for Young Scientists (to D.K.). This study was carried out as part of the Ground-Based Research Announcement for Space Utilization promoted by the Japan Space Forum.
Author contributions: D.K., Y.H., M.K., N.F., Y.M., and H.T. designed research; D.K., Y.H., M.K., N.F., Y.M., A.H., S.I., and H.T. performed research; A.H., S.I., H.F., M.T.M., M.T., and H.S. contributed new reagents/analytic tools; D.K., Y.H., M.K., N.F., Y.M., A.H., and H.T. analyzed data; and D.K., N.F., Y.M., and H.T. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SCR, SCARECROW; PnSCR, Pharbitis nil SCR; PnSCRm, mutant PnSCR; AtSCR, Arabidopsis SCR; pAtSCR, AtSCR promoter.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB200391).
References
- 1.Hoson, T., Nishitani, K., Miyamoto, K., Ueda, J., Kamisaka, S., Yamamoto, R. & Masuda, Y. (1996) J. Exp. Bot. 47, 513-517. [DOI] [PubMed] [Google Scholar]
- 2.Soga, K., Wakabayashi, K., Kamisaka, S. & Hoson, T. (2002) Planta 215, 1040-1046. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi, H. (1997) Planta 203, S164-S169. [DOI] [PubMed] [Google Scholar]
- 4.Kamada, M., Yamasaki, S., Fujii, N., Higashitani, A. & Takahashi, H. (2003) Planta 218, 15-26. [DOI] [PubMed] [Google Scholar]
- 5.Prasad, T. & Cline, M. (1987) Plant Physiol. 83, 505-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiss, J. Z. (2000) Crit. Rev. Plant Sci. 19, 551-573. [DOI] [PubMed] [Google Scholar]
- 7.Morita, M. T. & Tasaka, M. (2004) Curr. Opin. Plant Biol. 7, 712-718. [DOI] [PubMed] [Google Scholar]
- 8.Johnsson, A. & Heathcote, D. (1973) Zeitschr. Pflanzenphysiol. 70, 371-405. [Google Scholar]
- 9.Johnsson, A. (1977) Proc. R. Soc. London Ser. B 199, 505-512. [DOI] [PubMed] [Google Scholar]
- 10.Johnsson, A., Jansen, C., Engelmann, W. & Schuster, J. (1999) J. Grav. Physiol. 6, 9-12. [PubMed] [Google Scholar]
- 11.Johnsson, A. (1979) in Encyclopedia of Plant Physiology: Physiology of Movements, eds. Haupt, W. & Feinleib, M. E. (Springer, Berlin), Vol. 7, pp. 627-646. [Google Scholar]
- 12.Darwin, C. & Darwin, F. (1881) The Power of Movement in Plants (John Murray, London).
- 13.Israelsson, D. & Johnsson, A. (1967) Physiol. Plant. 20, 957-976. [DOI] [PubMed] [Google Scholar]
- 14.Brown, A. H. & Chapman, D. K. (1984) Science 225, 230-232. [DOI] [PubMed] [Google Scholar]
- 15.Yoshihara, T. & Iino, M. (2005) Plant Cell Environ. 28, 134-146. [DOI] [PubMed] [Google Scholar]
- 16.Hatakeda, Y., Kamada, M., Goto, N., Fukaki, H., Tasaka, M., Suge, H. & Takahashi, H. (2003) Physiol. Plant. 118, 464-473. [Google Scholar]
- 17.Fukaki, H., Wysocka-Diller, J., Kato, T., Fujisawa, H., Benfey, P. N. & Tasaka M. (1998) Plant J. 14, 425-430. [DOI] [PubMed] [Google Scholar]
- 18.Clough, S. J. & Bent, A. F. (1998) Plant J. 16, 735-743. [DOI] [PubMed] [Google Scholar]
- 19.Pysh, L. D., Wysocka-Diller, J., Camilleri, C., Bouchez, D. & Benfey, P. N. (1999) Plant J. 18, 111-119. [DOI] [PubMed] [Google Scholar]
- 20.Di Laurenzio, L., Wysocka-Diller, J., Malamy, J. E., Pysh, L., Helariutta, Y., Freshour, G., Hahn, M. G., Feldmann, K. A. & Benfey, P. N. (1996) Cell 86, 423-433. [DOI] [PubMed] [Google Scholar]
- 21.Wysocka-Diller, J., Helariutta, Y., Fukaki, H., Malamy, J. E. & Benfey, P. N. (2000) Development (Cambridge, U.K.) 127, 595-603. [DOI] [PubMed] [Google Scholar]
- 22.Fukaki, H., Fujisawa, H. & Tasaka, M. (1996) Plant Physiol. 110, 945-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tasaka, M., Kato, T. & Fukaki, H. (1999) Trends Plant Sci. 1, 103-107. [DOI] [PubMed] [Google Scholar]
- 24.Helariutta, Y., Fukaki, H., Wysocka-Diller, J., Nakajima, K., Jung, J., Sena, G., Hauser, M. & Benfey, P. N. (2000) Cell 101, 555-567. [DOI] [PubMed] [Google Scholar]
- 25.Benfey, P. N., Linstead, P. J., Roberts, K., Schiefelbein, J. W., Hauser, M. T. & Aeschbacher, R. A. (1993) Development (Cambridge, U.K.) 119, 57-70. [DOI] [PubMed] [Google Scholar]
- 26.Scheres, B., Di Laurenzio, L., Willemsen, V., Hauser, M.-T., Janmaat, K., Weisbeek, P. & Benfey, P. N. (1995) Development (Cambridge, U.K.) 121, 53-62. [Google Scholar]
- 27.Fukuda-Tanaka, S., Inagaki, Y., Yamaguchi, T., Saito, N. & Iida, S. (2000) Nature 407, 581 (brief communications). [DOI] [PubMed] [Google Scholar]
- 28.Hoshino, A., Johzuka-Hisatomi, Y. & Iida, S. (2001) Gene 265, 1-10. [DOI] [PubMed] [Google Scholar]
- 29.Tian, C., Wan, P., Sun, S., Li, J. & Chen, M. (2004) Plant Mol. Biol. 54, 519-532. [DOI] [PubMed] [Google Scholar]
- 30.Ellenberger, T. E., Brandl, C. J., Struhl, K. & Harrison, S. C. (1992) Cell 24, 1223-1237. [DOI] [PubMed] [Google Scholar]
- 31.Muangprom, A., Thomas, S. G., Sun, T. P. & Osborn, T. C. (2005) Plant Physiol. 137, 931-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamiya, N., Itoh, J., Morikami, A., Nagato, Y. & Matsuoka, M. (2003) Plant J. 36, 45-54. [DOI] [PubMed] [Google Scholar]
- 33.Kato, T., Morita, M. T., Fukaki, H., Yamauchi, Y., Uehara, M., Niihama, M. & Tasaka, M. (2002) Plant Cell 14, 33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morita, M. T., Kato, T., Nagafusa, K., Saito, C., Ueda, T., Nakano, A. & Tasaka, M. (2002) Plant Cell 14, 47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.