Abstract
Attachment of ubiquitin to substrate proteins is catalyzed by the three enzymes E1, E2 (ubiquitin conjugating [UBC]), and E3 (ubiquitin ligase). Forty-one functional proteins with a UBC domain and active-site cysteine are predicted in the Arabidopsis (Arabidopsis thaliana) genome, which includes four that are predicted or shown to function with ubiquitin-like proteins. Only nine were previously characterized biochemically as ubiquitin E2s. We obtained soluble protein for 22 of the 28 uncharacterized UBCs after expression in Escherichia coli and demonstrated that 16 function as ubiquitin E2s. Twelve, plus three previously characterized ubiquitin E2s, were also tested for the ability to catalyze ubiquitination in vitro in the presence of one of 65 really interesting new gene (RING) E3 ligases. UBC22, UBC19-20, and UBC1-6 had variable levels of E3-independent activity. Six UBCs were inactive with all RINGs tested. Closely related UBC8, 10, 11, and 28 were active with the largest number of RING E3s and with all RING types. Expression analysis was performed to determine whether E2s or E3s were expressed in specific organs or under specific environmental conditions. Closely related E2s show unique patterns of expression and most express ubiquitously. Some RING E3s are also ubiquitously expressed; however, others show organ-specific expression. Of all the organs tested, RING mRNAs are most abundant in floral organs. This study demonstrates that E2 diversity includes examples with broad and narrow specificity toward RINGs, and that most ubiquitin E2s are broadly expressed with each having a unique spatial and developmental pattern of expression.
Protein ubiquitination is the covalent attachment of the 76-amino acid eukaryotic molecule, ubiquitin, to substrate proteins. The fate of the ubiquitinated substrate depends upon the type of ubiquitin modification and the choice of ubiquitin lysyl residue used to form the attached polyubiquitin chain (Fang and Weissman, 2004; Sun and Chen, 2004) Ligation of a single ubiquitin molecule (monoubiquitination) has been linked to endocytosis and histone modification (Schnell and Hicke, 2003; Umebayashi, 2003). Proteins modified by the attachment of a polyubiquitin chain of four or more ubiquitins linked via ubiquitin lysyl residue 48 are typically targeted for degradation by the 26S proteasome (Thrower et al., 2000). In contrast, modification with a lysyl-63-linked polyubiquitin chain has been implicated in protein activation, stress response, and DNA damage repair (Hoege et al., 2002; Shi and Kehrl, 2003; Zhou et al., 2004).
Ubiquitin conjugation is a multistep reaction, sequentially involving three enzymes referred to as an E1 (ubiquitin-activating enzyme [UBA]), an E2 (ubiquitin-conjugating enzyme [UBC]), and an E3 (ubiquitin ligase; Glickman and Ciechanover, 2002). The first event in the cascade is the ATP-dependent formation of a thioester-linked ubiquitin by E1. Thioester-linked ubiquitin is then transferred to a cysteinyl residue of the E2 enzyme. An E3 enzyme facilitates the transfer of ubiquitin to a lysyl group on the substrate. E3 ligases mediate this step either through the formation of a ubiquitin thioester prior to transfer to the substrate or by noncovalent interaction with the E2 carrying thioester-linked ubiquitin. The E3 enzymes are the substrate recognition component and thus are an important determinant of specificity in the ubiquitination pathway.
E2s were originally defined as proteins capable of accepting ubiquitin from an E1 through a thioester linkage via a cysteinyl sulfhydryl group (Glickman and Ciechanover, 2002). The cysteinyl residue is found in a conserved region of approximately 140 to 150 amino acids called the UBC domain (Inter-Pro IPR000608). The UBC domain also interacts with the E3 enzyme and, in some cases, it also interacts with the substrate (Kalchman et al., 1996). Other conserved amino acids are also required for the formation of the E2-ubiquitin intermediate (Wu et al., 2003). The yeast (Saccharomyces cerevisiae) genome encodes 11 ubiquitin E2s and there are approximately 50 E2s in the human genome (Bachmair et al., 2001; Jiang and Beaudet, 2004). Previous analysis of the predicted Arabidopsis (Arabidopsis thaliana) proteome identified 37 proteins and one putative pseudogene (At1g35700) that contain a cysteinyl residue within a UBC domain (Bachmair et al., 2001). Additional characterized Arabidopsis proteins with UBC domains include RUB-conjugating enzyme RCE1 (At4g36800), which forms a thioester with the related to ubiquitin-like (RUB) protein RUB1 (At1g31340; Del Pozo and Estelle, 1999) and a closely related protein RCE2 (At2g18600), and SUMO-conjugating enzyme SCE1a (At3g57870) and a truncated protein SCE1b (At5g02240) thought to be a pseudogene (Kurepa et al., 2003). While no functional studies with AtSCE1a have been reported, a Nicotiana benthamiana UBC, 88% identical to AtSCE1a, can activate the ubiquitin-like protein small ubiquitin-related modifier (SUMO1) in vitro and can complement a yeast SUMO E2 mutant (Castillo et al., 2004). Compared to other UBC proteins, RCE1 and SCE1a have higher amino acid identity to human UBC domain-containing proteins, HsUBC12 and HsUBC9, respectively, that form thioester linkages with mammalian orthologs of RUB1 and SUMO. These data suggest that AtRCE1, AtRCE2, and SCE1a do not function in vivo as ubiquitin-conjugating enzymes, but as ubiquitin-like conjugating enzymes (Del Pozo and Estelle, 1999; Kurepa et al., 2003; Castillo et al., 2004).
Approximately 1,300 genes of the Arabidopsis genome are predicted to encode for E3 components (Smalle and Vierstra, 2004). The presence of the homology to the E6-AP C terminus (HECT), U-box, or really interesting new gene (RING) domain groups subdivides the Arabidopsis E3 ligases into three classes. These domains have been shown to be essential for E3 ligase function in protein ubiquitination (Lorick et al., 1999; Hatakeyama et al., 2001). The HECT domain class of ubiquitin ligases is unique in that it forms a thioester-linked ubiquitin E3 intermediate prior to transfer of the ubiquitin molecule to the substrate. The remaining classes, RING and U-box proteins, are thought to facilitate ubiquitination by functioning as scaffolds to bring the E2 with thioester-linked ubiquitin and substrate together. The Cys-rich, zinc ion coordinating the RING domain (Freemont et al., 1991; Freemont, 1993) is found in both simple and complex E3 ligases. Complex E3 ligases, such as the well characterized Skp1-Cullin-F-box-type ligase, have the substrate recognition and E2 binding on separate proteins, the F-box protein and the RING-containing protein RBX/ROC/HRT, respectively (Gagne et al., 2002; Kuroda et al., 2002; Lechner et al., 2002; Risseeuw et al., 2003). In contrast, with the simple RING E3 ligases (e.g. ABI3 interacting protein 2 AtAIP2 [Zhang et al., 2005] and SINA of Arabidopsis thaliana 5 AtSINAT5 [Xie et al., 2002]), both substrate and E2 binding occurs within a single protein.
The ubiquitination pathway modifies a diverse range of proteins, thus placing protein ubiquitination at the center of numerous cellular processes in all eukaryotic species. With over 5% of the Arabidopsis proteome predicted to be involved in the ubiquitination-26S proteasome pathway, it is not surprising that protein ubiquitination is postulated to be involved in many different aspects of plant growth and development (Smalle and Vierstra, 2004). The large number of potential ubiquitinating enzymes suggests that substrate-specific ubiquitination plays an essential role in cellular regulation in Arabidopsis. However, how the ubiquitination pathway confers specificity and how the pathway is regulated are not well understood in plants. To begin to understand the specificity or selectivity of the pathway, the components involved and how they interact must first be determined. To this end, we carried out an extensive search of the Arabidopsis genome to identify all potential ubiquitin E2 enzymes by both bioinformatics and activity assays. To further define the function of Arabidopsis ubiquitin E2 enzymes, we examined E2-E3 specificity in in vitro ubiquitination assays with the Arabidopsis RING family of E3 ligases. We also compared the expression profiles of UBC- and RING-encoding genes in different Arabidopsis organs and developmental stages using a 70-mer oligo microarray.
RESULTS
The Arabidopsis Genome Is Predicted to Encode 37 Ubiquitin E2 Proteins
Inspection of the Arabidopsis genome for predicted ubiquitin UBC domain-containing proteins identified a previously unannotated predicted protein (At3g24515) in addition to the 36 compiled either from previous activity assays or from predictions of the annotated genome at the time (Bachmair et al., 2001). Only nine of these have been demonstrated to be ubiquitin E2s through their ability to form a ubiquitin thioester or catalyze E1-dependent protein ubiquitination (summarized in Table I and refs. therein). Therefore, one of the purposes of this study was to determine which UBC domain-containing proteins function as bona fide ubiquitin-conjugating (as opposed to ubiquitin-like) enzymes through activity assays.
Table I.
Summary of UBC domain-containing proteins, their nomenclature, and activity as ubiquitin E2 enzymes
Previously unnamed UBC domain-containing Arabidopsis proteins were given UBC numbers and grouped together based on sequence similarity (see Fig. 1). InsolubleX, 6×His-tagged protein insoluble when expressed in E. coli; InsolubleY, 6×His-tagged protein insoluble when expressed in either E. coli or insect cells; InsolubleZ, UBC contains predicted transmembrane domain C terminal to UBC domain and protein is insoluble when expressed with predicted transmembrane domain, but soluble when expressed without it. References (as indicated in table by superscript numbers): 1, Sullivan and Vierstra, 1993; 2, Girod et al., 1993; 3, Van Nocker et al., 1996; 4, Bartling et al., 1993; 5, Criqui et al., 2002; 6, Yanagawa et al., 2004; 7, this study; 8, Girod and Vierstra, 1993; and 9, Van Nocker and Vierstra, 1993.
| Group No. | Gene UBC | AGI Code | Active in TEa | Active in Ubb | Comments | GenBank Accession No. |
|---|---|---|---|---|---|---|
| III | 1 | At1g14400 | Yescd,1,7 | Yes1,8,7 | E3-ine,7, E3-depf,1,8 | DQ027016 |
| III | 2 | At2g02760 | Yes7 | Yes7 | E3-in7 | DQ027017 |
| III | 3 | At5g62540 | ndg | Yes7 | E3-in7 | DQ027018 |
| IV | 4 | At5g41340 | Yesh,8,9 | Yes7 | E3-in7, E3-dep7 | DQ027019 |
| IV | 5 | At1g63800 | nd | Yes7 | E3-in7 | DQ027020 |
| IV | 6 | At2g46030 | nd | Yes7 | E3-in7 | DQ027021 |
| V | 7 | At5g59300 | Yesh,3 | nd | ||
| V | 13 | At3g46460 | Yesh,3 | nd | DQ027027 | |
| V | 14 | At3g55380 | Yesh,3 | nd | DQ027028 | |
| VI | 8 | At5g41700 | Yesh,2,d,7 | Yes2,7 | E3-dep7 | DQ027022 |
| VI | 9 | At4g27960 | Yesd,6 | nd | DQ027023 | |
| VI | 10 | At5g53300 | Yes7 | Yes7 | E3-dep7 | DQ027024 |
| VI | 11 | At3g08690 | Yes7 | Yes7 | E3-dep7 | DQ027025 |
| VI | 12 | At3g08700 | nd | nd | InsolubleX7 | DQ027026 |
| VI | 28 | At1g64230 | Yes7 | Yes7 | E3-dep7 | DQ027041 |
| VI | 29 | At2g16740 | nd | Yes7 | E3-dep7 | DQ027042 |
| VI | 30 | At5g56150 | nd | Yes7 | E3-dep7 | DQ027043 |
| VII | 15 | At1g45050 | Yesi,4 | Noj,7 | DQ027029 | |
| VII | 16 | At1g75440 | nd | No7 | DQ027030 | |
| VII | 17 | At4g36410 | nd | No7 | DQ027031 | |
| VII | 18 | At5g42990 | nd | No7 | DQ027032 | |
| VIII | 19 | At3g20060 | Yesd,5,7 | No | DQ027033 | |
| VIII | 20 | At1g50490 | nd | No | DQ027034 | |
| IX | 21 | At5g25760 | nd | nd | InsolubleY7 | DQ027035 |
| X | 22 | At5g05080 | nd | Yes7 | E3-in7 | DQ027036 |
| XI | 23 | At2g16920 | nd | nd | Not cloned | |
| XI | 24 | At2g33770 | nd | nd | InsolubleY7 | DQ027037 |
| XI | 25 | At3g15355 | nd | nd | InsolubleY7 | DQ027038 |
| XI | 26 | At1g53020 | nd | No7 | DQ027039 | |
| XII | 27 | At5g50870 | Yes7 | No7 | DQ027040 | |
| XIII | 31 | At1g36340 | nd | nd | InsolubleX7 | DQ027044 |
| XIV | 32 | At3g17000 | Yes7 | nd | InsolubleZ7 | DQ027045 |
| XIV | 33 | At5g50430 | nd | nd | InsolubleZ7 | DQ027046 |
| XIV | 34 | At1g17280 | nd | Yes7 | E3-dep7, InsolubleZ7 | DQ027047 |
| XV | 35 | At1g78870 | nd | Yes7 | E3-dep7 | DQ027048 |
| XV | 36 | At1g16890 | Yes7 | Yes7 | E3-dep7 | DQ027049 |
| XVI | 37 | At3g24515 | nd | No7 | DQ027050 |
A thioester linkage between the E2 and ubiquitin was observed (examples shown in Fig. 2).
Active in ubiquitination and indicates the E2 showed activity in the transfer of ubiquitin to a peptide linkage (see Fig. 4).
Yes, Activity has been demonstrated.
Ub-UBC conjugate eliminated under sulfhydryl-reducing conditions indicating Ub-E2 thioester linkage, as shown in reference.
E3-in, Ubiquitination of the E2 or other proteins that occurs in the absence of added E3 for E3-independent activity (for an E2 with strong activity, see Fig. 3).
E3-dep, Ubiquitination that requires E3 for E3-dependent (for examples, see Fig. 4).
nd, Protein was not assayed for activity.
Ub, E2 conjugate eliminated under sulfhydryl-reducing conditions, indicating Ub-E2 thioester linkage, but data not shown in reference.
Ub-E2 conjugate not eliminated after incubation under sulfhydryl-reducing conditions, but with 100 mm Lys.
No, No activity was detected.
Arabidopsis ubiquitin E2 proteins were initially named according to their identity to yeast UBC domain-containing proteins of which 11 of the 13 function with ubiquitin (Bachmair et al., 2001). Because there cannot be an association of all UBC numbers between the two species given that Arabidopsis has many more UBC domain-containing proteins than yeast, we classified Arabidopsis UBC proteins into subgroups based on their identity to each other. Phylogenetic analysis was performed, including all yeast UBC proteins that function with ubiquitin, RUB, or SUMO, as well as representative human E2s. Also included in this analysis were Arabidopsis ubiquitin-conjugating E2 enzyme variant (UEV) proteins that contain a UBC domain but lack the active-site cysteinyl residue (Sancho et al., 1998). These include constitutive photomorphogenic AtCOP10 and seven other UEV proteins indicated by Arabidopsis Genome Initiative (AGI) numbers (Fig. 1). Ubiquitin-fold modifier 1-conjugating enzyme (Ufc1) was included to serve as the outgroup. HsUfc1 was recently shown to form a thioester linkage through a conserved cysteinyl residue to the ubiquitin-like protein ubiquitin-fold modifier 1 (Ufm1; Komatsu et al., 2004). Phylogenetic analysis revealed that Arabidopsis UBC domain-containing proteins, excluding the UEV proteins, can be divided into 16 subgroups based on >65% bootstrap support (Fig. 1), with groups I and II functioning in RUB1 and SUMO conjugation pathways, respectively. UEV proteins form several of their own distinct groups, supporting their hypothesized divergence from UBCs with active-site Cys (Fig. 1). Uncharacterized UBC domain-containing proteins, with the exception of the UEV proteins, were given a UBC number based on similarity to previously characterized AtUBCs or to other members of the same subgroup (Table I; Fig. 1).
Figure 1.
Phylogenetic analysis of Arabidopsis E2s. Phylogenetic tree of the UBC domain from predicted UBC domain-containing proteins of Arabidopsis is shown. Included in the tree are all E2s from budding yeast (Sc, S. cerevisiae) and representative E2s from humans (Hs, Homo sapiens). Phylogenetic tree was generated using a full-heuristic search (PAUP, version 4.0), and Ufc1 from human and Arabidopsis was used as the outgroup (Komatsu et al., 2004). HsCroc-1 (UEV1) and ScSTP22 (VPS23) are ubiquitin-conjugating enzyme variants (Rothofsky and Lin, 1997; Li et al., 1999). Alignment was created from the core UBC domain as designated by Simple Modular Architecture Research Tool (http://smart.embl-heidelberg.de). The SUMO and RUB E2s as well as the Arabidopsis UBC-like UEV proteins are also included for comparison. Arabidopsis UEV proteins are listed by AGI number except for COP10 (Suzuki et al., 2002). The different Roman numeral designations indicate E2 subgroups (see Table I). Bootstrap values from 1,000 replications for each branch are shown. See Supplemental Figure 1 for protein sequence alignment used to generate the tree.
Most Arabidopsis UBC proteins shared highest similarity with another Arabidopsis protein, suggesting duplications; however, two Arabidopsis E2s displayed higher similarity to yeast and human proteins rather than to other Arabidopsis E2s (Bachmair et al., 2001; Fig. 1). AtUBC27 groups with ScUBC1 and HsUBCH1. AtUBC22 groups with a human protein, endemic pemphigus foliaceus (HsEPF5; Liu et al., 1992). Some groups did not have closely related yeast or human E2s. These are Arabidopsis UBC groups V, IX, XIII, and XVI, with Arabidopsis groups IX, XIII, and XVI consisting of a single member (Bachmair et al., 2001; Fig. 1).
Most of the Arabidopsis UBCs do not have additional characterized protein-protein interaction domains. The single exception is UBC27, which has a predicted ubiquitin-binding domain called a ubiquitin-associated domain at its C terminus. Instead, several of the putative E2s contain either an acidic extension, a basic extension, or a predicted transmembrane domain (Bachmair et al., 2001). UBC4 to 6 contain acidic C termini, UBC32 to 34 contain a C-terminal-predicted transmembrane domain, and UBC22 contains a basic, Lys-rich C terminus. UBC23 and UBC24 have large N-terminal regions with no defined domains; however, they show significant similarity to a human E2, APOLLON, that contains a baculoviral inverted repeat and was recently shown to function as a chimeric E2-E3 (Hao et al., 2004). The domain structure of UBC26 (At1g53020), which is predicted to contain three UBC domains (Bachmair et al., 2001), was not detected in any other eukaryotic genome. However, multiple cDNA sequences found in GenBank contain only the N-terminal domain, suggesting that the other two domains are not part of the expressed open reading frame (ORF). No product was obtained when The Arabidopsis Information Resource (TAIR) prediction with three domains was used to design primers to isolate a cDNA via reverse transcription (RT)-PCR. Whether these other two UBC domains are expressed remains unresolved. The single expressed UBC domain of UBC26 was used in our studies. The remaining UBC proteins, with the exception of groups VI, IX, and XIV, consist basically of only the UBC domain with short extensions of unknown function.
AtUBC8 clades strongly with seven other Arabidopsis E2s: UBC9 to 12 and 28 to 30 (Fig. 1), which form the largest Arabidopsis E2 subgroup, subgroup VI. AtUBC9 to 11 and 28 are very similar to UBC8, with 92% to 96% amino acid identity. UBC29 and 30 show 87% identity to UBC8, while UBC12 shows 78% identity to UBC8 (Fig. 1). These proteins are more similar to human HsUBC5a to c and yeast ScUBC4 and 5 than to other Arabidopsis UBCs (Fig. 1).
Most Arabidopsis UBC Domain-Containing Proteins Function as Ubiquitin E2s
The ability of seven uncharacterized UBC domain-containing proteins to carry thioester-linked ubiquitin was demonstrated (Fig. 2) and is summarized in Table I. For comparison, AtUBC1 (Sullivan and Vierstra, 1993), AtUBC8 (Girod et al., 1993), and AtUBC19 (Criqui et al., 2002) were included to demonstrate that our expression and purification procedure produced active protein. AtUBC2, 10, 11, 27, 28, 32, and 36 formed adducts with ubiquitin that were lost in the presence of a thiol-reducing agent, indicating that a thioester linkage was formed between ubiquitin and E2 (Fig. 2). These results identify these UBC domain-containing proteins as ubiquitin E2s.
Figure 2.
Thioester formation of select Arabidopsis E2s. A, UBC27 and UBC36 form DTT-sensitive ubiquitin adducts. Immunoblots with anti-ubiquitin and anti-6×His antibodies show the presence of a DTT-sensitive ubiquitin adduct for UBC36 and UBC27. B, Ubiquitin-conjugating enzymes from a variety of subgroups form DTT-sensitive ubiquitin adducts. Reactions were split after 5-min incubation at 37°C and treated with DTT or 8 m urea (−DTT). Reactions were resolved by SDS-PAGE and western blots were performed with indicated antibodies.
Another approach to demonstrate ubiquitin E2 activity is to determine whether a UBC domain-containing protein catalyzes E1-dependent protein ubiquitination. Previously uncharacterized Arabidopsis UBCs were expressed in Escherichia coli and tested for their ability to catalyze transfer of ubiquitin to substrate proteins. We were able to test 25 UBC domain-containing proteins for activity; however, we could not produce sufficient soluble protein to assay five others. UBC12, 21, 24, 25, and 31 were insoluble after expression in E. coli and UBC21, 24, and 25 were also insoluble when expressed in cultured insect cells, precluding any activity assays for these five (Table I). Several UBC proteins were sufficiently active in an E3-independent manner under our in vitro assay conditions to prevent their analyses in E3-dependent ubiquitination assays. UBC1 to 6 (data not shown), 20, and 22 (see below) transferred ubiquitin to proteins dependent upon E1, but independent of an E3 ubiquitin ligase. UBC19 exhibited some E3-independent ubiquitination, but it was low enough such that it would not mask E3-dependent activity (Fig. 4; data not shown). In the E3-dependent ubiquitination assays, the previously uncharacterized UBCs, UBC10, 11, 28, 29, 30, 34, 35, and 36, were active in catalyzing polyubiquitination with one or more RING E3 ligases (Fig. 4; Table II; Supplemental Table III), demonstrating that they function as ubiquitin E2s (see below).
Figure 4.
In vitro ubiquitination assays. Each GST-RING protein (E3) was tested against various E2s in in vitro ubiquitination assays to determine the E2-E3 specificity. Representative anti-ubiquitin blots from the ubiquitination assays are shown. Unconjugated ubiquitin migrates at 5.5 kD and has run off the bottom of the gel. Arabidopsis E2 enzymes used in each assay are indicated above each lane by their UBC number and minus (−) indicates the absence of any E2 enzyme. E2-E3 activity is confirmed by the presence of a smear of ubiquitinated proteins, as indicated by plus (+) below each lane, detected by anti-ubiquitin antibodies. The absence of a smear of ubiquitinated proteins greater than the no-E2 lane indicates that the E2-E3 combination used was not active, as indicated by minus (−) below each lane.
Table II.
E3-E2 specificity as determined by in vitro ubiquitination assays
Each E3 or GST-RING protein was tested in in vitro ubiquitination assays with a number of Arabidopsis E2s from different subfamilies as described and defined by Stone et al. (2005). Protein ubiquitination was observed (+) for a number of E2-E3 combinations. Two instances occurred where activity was inconsistent (±). For other E2-E3 combinations, protein ubiquitination was not detected (−). E2-E3 combinations that were not determined (nd) are indicated.
| E3
|
RING Type
|
E2 (UBC)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 10 | 11 | 28 | 29 | 30 | 19 | 34 | 35 | 36 | ||
| At1g02860 | HCa | − | − | − | − | − | − | − | − | − | − |
| At1g12760 | H2 | + | + | + | + | + | − | − | − | − | − |
| At1g14260 | v | + | + | + | + | − | − | − | − | − | − |
| At1g18760 | D | + | + | + | + | − | − | − | − | − | − |
| At1g22500 | H2 | + | + | + | + | + | − | − | − | − | − |
| At1g50440 | v | + | + | + | − | − | ± | − | − | − | − |
| At1g61620 | HCa | − | − | − | − | − | − | − | − | − | − |
| At1g63900 | HCa | + | + | + | + | + | − | − | − | − | − |
| At1g65430 | HCb | + | + | + | − | − | − | − | − | − | − |
| At1g68180 | H2 | + | + | + | − | + | − | − | + | nd | − |
| At1g74410 | H2 | − | − | − | − | − | − | − | − | − | − |
| At2g18670 | H2 | − | − | − | − | − | − | − | − | − | − |
| At2g22690 | S/T | − | − | − | − | − | − | − | − | − | − |
| At2g28530 | C2 | + | − | + | − | − | − | − | − | nd | − |
| At2g28840 | HCa | − | − | − | − | − | − | − | − | − | − |
| At2g42360 | H2 | + | + | + | + | + | + | − | − | + | + |
| At2g44330 | H2 | + | + | + | + | + | + | − | − | − | − |
| At2g47700 | H2 | + | + | + | + | + | − | − | − | + | + |
| At3g05250 | HCa | + | + | + | + | − | − | − | − | − | − |
| At3g05545 | H2 | + | ± | + | − | − | − | − | − | − | − |
| At3g06330 | v | + | + | + | + | + | − | − | − | − | − |
| At3g09760 | v | + | + | + | − | − | − | − | − | nd | − |
| At3g29270 | HCa | + | + | + | + | + | − | − | − | − | − |
| At3g45555 | HCb | − | − | − | − | − | − | − | − | nd | − |
| At3g47160 | HCa | + | + | + | − | − | − | − | − | − | − |
| At3g48070 | C2 | + | + | + | − | − | − | − | − | nd | − |
| At4g10160 | H2 | + | + | + | − | − | − | − | + | nd | − |
| At4g14220 | H2 | + | + | + | + | + | − | − | − | + | + |
| At4g27470 | HCa | + | + | + | + | + | − | − | − | − | − |
| At5g20910 | H2 | + | + | + | + | + | + | − | − | − | − |
| At5g37270 | H2 | + | + | + | + | + | − | − | − | + | + |
| At5g38070 | v | + | + | + | − | − | − | − | − | − | − |
| At5g42200 | H2 | + | + | + | + | + | − | − | − | − | − |
| At5g53910 | D | + | + | + | − | − | − | − | − | nd | − |
Three groups of UBC proteins tested were not active under any assay conditions. All four members of group VII, UBC15 to 18, were expressed and soluble, but exhibited no self-ubiquitination, E3-independent, or E3-dependent ubiquitination (Fig. 4; data not shown; Table II). No similar evidence of any activity was obtained for UBC26 and 37. UBC37 was proteolyzed extensively when expressed in bacteria (data not shown).
In summary, at least one representative from nine of the 14 UBC protein subgroups demonstrated E2 activity. Three subgroups had one member that produced sufficient soluble protein to test for activity, but did not exhibit activity: UBC26 in subgroup XI, UBC37 in subgroup XVI, and all members of subgroup VII. Two other subgroups could not be tested because they did not produce sufficient protein for ubiquitination assays (UBC21 and UBC31).
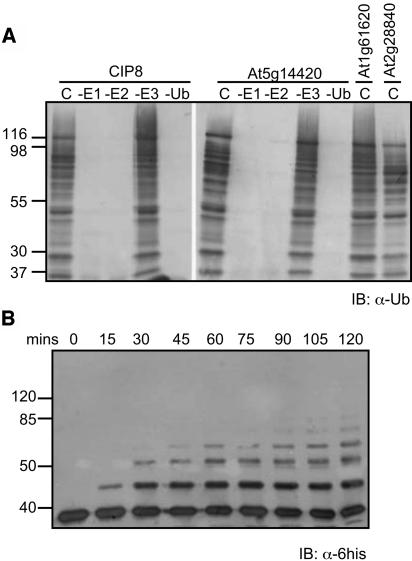
UBC22 Strongly Promotes Ubiquitination Independent of an E3 Ligase
UBC22 consistently showed strong E3-independent activity in in vitro ubiquitination assays when provided with E1 (Fig. 3). The pattern of ubiquitination observed for 6×His-tagged UBC22 (His-UBC22) does not change with the addition of a number of different types of glutathione S-transferase (GST)-RING E3s (Fig. 3). Conversion of the UBC22 catalytic Cys to Ala results in a loss of protein ubiquitination, thus indicating that the observed activity requires an active UBC22 (data not shown). To determine whether His-UBC22 is self-ubiquitinated, a time-course assay was conducted, followed by western blotting with anti-6×His antibodies to visualize accumulation of His-UBC22 linked to one or more ubiquitin moieties. Figure 3B shows the accumulation of a higher Mr UBC22 protein over time, indicating the ubiquitination of His-UBC22.
Figure 3.
Biochemical analysis of UBC22 activity. A, UBC22 shows polyubiquitination activity independent of the presence of an E3. The pattern of ubiquitination is identical between reactions lacking an E3 and those with the indicated E3. CIP8 and AGI names (e.g. At5g14420) represent E3 ligases added to the reaction. C (complete) indicates a reaction containing all the necessary components: E1, E2, E3, ubiquitin, and ATP. B, Processive addition of ubiquitin moieties to UBC22. Immunoblot with anti-6×His antibodies shows addition of multiple ubiquitins to UBC22 over times indicated.
Specificity of E2-E3 Polyubiquitination
Previous work in our laboratory tested the ability of recombinant RING E3 ligases expressed as GST fusions to catalyze polyubiquitination in vitro with the 6×His-tagged recombinant AtUBC8 (Stone et al., 2005). While the majority of RING proteins were active in this assay, 19 of the 64 RING proteins tested were inactive. There are several reasons for this inactivity. One possibility is that these RING proteins do not function with AtUBC8, the only E2 tested, but instead with other UBC proteins. Therefore, we determined the ability of RING proteins to catalyze ubiquitination in vitro with other UBC proteins representative of the E2 diversity in Arabidopsis. In addition, we asked whether there was any specificity between the RING domain type and the E2 subgroup in catalyzing polyubiquitination.
The 19 GST-RING proteins inactive with AtUBC8 were tested both with other members of the AtUBC8 subgroup as well as with more diverged UBCs. Two GST-RING proteins, At2g15580 and At2g28840, produced variable results with very low levels of higher Mr ubiquitinated species, so no conclusions regarding the activity of these two were made. The remaining 17 were not active with any of the E2s tested (Table II).
The RING domain is required for E2 interaction and Arabidopsis encodes three canonical RING domain types, as well as five modified RING domain types (Stone et al., 2005). To determine whether a particular RING domain functions with a specific E2 or E2 group, 46 RING proteins active with AtUBC8 (Stone et al., 2005) representing all RING domain types, including the previously characterized CIP8 (Hardtke et al., 2002), were tested for activity with a spectrum of E2s. Fifteen E2s from six different subgroups were used in the analysis. Representative blots illustrating the results obtained from in vitro ubiquitination assays are shown in Figure 4 and a subset of the data is summarized in Table II. All results obtained from our in vitro assays are shown in Supplemental Table III. Of those E2s that showed activity in these assays, UBC34 (group XIV) had the highest specificity because its activity was only observed with two E3s (At1g68180 and At4g10160), both RING-H2 proteins (Fig. 4; Table II). UBC35 and/or 36 (group XV) displayed activity with only seven of the 45 active RING E3s and were typically both active with the same RING subtype, either RING-HCa or RING-H2 types (Fig. 4; Table II).
The most generic E2s were members of the UBC8 group. This group was the only group active with both canonical and modified RING types. The relative order of activity was UBC8 = UBC10 = UBC11 > UBC28 > UBC29 > UBC30 (Supplemental Table III), with UBC30 active with the least number of E3s (eight) and UBC8, 10, and 11 active with the most (46, 44, and 46, respectively). UBC8, 10, and 11 were typically active with the same E3s, although there are a few exceptions. Two E3s active with UBC8 were found to be inactive or have questionable activity with UBC10 (At2g28530 and At3g05545; Table II).
Expression of UBCs in Arabidopsis Organs
To determine in which organs a particular UBC might function, the relative level of expression of 33 E2s on oligoarrays in selected organs and under different developmental conditions was determined as described previously (Ma et al., 2005). All eight members of the UBC8 family exhibited significant, but varying, levels of expression within each organ and environmental condition examined (Fig. 5). UBC10 was found to have a high level of expression in all organs, with the highest levels in rosette leaves, roots, and petals (Fig. 5, right). Similar to UBC10, UBC8 displayed a consistently high level of expression in most organs, with significantly higher expression in rosette leaves and petals than all other organs and conditions tested (Fig. 5, middle). UBC9, 11, 28, and 29 also display significant levels of expression in all organs examined (Fig. 5, left). The expression levels of UBC12 and 30 are extremely low in all organs. UBC11 is expressed mainly in petals with an approximately 5-fold higher level of expression than observed in rosette leaves. UBC28 is expressed at a consistent level between rosette leaves, stamens, and petals, while UBC29 expression is significantly lower in rosette leaves than in cotyledons, roots, sepals, and petals.
Figure 5.
Expression analysis of the UBC8 family of ubiquitin-conjugating enzymes. Relative expression level of UBC8 family members in the indicated organs. Determination of relative expression levels of each E2 gene is as described in “Materials and Methods.” Supplemental Table I contains a complete list of Arabidopsis UBC expression levels in all organ types examined. RL, Rosette leaves; RD, roots in dark-grown seedlings; RW, roots in light-grown seedlings; CD, cotyledons in dark-grown seedlings; CW, cotyledons in light-grown seedlings; SE, sepals; PE, petals; SM, stamens; and P1B, pistils 1 d before pollination.
These data were compared to that compiled at Genevestigator (Zimmerman et al., 2004; https://www.genevestigator.ethz.ch). The expression patterns of UBC28, 29, and 30 were, in general, consistent with the oligoarray data. Similarly, UBC12 expression was very low. In contrast, UBC8, 9, and 11 differed slightly from the data in this study. While UBC8 showed strong expression in leaf tissue, it did not show equivalently strong expression in petals. UBC9 and 11 showed a more general expression pattern.
The expression analyses for the other E2 subgroups collectively show expression in most organ and environmental conditions examined (Supplemental Table I). A predicted pseudogene with a UBC domain, At1g35700, showed a relative expression level that was extremely low in all organs (Supplemental Table I). These data give further support to the conclusion that At1g35700 is a pseudogene and was excluded from the UBC nomenclature. Data obtained in our study for representative UBC genes are shown in Supplemental Figure 2A; corresponding data obtained from Genevestigator are included in Supplemental Figure 2B. Interestingly, none of the members within a subgroup showed coordinated expression, suggesting complex and distinct regulation of UBC mRNA levels.
Analysis of stress-response data from Genevestigator (https://www.genevestigator.ethz.ch) shows that several E2 mRNAs were up-regulated at least 3-fold in response to various stimuli. Syringolin, a cell death-inducing chemical, induced UBC3, 11, 13, and 27 (data not shown). Several E2 mRNAs were induced by biotic stresses, including UBC17, 20, and 31. The herbicide isoxaben induced UBC20 and 22. UBC6 was induced in response to senescence. UBC16 was induced in response to 6-benzyl adenine and cycloheximide. UBC24 was induced under low nitrogen conditions. UBC31 was induced in high Glc/Suc conditions. UBC32 was induced in abscisic acid treatment and osmotic stress. Once again, distinct regulation of individual members of each UBC subgroup is seen since none showed coordinated changes in mRNA abundance.
Expression Profiling of the Arabidopsis RING-Type E3 Ligases
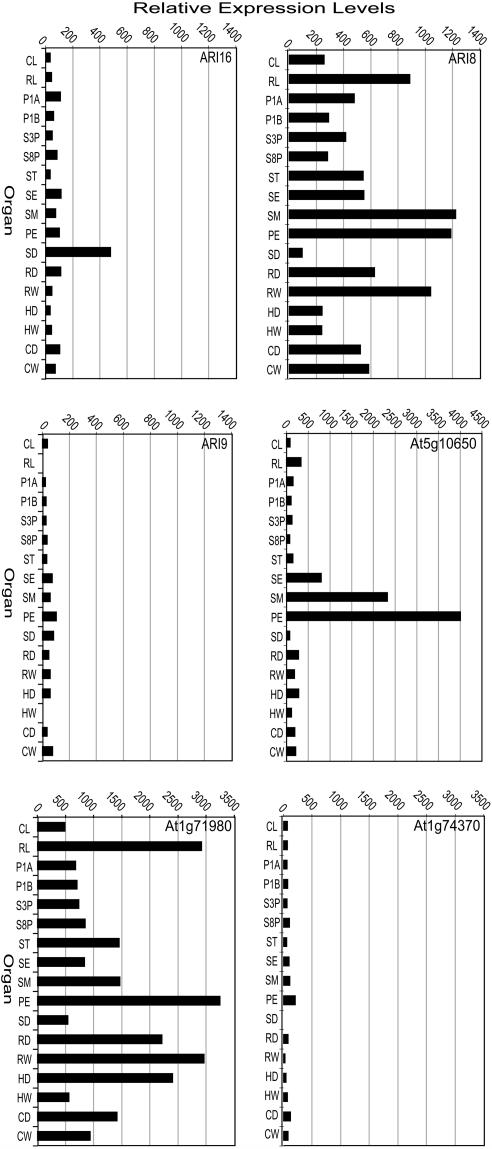
The large number of ubiquitin ligases account for the majority of ubiquitination components encoded by the Arabidopsis genome. The translated Arabidopsis genome is predicted to contain over 450 RING-type E3 ligases that can be grouped into eight subgroups based on the type of RING domain (Stone et al., 2005). To verify that the currently annotated RING genes (Stone et al., 2005) are transcribed, we examined the expression level of the RING E3 family in the same organ samples (Supplemental Table II). Of the 469 genes predicted to encode for RING domain-containing proteins (RING genes), 430 were analyzed on the same microarrays and all 430 could be detected to some extent in at least one of the 17 organs examined. The percentage of RING genes expressed varied from one organ to another, indicating that global RING gene expression was enriched in specific organs (Fig. 6A). Eighty-nine percent of RING genes examined were expressed in seeds, while over 99% of the total RING genes on the array were expressed in floral organs such as petals, stamens, and sepals. All 430 genes examined were expressed at some level in roots of dark-grown seedlings, hypocotyls of light-grown seedlings, and cotyledons of both dark- and light-grown seedlings. Over one-half of all RING genes displayed moderate levels of expression in each organ (Fig. 6A). Only seven samples had a small number (1–13) of RING genes expressing in the highest category (>5,000): cauline and rosette leaves, petals, cultured cells, light-grown roots, and light- and dark-grown cotyledons. Organs with greater than 25 RING genes in the next highest level of expression (1,001–5,000) are also rosette leaves, petals, cultured cells, light-grown roots, light- and dark-grown cotyledons, and, additionally, stamens and dark-grown roots (Fig. 6A). Petals had the largest number of RING genes (85) with the two highest categories of expression, while only two RING genes exhibited similar levels of expression in seeds and pistils 1 d after pollination (Fig. 6, A and B). Similarly, additional organs also had a low number (4–12) of highly expressed RING genes, such as hypocotyls, siliques, and sepals. Interestingly, many of the same RING genes account for the high or low levels of expression in almost all organs. For example, At1g71980 has above-average level of expression in all organs, while At1g74370 has a low level of expression in all organs examined (Fig. 7).
Figure 6.
Expression analysis of the Arabidopsis RING genes. A, Relative expression levels of RING genes in each organ as well as in cultured cells. Expression levels of RING genes in roots, cotyledons, and hypocotyls of light- and dark-grown seedlings were also determined. Number as well as percentage of RING genes with expression levels within the indicated range are given. B, Average level of expression of RING genes in each organ. The number of genes with above-average expression levels is shown. Determination of relative expression levels of each RING gene is as described in “Materials and Methods.” C, Average level of UBC expression in each organ type. CL, Cauline leaves; RL, rosette leaves; P1B, pistil 1 d before pollination; P1A, pistil 1 d after pollination; CW, cotyledons in light-grown seedlings; CD, cotyledons in dark-grown seedlings; HW, hypocotyl in light-grown seedlings; HD, hypocotyl in dark-grown seedlings; RW, roots in light-grown seedlings; RD, roots in dark-grown seedlings; SD, seed; PE, petal; SM, stamen; SE, sepals; ST, stem; S8P, siliques 8 d after pollination; S3P, siliques 3 d after pollination; CC, cultured cells.
Figure 7.
Relative expression levels of representative RING genes. Expression levels of previously characterized RING genes, ARI8, ARI9, and ARI16, are shown. At5g10650 is expressed predominantly in floral organs. This type of organ-specific pattern of expression is observed for a significant number of RING genes. At1g74370 and At1g71980 illustrate, respectively, the low and high levels of expression observed for a number of RING genes. Determination of relative expression levels of each RING gene is as described in “Materials and Methods.” See Supplemental Table II for relative expression levels of all RING genes analyzed. Sample abbreviations are as designated in Figure 6.
To get some insight into the relative activity of ubiquitin pathway enzymes, the mRNA levels of the UBCs and RING E3s in different organs and developmental stages were compared. The average expression level of the UBC genes reflects that of the RING genes, with similarly high levels of expression observed in organs such as rosette leaves, petals, and roots (compare Fig. 6, B and C). The majority of RING and UBC genes examined display low levels of expression in a number of organs, including seeds, pistils, siliques, hypocotyls, and cauline leaves. This type of expression pattern is illustrated by UBC1, 6, 27, and 35 (Supplemental Fig. 2A) and RING genes At1g71980 and ARI8 (Fig. 7). With the exception of the RING-D genes, this pattern of expression is observed for the majority of RING genes regardless of RING domain type. The RING genes encoding RING-D domain-containing proteins exhibited the lowest average expression levels in all organs and were expressed predominantly in seeds and petals (data not shown).
To assist in validating the relative expression levels revealed by our analysis, we determined whether our results correlated with previously published data on the expression levels of known RING genes. To do this, we examined the expression levels of the ARI RING gene family whose mRNA abundance was determined previously (Mladek et al., 2003). The results we obtained correlated with the previously published expression data. For example, ARI8 expression was detected in all organs and developmental conditions (Fig. 7). Similar to the majority of RING genes, ARI8 exhibited a higher level of expression in rosette leaves, stamens, petals, and roots. Previous studies detected very low levels of ARI9 expression below the level required for their quantitation (Mladek et al., 2003). In this study, ARI9 levels were also barely detectable (Fig. 7). ARI16 expression was reported to be specific to siliques (Mladek et al., 2003). A direct comparison to our data is difficult because the developmental stage of this sample is not known. Our analysis showed that there is no silique-specific expression early in silique maturation, but that ARI16 is predominantly expressed in seeds (Fig. 7). This result could be consistent with previous data if ARI16 expression is strong in developing seeds at later stages of silique development and is maintained in the mature seed.
DISCUSSION
The ubiquitin E1, E2, and E3 ligase enzymes are the enzymatic core of the ubiquitination pathway. The E2-E3 complex interacts with the substrate, catalyzing ubiquitin addition to the substrate. Thus, the expression, activity, localization, and selectivity of ubiquitin E2s and E3s are important parameters that can serve to regulate ubiquitination. The E2-E3 combination used in the ubiquitination reaction can also influence the fate of the substrate through determining the extent and nature of ubiquitin addition. The majority of substrates observed to date are modified by the attachment of a Lys-48-linked ubiquitin chain, which targets the substrate for degradation by the 26S proteasome. Substrates modified with other types of polyubiquitin chains linked via ubiquitin Lys-6, -11, -29, and -63, or modified with a single ubiquitin, face different or unknown fates. For example, the heterodimeric E2 UBC13/methyl methane sulfonate sensitivity 2 functions with the RING E3 TNF receptor-associated factor 6 to catalyze the formation of Lys-63 polyubiquitin chains involved in protein activation (Deng et al., 2000). Monoubiquitination of histone H2B via the UBC2/radiation insensitive 6-Brefeldin A sensitivity 1 E2-E3 pair is required for subsequent methylation of specific Lys on histone H3 (Wood et al., 2003). The function of other ubiquitin linkages in vivo is currently unknown. Specific E2-E3 combinations are also involved in specific functions. In yeast, UBC2/Rad6 along with the RING-type E3s Rad5 and Rad18 are implicated in DNA repair (Jentsch et al., 1987; Bailly et al., 1997). Therefore, identifying and characterizing the components and how they interact in catalyzing ubiquitination is critical for the understanding of E2-E3 combinations required for certain processes.
In vitro ubiquitination assays with RING E3 ligases, while operating without a physiological substrate, appear to be accurate reflections of in vivo activity. The requirements for in vivo and in vitro activity, where compared, appear to be identical. For example, mutations in the mammalian RING proteins Mdm2 (Fang et al., 2000) and Praja1 (Sasaki et al., 2003) and the Arabidopsis RING SINAT5 (Xie et al., 2002) that affect in vitro activity also affect in vivo activity. With the aim toward understanding E2-E3 specificity, we have expressed in E. coli multiple UBC domain-containing proteins from Arabidopsis, demonstrated that many function as ubiquitin E2 enzymes, and, using in vitro ubiquitination assays, assessed E2-E3 specificity using a number of different types of RING domain-containing proteins.
The E2 enzymes showed little specificity toward different types of RING domain-containing proteins. UBC8, 10, 11, and 28, which show considerable protein sequence similarity to each other, function in vitro with a large number of RING E3 enzymes assayed, including RING E3s with modified RING domains. The high level of expression observed for UBC8, 10, 11, and 28 correlates with the breadth of activity and suggests that these proteins may perform a general ubiquitination function in vivo. Other members of the UBC8 family were not as promiscuous. UBC29 and 30 did not show activity with any of the E3s that contain modified RING domains and only functioned with a fraction of the other RING E3s. UBC29 and 30 are not as similar to UBC8 as other family members and this may account for restricted activity of these E2 enzymes. The changes in the UBC domain of UBC29 and 30 may prevent them from interacting with the modified RING domains (Stone et al., 2005). Alternatively, or in combination with the above suggestion, the spacing variations and amino acid substitutions within the modified RING domains may also impair the interaction with the E2 UBC domain.
Greater specificity is observed for UBC35 and 36; both E2s functioned mainly with E3s containing RING-H2 domains. Although UBC35 and 36 are highly similar and functioned with the same set of RING E3s in vitro, their expression patterns are different. Therefore, each E2 may promote protein ubiquitination in different organs or at different times during development. UBC34 functioned with only a few RING-H2 domains. This may be due to the amino acid sequence of the UBC domain that may only allow enhancement of ubiquitin transfer in combination with certain RING domains. In addition, UBC34 shares the greatest identity with E2s known to be endoplasmic reticulum (ER)-associated via their C-terminal transmembrane domain. However, it remains to be determined whether AtUBC34 or the RING proteins it works with in vitro actually localize to the ER and whether it serves a role in ER-associated degradation. The RING-H2 is the most prevalent type of RING protein, so it is not surprising that the majority of E2s function with proteins containing this type of RING domain.
Another interesting observation with E2-E3 activity concerns the types of E2s we found to be active in our study. E2s of group VI and XV showed activity with the greatest number of E3s in this study. Members of these groups consist only of the core catalytic UBC domain. The remainder of the E2s of Arabidopsis, with the exception of UBC21, contains extensions of varying lengths outside the UBC domain or within the catalytic core itself, as in the case of group V members (for review, see Bachmair et al., 2001). These extra sequences outside the UBC domain may serve as a regulatory mechanism to preclude promiscuous activity with inappropriate E3s. Alternatively, these sequences could allow additional protein-protein interactions with other components required for ubiquitin transfer. Previous work with UBCH10 has shown that the E2 associates with the cullin subunit anaphase-promoting complex (APC2) and not with the RING protein (APC11) and requires APC2 in addition to APC11 to facilitate ubiquitination (Tang et al., 2001). This suggests that additional elements outside the RING domain-UBC domain interaction may be required for ubiquitination to occur. However, the region of UBCH10 required for this direct interaction remains to be determined.
Limited studies of E2 activity with other types of E3 ligases have been performed. Mudgil et al. (2004) did not find a clear subgroup of E2s that showed preference for the U-box proteins tested. One U-box protein, AtPUB38, was active with AtUBC8, but not with AtUBC7, while another U-box protein, AtPUB18, had the opposite activity (Mudgil et al., 2004). This finding is also supported by analysis of mammalian U-box proteins where, of the five tested, three were active with human E2s, hUBC4 and hUBC5c, but two others were active with other UBCs (Hatakeyama et al., 2001). Arabidopsis HECT E3 ligases have been shown to be active with members of the Arabidopsis UBC8 subgroup (Bates and Vierstra, 1999), although the testing of other E2s was not extensive.
The E2 enzymes that did not function in our in vitro assays with RING E3s can be divided into two classes, those that showed some E3-independent activity and those that did not. No E2 activity at all was observed for the latter class and the possible reasons for this are multiple. These proteins may not fold properly after expression in E. coli; they may require another type of E3 other than the RING types tested here; they may function with only a specific RING protein not yet tested; or they may be unable to transfer ubiquitin to nonphysiological substrates. Another possibility is that they may require cofactors such as UEVs that are not present in the in vitro assay to promote protein ubiquitination. The fact that these E2s may not conjugate ubiquitin, but instead conjugate a ubiquitin-like protein and require a different E1, should also be considered.
For the former class, the E3-independent conjugation observed for a number of E2s suggests that these enzymes are capable of E1-dependent ubiquitin thioester formation. However, the inability of these E2s to catalyze E3-dependent polyubiquitination could be because they may not be able to function with the RING E3s tested, may not be able to transfer to a nonphysiological substrate, or may require a cofactor or UEV for E3-dependent activity. Self-ubiquitination of one E2, AtUBC22, was easily detected and occurred both in the presence and absence of a RING E3. Whether self-ubiquitination of AtUBC22 or other E2s represents an additional level of regulation in vivo in Arabidopsis is not known. However, there are a few examples from other organisms that suggest self-ubiquitination may serve an in vivo function. Yeast Cdc34p self-ubiquitination is thought to regulate its own levels (Skowyra et al., 1999), and the Drosophila E2 Vihar E2-C of APC is degraded during mitosis to slow cyclin B degradation (Mathe et al., 2004).
To examine whether ubiquitin pathway enzyme expression at the mRNA level was coordinated, we analyzed E2 expression patterns along with that of over 400 RING E3 genes. Overall, specific UBC genes show a generic expression pattern with no UBC gene showing a 3-fold higher level of expression in a single specific organ based on our microarray analysis or data available through Genevestigator (Zimmerman et al., 2004). RING genes, in contrast, contained many examples whose expression was very specific and limited. At5g01520 and At1g23980 are expressed at over 3-fold greater levels in flowers and seeds, respectively, than any other organ examined. Surprisingly, expression of the UBC and RING genes in pistils, siliques, and seeds was reduced compared to other organs. The low expression of the ubiquitination enzymes may reflect a tighter regulation of UBC and RING genes in these organs. Another possibility is that the E2 and/or E3 enzymes utilized in these organs may have broad specificity. Alternatively, the total level of protein ubiquitination that occurs within seeds, for example, may be reduced. Therefore, the requirement for ubiquitinating enzymes would be reduced. The expression we observed for both UBC and RING genes generally correlates with previously reported expression data (Thoma et al., 1996; Jensen et al., 1998; Mladek et al., 2003).
The ubiquitination system is hierarchical in that few E2 enzymes (dozens) exist in comparison to the large number of E3s (hundreds). In the case of Arabidopsis, two E1s and as many as 34 to 37 ubiquitin E2 enzymes are utilized by hundreds of different E3 enzymes to facilitate ubiquitination of their cognate target proteins. Therefore, it would not be surprising to find a requirement for additional proteins to help guide the specificity of E2-E3 interactions.
MATERIALS AND METHODS
Plant Material
Seeds from Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) were either sown on soil and grown under photoperiodic cycles of 16 h light and 8 h dark at 16°C with 50% relative humidity or seeds surface sterilized with 30% (v/v) bleach and 0.1% (v/v) Triton X-100 were grown on 1% (w/v) agar with 1× Murashige and Skoog and 1% (w/v) Suc under continuous light.
Identification of Arabidopsis E2 Enzymes
The UBC domain of Arabidopsis UBC8 was used in BLAST searches against the complete nonredundant Arabidopsis genome (TAIR, April 16, 2003; http://www.arabidopsis.org). The Simple Modular Architecture Research Tool database was used to analyze retrieved sequences (version 4.0, May 28, 2004; http://smart.embl-heidelberg.de) followed by manual inspection to confirm the presence of the complete UBC domain. The previously named UBC12 (Girod et al., 1993) corresponded to a cDNA for UBC10, so UBC12 here refers to a previously uncharacterized ORF that is closely related to UBC8.
Sequence Analysis
The ClustalX program was used to generate an alignment of the UBC protein sequence. The alignment was generated using a PAM350 protein matrix, with gap opening and gap extension penalty parameters of 35.0 and 0.75, respectively, in pairwise alignment and 15.0 and 0.3, respectively, in the multiple alignments (Thompson et al., 1997). MacClade sequence editor (Sinauer Associates) was used to manually edit the alignment (Supplemental Fig. 1). The rooted phylogenetic trees were created by PAUP* (Phylogenetic Analysis Using Parsimony, version 4.0; Sinauer Associates) using the heuristic search method with 1,000 bootstrap replicates.
Microarray Hybridization and Expression Analysis
The dataset described in Ma et al. (2005) was used in the analyses presented here. Data are represented as mean normalized intensity value after subtraction of the value from a set of 192 negative control oligos contained on the same slide (Ma et al., 2005). Relative expression was classified as low (<50), moderate (50–1,000), high (1,001–5,000), and highest (>5,000).
Cloning of Arabidopsis UBCs and RING E3s
Arabidopsis E2 cDNAs were cloned by RT reactions followed by PCR to amplify the predicted ORF for each UBC. RNA isolated from either Arabidopsis ecotype Col-0 10-d-old seedlings or floral tissue from 6- to 7-week-old plants was used. The Qiagen RNeasy plant RNA extraction kit was used to isolate total RNA, according to the manufacturer's instructions. Coding regions for 35 UBCs were first introduced into the Gateway entry vector, pDONR (Invitrogen), and the DNA sequence was determined. Attempts to isolate cDNA for UBC23 failed. Sequences of each UBC cDNA were compared to the predicted ORF available on TAIR (http://www.arabidopsis.org), the Arabidopsis genome annotation database. Sequences obtained for UBC7 were different from TAIR predictions, as previously reported (Bachmair et al., 2001). UBC37 has an incorrect nucleotide prediction listed in TAIR. This results in a codon change from Ser to Leu at nucleotide position 1,193. UBC26 was isolated based on cDNAs predicting only the N-terminal UBC domain to be expressed. TAIR suggests the presence of three UBC domains encoded by the computer-predicted cDNA. All other UBCs isolated matched TAIR-predicted ORFs and the sequences obtained are available from GenBank (Table I). cDNAs determined to be correct were introduced into the Gateway-compatible pDEST17 vector to allow for recombinant 6×His-tagged protein to be produced in and purified from Escherichia coli. Truncations of UBC32, 33, and 34 were made to remove C-terminal transmembrane domains to improve solubility. For mutation of the conserved Cys of UBC22, site-directed mutagenesis (Stratagene) was used to change the Cys codon to an Ala codon. pDONR vector containing the UBC22 cDNA was used as a PCR template. After verification by DNA sequencing, the mutated UBC22 cDNA was then cloned, via the Gateway system, into the pDEST17 vector. The RING E3 cDNA GST expression vectors were as described in Stone et al. (2005).
Protein Expression, Purification, in Vitro Ubiquitination Assays, and Western-Blot Analysis
6×His UBC fusions were expressed in E. coli strain BL21 AI or BL21-pLysS. Transformed cells were grown at 37°C for 2 to 3 h or to an OD600 of 0.4 to 0.6 before induction with 0.2% Ara or 0.5 to 1.0 mm isopropylthio-β-galactoside, respectively, for 2 to 3 h at 37°C. Cells were harvested by centrifugation and lysed in lysis buffer containing 25 mm Tris-HCl, pH 7.5, 500 mm NaCl, 0.01% Triton X-100, and 5 mm imidazole. For purification, nickel-nitrilotriacetic acid agarose (Qiagen) was added to cleared lysates and incubated for 1 h at 4°C. Beads were then washed four times with wash buffer containing 25 mm Tris-HCl, pH 7.5, 300 mm NaCl, 0.01% Triton X-100, and 5 mm imidazole. 6×His fusion proteins were eluted with elution buffer containing 25 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 0.01% Triton X-100 supplemented with 300 mm imidazole. Glycerol was added to the eluted protein to a final concentration of 40%. Proteins were stored at −80°C until needed.
GST-RING fusions were expressed and purified from bacterial extracts as described in Stone et al. (2005). SDS-PAGE electrophoresis followed by Coomassie Blue staining and Bradford assays (Bio-Rad) were used to quantify purified proteins. Ubiquitination assays were carried out as previously described (Stone et al., 2005).
Thioester Assay
Thioester assays were performed in a total reaction volume of 30 μL, consisting of 50 mm Tris-HCl, pH 7.4, 10 mm MgCl2, 10 mm ATP, 100 ng rabbit E1 (Boston Biochem), 500 ng of recombinant E2s (2 μg for AtUBC32), and 10 μg ubiquitin (Boston Biochem). Reactions were split after incubation for 5 min at 37°C and terminated by SDS sample buffer with dithiothreitol (DTT) or 8 m urea sample buffer without DTT.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers DQ027017 to DQ027050 (see Table I).
Acknowledgments
We would like to thank Christina Tan for assistance with the ubiquitination assays and Mandy Hsia for recombinant E1; Andy Troy and Michael Kerber for their assistance with E2 analysis; and Jemma Jowett and Kate Dreher for comments on the manuscript, as well as other members of the Callis laboratory for helpful discussions.
This work was supported by the National Science Foundation (2010 grant nos. MCB–00115870 and MCB–0519970 to J.C. and X.-W.D.); by the National Institutes of Health (grant no. GM–47850 to X.-W.D. and grant no. GM0007377–27 to E.K.); and by the Natural Sciences and Engineering Research Council of Canada and the International Human Frontier Science Program Organization fellowships (to S.L.S.). Y.J. was the recipient of a Yale University Joseph F. Cullman Jr. fellowship and L.M. was a long-term postdoctoral fellow of the Human Frontier Science Program.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Judy Callis (jcallis@ucdavis.edu).
The online version of this article contains Web-only data.
References
- Bachmair A, Novatchkova M, Potuschak T, Eisenhaber F (2001) Ubiquitylation in plants: a post-genomic look at a post-translational modification. Trends Plant Sci 6: 463–470 [DOI] [PubMed] [Google Scholar]
- Bailly V, Lauder S, Prakash S, Prakash L (1997) Yeast DNA repair proteins Rad6 and Rad18 form a heterodimer that has ubiquitin conjugating, DNA binding, and ATP hydrolytic activities. J Biol Chem 272: 23360–23365 [DOI] [PubMed] [Google Scholar]
- Bartling D, Rehling P, Weiler EW (1993) Functional expression and molecular characterization of AtUBC2-1, a novel ubiquitin-conjugating enzyme (E2) from Arabidopsis thaliana. Plant Mol Biol 23: 387–396 [DOI] [PubMed] [Google Scholar]
- Bates PW, Vierstra RD (1999) UPL1 and 2, two 405 kDa ubiquitin-protein ligases from Arabidopsis thaliana related to the HECT-domain protein family. Plant J 20: 183–195 [DOI] [PubMed] [Google Scholar]
- Castillo AG, Kong LJ, Hanley-Bowdoin L, Bejarano ER (2004) Interaction between a geminivirus replication protein and the plant sumoylation system. J Virol 78: 2758–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criqui MC, de Almeida Engler J, Camasses A, Capron A, Parmentier Y, Inze D, Genschik P (2002) Molecular characterization of plant ubiquitin-conjugating enzymes belonging to the UbcP4/E2-C/UBCx/UbcH10 gene family. Plant Physiol 130: 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo C, Estelle M (1999) The Arabidopsis cullin AtCUL1 is modified by the ubiquitin-related protein RUB1. Proc Natl Acad Sci USA 96: 15342–15347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang LY, Braun A, You JX, Slaughter C, Pickart C, Chen ZJ (2000) Activation of the I kappa B kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103: 351–361 [DOI] [PubMed] [Google Scholar]
- Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM (2000) Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem 275: 8945–8951 [DOI] [PubMed] [Google Scholar]
- Fang S, Weissman AM (2004) A field guide to ubiquitylation. Cell Mol Life Sci 61: 1546–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont PS (1993) The RING finger: a novel protein sequence motif related to the zinc finger. Ann N Y Acad Sci 684: 174–192 [DOI] [PubMed] [Google Scholar]
- Freemont PS, Hanson IM, Trowsdale J (1991) A novel cysteine-rich sequence motif. Cell 64: 483–484 [DOI] [PubMed] [Google Scholar]
- Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD (2002) The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci USA 99: 11519–11524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod PA, Carpenter TB, van Nocker S, Sullivan ML, Vierstra RD (1993) Homologs of the essential ubiquitin conjugating enzymes UBC1, 4, and 5 in yeast are encoded by a multigene family in Arabidopsis thaliana. Plant J 3: 545–552 [DOI] [PubMed] [Google Scholar]
- Girod PA, Vierstra RD (1993) A major ubiquitin conjugation system in wheat germ extracts involves a 15-kDa ubiquitin-conjugating enzyme (E2) homologous to the yeast UBC4/UBC5 gene products. J Biol Chem 268: 955–960 [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428 [DOI] [PubMed] [Google Scholar]
- Hao Y, Sekine K, Kawabata A, Nakamura H, Ishioka T, Ohata H, Katayama R, Hashimoto C, Zhang X, Noda T, et al (2004) Apollon ubiquitinates SMAC and caspase-9, and has an essential cytoprotection function. Nat Cell Biol 6: 849–860 [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Okamoto H, Stoop-Myer C, Deng XW (2002) Biochemical evidence for ubiquitin ligase activity of the Arabidopsis COP1 interacting protein 8 (CIP8). Plant J 30: 385–394 [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI (2001) U box proteins as a new family of ubiquitin-protein ligases. J Biol Chem 276: 33111–33120 [DOI] [PubMed] [Google Scholar]
- Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S (2002) RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419: 135–141 [DOI] [PubMed] [Google Scholar]
- Jensen RB, Jensen KL, Jespersen HM, Skriver K (1998) Widespread occurrence of a highly conserved RING-H2 zinc finger motif in the model plant Arabidopsis thaliana. FEBS Lett 436: 283–287 [DOI] [PubMed] [Google Scholar]
- Jentsch S, McGrath JP, Varshavsky A (1987) The yeast DNA repair gene RAD6 encodes a ubiquitin-conjugating enzyme. Nature 329: 131–134 [DOI] [PubMed] [Google Scholar]
- Jiang YH, Beaudet AL (2004) Human disorders of ubiquitination and proteasomal degradation. Curr Opin Pediatr 16: 419–426 [DOI] [PubMed] [Google Scholar]
- Kalchman MA, Graham RK, Xia G, Koide HB, Hodgson JG, Graham KC, Goldberg YP, Gietz RD, Pickart CM, Hayden MR (1996) Huntingtin is ubiquitinated and interacts with a specific ubiquitin-conjugating enzyme. J Biol Chem 271: 19385–19394 [DOI] [PubMed] [Google Scholar]
- Komatsu M, Chiba T, Tatsumi K, Iemura S, Tanida I, Okazaki N, Ueno T, Kominami E, Natsume T, Tanaka K (2004) A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J 23: 1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa J, Walker JM, Smalle J, Gosink MM, Davis SJ, Durham TL, Sung DY, Vierstra RD (2003) The small ubiquitin-like modifier (SUMO) protein modification system in Arabidopsis. Accumulation of SUMO1 and -2 conjugates is increased by stress. J Biol Chem 278: 6862–6872 [DOI] [PubMed] [Google Scholar]
- Kuroda H, Takahashi N, Shimada H, Seki M, Shinozaki K, Matsui M (2002) Classification and expression analysis of Arabidopsis F-box-containing protein genes. Plant Cell Physiol 43: 1073–1085 [DOI] [PubMed] [Google Scholar]
- Lechner E, Xie D, Grava S, Pigaglio E, Planchais S, Murray JA, Parmentier Y, Mutterer J, Dubreucq B, Shen WH, et al (2002) The AtRbx1 protein is part of plant SCF complexes, and its down-regulation causes severe growth and developmental defects. J Biol Chem 277: 50069–50080 [DOI] [PubMed] [Google Scholar]
- Li Y, Kane T, Tipper C, Spatrick P, Jenness DD (1999) Yeast mutants affecting possible quality control of plasma membrane proteins. Mol Cell Biol 19: 3588–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Diaz LA, Haas AL, Giudice GJ (1992) cDNA cloning of a novel human ubiquitin carrier protein. An antigenic domain specifically recognized by endemic pemphigus foliaceus autoantibodies is encoded in a secondary reading frame of this human epidermal transcript. J Biol Chem 267: 15829–15835 [PubMed] [Google Scholar]
- Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM (1999) RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA 96: 11364–11369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Sun N, Liu X, Jiao Y, Zhao H, Deng XW (2005) Organ-specific expression of Arabidopsis genome during development. Plant Physiol 138: 80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathe E, Kraft C, Giet R, Deak P, Peters JM, Glover DM (2004) The E2-C vihar is required for the correct spatiotemporal proteolysis of cyclin B and itself undergoes cyclical degradation. Curr Biol 14: 1723–1733 [DOI] [PubMed] [Google Scholar]
- Mladek C, Guger K, Hauser MT (2003) Identification and characterization of the ARIADNE gene family in Arabidopsis: a group of putative E3 ligases. Plant Physiol 131: 27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgil Y, Shiu SH, Stone SL, Salt JN, Goring DR (2004) A large complement of the predicted Arabidopsis ARM repeat proteins are members of the U-box E3 ubiquitin ligase family. Plant Physiol 134: 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, Estelle M, Somers DE, Crosby WL (2003) Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J 34: 753–767 [DOI] [PubMed] [Google Scholar]
- Rothofsky ML, Lin SL (1997) CROC-1 encodes a protein which mediates transcriptional activation of the human FOS promoter. Gene 195: 141–149 [DOI] [PubMed] [Google Scholar]
- Sancho E, Vilá MR, Sánchez-Pulido L, Lozano JJ, Paciucci R, Nadal M, Fox M, Harvey C, Bercovich B, Loukili N, et al (1998) Role of UEV-1, an inactive variant of the E2 ubiquitin-conjugating enzymes, in in vitro differentiation and cell cycle behavior of HT-29-M6 intestinal mucosecretory cells. Mol Cell Biol 18: 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong D-H, An G, Kitano H, Ashikari M, et al (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F- box mutant. Science 299: 1896–1899 [DOI] [PubMed] [Google Scholar]
- Schnell JD, Hicke L (2003) Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem 278: 35857–35860 [DOI] [PubMed] [Google Scholar]
- Shi CS, Kehrl JH (2003) Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J Biol Chem 278: 15429–15434 [DOI] [PubMed] [Google Scholar]
- Skowyra D, Koepp DM, Kamura T, Conrad MN, Conaway RC, Conaway JW, Elledge SJ, Harper JW (1999) Reconstitution of G1 cyclin ubiquitination with complexes containing SCFGrr1 and Rbx1. Science 284: 662–665 [DOI] [PubMed] [Google Scholar]
- Smalle J, Vierstra RD (2004) The ubiquitin 26s proteasome proteolytic pathway. Annu Rev Plant Physiol Plant Mol Biol 55: 555–590 [DOI] [PubMed] [Google Scholar]
- Stone SL, Hauksdottir H, Troy A, Herschleb J, Kraft E, Callis J (2005) Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol 137: 13–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan ML, Vierstra RD (1993) Formation of a stable adduct between ubiquitin and the Arabidopsis ubiquitin-conjugating enzyme, AtUBC1+. J Biol Chem 268: 8777–8780 [PubMed] [Google Scholar]
- Sun L, Chen ZJ (2004) The novel functions of ubiquitination in signaling. Curr Opin Cell Biol 16: 119–126 [DOI] [PubMed] [Google Scholar]
- Suzuki G, Yanagawa Y, Kwok SF, Matsui M, Deng X-W (2002) Arabidopsis COP10 is a ubiquitin-conjugating enzyme variant that acts together with COP1 and the COP9 signalosome in repressing photomorphogenesis. Genes Dev 16: 554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Li B, Bharadwaj R, Zhu H, Ozkan E, Hakala K, Deisenhofer J, Yu H (2001) APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol Biol Cell 12: 3839–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma S, Sullivan ML, Vierstra RD (1996) Members of two gene families encoding ubiquitin-conjugating enzymes, AtUBC1-3 and AtUBC4-6, from Arabidopsis thaliana are differentially expressed. Plant Mol Biol 31: 493–505 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrower JS, Hoffman L, Rechsteiner M, Pickart CM (2000) Recognition of the polyubiquitin proteolytic signal. EMBO J 19: 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umebayashi K (2003) The roles of ubiquitin and lipids in protein sorting along the endocytic pathway. Cell Struct Funct 28: 443–453 [DOI] [PubMed] [Google Scholar]
- Van Nocker S, Vierstra RD (1993) Multiubiquitin chains linked through lysine 48 are abundant in vivo and are competent intermediates in the ubiquitin proteolytic pathway. J Biol Chem 268: 24766–24773 [PubMed] [Google Scholar]
- Van Nocker S, Walker JM, Vierstra RD (1996) The Arabidopsis thaliana UBC7/13/14 gene encode a family of multiubiquitin chain-forming E2 enzymes. J Biol Chem 271: 12150–12158 [DOI] [PubMed] [Google Scholar]
- Wood A, Schneider J, Dover J, Johnston M, Shilatifard A (2003) The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J Biol Chem 278: 34739–34742 [DOI] [PubMed] [Google Scholar]
- Wu PY, Hanlon M, Eddins M, Tsui C, Rogers RS, Jensen JP, Matunis MJ, Weissman AM, Wolberger CP, Pickart CM (2003) A conserved catalytic residue in the ubiquitin-conjugating enzyme family. EMBO J 22: 5241–5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Guo HS, Dallman G, Fang SY, Weissman AM, Chua NH (2002) SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature 419: 167–170 [DOI] [PubMed] [Google Scholar]
- Yanagawa Y, Sullivan JA, Komatsu S, Gusmaroli G, Suzuki G, Yin J, Ishibashi T, Saijo Y, Rubio V, Kimura S, et al (2004) Arabidopsis COP10 forms a complex with DDB1 and DET1 in vivo and enhances the activity of ubiquitin conjugating enzymes. Genes Dev 18: 2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Garreton V, Chua H-H (2005) The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev 19: 1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wertz I, O'Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM (2004) Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature 427: 167–171 [DOI] [PubMed] [Google Scholar]
- Zimmerman P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]