Abstract
ADP-glucose pyrophosphorylase (AGPase) is a key regulatory enzyme in starch biosynthesis. However, plant AGPases differ in several parameters, including spatial and temporal expression, allosteric regulation, and heat stability. AGPases of cereal endosperms are heat labile, while those in other tissues, such as the potato (Solanum tuberosum) tuber, are heat stable. Sequence comparisons of heat-stable and heat-labile AGPases identified an N-terminal motif unique to heat-stable enzymes. Insertion of this motif into recombinant maize (Zea mays) endosperm AGPase increased the half-life at 58°C more than 70-fold. Km values for physiological substrates were unaffected, although Kcat was doubled. A cysteine within the inserted motif gives rise to small subunit homodimers not found in the wild-type maize enzyme. Placement of this N-terminal motif into a mosaic small subunit containing the N terminus from maize endosperm and the C terminus from potato tuber AGPase increases heat stability more than 300-fold.
ADP-Glc pyrophosphorylase (AGPase) is ubiquitous in starch-synthesizing plant tissues and plays an essential role in glycogen biosynthesis in bacteria (for review, see Preiss and Romeo, 1994; Preiss and Sivak, 1996). AGPase catalyzes the initial step in the starch biosynthetic pathway by converting ATP and α-Glc-1-P (G-1-P) to ADP-Glc and pyrophosphate (for review, see Hannah, 1997). Seminal evidence for the crucial role AGPase plays in starch synthesis was derived from transgenic experiments in which altered AGPases were expressed in plants. An increase in starch synthesis was found in potato (Solanum tuberosum) tubers (Stark et al., 1992) and seeds of wheat (Triticum aestivum; Smidansky et al., 2002), rice (Oryza sativa; Smidansky et al., 2003), and maize (Zea mays; Giroux et al., 1996; T.W. Greene and L.C. Hannah, unpublished data). Accordingly, the stability and regulatory properties of AGPase have been subjects of intense study.
Plant AGPases are heterotetramers composed of two small subunits and two large subunits. While the two subunits are not interchangeable, both evolved from a common progenitor (Bae et al., 1990; Bhave et al., 1990) and each exhibits a low level of activity when expressed alone in an Escherichia coli expression system (Iglesias et al., 1993; Burger et al., 2003). Mutations affecting catalytic and allosteric properties of AGPase map to both subunits (Cross et al., 2004, 2005; Hwang et al., 2005).
Modulation of plant AGPase activity can involve several mechanisms: allosteric regulation by small effecter molecules, thermal inactivation, and reductive activation. AGPase is activated by 3-phosphoglyceric acid (3-PGA) and inhibited by inorganic phosphate (Pi) in many plant tissues. 3-PGA also can inhibit activity at high concentrations (Hwang et al., 2005). Several AGPases, such as the potato tuber enzyme, are fully stable at 70°C (Sowokinos and Preiss, 1982; Okita et al., 1990), whereas others are quite labile. The maize endosperm AGPase loses 96% of its activity when heated at 57°C for 5 min (Hannah et al., 1980).
Temperature extremes are responsible for reduced grain yield in many cereal crops of worldwide importance, such as maize, wheat, and rice (Singletary et al., 1994). AGPase is one of the enzymes most profoundly affected by elevated temperature (Singletary et al., 1993, 1994). Since AGPase is rate limiting in starch biosynthesis, high temperatures adversely affect starch production and, in turn, yield.
Heat stability and reductive activation of the potato tuber AGPase involve a small subunit N-terminal Cys (Ballicora et al., 1995). This Cys enhances stability of the potato tuber AGPase at high temperatures, presumably by formation of a disulfide bridge between the two small subunits (Fu et al., 1998; Ballicora et al., 1999). The presence of this disulfide bridge was also observed in the crystal structure of an allosterically inhibited potato small subunit homotetramer (Jin et al., 2005). Although the disulfide bridge conveys enzyme stability, it can have negative effects on enzyme activity. A two-step process has been proposed for maximal enzyme activity levels in the absence of 3-PGA. First, the disulfide bridge must be reduced and then an ADP-Glc-induced conformational change must occur (Fu et al., 1998; Tiessen et al., 2002). However, the specific activity of the reductively activated enzyme is 13-fold lower in the ADP-Glc synthesis reaction than that observed in the presence of 3-PGA (Fu et al., 1998).
Comparison of heat-stable and heat-labile AGPases (Hannah et al., 2001) identified a conserved amino acid motif in the N terminus of the small subunit of heat-stable enzymes, designated QTCL (containing Gln, Thr, Cys, and Leu). This motif contains the Cys residue described above. This motif is absent in the heat-labile AGPases of the maize and barley (Hordeum vulgare) endosperms as well as other cereal seeds. Here, the QTCL motif of heat-stable AGPases and variants thereof were placed into the maize endosperm AGPase expressed in E. coli. The Cys causes formation of an intermolecular small subunit disulfide bond and greatly enhances heat stability apparently by stabilizing heterotetramers. The presence of the QTCL motif in the maize small subunit does not alter binding of physiological substrates or the physiological inhibitor, Pi; however, it does double Kcat and increases sensitivity to the activator, 3-PGA. Placement of the heat-stable motif into a recently described (Boehlein et al., 2005), Pi-insensitive mosaic subunit derived from maize and potato revealed complex interactions within the small subunit. While incorporation of the QTCL motif gave rise to an AGPase 300 times more heat stable than wild type, the Pi insensitivity of the maize/potato mosaic was nullified by the QTCL motif.
RESULTS
Identification of Motifs Important in Heat Stability
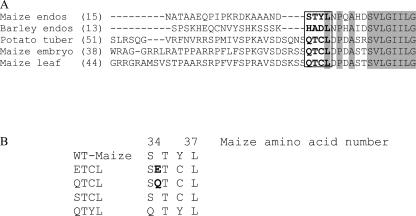
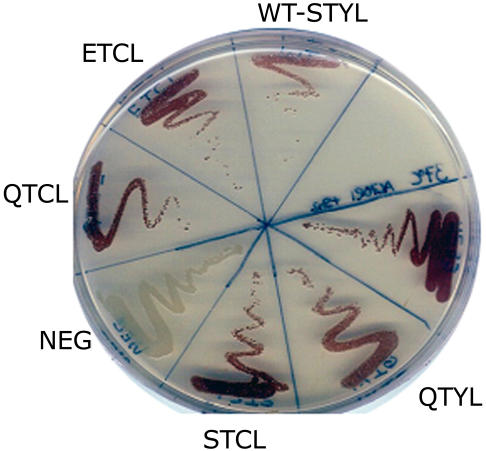
Sequence alignments of heat-stable and heat-labile AGPase small subunits revealed pronounced sequence conservation (Hannah et al., 2001). However, significant sequence variation occurs in both termini. Of particular interest was the N-terminal motif QTCL (Fig. 1A). This sequence motif occurs only in the heat-stable AGPases shown in Figure 1 and Hannah et al. (2001). The Cys residue in this motif has been implicated in conferring heat stability to the potato tuber AGPase (Ballicora et al., 1999). To establish whether this motif bestows heat stability in the otherwise heat-labile maize endosperm AGPase, four variants were created in the N terminus of the maize endosperm small subunit (Fig. 1B). The mutation STCL replaces the native endosperm Tyr residue with a Cys. The mutation termed QTCL adds a Gln residue and changes the Tyr residue to a Cys. To evaluate any alteration caused by the Gln insertion, two additional constructs were created, ETCL and QTYL. Each recombinant AGPase small subunit plus a wild-type maize endosperm large subunit on a separate, compatible vector was expressed in an E. coli mutant harboring an inactive AGPase. Functional AGPase encoded by the plasmids complements the mutant E. coli, resulting in glycogen production. This is easily detected by brown staining of colonies following exposure to iodine vapors. All variant small subunits were functional in E. coli (Fig. 2).
Figure 1.
Alignment of small subunits and created mutations. A, Position of the first amino acid shown is given in parentheses following the small subunit origin. Sequence numbering is based on the maize endosperm small subunit. Maize and barley endosperm are heat sensitive while maize embryo, maize leaf, and potato tuber are heat stable. Amino acids examined here are in bold and boxed. Shaded areas indicated highly conserved amino acids. B, Changes in the N termini of the various mutants are listed. Inserted amino acids are in bold.
Figure 2.
Staining of mutations. Cells were grown overnight and then exposed to iodine vapors for 1 min. Complementation is observed by the production of brown staining. The positive control (WT) is wild-type maize endosperm AGPase, and the negative control (NEG) is cells containing empty vectors.
In agreement with the in vivo-produced glycogen, AGPase activity levels of the variants were comparable to that of the recombinant maize enzyme (Table I). AGPase activity from whole cell extracts was measured in both the forward and backward direction. Activity levels of the variants were moderately increased compared to the recombinant wild-type enzyme.
Table I.
Activity of small subunit mutations compared to the wild-type maize endosperm AGPase in crude preparationsa
| Sample | Assay A (Forward) | Assay B (Reverse) |
|---|---|---|
| STYL (wild type) | 100 | 100 |
| ETCL | 123 ± 25 | 130 ± 5 |
| QTCL | 170 ± 18 | 165 ± 7 |
| STCL | 135 ± 5 | 150 ± 5 |
| QTYL | 120 ± 5 | 135 ± 21 |
Results are the averages of three independent experiments for each genotype. Assays within each experiment were performed in triplicate. The forward activity was measured in the direction of ADP-Glc synthesis in the presence of 10 mm 3-PGA. The nonradioactive reverse assay was used (see “Materials and Methods”).
To determine whether any of the mutations conferred heat stability, whole cell extracts were incubated for 6 min at 58°C, and the percentage of AGPase activity remaining was determined. All constructs containing the added Cys (ETCL, QTCL, and STCL) exhibited significant increases in heat stability. Cys-containing variants retained 30% to 50% of AGPase activity (Table II), compared to only 2% for those without the Cys (wild type and QTYL). In addition, the Gln within this motif may aid in enhancing heat stability of mosaic subunits containing the added Cys.
Table II.
Percent heat stability of small subunit mutations in crude preparationa
| Sample | Assay A (Forward) | Assay B (Reverse) |
|---|---|---|
| STYL (wild type) | 2.4% ± 0.8 | 0.9% ± 1.2 |
| ETCL | 31.7% ± 2.5 | 44.5% ± 17.7 |
| QTCL | 50.0% ± 7.2 | 68.5% ± 0.7 |
| STCL | 44.3% ± 1.5 | 55.0% ± 5.7 |
| QTYL | 1.7% ± 1.1 | 1.4% ± 2.0 |
Results are the averages of at least two independent experiments. Enzyme preparations were placed at 58°C for 6 min then submerged in ice prior to assay. Percentage heat stability is activity remaining after heat treatment divided by activity before heating.
Kinetic Analysis
Because of its increased activity and heat stability, detailed kinetic analysis was performed on the QTCL variant. Recombinant QTCL and wild-type enzymes were purified as described previously (Boehlein et al., 2005). Km and Vmax values for ATP and for G-1-P were determined for both enzymes in the presence of 3-PGA (Table III, first two entries). An inspection of the Km values shows that the QTCL alteration has little to no effect on these parameters, indicating that this mutation does not have a direct role in substrate binding or catalysis. The alteration does affect catalytic efficiency as evidenced by the doubling of Vmax of the purified enzyme. An increased Vmax without changes in Km values was also noted in the absence of 3-PGA (data not shown).
Table III.
Kinetic parameters of purified QTCL and wild-type AGPases
Reactions were run with 10 mm 3-PGA.
| Mutant
|
ATP
|
G-1-P
|
||||
|---|---|---|---|---|---|---|
| Km | Vmax | Kcat/Km | Km | Vmax | Kcat/Km | |
| mm | μmolmin−1mg−1 | mm | μmolmin−1mg−1 | |||
| Wild typea | 0.12 ± 0.003 | 26.7 ± 0.22 | 0.85 × 106 | 0.058 ± 0.001 | 23.2 ± 0.15 | 1.45 × 106 |
| QTCL | 0.10 ± 0.009 | 48.6 ± 1.18 | 1.82 × 106 | 0.042 ± 0.003 | 43.3 ± 0.65 | 3.77 × 106 |
| MPa | 0.10 ± 0.006 | 37.3 ± 0.65 | 1.41 × 106 | 0.050 ± 0.002 | 31.7 ± 0.34 | 2.3 × 106 |
| MP-QTCL | 0.09 ± 0.003 | 49.1 ± 0.52 | 1.91 × 106 | 0.051 ± 0.003 | 43.9 ± 0.67 | 3.15 × 106 |
Data were taken from Boehlein et al. (2005) and are included here for comparison.
Allosteric properties of QTCL were also examined. Since the maize endosperm AGPase is activated by 3-PGA and deactivated by Pi, the Ka value for 3-PGA and the Ki value for Pi were determined. Resulting data show that the QTCL motif causes a small, but significant, increase in 3-PGA sensitivity (Table IV). The extent of phosphate modulation was determined in the presence of 3-PGA. Comparable Ki values for QTCL and wild-type recombinant AGPase were detected.
Table IV.
Activation and inhibition of QTCL and wild type
Assays were in the forward direction (assay C) using standard reaction conditions
| Mutant
|
3-PGA | Pi |
|---|---|---|
| Ka | Ki | |
| Wild typea | 0.17 ± 0.015 | 2.96 ± 0.12 |
| QTCL | 0.11 ± 0.013 | 2.62 ± 0.10 |
| MPa | 0.03 ± 0.008 | 12.28 ± 0.73 |
| MP-QTCL | 0.10 ± 0.014 | 2.87 ± 0.08 |
Data were previously published in Boehlein et al. (2005). Ki for phosphate was determined in the presence of 2.5 mm 3-PGA.
QTCL Disulfide Bridge Formation
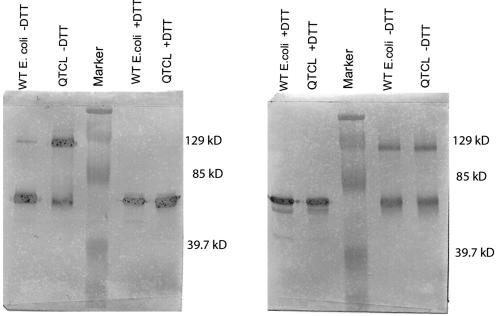
The Cys of QTCL has been shown to be involved in disulfide bridge formation between the two small subunits (Jin et al., 2005). Because the wild-type maize endosperm lacks this Cys and the QTCL variant contains it, both were monitored for disulfide bond formation. Purified recombinant wild-type and QTCL AGPases were subjected to a nondenaturing SDS-PAGE analysis, blotted, and probed with antibodies against the large or small AGPase subunit. Proteins recognized by these antibodies are approximately 50 and 100 kD, the sizes of monomers and dimers of the two subunits, respectively (Fig. 3). Exposure to dithiothreitol (DTT) prior to electrophoresis abolishes the 100-kD proteins and intensifies the 50-kD band. We conclude that disulfide bridges maintain dimers not only of the small subunit, but also of the large subunit. Heterotetrameric AGPase was not observed in the presence or absence of DTT on the SDS gels.
Figure 3.
Disulfide bridge formation. A nonreducing 10% SDS-PAGE gel followed by a western transfer was performed to investigate the formation of the disulfide bridge. A, Western blot developed with polyclonal small subunit. B, Western blot probed with a polyclonal large subunit antibody. Both antibodies recognize a dimer in QTCL and wild-type AGPase that depolymerizes in the presence of DTT. The small subunit dimer of QTCL is substantially more intense than that of wild type.
The presence of QTCL in the maize small subunit does not alter the size or amounts of proteins recognized by the large subunit antibody (Fig. 3B); however, its presence dramatically enhances the amount of the small subunit in the 100-kD protein at the expense of the 50-kD protein. Because the dimer involving the small subunit is abolished by the reducing agent and is enriched by the addition of the Cys in the N terminus, a disulfide bridge involving the small subunit is involved in dimerization. The low-intensity 100-kD band detected with the small subunit polyclonal antibody in wild type was shown to be nonspecific binding. This band is absent when an identical blot is developed with a monoclonal small subunit antibody (data not shown). We suspect that the cross-reaction of the polyclonal small subunit antibody with the large subunit is due to sequence conservation between the subunits.
Incorporation of the QTCL motif into the small subunit promotes dimer formation between small subunits but not between small and large subunits. Perusal of the two blots in Figure 3 shows that QTCL enhances the amount of the dimer detected with the small subunit antibody but not the dimer detected with large subunit antibody. Were heterodimers enhanced by the QTCL mutation, an increased intensity of both dimers would have been seen. We also identified a disulfide bond involving the large subunit in our purified extracts.
Activation of QTCL by DTT in the Absence of 3-PGA
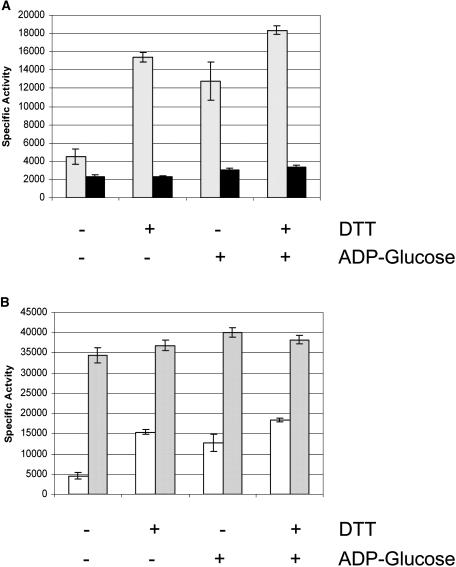
Reduction of the Cys residue in the QTCL motif may represent a form of physiological control of the potato tuber AGPase (Ballicora et al., 2000; Tiessen et al., 2002). In the absence of 3-PGA, potato tuber AGPase is activated by preincubation with DTT and the substrate ADP-Glc (Fu et al., 1998). Accordingly, DTT and substrate modulation of recombinant maize endosperm AGPase containing or lacking the QTCL small subunit motif was investigated. Activities of the two AGPases were measured after preincubation with 3 mm DTT and/or 2.0 mm ADP-Glc (Fig. 4A). Preincubation of the QTCL variant with DTT and/or ADP-Glc enhanced activity almost 3-fold; however, wild-type AGPase was only slightly affected. While the greatest enhancement of QTCL occurred when both DTT and ADP-Glc were present, the modulating effects of the two are not additive (Fig. 4A). Interestingly, activation by DTT and/or ADP-Glc is not obligatory for maximal activity since the presence of saturating concentrations of the activator 3-PGA during the reaction more than compensates for preincubation activation (Fig. 4B).
Figure 4.
Reduction of the disulfide bridge. Reactions were incubated for 15 min at room temperature in 20 μL of a mixture containing 100 mm HEPES, pH 7.4, 0.2 mg/mL BSA, 5 mm MgCl2, and the specified treatment listed. Following preincubation, reactions were performed using assay B with the following modification. The ADP-Glc concentration was adjusted from 2.0 mm so that the final concentration in all assays was 1.4 mm. The concentration of DTT used was 3.0 mm. Each assay was performed from 0 to 6 min and the slope of the velocity versus time plot was used to calculate the specific activity. A, Activity (μmol min−1 mg−1) in the absence of 3-PGA. Black bars represent wild-type and cross-hatching QTCL. B, Activity (μmol min−1 mg−1) of QTCL in the absence (white bars) or presence (gray bars) of 10 mm 3-PGA.
QTCL Heterotetramer Formation
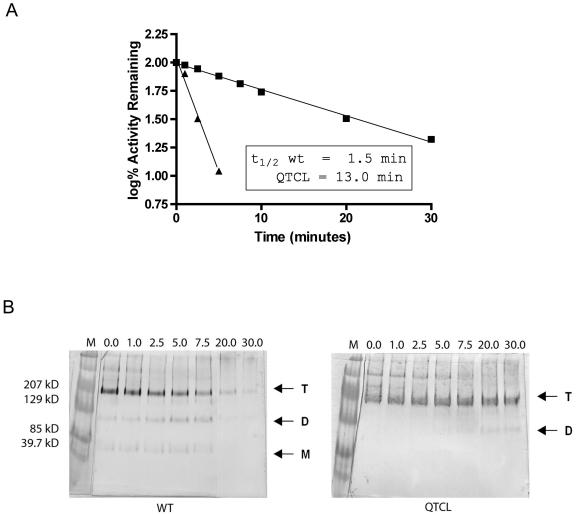
Addition of QTCL to the N terminus of the small subunit confers substantial heat stability to the recombinant maize endosperm AGPase and enhances homodimer formation, as shown above. Next, the aggregation state of purified AGPase was monitored at various stages of heat inactivation (Fig. 5). Purified recombinant AGPases were incubated at 42°C for varying times and the levels of activity and their aggregation states were determined. The half-life of AGPase activity was increased approximately 10-fold at this temperature by addition of the QTCL motif, from 1.50 min to 13.0 min (Fig. 5A). Blue native (BN)-PAGE gels were used to investigate the aggregation state of the enzyme as a function of inactivation (Fig. 5B). Both AGPases exist primarily in the heterotetrameric state before exposure to elevated temperature. However, associated with the rapid loss of activity in wild type is the production of monomers and dimers. The heterotetrameric band is virtually abolished by 20 min, and the only proteins detected after this time are high-Mr aggregates. In contrast, the QTCL enzyme remains predominantly as a heterotetramer, even after a 30-min heat treatment. Note that bovine serum albumin (BSA) was not added to these samples to stabilize the activity of the enzymes; therefore, the half-life of QTCL at 42°C appears to be less than when incubated at 58°C. When this experiment was repeated with BSA, the half-life for the wild-type enzyme was approximately 4.5 min and the half-life for the QTCL mutant could not be determined due to its long stability over the course of the 45-min experiment (data not shown).
Figure 5.
Heat stability of purified QTCL at 42°C. Enzymes were placed in a water bath at 42°C for varying times and then cooled on ice. Each enzyme was assayed for 10 min in the forward direction in the presence of 10 mm 3-PGA. Reactions were started with 0.065 μg of enzyme. Data were plotted as log% activity versus time (min), and the inactivation constant t1/2 was calculated as follows: slope = −k/(2.3). t1/2 is calculated from the equation k = 0.693/t1/2. A, Inactivation time course at 42°C. Symbols are as follows: ▪, QTCL mutant; and ▴, wild-type enzyme. B, BN-PAGE gel transferred and probed with small subunit antibody at the time points from A. T, The approximately 220-kD tetramer; D, the approximately 100-kD dimer; and M, the approximately 50-kD monomer.
Further Small Subunit Modifications
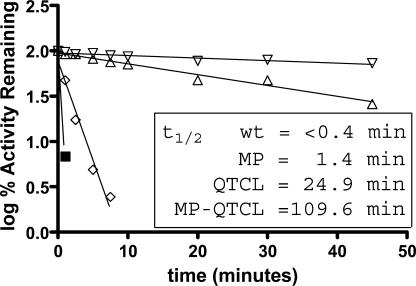
Boehlein et al. (2005) recently reported an AGPase small subunit mosaic that contains amino acids 1 to 199 of the maize small subunit and 200 to 475 from the potato small subunit (MPss). Compared to the recombinant wild-type maize endosperm AGPase, this mosaic was recalcitrant to phosphate inhibition and exhibited increased heat stability. The half-life was 18 min at 42°C or 4 times greater than wild type. To determine whether there was an additive effect in heat stability, the QTCL mutation was placed into MPss. This new variant, termed QTCL-MP, was kinetically indistinguishable from the QTCL mutation (Table III). Interestingly, the increased 3-PGA sensitivity and decreased Pi inhibition caused by substituting the terminal 275 potato tuber small subunit amino acids for their counterparts of maize were nullified by the N-terminal QTCL mutation (Table IV). The QTCL-MP mutation is approximately 5 times more heat stable than QTCL and over 300 times more stable than the recombinant wild-type maize endosperm enzyme (Fig. 6).
Figure 6.
Heat stability of purified QTCL, wild type, and QTCL-MP at 55°C. Reactions were carried out as described in Figure 5, except temperature was raised to 55°C. ▪, Wild type; ⋄, MP; ▵, QTCL; ∇, QTCL-MP.
DISCUSSION
Here, we report recombinant maize endosperm AGPases with greatly enhanced heat stability. Substitution of a motif that we term QTCL from small subunits of heat-stable AGPases into the otherwise heat-labile maize endosperm AGPase causes a 70-fold increase in heat stability at 55°C. In addition, this substitution conditioned two other changes that may be beneficial in the cereal endosperm. The turnover number of the enzyme was doubled and sensitivity to the activator 3-PGA was increased. Significantly, inhibition caused by phosphate was not altered, nor were Km values for the physiologically important substrates, G-1-P and ATP.
Further enhancement of maize endosperm AGPase heat stability was accomplished by placement of the QTCL motif into a modified small subunit described recently by Boehlein et al. (2005). This mosaic subunit, termed MPss, contains amino acids 1 to 199 from the maize endosperm small subunit and amino acids 200 to 475 from the potato tuber subunit. Whereas substitution of MPss for the maize small subunit increases heat stability 4-fold, placement of QTCL into MPss increases heat stability over 300-fold.
Previous work with the potato tuber AGPase (Fu et al., 1998) identified this Cys as being important in enzyme stability. The Cys residue in the potato tuber AGPase is also involved in the reductive activation of the enzyme. This is a two-part mechanism involving substrates and a reducing agent (Fu et al., 1998). Here we show that placement of this Cys into the recombinant maize endosperm AGPase gives rise to activation kinetics similar, although not identical, to those described for the potato AGPase. In the absence of 3-PGA, preincubation of the QTCL mutant with the substrate ADP-Glc and DTT gives rise to a 3.75-fold increase in activity. This does not occur with the wild-type maize endosperm enzyme, nor does it occur with either enzyme in the presence of the activator 3-PGA. One notable difference does distinguish activation of the maize AGPase from the reported potato tuber observations. Whereas preincubation of the potato enzyme with DTT reduces activity, the Cys-containing maize endosperm enzyme is activated by DTT preincubation at room temperature.
We exploited a BN gel system recently modified by us for AGPases (Boehlein et al., 2005) to monitor changes in aggregation states of AGPase caused by the Cys substitution, as well as changes induced by high temperatures. In the absence of SDS and reducing agents, different polymers of AGPase ranging from monomers to higher order polymers can be detected. Polymers involving disulfide bridges are detected in the absence of DTT on nonreducing SDS gels, whereas AGPase monomeric subunits are seen when samples contained DTT. This disulfide bridge does not form in the wild-type maize small subunit, which lacks the QTCL motif. The formation of the disulfide bridge is interesting since potato and maize bear little similarity in the extreme N terminus, with maize having a 25-amino acid extension.
A fundamental difference distinguishes the maize endosperm and the potato tuber AGPases. Whereas disulfide bridges form only between small subunits in the wild-type potato tuber AGPase (Fu et al., 1998), here we show that disulfide bridge formation occurs only between large subunits in the recombinant wild-type maize endosperm enzyme. In view of this, it is noteworthy that hybrid AGPases involving maize and potato subunits exhibit vastly different properties. Whereas the hybrid involving the potato small subunit with the maize large subunit (Pss/Mls) is more active than either parental AGPase, the hybrid involving the maize small subunit with the potato large subunit (Mss/Pls) exhibits barely detectable activity as judged by glycogen production in E. coli (Cross et al., 2004, 2005). The Pss/Mls hybrid could potentially form disulfide bridges both between the small subunits and between the large subunits, whereas Mss/Pls lacks the ability to form these disulfide bridges. In addition, it is interesting to note that a variant of the Mss/Pls enzyme that contains 55 potato-derived amino acids substituted into maize [Mos(1-321,377-457)] produces copious amounts of glycogen in E. coli. However, the AGPase produced by this variant dissociates rapidly following extraction (Cross et al., 2005). These observations suggest that the ability to form at least one disulfide bridge has been selected over evolutionary time. Due to the interchangeability of the subunits early in evolution, the ability to form a disulfide bridge was selected in the large subunit of the maize endosperm (and likely other cereal seed AGPases), whereas the small subunit in potato and likely other AGPases harbor the Cys residues involved in bond formation.
Mutations in the small subunit of maize AGPase are not the only alterations that lead to increased heat stability. Greene and Hannah (1998), through mutation analysis, identified a large subunit heat-stable variant of the maize endosperm. This heat-stable mutation, termed HS33, functions by strengthening subunit interactions forming the heterotetramer. While HS33 enhances heat stability when combined with the wild-type small subunit, the enzyme containing HS33 is much less heat stable than the variants reported here.
A detailed analysis of heat-induced activity loss and enzyme aggregation state shows that the QTCL variant not only enhances enzyme stability, but also aids in maintenance of the tetrameric state. A comparison of heat-induced changes in the aggregation state of wild type and QTCL shows that the QTCL enzyme remains as a heterotetramer much longer than does the wild-type enzyme. In addition, it appears that activity is lost in both wild type and QTCL before loss of the heterotetramer.
Several roles for the N-terminal QTCL motif of the small subunit have now been identified. In addition to its role in disulfide bridge formation and enhanced heat stability, the data presented here show that it plays a significant role in Pi sensitivity. We (Boehlein et al., 2005) reported earlier that substitution of potato sequences into the carboxyl-terminal region of the small subunit effectively removed Pi inhibition. Here we show that incorporation of the QTCL motif into the Pi-insensitive enzyme restores Pi sensitivity. Hence, the QTCL motif plays an important role in modulating AGPase activity.
The lack of this important motif in cereal endosperms of worldwide importance is an interesting observation. As noted previously (Geigenberger et al., 2005), the absence of this Cys indicates redox insensitivity. Here we show that its presence in the maize endosperm AGPase greatly enhances heat stability. It is possible that the QTCL motif has been under negative selection pressure in cereal seeds. Earlier, Greene and Hannah (1998) speculated that, under adverse temperature conditions, the heat lability of the endosperm AGPase might favor greater carbon flow into the embryo rather than the endosperm. This possibly enhances seed viability under adverse conditions. It is also interesting to note that expression in Arabidopsis leaves of the wild-type small subunit protein conjoined to a chloroplast transit peptide functionally substitutes for the endogenous small subunit gene (J. Zou, K. Folta, and L.C. Hannah, unpublished data). Hence, because the Arabidopsis small subunit contains the QTCL, this motif and the associated heat stability and reductive activation are not obligatory for normal starch synthesis in the Arabidopsis leaf.
Starch production in cereals relies heavily on the function of AGPase. Thus, modifications of this enzyme can lead to enhancement or reduction in starch content (Stark et al., 1992; Giroux and Hannah, 1994; Giroux et al., 1996; Smidansky et al., 2002, 2003; T.W. Greene and L.C. Hannah, unpublished data). Since yield loss is associated with temperature stress (Singletary et al., 1993, 1994), a superior AGPase with increased heat stability would be beneficial to cereal crop production. Therefore, further testing of QTCL or QTCL-MP variants in transgenic plants will determine whether the enhanced catalytic activity and heat stability seen in E. coli translates to increases in starch synthesis.
MATERIALS AND METHODS
Site-Directed Mutagenesis
Mutations in the maize (Zea mays) small subunit were created essentially as described by Horton et al. (1993). PCR-mutagenized DNA fragments of the maize endosperm small subunit were digested with NcoI and KpnI. These were used to replace the equivalent wild-type region of small subunit in an expression vector. Mutations were verified by sequencing. The vector was transformed into the Escherichia coli strain AC70R1-504, which also contained the wild-type large subunit coding region on a compatible expression vector (Giroux et al., 1996). The AC70RI-504 cell line contains a mutation that renders the strain incapable of producing bacterial AGPase (Iglesias et al., 1993).
Growth and Purification of Maize AGPase from E. coli
Protein inductions were as described by Burger et al. (2003), except that induction was performed for 3 h at room temperature. Following cell harvest by centrifugation, cell pellets were stored at −80°C.
Enzyme Purification
Purification of the wild type and the QTCL variant AGPase was done as described by Boehlein et al. (2005). For crude extracts, the bacterial pellets were resuspended in 1.0 mL of extraction buffer (50 mm HEPES, pH 7.5, 200 mm KCl, 10 mm MgCl2, 2.5 mm EDTA, and 5% Suc) containing 20% ammonium sulfate, 50 μg/mL lysozyme, 1 μg/mL pepstatin, 1 μg/mL leupeptin, 1 mm phenylmethylsulfonyl fluoride, 10 μg/mL chymostatin, and 1 mm benzamidine. The lysate was maintained on ice and sonicated three times for 10 s each. The sample was centrifuged for 5 min at 12,500 rpm at 4°C and the supernatant was conserved on ice. Solid ammonium sulfate was added to 45% saturation and the sample was centrifuged for 5 min at 12,500 rpm at 4°C. The pellet was resuspended in extraction buffer containing protease inhibitors and stored on ice. The concentration of the crude protein extract was determined using the Bio-Rad protein assay using BSA as a standard.
Assay A (Forward Direction, Radioactive)
For heat-stability measurements, the enzymes were diluted to 1.0 μg/μL and divided into two tubes. A single tube remained on ice while the second tube was placed at 58°C for 6 min with occasional gentle agitation. AGPase activity of the crude extracts was determined in the direction of ADP-Glc synthesis as described in Burger et al. (2003) with a reaction time of 5 min. Reactions were started by the addition of the enzyme. The value reported within an experiment is the average from triplicate samples.
Assay B (Reverse Direction, Nonradioactive)
A nonradioactive endpoint assay was used to measure G-1-P produced by coupling synthesis to NADH production using phosphoglucomutase and Glc-6-P dehydrogenase as described by Boehlein et al. (2005). The temperature of all the assays was 37°C unless otherwise specified. Standard reaction mixtures contained 100 mm MOPS HCl, pH 7.4, 0.4 mg/mL BSA, 5 mm MgCl2, 1 mm ADP-Glc, 20 mm 3-PGA, 1 mm sodium pyrophosphate, and enzyme in 100-μL reaction volume. Reactions were incubated for 5 min and terminated by boiling in a water bath for 1 min. After termination, 330 μL of water were added, followed by 70 μL of a development mixture containing a final concentration of 100 mm MOPS HCl, pH 7.4, 0.1 mg/mL BSA, 7 mm MgCl2, 0.6 mm NAD, 1 unit Glc-6-P dehydrogenase, and 1 unit phosphoglucomutase. Reactions were centrifuged for 5 min and then the absorbance read at 340 nm. Amount of G-1-P produced was calculated from a standard curve using freshly prepared G-1-P instead of enzyme. Assay tubes were prewarmed to 37°C prior to assaying. All assays were initiated by the addition of enzyme. Specific activity is defined as a unit/mg protein. Purification was always monitored using the reverse assay.
Assay C (Forward Reaction, Nonradioactive)
A nonradioactive endpoint assay was used to determine the amount of inorganic pyrophospate produced by coupling it to a decrease in NADH using a pyrophosphate reagent (Sigma P-7275) as previously described (Boehlein et al., 2005). Standard reaction mixtures contained 50 mm HEPES, pH 7.4, 15 mm MgCl2, 4.0 mm ATP, and 4.0 mm G-1-P in 200 μL. 3-PGA was added at varying amounts, as specified. Reactions were terminated after 10 min by boiling in a water bath for 1 min. The reactions were developed by adding 300 μL of pyrophosphate reagent (one bottle diluted with 22.5 mL water) to each assay and then the absorbance read at 340 nm. Change in absorbance between the blank and the reaction was used to calculate the amount to inorganic pyrophospate produced. All reactions were linear with time and enzyme concentration. Assay tubes were prewarmed to 37°C prior to assay and were initiated by enzyme addition.
Enzyme Kinetics
Determination of Km and Vmax
Reactions were performed in the presence of 10 mm 3-PGA using assay C. The reactions were performed in triplicate and started by the addition of 0.043 μg of purified wild-type AGPase and 0.022 μg of QTCL or QTCL-MP enzyme. The amount of ATP or G-1-P varied from 0 to 3 mm.
Determination of Ka and Ki
To determine the activation constant for the recombinant maize wild-type AGPase (0.043 μg), QTCL (0.0217 μg) and QTCL-MP (0.0217 μg) of purified AGPase was incubated for 10 min using assay C. 3-PGA concentrations ranged from 0 to 5.0 mm. The value of the Ki for Pi was calculated in the presence of 2.5 mm 3-PGA and varied from 0 to 15 mm. Curves were fit using Graph Pad Prism nonlinear or linear regression. Activity was not detectable in the absence of 3-PGA at these protein concentrations.
Heat Stability
The half-life of wild type and QTCL at 42°C was determined by desalting enzyme in 50 mm HEPES, pH 6.5, 5.0 mm MgCl2, 0.5 mm EDTA. Heat was applied to desalted enzyme (0.065 mg/mL) and, at the appropriate time, enzyme was withdrawn from the tube and placed on ice. This enzyme was used for activity assays and BN gels. All reactions were carried out in triplicate using assay C with the addition of 10 mm 3-PGA. Both SDS-PAGE and BN-PAGE gels were prepared as outlined (Boehlein et al., 2005). Briefly, the gradient for the BN-PAGE was 5% to 18%. The buffers used are according to Schagger et al. (1994) with minor modifications. Two types of cathode buffer were prepared, one contained 0.002% Coomassie G and the other lacked Coomassie G. Aminocaproic acid was not used in the gel buffer. The gels were run at 4°C for 20 min at 100 V in cathode buffer containing Coomassie G. The voltage then was increased to 200 V for an additional 20 min. Finally, the gel was transferred to cathode buffer without Coomassie and run at 200 V until the dye front was off the gel. The gel was equilibrated in cold 1× transfer buffer (25 mm Tris base, 192 mm Gly, and 20% methanol) plus 1% SDS. A standard western transfer procedure was performed using polyvinylidene difluoride membrane with a 0.2 μm pore size (Bio-Rad). The molecular mass markers were as follows: thyroglobulin, 669,000 kD; ferritin, 440,000 kD; catalase, 232,000 kD; lactate dehydrogenase, 140,000 kD; and BSA, 67,000 kD (Amersham-Pharmacia Biotech electrophoresis calibration kit). Each lane contained approximately 0.87 μg of purified enzyme. All BN-PAGE gel western blots were developed with polyclonal antibodies against the maize AGPase small subunit.
Heat stability experiments at 55°C were performed as described above, except each enzyme was desalted into 50 mm HEPES, pH 7.4, 5 mm MgCl2, 0.5 mm EDTA followed by the addition of BSA (0.5 mg/mL). Data were plotted as log% activity versus time, and the inactivation constant t1/2 was calculated as follows: slope = −k/(2.3). t1/2 is calculated from the equation k = 0.693/t1/2.
Nonreducing 10% SDS-PAGE gels were prepared according to standard procedures (Sambrook et al., 1989). Approximately 0.43 μg of purified enzyme was loaded in each lane, and western blots were developed using polyclonal antibodies raised against the small and large subunits of the maize AGPase.
Disulfide Bridge Reduction
Enzymes were desalted into 50 mm HEPES, pH 7.4, 5 mm MgCl2, and 0.5 mm EDTA. Protein concentrations were determined and BSA was added to a final concentration of 0.5 mg/mL to stabilize the desalted enzyme. Enzymes were incubated for 15 min at room temperature in a mixture containing 100 mm HEPES, pH 7.4, 0.2 mg/mL BSA, 5 mm MgCl2, and the appropriate treatment in a 20-μL volume. Following this incubation, reactions were performed using assay B with the following modification. The final concentration in samples with DTT was 3.0 mm and the ADP-Glc concentration was adjusted so that the final concentration in the assay was always 1.4 mm. Each assay was performed for 2, 3, 4, 5, and 6 min and the slope of the plot of velocity versus time was used to calculate the specific activity.
Acknowledgments
We thank Dr. Jon Stewart, Dr. Thomas Greene, and members of the Hannah Laboratory for many useful comments and discussions.
This work was supported by the National Science Foundation (IBN–9316887, IBN–960416, IBN–9982626, IBN–0444031, and MCB–9420422), the U.S. Department of Agriculture Competitive Grants Program (94–37300–453, 9500836, 95–37301–2080, 9701964, 97–36306–4461, 98–01006, and 2000–01488), and the Florida Agricultural Experiment Station (Journal Series no. R–10889).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: L. Curtis Hannah (hannah@mail.ifas.ufl.edu).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.067637.
References
- Bae J, Giroux M, Hannah LC (1990) Cloning and molecular characterization of the brittle-2 gene of maize. Maydica 35: 317–322 [Google Scholar]
- Ballicora MA, Frueauf JB, Fu Y, Schurmann P, Preiss J (2000) Activation of the potato tuber ADP-glucose pyrophosphorylase by thioredoxin. J Biol Chem 275: 1315–1320 [DOI] [PubMed] [Google Scholar]
- Ballicora MA, Fu Y, Frueauf JB, Preiss J (1999) Heat stability of the potato tuber ADP-glucose pyrophosphorylase: role of Cys residue 12 in the small subunit. Biochem Biophys Res Commun 257: 782–786 [DOI] [PubMed] [Google Scholar]
- Ballicora MA, Laughlin MJ, Fu Y, Okita TW, Barry GF, Preiss J (1995) Adenosine 5′-diphosphate-glucose pyrophosphorylase from potato tuber. Significance of the N terminus of the small subunit for catalytic properties and heat stability. Plant Physiol 109: 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave M, Lawrence S, Barton C, Hannah LC (1990) Identification and molecular characterization of shrunken-2 cDNA clones of maize. Plant Cell 2: 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehlein SK, Sewell AK, Cross J, Stewart JD, Hannah LC (2005) Purification and characterization of adenosine diphosphate glucose pyrophosphorylase from maize/potato mosaics. Plant Physiol 138: 1552–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger BT, Cross JM, Shaw JR, Caren JR, Greene TW, Okita TW, Hannah LC (2003) Relative turnover numbers of maize endosperm and potato tuber ADP-glucose pyrophosphorylases in the absence and presence of 3-phosphoglyceric acid. Planta 217: 449–456 [DOI] [PubMed] [Google Scholar]
- Cross JM, Clancy M, Shaw JR, Boehlein SK, Greene TW, Schmidt RR, Okita TW, Hannah LC (2005) A polymorphic motif in the small subunit of ADP-glucose pyrophosphorylase modulates interactions between the small and large subunits. Plant J 41: 501–511 [DOI] [PubMed] [Google Scholar]
- Cross JM, Clancy M, Shaw JR, Greene TW, Schmidt RR, Okita TW, Hannah LC (2004) Both subunits of ADP-glucose pyrophosphorylase are regulatory. Plant Physiol 135: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Ballicora MA, Leykam JF, Preiss J (1998) Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J Biol Chem 273: 25045–25052 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Kolbe A, Tiessen A (2005) Redox regulation of carbon storage and partitioning in response to light and sugars. J Exp Bot 56: 1469–1479 [DOI] [PubMed] [Google Scholar]
- Giroux MJ, Hannah LC (1994) ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize. Mol Gen Genet 243: 400–408 [DOI] [PubMed] [Google Scholar]
- Giroux MJ, Shaw J, Barry G, Cobb BG, Greene T, Okita T, Hannah LC (1996) A single mutation that increases maize seed weight. Proc Natl Acad Sci USA 93: 5824–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene TW, Hannah LC (1998) Enhanced stability of maize endosperm ADP-glucose pyrophosphorylase is gained through mutants that alter subunit interactions. Proc Natl Acad Sci USA 95: 13342–13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah L, Tuschall D, Mans R (1980) Multiple forms of maize endosperm ADP-glucose pyrophosphorylase and their control by Shrunken-2 and Brittle-2. Genetics 95: 961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah LC (1997) Starch synthesis in the maize endosperm. In BA Larkins, IK Vasil, eds, Advances in Cellular and Molecular Biology of Plants, Vol 4. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 375–405
- Hannah LC, Shaw JR, Giroux MJ, Reyss A, Prioul JL, Bae JM, Lee JY (2001) Maize genes encoding the small subunit of ADP-glucose pyrophosphorylase. Plant Physiol 127: 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R, Steffan N, Pullen J, Hunt H, Cai Z, Pease L (1993) Gene splicing by overlap extension. In J Abelson, M Simon, eds, Methods in Enzymology. Academic Press, New York, pp 270–280 [DOI] [PubMed]
- Hwang SK, Salamone PR, Okita TW (2005) Allosteric regulation of the higher plant ADP-glucose pyrophosphorylase is a product of synergy between the two subunits. FEBS Lett 579: 983–990 [DOI] [PubMed] [Google Scholar]
- Iglesias AA, Barry GF, Meyer C, Bloksberg L, Nakata PA, Greene T, Laughlin MJ, Okita TW, Kishore GM, Preiss J (1993) Expression of the potato tuber ADP-glucose pyrophosphorylase in Escherichia coli. J Biol Chem 268: 1081–1086 [PubMed] [Google Scholar]
- Jin X, Ballicora MA, Preiss J, Geiger JH (2005) Crystal structure of potato tuber ADP-glucose pyrophosphorylase. EMBO J 24: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T, Nakata P, Anderson J, Sowokinos J, Morell M, Preiss J (1990) The subunit structure of potato tuber ADPglucose pyrophosphorylase. Plant Physiol 93: 785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J, Romeo T (1994) Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog Nucleic Acid Res Mol Biol 47: 299–329 [DOI] [PubMed] [Google Scholar]
- Preiss J, Sivak M (1996) Starch synthesis in sinks and sources. In E Zamski, ed, Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. Marcel Dekker, New York, pp 139–168
- Sambrook J, Fritsch E, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schagger H, Cramer W, von Jagow G (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem 217: 220–230 [DOI] [PubMed] [Google Scholar]
- Singletary G, Banisadr R, Keeling P (1993) Decreased starch synthesis in heat stressed maize kernels results from reduced ADPG-pyrophosphorylase and starch synthase activities. Plant Physiol (Suppl) 102: 6 [Google Scholar]
- Singletary G, Banisadr R, Keeling P (1994) Heat stress during grain filling in maize: effects of carbohydrate storage and metabolism. Aust J Plant Physiol 21: 829–841 [Google Scholar]
- Smidansky ED, Clancy M, Meyer FD, Lanning SP, Blake NK, Talbert LE, Giroux MJ (2002) Enhanced ADP-glucose pyrophosphorylase activity in wheat endosperm increases seed yield. Proc Natl Acad Sci USA 99: 1724–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidansky ED, Martin JM, Hannah LC, Fischer AM, Giroux MJ (2003) Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase. Planta 216: 656–664 [DOI] [PubMed] [Google Scholar]
- Sowokinos J, Preiss J (1982) Pyrophosphorylases in Solanum tuberosum. III. Purification, physical, and catalytic properties of ADPglucose pyrophosphorylase in potatoes. Plant Physiol 69: 1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D, Timmerman K, Barry G, Preiss J, Kishore G (1992) Regulation of the amount of starch in plant tissues by ADP glucose pyrophosphorylase. Science 258: 287–291 [DOI] [PubMed] [Google Scholar]
- Tiessen A, Hendriks JH, Stitt M, Branscheid A, Gibon Y, Farre EM, Geigenberger P (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14: 2191–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]