Abstract
We assessed the effects of brefeldin A (BFA) on pollen tube development in Picea meyeri using fluorescent marker FM4-64 as a membrane-inserted endocytic/recycling marker, together with ultrastructural studies and Fourier transform infrared analysis of cell walls. BFA inhibited pollen germination and pollen tube growth, causing morphological changes in a dose-dependent manner, and pollen tube tip growth recovered after transferring into BFA-free medium. FM4-64 labeling showed typical bright apical staining in normally growing P. meyeri pollen tubes; this apical staining pattern differed from the V-formation pattern found in angiosperm pollen tubes. Confocal microscopy revealed that exocytosis was greatly inhibited in the presence of BFA. In contrast, the overall uptake of FM4-64 dye was about 2-fold that in the control after BFA (5 μg mL−1) treatment, revealing that BFA stimulated endocytosis in a manner opposite to the induced changes in exocytosis. Transmission electron microscopic observation showed that the number of secretory vesicles at the apical zone dramatically decreased, together with the disappearance of paramural bodies, while the number of vacuoles and other larger organelles increased. An acid phosphatase assay confirmed that the addition of BFA significantly inhibited secretory pathways. Importantly, Fourier transform infrared microspectroscopy documented significant changes in the cell wall composition of pollen tubes growing in the presence of BFA. These results suggest that enhanced endocytosis, together with inhibited secretion, is responsible for the retarded growth of pollen tubes induced by BFA.
The pollen tube is a highly polarized plant cell with a rapidly growing tip that is specialized to deliver genetic material from the site of pollination on the flower stigma to the site of fertilization at the ovule (Hepler et al., 2001). Polarized pollen tube growth results from continued fusion with the plasma membrane by secretory vesicles derived from the Golgi apparatus. This process provides new plasma membrane and cell wall components, and remodels the cell wall composition (Mascarenhas, 1993). The quantity of membrane delivered to the cell tip by exocytosis is in excess of that required for the pollen tube growth rate, suggesting an underlying recycling process (Parton et al., 2001; Camacho and Malhó, 2003). Obviously, a delicate balance between the exocytosis of cell wall components, cell wall assembly, and endocytosis is essential for pollen tube growth (Parton et al., 2001). However, the precise mechanisms involved in the regulation of exocytosis, endocytosis, and vesicle recycling in growing pollen tubes remain speculative.
Exocytosis is a general term used to denote vesicle fusion at the plasma membrane, and it is the final step in the secretory pathway that typically begins in the endoplasmic reticulum (ER), passes through the Golgi apparatus, and ends at the outside of the cell (Battey et al., 1999). Endocytosis is postulated to counterbalance membrane secretion (Emans et al., 2002). However, the growing number of plasma membrane proteins and cell wall molecules, such as pectins and xyloglucans, accomplish recycling via endocytosis, followed by exocytosis from secretory endosomes (Baluška et al., 2002, 2005; Šamaj et al., 2004, 2005). Growing pollen tubes exhibit higher endocytosis and/or exocytosis activity in the apical region (Parton et al., 2001, 2003; Camacho and Malhó, 2003). Rapid tip growth in angiosperm pollen tubes has been studied extensively (Derksen et al., 1995) and is characterized by a particular type of cytoplasmic organization, i.e. the specific accumulation of secretory vesicles, clathrin, and clathrin-coated pits at the tube tip (Derksen et al., 2002). However, pollen tubes of gymnosperms differ from those of angiosperms in many important characteristics (Derksen et al., 1995; Wang et al., 2003). Gymnosperm pollen tubes show natural ramification, and a slow, mostly Brownian-like, movement of the organelles. They have little tip-to-base zonation of large organelles. The cytoplasm contains numerous starch grains, and few microtubules and actin filaments are found in the cortex. Outside the tip, the mitochondrial density increases toward the periphery, giving rise to rows of mitochondria along the tube wall. Unlike in angiosperms, the Golgi does not show any specific zonation or accumulation in the gymnosperm tube (De Win et al., 1996). In addition, the initiation of germination and the maintenance of pollen tube elongation in gymnosperms depend on continuous protein synthesis (Fernando et al., 2001; Hao et al., 2005). These different physical characteristics may be reflected by another type of cytoplasmic organization than that known in angiosperm pollen tubes.
The inhibitor brefeldin A (BFA) is a metabolite produced by fungi that is used for the study of endomembrane vesicle flow in eukaryotic cells (Rojas et al., 1999). It affects membrane traffic in the animal secretory and endocytic pathways (Lippincott-Schwartz et al., 1991). Increasing evidence documents striking and reversible effects of BFA on secretion from both plant Golgi apparatus and endosomes, making it a useful tool in following the secretory pathway in plant cells (Baluška et al., 2002; Šamaj et al., 2004). In meristematic root cells, BFA inhibits exocytosis but allows endocytosis (Baluška et al., 2002, 2005). Using fluorescent marker FM1-43, Emans et al. (2002) reported that BFA stimulated temperature-dependent endocytosis in BY-2 suspension cells. Moreover, studies on tobacco (Nicotiana tabacum) pollen tubes established that BFA blocks the secretion of cell wall material, resulting in growth arrest (Rutten and Knuiman, 1993). Additionally, BFA inhibits root hair tip growth, accompanied by the disappearance of the clear zone, depletion of secretory vesicles, and the simultaneous relocation of actin and mitogen-activated protein kinase (Šamaj et al., 2002). However, there is controversy regarding the effects of BFA on endocytosis (Prydz et al., 1992; Baluška et al., 2002), and no study has systematically compared differences in the endocytic pathways to changes in the cell wall components in response to BFA treatment. Such findings may be important for bridging existing information among the biochemical, physiological, and cellular levels.
The goal of this investigation was to evaluate the effects of BFA on the endocytosis and the abundance and distribution of secretory vesicles at the apical region during pollen tube development in the gymnosperm Picea meyeri using several independent methods. In addition, the chemical components of the tube wall and the ultrastructure of the pollen tubes were analyzed to gain insight into the structural basis of the observed effects.
RESULTS
Pollen Germination and Pollen Tube Growth
Much variation was observed in the germination rate of pollen grains incubated in medium containing various concentrations of BFA. Pollen began to germinate after 6 h in control culture medium and reached its maximum germination ratio of 85% after 36 h (Table I; Fig. 1A). Little difference was observed in the germination rate with the addition of 1 μg mL−1 BFA to the culture medium. However, with the addition of 5 μg mL−1 BFA, the average pollen germination rate was significantly lower, at 48% (P < 0.05). The pollen grain germination process was severely retarded when treated with higher concentrations of BFA (Table I; Fig. 1B).
Table I.
Dose-dependent effects of BFA on P. meyeri pollen germination and pollen tube growth
The germinated pollen grains were counted after 36-h culture, and only those pollen tubes with their length longer than the diameter of pollen grain were considered germinated.
| BFA
|
Germination Rate
|
Pollen Tube Length
|
|||||
|---|---|---|---|---|---|---|---|
| 6 h | 12 h | 18 h | 24 h | 30 h | 36 h | ||
| μg mL−1 | % | μm | |||||
| 0 | 84.5 ± 3.4 | 45.0 ± 1.8 | 91.5 ± 3.6 | 187.7 ± 5.6 | 262.5 ± 8.4 | 300.9 ± 9.1 | 315.9 ± 9.7 |
| 1 | 83.0 ± 3.3 | 43.0 ± 1.9 | 89.8 ± 4.5 | 185.9 ± 5.5 | 250.2 ± 7.9 | 265.7 ± 9.0 | 265.2 ± 9.5 |
| 3 | 68.1 ± 2.7 | 39.5 ± 1.6 | 85.3 ± 4.3 | 151.3 ± 5.5 | 175.0 ± 7.3 | 169.2 ± 8.4 | 165.1 ± 8.2 |
| 5 | 48.3 ± 1.9 | 39.2 ± 1.8 | 78.5 ± 3.8 | 80.5 ± 3.6 | 82.3 ± 4.0 | 83.0 ± 4.1 | 84.8 ± 4.2 |
| 8 | 8.2 ± 0.4 | 38.1 ± 1.8 | 39.5 ± 1.8 | 45.2 ± 2.2 | 43.7 ± 2.1 | 49.0 ± 2.4 | 48.0 ± 2.3 |
Figure 1.
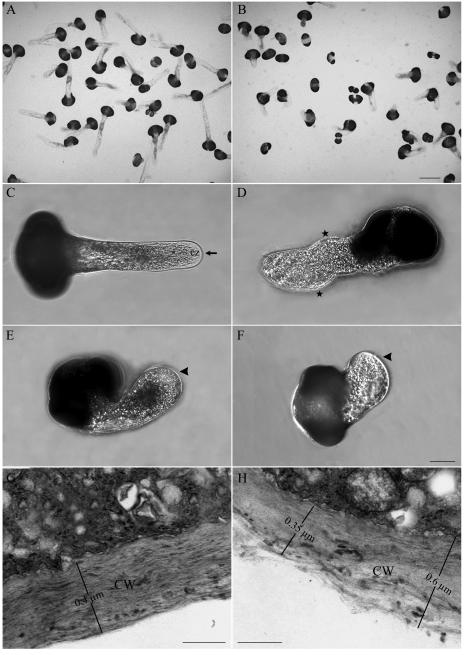
Effects of BFA on pollen germination and pollen tube morphology. A, Healthy P. meyeri pollen tubes cultured in standard medium for 36 h, showing good germination and many long pollen tubes with normal shape. B, P. meyeri pollen tubes cultured in medium containing 5 μg mL−1 BFA for 36 h, showing poor germination and a few short tubes with morphological abnormalities. C, Micrograph of a control P. meyeri pollen tube cultured for 20 h, showing a regularly shaped pollen tube of constant diameter and a clear zone at apical region. D to F, Typical examples of pollen tubes in the presence of BFA for 20 h. D, Pollen tube incubated in 1 μg mL−1 BFA; stars indicate changes in growth direction (wavy pattern). E, Pollen tube incubated in 5 μg mL−1 BFA, showing a swelled tube tip (arrowhead). F, Most of the pollen grains cultured in 8 μg mL−1 BFA, showing aberrant protrusions, which were not regarded as germinated. G, Details of cell wall structure in control pollen tube. The cell wall was uniform, compact, and 0.4 μm thick. H, Details of cell wall structure in BFA-treated pollen tube. The cell wall became more loosely packed, showing the cell wall thickness from 0.35 to 0.6 μm in the presence of BFA. cz, Clear zone; CW, cell wall. Bars in A and B = 100 μm; in C to F = 50 μm; and in G and H = 0.3 μm.
When cultured in standard medium, pollen tubes were long, with uniform diameter and a clear zone at the apical region (Fig. 1C). The addition of BFA led to obvious morphological changes, including changes in growth direction (wavy growth pattern), an increase in the tube diameter, and variable changes in the tube tip, such as more pointed tips or tip swelling (Fig. 1, D–F). With increasing incubation durations, the BFA-treated pollen tubes ruptured, indicating that they were more fragile. Treatment with 1 μg mL−1 BFA decreased the pollen tube growth rate to 7.8 μm h−1. When the BFA concentration was increased to 5 μg mL−1, the average pollen tube growth rate dropped to 2.5 μm h−1, in comparison with 16.2 μm h−1 observed in the control during 30 h of growth after germination. With increasing BFA concentrations, the inhibitory effect on pollen tube growth was more pronounced (Table I). Most of the pollen grains cultured in the presence of 8 μg mL−1 BFA did not germinate. Although the pollen grains were observed to have short protrusions, they cannot be considered as germinated since their length was shorter than the pollen grain diameter (Fig. 1F). Thus, BFA inhibited pollen tube growth in a dose-dependent manner. To determine whether the effects of BFA were reversible, pollen grains were first cultured in media supplemented with 5 μg mL−1 or 8 μg mL−1 BFA for 18 h, and were then transferred into BFA-free medium. Our results showed that for samples previously treated with 5 μg mL−1 or 8 μg mL−1 BFA, not only did germination rate increase but also pollen tubes continued to elongate when transferred into BFA-free medium (Supplemental Fig. 1).
FM4-64 Staining Distribution Pattern
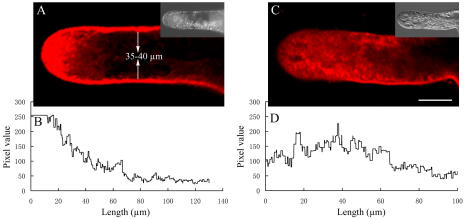
In median confocal optical sections (Fig. 2A), FM4-64 consistently produced a distinct peripheral and bright apical staining, which was distributed in the extreme apical (15–20 μm) region in normally growing P. meyeri pollen tubes. The corresponding quantitative image revealed a model of a sharply defined high signal in the extreme apex; however, beyond this region, FM4-64 staining was generally and significantly weakened (Fig. 2B). Peripheral staining was shown to be plasma membrane, and not associated with the cell wall, as shown by plasmolysis with 100 μm sorbitol in the presence of FM4-64. Pretreatment of pollen tubes with 500 μm sodium azide impaired dye uptake: FM4-64 fluorescence could only be observed at plasma membrane, but the dye was not internalized over time (Supplemental Fig. 2).
Figure 2.
Confocal images of FM4-64 staining in pollen tubes of P. meyeri. A, A median focal plane confocal optical section, showing a typical FM4-64 staining pattern in a growing pollen tube; the bright field at one-third size appears as an insert. B, Pixel values along a central transect through the fluorescence image in A. C, A median focal plane confocal optical section, showing a dispersed and disrupted FM4-64 staining pattern in BFA-treated (5 μg mL−1) pollen tube; the bright field at one-third size appears as an insert. D, Pixel values along a central transect through the fluorescence image in C.
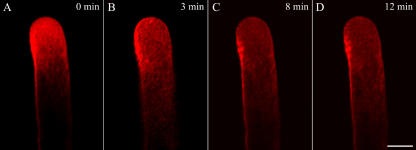
For pollen tubes cultured in medium containing 5 μg mL−1 BFA, the FM4-64 membrane-staining pattern at the apical region was disrupted. Extreme apical staining was more diffuse and less well localized, no longer exhibiting the bright region at the apical clear zone that is characteristic of normally growing pollen tubes (Fig. 2C). The corresponding quantitative image showed a broader distribution of FM4-64 staining (Fig. 2D). Furthermore, a typical FM4-64 staining pattern in a normal pollen tube could be rapidly dispersed by short-term treatment with BFA (Fig. 3). Within 10 to 15 min, apical FM4-64 staining was largely redistributed from the apex and became continuously scattered over more subapical regions (Fig. 3, B and C). Ultimately, the apical bright FM4-64 staining pattern disappeared with increasing time of exposure to BFA (Fig. 3D).
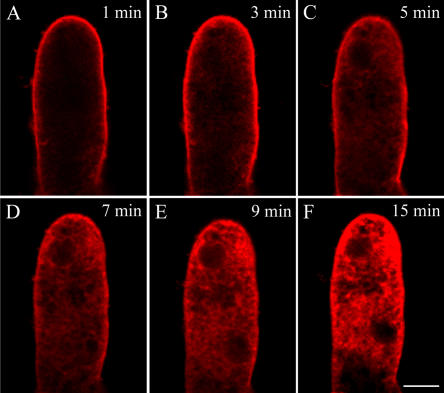
Figure 3.
Time course of disruption in a normal typical FM4-64 staining induced by 5 μg mL−1 BFA. BFA was applied directly to growing pollen tubes on thin gel layers in 70 μL of 115% to 120% liquid medium. A, A typical FM4-64 distribution pattern in a normal growing pollen tube of P. meyeri. B, The changes of FM4-64 staining at 3 min after the addition of BFA, showing the FM4-64 fluorescence tended to be scattered. C and D, The changed FM4-64 staining distribution with increasing time, showing FM4-64 fluorescence became more dispersed and almost distributed in the whole pollen tube after 12 min of BFA treatment. Bar = 25 μm.
The Time Course of FM4-64 Internalization
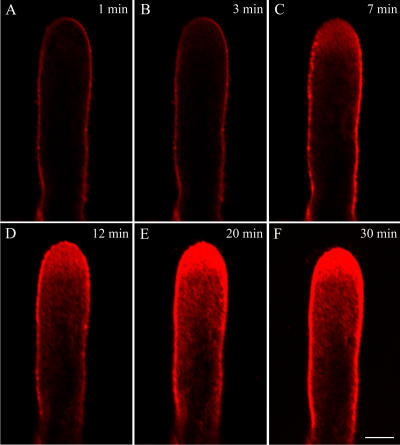
The uptake of FM4-64 into P. meyeri pollen tubes followed a strict time sequence (Fig. 4, A–F). Staining associated with the plasma membrane became obvious immediately after dye application to the germination medium (Fig. 4A). This was followed by internalization of the dye, mainly at the apical region (Fig. 4, C and D). Within several minutes, the typical staining pattern of FM4-64 dye was observed, i.e. bright staining of the entire apical region that extended to the subapical region with very weak staining (Fig. 4, E and F), rather than the inverted cone shape typically observed in angiosperms. The apical fluorescent region corresponds to the so-called clear zone, a region filled with secretory vesicles but lacking any larger organelles.
Figure 4.
FM4-64-uptake time course in a growing P. meyeri pollen tube. A to F, Median confocal fluorescence images at increasing times after addition of FM4-64 (2 μm in 115% standard medium). To avoid osmotic perturbation, pollen tubes were pretreated with 115% medium before dye application. The rapid uptake suggests an extremely high rate of endocytosis and membrane traffic; the bright region suggests there is an accumulation of secretory vesicles in the apical zone. Bar = 25 μm.
In BFA-treated pollen tubes, the FM4-64 internalization continued even more rapidly, and the dye internalization occurred throughout almost the whole pollen tube (Fig. 5, A–F). However, the bright apical FM4-64 staining pattern did not occur. With increasing time after FM4-64 dye application, only patch-like and dispersed fluorescence of stained material emerged over most of the pollen tube (Fig. 5F). To quantify the effect of BFA on FM4-64 dye internalization, pollen tubes cultured for 18 h in control medium were pretreated with BFA, latrunculin B, or sodium azide for 60 min, and, subsequently, FM4-64 was internalized for 12 min. The images in Figure 6 (A–H) show that the FM4-64 staining pattern was disrupted by treatment with BFA (Fig. 6C), latrunculin B (Fig. 6E), and sodium azide (Fig. 6G) compared to the control (Fig. 6A). Moreover, quantitative data suggest that BFA stimulated the total uptake of FM4-64 dye (Fig. 6D). In contrast, latrunculin B inhibited the internalization of FM4-64 dye (Fig. 6F), and sodium azide blocked FM4-64 internalization almost completely (Fig. 6H) compared to the control (Fig. 6B). This fluorimetric quantification showed that FM4-64 uptake was stimulated about 2-fold by BFA and inhibited about 0.48-fold by latrunculin B, while almost no dye was internalized after the sodium azide treatment (Fig. 7).
Figure 5.
Time course of changes in dye distribution of FM4-64-loaded P. meyeri pollen tubes treated with 5 μg mL−1 BFA. A to F, Confocal fluorescence images of a BFA-treated pollen tube at different times, showing BFA treatment did not inhibit the internalization of FM4-64 dye through endocytosis but the dispersed FM4-64 distributing pattern indicating BFA inhibited secretory vesicle accumulation at tube tip. Bar = 25 μm.
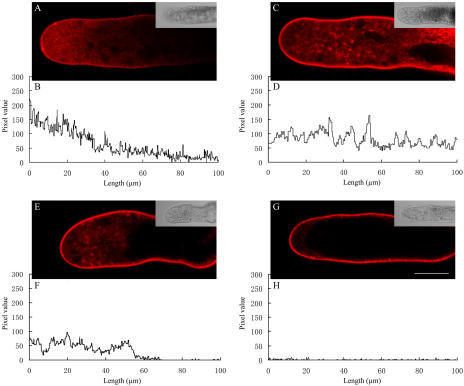
Figure 6.
Confocal images showing uptake of 2 μm FM4-64 into control and inhibitor-treated pollen tubes of P. meyeri within 12 min. All the pixel values did not include the peripheral region. A, A median focal plane confocal optical section of a control pollen tube; the bright field at one-third size appears as an insert. B, Pixel values along a central transect through the fluorescence image in A. C, A median focal plane confocal optical section of a pollen tube pretreated with 5 μg mL−1 BFA for 60 min; the bright field at one-third size appears as an insert. D, Pixel values along a central transect through the fluorescence image in C, showing that BFA stimulated the internalization of FM4-64 dye. E, A median focal plane confocal optical section of a pollen tube pretreated with 1 μm latrunculin B for 60 min; the bright field at one-third size appears as an insert. F, Pixel values along a central transect through the fluorescence image in E, showing latrunculin B inhibited the internalization of FM4-64 dye. G, A median focal plane confocal optical section of a pollen tube pretreated with 500 μm sodium azide for 60 min; the bright field at one-third size appears as an insert. H, Pixel values along a central transect through the fluorescence image in G, showing sodium azide blocked internalization of FM4-64 dye almost entirely. Bar = 25 μm.
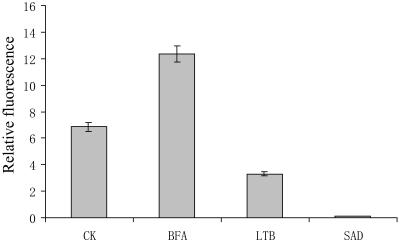
Figure 7.
FM4-64 internalization in control and inhibitor-treated pollen tubes showing that dye uptake is stimulated by BFA (5 μg mL−1) inhibited by latrunculin B (1 μm) and sodium azide (500 μm). Cell-associated FM4-64 fluorescence was quantified after a 12-min internalization of the dye and normalized to control pollen tubes labeling. Data shown are means ± sd and are representative of three experiments, each containing three individual measurements. CK, Control; LTB, latrunculin B; SAD, sodium azide.
Ultrastructural Changes in Organelles and the Production of Secretory Vesicles
A zone filled mainly with secretory vesicles (clear zone) could be distinguished from the streaming cytoplasm at the very tip of pollen tubes growing in control medium. In the zones behind the very tip, many larger organelles such as Golgi stacks, ER, and mitochondria, as well as many small round-shaped vacuoles, were well resolved (Fig. 8A). Some of the secretory vesicles fusing with plasma membrane, and paramural bodies (PBs), probably resulting from fusion of multivesicular bodies (MVBs) with the plasma membrane, were observed at higher magnification (Fig. 8, C and E). Most Golgi stacks in the control pollen tubes consisted of four to seven flattened cisternae with a distinct cis-to-trans polarity, and numerous secretory vesicles were present around them (Fig. 8F).
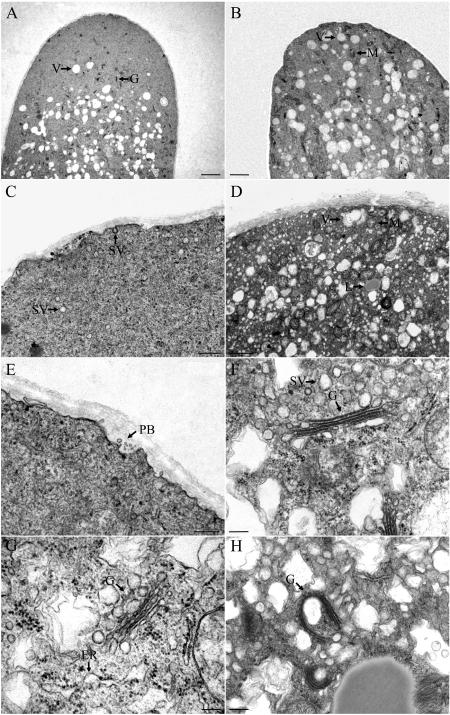
Figure 8.
Electron micrographs in control and BFA-treated (5 μg mL−1) pollen tubes. A, Tip region of a control pollen tube, showing the apical clear zone. B, Tip region of a BFA-treated pollen tube, showing the apical clear zone was occupied by many organelles, e.g. mitochondria and vacuoles. C, Magnified picture of a control pollen tube tip region, from where many vesicles could be observed; some of them were fusing with, or releasing from, the plasma membrane. D, Tip region of a BFA-treated pollen tube. No PBs and secretory vesicles could be observed, but many other types of vesicles and large organelles, such as mitochondria, vacuoles, and lipid bodies, appeared. E, Another apical zone in control pollen tube, indicating a typical PB (arrow). F, Golgi apparatus in control pollen tubes, showing the Golgi stacks contain four flattened cisternae with a distinct cis-trans polarity with numerous vesicles attached to them. G, Abnormal arrangements of the Golgi stacks in BFA-treated pollen tubes, indicating the disassembly of the Golgi apparatus accompanied by vesiculation of trans-side (arrow), less Golgi-derived vesicles, as well as the ER dilations (arrow). H, Another abnormal arrangement of Golgi apparatus induced by BFA treatment. Golgi cisternae appeared abnormally stacked and curved, engulfing some large vesicles. Bar = 2 μm in A and B; 1 μm in C and D; 0.5 μm in E; and 0.2 μm in F, G, and H. SV, Secretory vesicles; M, mitochondrion; V, vacuole; L, lipid body; G, Golgi apparatus; PB, paramural body.
Much variation was observed when pollen grains were incubated in the medium containing BFA. Treatment with 5 μg mL−1 BFA drastically decreased the number of secretory vesicles and increased the number of small vacuoles at pollen tube tips, and other larger organelles such as mitochondria and lipid bodies could be observed at the very apical region (Fig. 8, B and D). Upon BFA treatment, Golgi stacks disassembled or tended to be stacked and curved at the trans face, while the number of secretory vesicles surrounding them drastically decreased (Fig. 8, G and H). The ER became swollen with the detachment of ribosomes from the rough ER (Fig. 8G). However, no significant differences were observed between BFA-treated and normal pollen tubes in terms of mitochondria (data not shown). In addition, a more loosely packed cell wall, 0.35 to 0.6 μm thick, was produced (Fig. 1H) in the treated than in the control pollen tubes, which showed a uniform cell wall thickness of 0.4 μm (Fig. 1G).
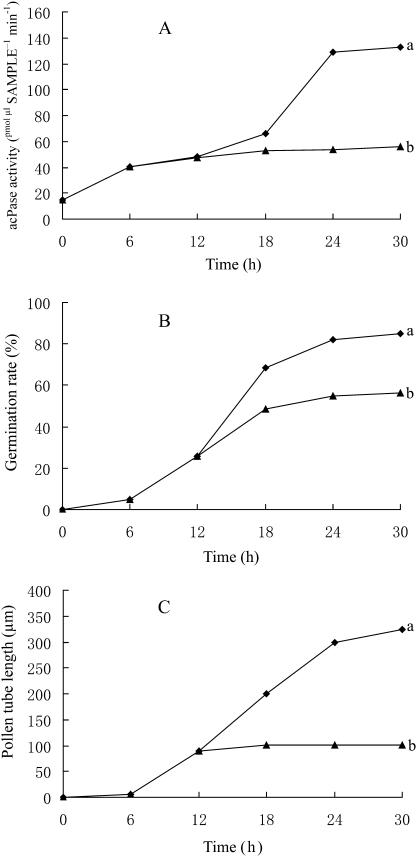
Activity of Secreted Acid Phosphatase
The effects of BFA on the acid phosphatase (acPase) activity are plotted in Figure 9 and show the average of three experiments. The acPase activity increased as the washed pollen grains began to germinate and pollen tubes elongated in the germination medium (Fig. 9, A, a, B, a, and C, a). There were almost parallel increases in acPase activity and pollen tube length over time (Fig. 9, A, a, and C, a). When 5 μg mL−1 BFA was added to the medium after 12 h of incubation, the export of acPase activity (Fig. 9A, b) was immediately inhibited, along with the inhibition of tube elongation (Fig. 9C, b) and pollen germination (Fig. 9B, b). Pollen tube growth and secretion of active acPase were almost completely inhibited over time with BFA treatment (Fig. 9, A, b, and C, b), whereas pollen germination was less affected (Fig. 9B, b).
Figure 9.
A typical inhibitor experiment showing the effects of BFA on acPase activity (A), germination frequency (B), and mean tube length (C). Pollen grains were incubated in germination medium. After 12 h, BFA (5 μg mL−1) was added to one-half of the pollen culture (▴), whereas the other half served as a control (♦). All three parameters were determined every 6 h. The data points represent means with sds of three independent experiments.
Changes in Chemical Components
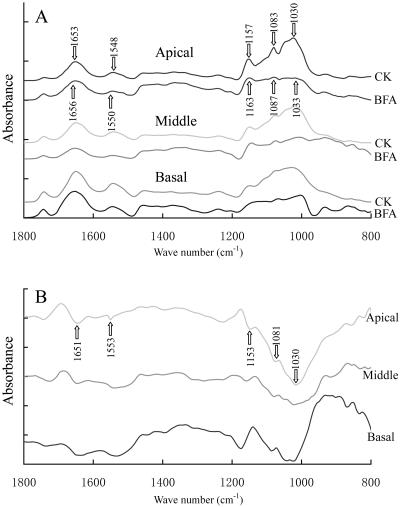
Fourier transform infrared (FTIR) spectra were analyzed in apical, middle, and basal regions of pollen tubes. FTIR spectra of pollen tubes growing in control medium showed similar patterns among the three regions analyzed, although the proportion of proteins to polysaccharides varied among different regions within an individual pollen tube. The absorption bands occurred at 1,650 cm−1 and 1,550 cm−1, corresponding to amide I and amide II of proteins (McCann et al., 1994), while a peak at 1,744 cm−1 corresponded to saturated esters (McCann et al., 1994), and polysaccharides absorbed at 1,200 to 900 cm−1 (McCann et al., 1994; Fig. 10A).
Figure 10.
FTIR spectra obtained from the apical region, middle region, and basal region of P. meyeri pollen tubes cultured for 20 h. A, FTIR spectra obtained from the tip, middle, and basal regions of pollen tubes cultured in standard medium (CK) or in medium containing 5 μg mL−1 BFA (BFA) revealed that BFA treatment induced displacements of the peaks or changes of absorbance. B, Difference spectra generated by digital subtraction of spectra CK from spectra BFA, showing that the content of proteins and polysaccharides decreased after BFA treatment and that the reduction in polysaccharide was most obvious in apical region.
Treatment with 5 μg mL−1 BFA induced displacement of the peaks or changes in absorbance. The difference spectra generated by digital subtraction of the spectra of control tube walls from the spectra of BFA-treated tube walls (Fig. 10B) showed that both the amide-stretching bands and the carbohydrate bands were dramatically reduced after BFA treatment in all three regions analyzed. In contrast to a small reduction in protein content, there was a marked decrease in polysaccharide content among the three regions. Further, the reduction of polysaccharides in the newly formed tip region was far more obvious than in the already formed middle and basal regions.
DISCUSSION
BFA Retarded Pollen Tube Development
The fungal metabolite BFA, which has been shown to interfere with protein transfer through the endomembrane system, is an inhibitor of the secretory pathway of intracellular protein transfer and mainly induces dysfunction of the Golgi stacks (Nebenführ et al., 2002). Lanubile et al. (1997) reported that BFA affects the synthesis and transport of cell wall polysaccharides and proteins in pea (Pisum sativum) root seedlings. Additionally, the comparison of FM1-43 uptake in normal and BFA-treated tobacco suspension cells showed that BFA can stimulate endocytosis (Emans et al., 2002). However, the reliability of FM1-43 as an endocytic tracer for plant cells has not been confirmed (Meckel et al., 2004; Šamaj et al., 2005).
Little information about the effects of BFA on the secretory pathway during pollen germination and pollen tube growth has been reported. Rutten and Knuiman (1993) reported that the secretion and pollen tube growth were two separate processes. In this study, BFA not only reduced pollen germination rates (Table I; Fig. 1B) and retarded pollen tube growth (Table I), but also resulted in abnormalities in pollen tube shape (Fig. 1, D–F). The recovery experiments indicated that the effects of BFA were reversible, clearly documenting the concentrations of BFA used in this study are within a physiological range (Supplemental Fig. 1). The results presented here indicated that the pollen tube growth was closely linked to the cellular process of the secretory pathway, the disruption of which led to the pollen tube growth arrest. However, it should be noted that, for P. meyeri, only concentrations of BFA greater than 1 μg mL−1 exerted a significant effect. This concentration is much higher than that reported for angiosperms, e.g. 0.1 μg mL−1 of BFA in the pollen tubes of tobacco (Rutten and Knuiman, 1993). This large discrepancy may be due to differences in the plant species examined, and may also reflect alterations in the activity of the Golgi apparatus.
BFA Disrupted Secretory Vesicle Accumulation at the Pollen Tube Apex
Regulated secretory vesicle delivery, distribution, vesicle fusion, and rapid membrane recycling can be tracked in living cells using FM4-64 dye because this dye is nontoxic and water soluble (Šamaj et al., 2005). For studies of plant and fungal cells, FM4-64 is usually preferred to FM1-43 because of its superior brightness, greater contrast, and higher photostability, and, more importantly, unlike FM1-43, the dye is not retained in cell walls (Bolte et al., 2004). Because the dye stains membranes in an activity-dependent manner, it has been found increasingly useful in exploring the endocytosis and secretory mechanisms in a variety of biological models (Smith and Betz, 1996). The present experiment of plasmolysis with sorbitol and the effect of sodium azide on FM4-64 uptake (Supplemental Fig. 2) confirmed earlier reports that FM4-64 staining was plasma membrane rather than cell wall associated, and its internalization occurred via an endocytic mechanism instead of passive diffusion (Parton et al., 2001, 2003; Camacho and Malhó, 2003). The median confocal optical images of FM4-64 fluorescence in normally growing P. meyeri tubes consistently revealed bright peripheral and apical staining (Fig. 2A). The FM4-64 staining pattern detected in P. meyeri was different from the V-shaped apical staining reported in angiosperm species (Parton et al., 2001, 2003). In addition to the staining pattern, the proportion of the apical region stained by FM4-64 to the whole pollen tube in P. meyeri was much lower than that in lily (Lilium longiflorum) pollen tubes. In P. meyeri, the ratio of the length of the FM4-64-labeled apex region (15–20 μm) to the pollen tube diameter (35–40 μm) was about 1:2 in comparison with the corresponding parameter (1:1) in lily pollen tubes, in which both the length of the FM4-64 staining region and the pollen tube diameter were about 15 to 20 μm (Parton et al., 2001, 2003). Because FM4-64 staining corresponds strikingly to the location and distribution of secretory vesicles at the apical region (Lancelle and Hepler, 1992; Derksen et al., 1995), we may conclude that the slow growth rate of P. meyeri pollen tubes was largely caused by the smaller region of secretory vesicles at the tip.
Previous studies have established that treatment with BFA can result in a particular reorganization of the cytoplasm at the pollen tube apex (Parton et al., 2003). Our experiment with FM4-64 indicated that the typical bright FM4-64 staining pattern that appeared at the apical region in normal pollen tubes is lost in BFA-treated pollen tubes, and is replaced by a scattered and dispersed distribution of the dye (Fig. 2, C and D). This implies that the distribution and number of secretory vesicles at the tip region varied after BFA treatment. The time course of dissociation and dissipation of a normal apical FM4-64 staining pattern after the addition of BFA in P. meyeri revealed a dynamic process; BFA can rapidly disperse and reduce the abundance of secretory vesicles located in the tube apex (Fig. 3). The decreased number and dispersed distribution of secretory vesicles in the apical region indicated that vesicle secretion became less active after BFA treatment, which likely resulted from the effect of BFA on the production of secretory vesicles and/or vesicle delivery to the apical region. Vesicle trafficking in plants is dependent on the actin cytoskeleton, and previous reports have shown that BFA affects both actin organization and actin-dependent endosomal motility within the growing tips of root hairs (Šamaj et al., 2002; Voigt et al., 2005). The phenotype of wavy pollen tubes with increased diameter produced upon BFA treatment (Fig. 1, D–F) that was observed in our experiment was possibly caused by the improper delivery to and depletion of secretory vesicles from the clear zone of the pollen tubes. Nevertheless, we did not detect BFA-induced vesicular aggregation, as was found in BFA-treated lily pollen tubes (Parton et al., 2003), probably because of the different type of cytoplasmic streaming found in conifer and angiosperm pollen tubes (De Win et al., 1996; Lazzaro et al., 2003).
BFA Stimulated Endocytosis
There have been reports of endocytosis associated with tip growth in the development of pollen tubes, root hairs, and fungal hyphae (Derksen et al., 2002; Read and Kalkman, 2003; Voigt et al., 2005). Studies on yeast (Saccharomyces cerevisiae), tobacco BY-2 cells, and lily pollen tubes have indicated that FM dyes were taken up by the endocytic pathway (Parton et al., 2001; Emans et al., 2002). In this study, we found that FM4-64 dye was internalized to induce a typical bright staining pattern at the apical region (Fig. 4) within 30 min, indicating that endocytosis occurred during normal pollen tube growth in P. meyeri, similar to reports from other species (Parton et al., 2001; Camacho and Malhó, 2003). In BFA-treated pollen tubes, FM4-64 dye uptake was more rapid, probably because the dye internalization occurred at broader range, in spite of dispersed staining (Fig. 5), implying that BFA treatment stimulated endocytosis in contrast to untreated pollen tubes. Furthermore, latrunculin B and sodium azide inhibited and blocked FM4-64 dye internalization, respectively, while BFA obviously stimulated endocytosis (Fig. 6, A–H). This conclusion was also strongly supported by a fluorimetric quantitative analysis (Fig. 7). Because endocytosis is postulated to counterbalance membrane secretion (Emans et al., 2002), we conclude that decreased tube growth is likely a direct consequence of increased endocytosis and inhibited secretion from both Golgi stacks and endosomes. Stimulated apical endocytosis after BFA treatment, through its action on an ADP ribosylation-guanine nucleotide exchange factor involved in endocytosis, was indeed reported earlier in tobacco BY-2 suspension cells exposed to FM1-43 (Emans et al., 2002).
BFA Disassembled the Golgi Apparatus
Tip growth in pollen tubes requires the integrity of the secretory system (Moscatelli and Cresti, 2001). During tube growth, secretory vesicles derived from the Golgi apparatus and/or from the early/recycling endosomes (Geldner et al., 2003; Murphy et al., 2005) transport components needed for cell wall expansion (Camacho and Malhó, 2003). In this experiment, we observed that secretory vesicles dominated the apex in P. meyeri pollen tubes, and their distribution corresponded strikingly to the FM4-64 staining pattern in both location and distribution, as described previously. Because many of the secretory vesicles received the internalized endocytic tracer FM4-64 within a few minutes of exposure, we propose that many of these secretory vesicles are derived from the early/recycling endosomes, as reported for the apices of tip-growing root hairs (Voigt et al., 2005). However, it is important to note that the vesicle distribution at the extreme apical region shows a lower density (Fig. 8C) than that found in the V-shaped vesicle accumulation in angiosperm pollen tubes (Parton et al., 2001, 2003) and also differed from other coniferous species, in which many other large organelles are detected in the apical region of the pollen tube (Hao et al., 2005). Deducing from the facts that the pollen tube growth rate in P. meyeri (16 μm h−1) ranks between that of the lily (390 μm h−1) and other gymnosperm species (1 μm h−1; De Win et al., 1996; Yang et al., 1999; Hao et al., 2005), we speculate that the pollen tube growth rate mainly depends on the particular cellular organization of the apical region. In the presence of BFA (5 μg mL−1), the secretory vesicles rapidly vanished from the very tip, while many small vacuoles and mitochondria, as well as some lipid bodies and various types of vesicles, appeared in the apical region (Fig. 8, B and D). Furthermore, the number of secretory vesicles at the trans-Golgi side decreased because of the structural disintegration of the Golgi apparatus (Fig. 8, G and H), suggesting that BFA inhibited the production of secretory vesicles by disorganizing the Golgi apparatus and trans-Golgi network. The ER also dilated after BFA treatment (Fig. 8G). Because the pollen tube growth rate depends on the supply of new material to the cell walls (Derksen et al., 1995), the disorder inflicted on the secretory system (ER, Golgi apparatus, trans-Golgi network) by treatment with BFA inevitably retarded the elongation of the pollen tubes, as discussed previously.
The PB, a general term used to describe all membrane systems associated with the plasma membrane, comprising a group of membranous elements situated between the plant cell wall and the plasma membrane, is involved in exocytosis and cell wall deposition (Marchant and Robards, 1968). Because PBs possessed vesicles similar to the internal vesicles of MVBs, they may eventually originate from fusions of MVBs, representing endosomes with apical plasma membranes (Tse et al., 2004). In this study, we noted that many PBs appeared in the normal growing pollen tube apex (Fig. 8E), suggesting that active exocytosis of MVBs occurred during normal pollen tube growth. However, in the BFA-treated pollen tube apical region, no PBs were observed (Fig. 10D), providing strong evidence that BFA disrupted the secretory activity of the MVBs, which may contribute to pollen tube wall deposition during pollen tube elongation (Derksen et al., 1995).
BFA Lowered AcPase Activity
In plant cells, secretion can be measured by monitoring the release of secretory products in cell cultures (Ibrahim et al., 2002). For example, the growth of yeast is accompanied by the local secretion of acPase at the growing site of the cell (Field and Schekman, 1980). Based on a study of lily pollen tube development, Ibrahim et al. (2002) suggested that the secreted acPase activity released via the secretory pathway may serve as a useful indicator of exocytotic activity in terms of the conspicuous correlation between acPase secretion and tube growth. The present experiment showed that secreted acPase activity was strongly correlated with pollen grain germination and tube growth in P. meyeri. With the development of normal pollen tubes, the acPase activity increased (Fig. 9), suggesting that acPase was secreted during P. meyeri pollen tube growth, and its secretory activity increased with the elongation of pollen tubes. In the presence of BFA, the export of acPase activity (Fig. 9A, b) was dramatically inhibited, along with the inhibition of pollen germination (Fig. 9B, b) and pollen tube growth (Fig. 9C, b), suggesting that the cellular process leading to secretion was disrupted by BFA. Because acPase was probably secreted together with the cell wall material at the tube tip from where it diffuses into the medium (Ibrahim et al., 2002), it seems reasonable to conclude that the inhibition of production of secretory vesicles induced by BFA led to the suppression of acPase secretion.
BFA Altered the Tube Cell Wall Composition and Structure
Pollen tube elongation requires the insertion of many polysaccharides and structural proteins into the tube walls (Mascarenhas, 1993). Cell wall proteins, polysaccharides, and other components of the wall matrix are synthesized in the ER-Golgi system and transported into the apoplastic space via secretory vesicles (Moore et al., 1991). Interference with the production and transport of secretory vesicles results in the modified secretion of cell wall components (Cheung et al., 2002). FTIR microspectroscopy is a reliable and highly reproducible assay used to study cell wall composition (McCann et al., 1994) and pollen tube walls cultured in different media (Yang et al., 1999; Wang et al., 2003). Using FITR analysis, we confirmed that proteins were present in the pollen tube walls of P. meyeri (Fig. 10A), as has been reported for other species (Yang et al., 1999). The difference spectra (Fig. 10B) showed that the protein and polysaccharide contents along the whole tube walls were reduced when treated with BFA, directly demonstrating the inhibitory effect of BFA on tube wall deposition during pollen tube growth. Furthermore, the polysaccharide content decreased more obviously than that of protein, particularly at the apical region (Fig. 10B). This explains the differential sensitivity to BFA treatment, at the Golgi apparatus, toward the synthesis of cell wall polysaccharides and the machinery of vesicular transport of proteins to the wall. The BFA-induced modifications in the composition of pollen tube walls were also reflected by the noncompact and disordered wall structure (Fig. 1H).
It has been reported that cell walls at the apical region are highly enriched with pectin (Li et al., 2002). The FTIR analysis described here showed that polysaccharides decreased more obviously in the newly formed tip region than in the already formed regions after treatment with BFA. Because most of the bands in the polysaccharide region between 1,200 and 900 cm−1 are the vibrations associated with the sugar monomers of pectin (Chen et al., 1997), we conclude that the prominent decrease in polysaccharide content at the apical region was caused by a decrease in pectin content. Cell wall pectins cross-linked with boron and calcium are internalized into plant cells (Baluška et al., 2002, 2005) and apparently recycled via early/recycling endosomes (Baluška et al., 2002; Šamaj et al., 2004). Thus, we propose that BFA-induced aberrant cell walls are the result not only of inhibited secretion but also of enhanced endocytosis of cell wall pectins in the tip region. Further studies should test this scenario.
In summary, our results clearly showed that BFA retarded pollen tube development; the effects of BFA were reversible because pollen tubes can recover their tip growth after the removal of BFA. BFA treatment inhibited exocytosis by disrupting the distribution and reducing the abundance of secretory vesicles at the apical region through disorganizing the Golgi apparatus, trans-Golgi network, and early/recycling endosomes. Importantly, endocytosis was stimulated, as revealed by confocal microscopy and fluorimetry measurements, in a manner opposite to the inhibition of exocytosis in the presence of BFA. The results also demonstrated that the distribution pattern and abundance of secretory vesicles found at the pollen tube apical region in P. meyeri differ from the V-formation of vesicle accumulation in angiosperms, using both ultrastructural examination and fluorescent marker FM4-64 labeling. FTIR analysis further demonstrated that the changes in the cell wall composition could clearly be attributed not only to inhibited exocytosis but also to the enhanced uptake of cell wall components (especially pectins) through increased endocytosis. Based on these results, we conclude that enhanced endocytosis, together with inhibited secretion, is responsible for the abnormal morphology and growth inhibition of pollen tubes induced by BFA, a phenomenon that has not been reported previously.
MATERIALS AND METHODS
Plant Materials
Cones with mature pollen were collected from trees of Picea meyeri Rehd. et Wils. growing in the Botanical Garden of the Institute of Botany, Chinese Academy of Sciences, prior to the beginning of the pollination season at the middle of April 2003, and were dried overnight at room temperature. The dry pollen was stored at −20°C until use.
Pollen Culture
Stored pollen was equilibrated at room temperature for 30 min and carefully suspended in 12% (w/v) Suc medium containing 0, 1, 3, 5, 8, or 10 μg mL−1 BFA, respectively. The pH of the media was adjusted to 6.4 with phosphate-buffered saline. The cultures were incubated on a shaker (100 rpm) at 25°C. For recovery experiments, samples exposed to the drug were gently centrifuged and transferred to drug-free medium. For confocal images, pollen grains were transferred to thin layers of 0.2% agar solidified culture medium after hydration for 30 min in liquid media. Thin gel layers were prepared according to Parton et al. (2001). Thin gel layers were sown with pollen suspension and incubated in a dark humid environment at room temperature.
Determination of Pollen Germination and Pollen Tube Growth
The germination rate was determined by checking at least 500 grains under a Nikon microscope. Pollen grains were considered as germinated when the pollen tube length was greater than the diameter of the pollen grain (Wang et al., 2003). To measure the mean tube length, images of pollen tubes cultured in the above media were taken at 6-h intervals, and the number of pollen tubes examined was at least 200 for each treatment. All experiments were performed at room temperature and in triplicate.
Confocal Microscopy
Loading cells with 2 μm FM4-64 dye was generally achieved by application during the imbibition of pollen grains by direct addition of dye solutions in 115% liquid medium to growing tubes on thin gel layers. BFA (5 μg mL−1), latrunculin B (1 μm), and sodium azide (500 μm) were applied directly to growing pollen tubes on thin gel layers in 70 μL of 115% to 120% liquid medium. Fluorescence from FM4-64 staining was detected using a Bio-Rad MRC 600 laser confocal scanning unit attached to an Optiphot microscope (Nikon). The samples were excited at 514 nm with a 25-mW argon ion laser operated at full power at an intensity of 3%, achieved by means of neutral-density filters, with a nearly closed pinhole and the gain adjusted to below level 7.00.
Fluorimetry Measurements
After 18-h culture, the pollen suspension of 20 mL was divided into four equal aliquots. Three of these aliquots were incubated with BFA (5 μg mL−1), latrunculin B (1 μm), or sodium azide (500 μm) for 60 min, whereas another aliquot served as a control. After 60-min incubation, all of these aliquots were supplemented with 2 μm FM4-64 for 12 min. Then, the aliquots were washed three times with control medium by centrifugation (at 1,000g for 5 min). Cell-associated fluorescence was quantitated by fluorimetry using an F-4500 fluorospectrometer (Japan). FM4-64 was excited at 514 nm (5-nm bandpass), and emission was detected at 580 nm (5-nm bandpass). Cell-associated fluorescence was normalized to the cell-associated fluorescence of control pollen tube labeling and the results of three measurements were averaged.
Electron Microscopy
For electron microscopy, pollen tubes incubated for 20 h in media containing 0 or 5 μg mL−1 BFA were fixed in 2.5% glutaraldehyde in 100 mm phosphate buffer, pH 7.2, for 2 h at room temperature. Pollen tubes were washed three times with this buffer, post-fixed with 1% OsO4 for 3 h in 100 mm phosphate buffer, pH 7.2. After washing with this buffer three times, pollen tubes were dehydrated in ethanol series and finally embedded in Spurr resin. Ultrathin sections of pollen tubes were mounted on Formvar coated grids and stained with 2% aqueous uranyl acetate and lead citrate, and observed with an electron microscope (JEOL 1210) at 80 kV.
Secreted AcPase Assay
Pollen grains of 50 mg were suspended in 50 mL of germination medium and gently shaken for 1 min. Pollen grains were washed five times by subsequently pelleting the grains, resuspending in fresh medium, and shaking for 1 min to remove cell wall-bound acPase. After this washing procedure, the pollen suspension was pipetted into petri dishes for germination. AcPase secretion was monitored by sampling aliquots every 6 h and analyzing them for acPase activity. After 30 h the pollen grains and tubes were pelleted and aliquots of the supernatant containing the secreted acPase activity were frozen and stored at −20°C. The activity of the acPase was measured according to Ibrahim et al. (2002) by measuring the release of p-nitrophenol from p-nitrophenyl phosphate. Samples of 100 μL were incubated with 400 μL of reaction buffer containing 100 mm acetic acid, pH 5.0, 5 mm p-nitrophenyl phosphate for 45 min at 30°C. The reaction was stopped by the addition of 200 μL of 500 mm NaOH, pH 9.8, and the concentration of p-nitrophenol was determined at 405-nm wavelength (U3000; Hitachi). All assays were performed as triplicates.
FTIR Analysis
Pollen tubes cultured either in the absence of BFA or supplemented with 5 μg mL−1 BFA for 20 h were collected and washed with deionized water three times. The samples were dried at room temperature on a barium fluoride window (13 mm diameter × 2 mm). Infrared spectra were obtained from the apical region, middle region, and basal region of pollen tubes, respectively, using a MAGNA 750 FTIR spectrometer (Nicolet) equipped with a mercury-cadmium-telluride detector. The spectra were recorded at a resolution of 8 cm−1 with 128 co-added interferograms and were normalized to obtain the relative absorbance.
Supplementary Material
Acknowledgments
We thank Drs. Mathew Benson and Shi-fu Wen for valuable discussions at the early stages of these experiments and technical assistance with FTIR microspectroscopy. We also thank Dr. Richard Turner for his patient correction of the draft of the manuscript. This is journal paper number 0503 of the Institute of Botany, Chinese Academy of Sciences.
This work was supported by the National Science Fund of China for Distinguished Young Scholars (30225005), and by grants from the European Union Research Training Network TIPNET (project no. HPRN–CT–2002–00265) obtained from Brussels; from Deutsches Zentrum für Luft- und Raumfahrt (Bonn); and from Grant Agency Vega (grant nos. 2/2011/22 and 2031), Bratislava, Slovakia.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jinxing Lin (linjx@ibcas.ac.cn).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.069765.
References
- Baluška F, Hlavacka A, Šamaj J, Palme K, Robinson DG, Matoh T, McCurdy DW, Menzel D, Volkmann D (2002) F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from Brefeldin A-induced compartments. Plant Physiol 130: 422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Liners F, Hlavacka A, Schlicht M, Van Cutsem P, McCurdy D, Menzel D (2005) Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 225: 141–155 [DOI] [PubMed] [Google Scholar]
- Battey NH, James NC, Greenland AJ, Brownlee C (1999) Exocytosis and endocytosis. Plant Cell 11: 643–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214: 159–173 [DOI] [PubMed] [Google Scholar]
- Camacho L, Malhó R (2003) Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J Exp Bot 54: 83–92 [DOI] [PubMed] [Google Scholar]
- Chen L, Wilson RH, McCann MC (1997) Infra-red microspectroscopy of hydrated biological systems: design and construction of a new cell with atmospheric control for the study of plant cell walls. J Microsc 188: 62–71 [Google Scholar]
- Cheung AY, Chen YH, Glaven RH, de Graaf BHJ, Vidali L, Hepler PK, Wu HM (2002) Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell 14: 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen J, Knuiman B, Hoedemaekers K, Guyon A, Bonhomme S, Pierson ES (2002) Growth and cellular organization of Arabidopsis pollen tubes in vitro. Sex Plant Reprod 15: 133–139 [Google Scholar]
- Derksen J, Rutten T, Lichtscheidl IK, Dewin AHN, Pierson ES, Rongen G (1995) Quantitative analysis of the distribution of organelles in tobacco pollen tubes: implications for exocytosis and endocytosis. Protoplasma 188: 267–276 [Google Scholar]
- De Win AHN, Knuiman B, Pierson ES, Geurts H, Kengen HMP, Derksen J (1996) Development and cellular organization of Pinus sylvestris pollen tubes. Sex Plant Reprod 9: 93–101 [Google Scholar]
- Emans N, Zimmermann S, Fischer R (2002) Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando DD, Owens JN, Yu XS, Ekramoddoullah AKM (2001) RNA and protein synthesis during in vitro pollen germination and tube elongation in Pinus monticola and other conifers. Sex Plant Reprod 13: 259–264 [Google Scholar]
- Field C, Schekman R (1980) Localized secretion of acid phosphatase reflects the pattern of cell surface growth in Saccharomyces cerevisiae. J Cell Biol 86: 123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Hao HQ, Li YQ, Hu YX, Lin JX (2005) Inhibition of RNA and protein synthesis in pollen tube development of Pinus bungeana by actinomycin D and cycloheximide. New Phytol 165: 721–730 [DOI] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Ibrahim H, Pertl H, Pittertschatscher K, Fadl-Allah E, El-Shahed A, Bentrup FW, Obermeyer G (2002) Release of an acid phosphatase activity during lily pollen tube growth involves components of the secretory pathway. Protoplasma 219: 176–183 [DOI] [PubMed] [Google Scholar]
- Lancelle SA, Hepler PH (1992) Ultrastructure of freeze-substituted pollen tubes of Lilium longiflorum. Protoplasma 167: 215–230 [Google Scholar]
- Lanubile R, Piro G, Dalessandro G (1997) Effect of Brefeldin A on the synthesis and transport of cell wall polysaccharides and proteins in pea root seedlings. J Exp Bot 48: 1925–1933 [Google Scholar]
- Lazzaro MD, Donohue JM, Soodavar FM (2003) Distribution of cellulose synthesis by isoxaben causes tip swelling and disorganizes cortical microtubules in elongating conifer pollen tubes. Protoplasma 220: 201–207 [DOI] [PubMed] [Google Scholar]
- Li YQ, Mareck A, Faleri C, Moscatelli A, Liu Q, Cresti M (2002) Detection and localization of pectin methylesterase isoforms in pollen tubes of Nicotiana tabacum L. Planta 214: 734–740 [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Tipper M, Amherdt LO, Klausner RD (1991) Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67: 601–616 [DOI] [PubMed] [Google Scholar]
- Marchant R, Robards AW (1968) Membrane systems associated with the plasmalemma of plant cells. Ann Bot 32: 457–471 [Google Scholar]
- Mascarenhas JP (1993) Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5: 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MC, Roberts JSK, Carpita NC (1994) Changes in pectin structure and localization during the growth of unadapted and NaCl-adapted tobacco cells. Plant J 5: 773–785 [Google Scholar]
- Meckel T, Hurst AC, Thiel G, Homann U (2004) Endocytosis against high turgor: intact guard cells of Vicia faba constitutively endocytose fluorescently labelled plasma membrane and GFP-tagged K+-channel KAT1. Plant J 39: 182–193 [DOI] [PubMed] [Google Scholar]
- Moore PJ, Sword KMM, Lynch MA, Staehelin LA (1991) Spatial organization of the assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants. J Cell Biol 112: 589–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli A, Cresti M (2001) Pollen germination and pollen tube growth. In SS Bhoiwani, WY Soh, eds, Current Trends in the Embryology of Angiosperms. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 33–65
- Murphy AS, Bandyopadhyay A, Holstein SE, Peer WA (2005) Endocytotic cycling of PM proteins. Annu Rev Plant Biol 56: 221–251 [DOI] [PubMed] [Google Scholar]
- Nebenführ A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol 130: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RM, Fischer-Parton S, Trewavas AJ, Watahiki MK (2003) Pollen tubes exhibit regular periodic membrane trafficking events in the absence of apical extension. J Cell Sci 116: 2707–2719 [DOI] [PubMed] [Google Scholar]
- Parton RM, Fischer-Parton S, Watahiki MK, Trewavas AJ (2001) Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci 114: 2685–2695 [DOI] [PubMed] [Google Scholar]
- Prydz K, Hansen SH, Sandvig K, van Deurs B (1992) Effects of brefeldin A on endocytosis, transcytosis and transport to the Golgi complex in polarized MDCK cells. J Cell Biol 119: 259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read ND, Kalkman ER (2003) Does endocytosis occur in fungal hyphae? Fungal Genet Biol 39: 199–203 [DOI] [PubMed] [Google Scholar]
- Rojas M, Owen TP Jr, Kathleen NL (1999) Brefeldin A inhibits secondary cell wall synthesis in developing tracheary elements of Zinnia elegans. Int J Plant Sci 160: 683–690 [Google Scholar]
- Rutten TLM, Knuiman B (1993) Brefeldin A effects on tobacco pollen tubes. Eur J Cell Biol 61: 247–255 [PubMed] [Google Scholar]
- Šamaj J, Baluška F, Voigt B, Schlicht M, Volkmann D, Menzel D (2004) Endocytosis, actin cytoskeleton, and signaling. Plant Physiol 135: 1150–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šamaj J, Ovecka M, Hlavacka A, Lecourieux F, Meskiene I, Lichtscheidl I, Lenart P, Salaj J, Volkmann D, Bögre L, et al (2002) Involvement of the mitogen-activated protein kinase SIMK in regulation of root hair tip growth. EMBO J 21: 3296–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šamaj J, Read ND, Volkmann D, Menzel D, Baluška F (2005) The endocytic network in plants. Trends Cell Biol 15: 425–433 [DOI] [PubMed] [Google Scholar]
- Smith CB, Betz WJ (1996) Simultaneous independent measurement of endocytosis and exocytosis. Nature 380: 531–534 [DOI] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang LW (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt B, Timmers ACJ, Šamaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H, et al (2005) Actin-propelled motility of endosomes is tightly linked to polar tip-growth of root hairs. Eur J Cell Biol 84: 609–621 [DOI] [PubMed] [Google Scholar]
- Wang QL, Lu LD, Wu XQ, Li YQ, Lin JX (2003) Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiol 23: 345–351 [DOI] [PubMed] [Google Scholar]
- Yang XD, Sun SQ, Li YQ (1999) Boron deficiency causes changes in the distribution of major polysaccharides of pollen tube wall. Acta Bot Sin 41: 1169–1176 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.