Abstract
Populus euphratica Olivier is known to exist in saline and arid environments. In this study we investigated the physiological mechanisms enabling this species to cope with stress caused by salinity. Acclimation to increasing Na+ concentrations required adjustments of the osmotic pressure of leaves, which were achieved by accumulation of Na+ and compensatory decreases in calcium and soluble carbohydrates. The counterbalance of Na+/Ca2+ was also observed in mature leaves from field-grown P. euphratica trees exposed to an environmental gradient of increasing salinity. X-ray microanalysis showed that a primary strategy to protect the cytosol against sodium toxicity was apoplastic but not vacuolar salt accumulation. The ability to cope with salinity also included maintenance of cytosolic potassium concentrations and development of leaf succulence due to an increase in cell number and cell volume leading to sodium dilution. Decreases in apoplastic and vacuolar Ca2+ combined with suppression of calcineurin B-like protein transcripts suggest that Na+ adaptation required suppression of calcium-related signaling pathways. Significant increases in galactinol synthase and alternative oxidase after salt shock and salt adaptation point to shifts in carbohydrate metabolism and suppression of reactive oxygen species in mitochondria under salt stress.
Salinity has a major impact on plant growth and productivity. Worldwide, almost 1 billion ha of land are affected by soil salinity (Szabolcs, 1994). Today, soil salinization is still increasing mainly because of unsuitable irrigation practices. To cope with this enormous problem, efforts are undertaken to enhance the salt tolerance of economically important plants by traditional plant breeding as well as biotechnological approaches. Appropriate strategies may include enhancing stress resistance of salt-sensitive plant species or using plants that naturally display high salt resistance (Flowers, 2003). For the latter option, the stress-tolerant tree Populus euphratica (Olivier) seems a promising candidate. Its native distribution ranges from the semiarid areas of northwest China to western Morocco (Browicz, 1977; Xu, 1988). It grows under unfavorable conditions such as saline and alkaline soils (Kang et al., 1996; Watanabe et al., 2001; Chen et al., 2002). Therefore, it has been used successfully for large-scale afforestation projects on saline desert sites in China (Wei, 1993). In addition to its beneficial ecological effects, the wood of P. euphratica is used for house construction and fuel wood, playing an important role in the local economy. Despite its multipurpose potential, little is known about the physiological mechanisms underlying its stress tolerance (Ma et al., 1997).
Salt (NaCl) imposes several kinds of stresses upon plants. It causes drastic changes in the osmotic water balance and increases the cellular concentration of deleterious ions, leading to membrane disorganization, ion toxicity, and oxidative stress (Hasegawa et al., 2000; Zhu, 2001). To cope with salinity, defense responses are activated at different levels. At the whole-plant level many nonhalophytes try to exclude salt (Greenway and Munns, 1980). However, this strategy is not important in P. euphratica, which showed no restriction of Na+ uptake into roots compared with salt-sensitive poplar species (Chen et al., 2001).
At the cellular level common metabolic answers to salt stress are the synthesis of stress-related enzymes like antioxidant systems, chaperons (e.g. salt-shock proteins), and compatible solutes (Hasegawa et al., 2000; Wang et al., 2003). While these responses are important to protect metabolic functions under increasing salt concentrations, a primary defense is the avoidance of excessive Na+ accumulation in the cytosol (Blumwald et al., 2000). At the mechanistic level our understanding of how plants signal salt stress has greatly increased in recent years. Transmission of the stress signal involves transient increases in cytosolic Ca2+ triggering downstream pathways by interacting with different sensors such as calmodulin (CaM), CaM domain protein kinases, and calcineurin B-like proteins (CBLs; Knight and Knight, 2001; Sanders et al., 2002). In the salt overly sensitive (SOS) pathway, which is crucial for salt adaptation (Zhu, 2001), Ca2+ binding to SOS3, a calcineurin-like protein (Liu and Zhu, 1998), affords association with the protein kinase SOS2 (Chinnusamy et al., 2004). The complex of SOS3-SOS2 stimulates SOS1, an NHX-type Na+/H+ antiporter regulating cytosolic Na+ concentrations (Chinnusamy et al., 2004). More recently, it was shown that SOS2 also affected CAX1, a vacuolar Ca2+/H+ antiporter, thereby linking cellular Ca2+ and Na+ transport (Cheng et al., 2004). However, CAX1 overexpression resulted in increased salt sensitivity (Cheng et al., 2004), whereas overexpression of SOS1 resulted in increased salt tolerance in various species, underlining the importance of sodium export out of the cell or into the vacuole for cytosolic ion homeostasis (Apse et al., 1999; Hamada et al., 2001; Shi et al., 2003). In P. euphratica additionally a NhaD-type Na+/H+ antiporter (PeNhaD1) has been characterized, whose transcription, in contrast to a salt-sensitive poplar, was maintained under salt stress (Ottow et al., 2005). Since complementation of salt-sensitive Escherichia coli mutants with PeNhaD1 restored their salt tolerance, a function of this antiporter in subcellular sodium compartmentation was suggested (Ottow et al., 2005).
A key issue in salt adaptation is osmotic adjustment. Salinity decreases soil water potential and thus leads to turgor loss in nonacclimated plants. To maintain water uptake, adjustment of the osmotic potential of the cells is required. Unlike stress signaling, which relies on rapid, transient changes of the messenger, osmoprotection is afforded by compensatory changes in bulk solutes. Typically involved in these responses are sugars, sugar alcohols, amino acids, organic acids, or inorganic ions (Munns, 2005). Plants with increased concentrations of Pro, mannitol, or other products of sugar metabolism displayed increased salt tolerance (Kishor et al., 1995; Karakas et al., 1997; Garg et al., 2002; Taji et al., 2002). However, the significance of these osmolytes in conferring increased salt tolerance by osmotic adjustment has been questioned (Blum et al., 1996). Sodium itself may act as an osmoticum (Munns, 2005), but it displaces other cations such as Ca2+ and K+, which are important for membrane integrity, ion selectivity, and which also function as plant osmolytes (Epstein, 1998; Tester and Davenport, 2003). Therefore, the question remains to what extent salt-resistant glycophytes such as P. euphratica can make use of sodium to increase their cellular osmotic potential to maintain water uptake or whether other compounds such as organic solutes fulfill this function.
P. euphratica can persist for decades and centuries in naturally hostile saline and arid environments. In this study we investigated changes in osmotic pressure in P. euphratica leaves in response to increasing salt stress and dissected the contribution of sugars, amino compounds, and inorganic ions to osmotic adjustment. We show that the ability to cope with salinity includes salt-induced development of leaf succulence, apoplastic sodium accumulation, Ca2+ depletion, and cytosolic K+ homeostasis. At the molecular level the expression of several genes known to be involved in salt or general stress responses in plants were investigated.
RESULTS
Salt Adaptation in P. euphratica Is Mainly Achieved by Osmotic Adjustment of Na+ Versus Ca2+ Levels and by Net Decreases in Organic Osmolytes
P. euphratica was acclimated to 400 mm NaCl by doubling the sodium concentration in the nutrient solution in weekly steps. These consecutive increases in NaCl changed the osmotic pressure of the nutrient solution by Δ1.6 MPa. Osmometric measurements showed that P. euphratica was able to adjust the osmotic pressure of leaves to levels just exceeding those of the nutrient solution, which is important to maintain water uptake and to prevent dehydration (Fig. 1).
Figure 1.
Changes in osmotic cell pressure in leaves of P. euphratica with increasing osmotic pressure of the nutrient solution. The osmotic pressure (Π) in the nutrient solution was increased weekly by increasing the sodium concentrations from 0, 25, 50, 100, 200, to 400 mm. Changes in Π of the nutrient solution were calculated according to Heyrovska (1996). Π of the cell sap was determined osmometrically (black triangles). To obtain changes in Π, the osmotic pressure of controls of 2.28 ± 0.03 MPa was subtracted from values measured with salt-stressed leaves. To predict changes in Π of the cell sap, the concentrations of cations, soluble carbohydrates, and amino acids were determined (see “Materials and Methods” and text). The changes in osmolytes were calculated by subtracting the sum of osmolytes of controls from those of salt-treated leaves. The resulting osmolyte concentration was related to foliar water content and converted to osmotic pressure using the van't Hoff's equation. Data were obtained by analyzing three independent samples each consisting of leaves from five trees (±sd).
To identify major components contributing to the physiological adjustment of P. euphratica to salt stress, nutrient elements, carbohydrates, and amino acid concentrations were determined in leaves (Figs. 2 and 3; Supplemental Fig. 1). Sodium increased from negligible concentrations in control plants to 915 μmol g−1 dry mass during acclimation to 400 mm NaCl in the nutrient solution (Fig. 2). This increase would correspond to a decrease in the osmotic pressure of the leaves of 2.96 MPa and thus exceed the pressure required for osmotic adjustment more than 1.5-fold. However, the overall tissue concentrations of abundant cations (Na+, K+, Ca2+, and Mg2+) increased altogether only by 534 μmol g−1 dry mass, because salt exposure resulted in pronounced decreases in Ca2+ (−68%) and moderate decreases in K+ (−17%, Fig. 2). The concentrations of Mg2+ (Fig. 2) and micronutrients (Fe2+, Mn2+, Cu2+, etc.) were negligible and unaffected by salt (data not shown).
Figure 2.
Changes in sodium (black squares), potassium (white circles), calcium (black circles), and magnesium (white squares) in leaves of P. euphratica exposed to increasing sodium concentrations in the nutrient solution. The sodium concentration of the nutrient solution was increased weekly. Leaves were harvested after 1-week exposure to the indicated Na concentration. The sum of cations (white triangles) was obtained by adding Na, Ca, K, and Mg concentrations. Data were obtained by analyzing three independent samples each consisting of leaves from five trees (± sd).
Figure 3.
Changes in soluble carbohydrates in leaves of P. euphratica exposed to increasing sodium concentrations in the nutrient solution. The sodium concentration of the nutrient solution was increased weekly. Leaves were harvested after 1 week of exposure to the indicated Na concentration. The sum of soluble carbohydrates (black squares) was obtained by adding Glc (white squares), Fru (white circles), and Suc concentrations (black circles). Data are means of three independent samples each consisting of leaves from five trees (± sd). Occasionally error bars are smaller than symbols.
Adaptation to moderate salt stress caused strong decreases in the foliar concentrations of Glc and Fru, whereas Suc remained almost unaffected (Fig. 3). Severe salt stress caused small increases in Suc (Fig. 3) but net decreases of 240 μmol g−1 dry mass in soluble carbohydrates (sum of Glc, Fru, and Suc; Fig. 4). The method employed here for carbohydrate analysis revealed a further unidentified peak, which did not change in response to salt and thus did not contribute to adjusting osmotic pressure.
Figure 4.
Changes in free amino compounds (white circles) and sum of osmolytes (black circles) in leaves of P. euphratica exposed to increasing sodium concentrations in the nutrient solution. The sum of free amino compounds (white circles) was obtained by adding NH4+, γ-amino butyric acid, Orn, citrulline, and 20 proteinogenic amino acids. The concentrations of individual amino compounds are shown in Supplemental Figure 1. Osmolytes were determined as the sum of cations from Figure 1, carbohydrates from Figure 2, and amino compounds, and used to calculate the predicted changes in Π shown in Figure 1. Data were obtained by analyzing three independent samples each consisting of leaves from five trees (± sd).
Free amino acids, ammonia, γ-amino butaric acid, and citrulline showed distinct changes in response to adaptation of poplar to increasing salt stress (Supplemental Fig. 1). But the overall concentration of these metabolites increased only by 50 μmol g−1 dry mass under severe salt stress (Fig. 4).
The net increase in all measured compounds was about 345 μmol g−1 dry mass (Fig. 4) and, thus, almost 3 times less than the increase in Na+ (Fig. 2). To find out whether this increase in measured osmolytes was sufficient to explain the observed change in osmotic pressure, we used the measured differences of osmotically active compounds between salt-treated and control plants and the water content of the leaves to predict changes in osmotic pressure employing van't Hoff's equation (see “Materials and Methods”). The calculated changes in osmotic pressure did not deviate significantly from measured values (Fig. 1). This suggests that our analyses covered the major solutes responsible for osmotic adjustment. This result is surprising because known osmoprotectants such as sugar alcohols or amino compounds such as Pro appear to play no key role in cell pressure adjustment in P. euphratica. In fact, metabolite profiling of sugar alcohols in P. euphratica leaves from different field sites revealed that the concentrations of these compounds were negligible compared with Fru, Glc, and Suc (Brosché et al., 2006). Our data show that protective metabolites such as Pro increased more than 100 times compared with controls (Supplemental Fig. 1). Nevertheless, comparing the maximum Pro concentration of about 3.5 μmol g−1 dry mass with overall increases in osmolytes of about 300 μmol g−1 dry mass, it is immediately apparent that Pro can contribute only marginally to osmotic pressure adjustment. Increases in a range similar to that of Pro were also observed for Trp, Val, and γ-amino butaric acid (Supplemental Fig. 1).
Our analysis clearly shows that the increase in sodium was much higher than required for osmotic adjustment. Therefore, balancing the osmotic pressure necessitates decreases and not increases in osmotically active components. The decreases in cations, especially in Ca2+, may also serve electric charge compensation. Screening the element composition of P. euphratica leaves in natural habitats (Tarim basin, Taklamakan desert, People's Republic of China) with differences in Na+ exposure, we found that Na+ accumulation resulted only in moderate increases in total cation contents and was correlated with significant decreases in Ca2+ concentrations (Fig. 5). This observation underlines the importance of the Na+/Ca2+ counterbalance for Na+ stress compensation.
Figure 5.
Changes in cation concentrations (white squares) and calcium (black squares) in P. euphratica with increasing sodium in the leaves. Cations were calculated as sum of sodium, potassium, calcium, and magnesium. Leaves were sampled at different field sites in the Tarim basin (Luntai county, Taklemakan desert, People's Republic of China) and used for element analysis. At each site, which differed in salt exposure, leaves were collected from five trees.
Salt Resistance of P. euphratica Is Associated with Apoplastic Na+ Accumulation But Vacuolar and Apoplastic Ca2+ Depletion
To determine the subcellular localization of the accumulated Na+ and of other cations, P. euphratica was grown for several weeks under saline conditions, and leaves were harvested for x-ray microanalysis after 3, 5, and 9 weeks. Without salt stress the cytosol displayed little and the vacuoles no detectable sodium at all (Fig. 6). When exposed to NaCl, sodium concentrations increased with exposure time in a compartment-specific manner. The strongest sodium accumulation occurred in cell walls (Fig. 6). In the cytosol and in vacuoles sodium accumulation was delayed and less pronounced than in the apoplast. This result shows that P. euphratica has the ability to protect its cytosol from excess sodium after a crucial threshold value had been reached by excluding Na+ in the extracellular space and, to a much smaller extent, to the vacuole. P. euphratica did not behave like typical halophytes, whose primary strategy is to store incoming sodium in the vacuole. The vacuolar sodium concentrations increased only after prolonged exposure and after cytosolic increases had occurred (Fig. 6). In both apoplast and vacuole, accumulation of Na+ resulted in decreases in Ca2+ (Fig. 6).
Figure 6.
Subcellular localization of cations in leaves of P. euphratica and changes during salt exposure. Plants were grown in soil and watered daily for 9 weeks with 150 mm NaCl in the nutrient solution. Samples for electron microscopy were collected at the beginning and after 3, 5, and 9 weeks of salt treatment, respectively. Measurements were carried out using a TEM and EDX microanalysis with a beam diameter of <200 nm in the palisade parenchyma cells. Bars indicate means of n > 10 ± sd. Scale bar indicates 500 nmol mm−3. Black bar = sodium, white bar = magnesium, hatched bar = potassium, crossed bar = calcium.
Since Na+ competes with K+ for uptake, salt exposure generally leads to diminished K+ concentrations (Hasegawa et al., 2000). Salt stress caused only a small decrease in K+ concentrations in leaves of P. euphratica (Fig. 2). A closer inspection at the subcellular level indicated that vacuolar but not cytosolic K+ concentrations were diminished under salt stress compared with controls (Figs. 6 and 7). This indicates that P. euphratica is able to protect the cytosol against the depletion of this physiologically important cation and may use the vacuole as a reservoir to supply K+ to the cytosol (Fig. 7).
Figure 7.
Relative subcellular potassium distribution in leaves of P. euphratica exposed to salt and in controls. Figures were calculated with data from Figure 5. The potassium concentration in the cytosol of controls was set to 1 and the concentrations in the other compartments and those determined after 9 weeks of salt stress were expressed relative to this value. Significant differences at P ≤ 0.05 are marked by an asterisk. White bar = controls, black bar = salt stress.
Salt Treatment Induces Leaf Succulence
Under saline conditions P. euphratica developed pronounced leaf succulence compared to controls (Fig. 8, A and B). Morphometric studies revealed a significant increase in leaf thickness (2- to 3-fold), whereas the lamina width remained unaffected (Table I). To determine whether enlarged leaf thickness was caused by swelling of cells or by changes in anatomy, the number of cell layers between the upper and lower epidermis and the cell size were determined. The number of cell layers increased more than 3-fold in salt-treated leaves compared to controls (Fig. 8C; Table I). The average area and perimeter of individual cells also increased significantly (Table I). Additionally, new cells with various unusual shapes were formed (Fig. 8C). Therefore, the overall appearance of the leaves changed from a plain to a dumbbell shape. In the center and on the edges of the leaves, the cells were much larger than palisade parenchyma cells in leaves of control plants. Due to their shape they were called elongated cells (Fig. 8C). Typical palisade and spongy parenchyma cells as observed in the controls were absent. Instead, isodiametric-like cells were observed exhibiting a homogeneous structure, being tightly packed. In combination with a reduction of intercellular spaces, this aberrant growth resulted in high cell densities. Both the bundle-sheath cells and the bundle-sheath extension cells increased in number and size (data not shown).
Figure 8.
Cross sections of P. euphratica leaves. Plants were irrigated daily with Long Ashton nutrient solution in the absence (A) or presence of 150 mm NaCl (B and C) for 9 weeks. Leaves were harvested and embedded for anatomical analysis. Cross sections of leaf tips are shown. Magnification was 25× (A and B) and 200× (C), respectively. IC, Isodiametric-like cells; EC, elongated cells; refer to the cell area measurements in Table I.
Table I.
Anatomical properties of leaf tips from P. euphratica plants treated for 9 weeks with 150 mm NaCl in comparison with control plants
PP, Palisade parenchyma; SP, spongy parenchyma; EC, elongated cells; IC, isodiametric-like cells. Significant differences for P ≤ 0.001 are marked by *** and for P < 0.05 by *.
| Parameter | Control | NaCl |
|---|---|---|
| Leaf thickness (mm; n = 10) | ||
| Basis | 0.780 ± 0.084 | 1.520 ± 0.192*** |
| Mid | 0.180 ± 0.084 | 0.640 ± 0.114*** |
| Lamina width (mm; n = 10) | 8.8 ± 3.56 | 9.2 ± 4.49*** |
| Cell layers (n = 10) | 12.5 ± 3.4 | 42.1 ± 8.8* |
| Area per cell (mm2; n = 45) | PP = 0.322 ± 0.09 | EC = 1.650 ± 1.21*** |
| SP = 0.244 ± 0.13 | IC = 0.413 ± 0.15*** | |
| Perimeter per cell (μm; n = 30) | PP = 96 ± 11 | EC = 245 ± 68*** |
| SP = 66 ± 16 | IC = 94 ± 23*** |
Salt Shock Induces Stronger Expression of and More Stress-Related Genes in P. euphratica Leaves Than Salt Adaptation
The molecular basis for the ability of P. euphratica to cope with high salt concentrations is not known. Therefore, we investigated changes in transcript levels of various genes known to be involved in salt or general stress signaling or adaptation, including members of Ca2+-regulated pathways, redox control, reactive oxygen formation and detoxification, etc. (Table II). The clones were obtained from a stress-induced cDNA library of P. euphratica (Brosché et al., 2006). To distinguish between responses to salt adaptation or shock, young P. euphratica trees were either gradually acclimated to increasing NaCl concentrations up to 200 mm or exposed to salt shock by adding 200 mm NaCl 24 h before harvest.
Table II.
Relative changes in gene expression after salt shock or salt adaptation in P. euphratica leaves
Trees were grown hydroponically in climatized growth cabinets and adapted in weekly steps to increasing salt concentrations (25 mm, 50 mm, 100 mm, and 200 mm NaCl). For the shock treatment 200 mm NaCl was added 24 h before harvest. Transcript levels were analyzed by dot-blot analyses and normalized to actin. 18S rRNA and tubulin were also spotted and showed no significant differences relative to actin. Significant differences were determined by ANOVA and a multiple range test (four replicates per filter, four biological samples). Relative expression was calculated as stress (S)/control (C). P values ≤ 0.05 indicate significant differences. AC, Accession number.
| Putative Function | Gen-AC | Shock S/C | P (t Test) | Adapted S/C | P (t Test) |
|---|---|---|---|---|---|
| Galactinol synthase (GolS-1) | AJ769227 | 7.0548 | 0.0014 | 2.9575 | 0.0009 |
| Alternative oxidase | AJ771714 | 3.1191 | 0.0015 | 1.8395 | 0.0209 |
| 1-Aminocyclopropane-1-carboxylate oxidase | AJ770898 | 0.3833 | 0.0201 | 0.3607 | 0.0152 |
| CBL 10 | AJ780241 | 0.4119 | 0.0109 | 0.5648 | 0.0364 |
| Glutathione peroxidase | AJ778382 | 2.0810 | 0.0036 | 1.4172 | 0.2173 |
| Acyltransferase-like protein | AJ774576 | 1.8083 | 0.0285 | 1.2843 | 0.1278 |
| Polyamine oxidase | AJ777563 | 1.4152 | 0.0506 | 1.0399 | 0.7431 |
| Cu/Zn SOD, chloroplastic form | AJ767460 | 1.3279 | 0.0058 | 0.9263 | 0.4184 |
| Gibberellin-regulated protein GASA3 precursor | AJ778881 | 0.6564 | 0.0242 | 1.2349 | 0.4282 |
| Dehydration-responsive protein RD22 | AJ773118 | 1.4831 | 0.0737 | 1.3899 | 0.0937 |
| NADPH oxidase/respiratory burst oxidase protein | AJ774827 | 2.1945 | 0.0759 | 1.2046 | 0.4815 |
| Lipoxygenase B (EC 1.13.11.12) | AJ771712 | 1.7110 | 0.1001 | 1.4946 | 0.3395 |
| Germin | AJ775763 | 2.6852 | 0.1257 | 1.4529 | 0.4129 |
| Peroxidase | AJ776277 | 1.4452 | 0.1768 | 1.0652 | 0.8251 |
| 2-Cys peroxiredoxin BAS1, chloroplast precursor | AJ778007 | 1.2769 | 0.2023 | 0.7940 | 0.1541 |
| Unknown protein, Arabidopsis | AJ770289 | 0.7882 | 0.3017 | 0.8912 | 0.6128 |
| CaM | AJ769651 | 1.2220 | 0.4562 | 0.8886 | 0.5022 |
| Δ1-pyrroline-5-carboxylate synthetase (P5CS) | AJ772704 | 1.1779 | 0.5346 | 1.3558 | 0.3320 |
| Tubulin | AJ775509 | 1.4541 | 0.1415 | 1.0454 | 0.8016 |
| 18S rRNA | AJ775618 | 1.0040 | 0.9785 | 0.8396 | 0.1607 |
| Actin | AJ775312 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
Salt shock resulted in changes in the transcript levels of half of the stress genes tested here compared with controls (nine out of 19; Table II), twice as many as salt adaptation (Table II). In both salt treatments, transcripts for galactinol synthase (GolS-1) and alternative oxidase were significantly increased and those for 1-aminocyclopropane-1-carboxylate oxidase (ethylene formation) and CBL 10 (calcium signaling) decreased, though to a stronger extent after salt shock compared with salt adapted leaves (Table II). After salt shock, elevated transcript levels were also observed for glutathione peroxidase, acyltransferase-like protein, polyamine oxidase, and Cu/Zn superoxide dismutase (SOD; chloroplastic form) and decreases for a putative gibberellin-regulated protein GASA3 precursor (Table II). It is noteworthy that a range of other transcripts, e.g. for enzymes involved in hydrogen peroxide formation (NADPH oxidase, germin), redox control (peroxidase, peroxiredoxin), Pro synthesis (P5CS), and drought response (RD22), were not significantly affected by salt.
DISCUSSION
Salt Resistance of P. euphratica Is Based on Physiological Salt Avoidance and Potassium Homeostasis
To protect the highly sensitive biosynthetic apparatus of the cell against excess sodium, cytosolic Na+ must be kept at low, tolerable concentrations. Most commonly this is achieved in nonhalophytes by sequestering toxic amounts of Na+ in vacuoles employing Na+/H+ antiporters (Blumwald et al., 2000). P. euphratica, however, followed a different strategy. While the cytoplasmic Na+ concentration increased gradually and only moderately, the cells obviously had the ability to maintain ion homeostasis by excluding Na+ from the cytosol and storing it primarily in cell walls and not in the vacuole (Fig. 6). Na+ export from the cytosol into the apoplast is most likely carried out by a homolog of the plasma membrane-bound SOS1 (Shi et al., 2000). An NHX homolog has also been demonstrated in P. euphratica but Na+ export may additionally involve PeNhaD1, which carries a signal peptide for the secretory pathway and whose expression was maintained under salt stress (Ottow et al., 2005).
Previously, the capability of P. euphratica to tolerate high salt concentrations has mainly been ascribed to root-born processes such as limited ion loading into the xylem during radial transport, thereby restricting axial transport (Chen et al., 2002). Sodium accumulation in leaves was slower in P. euphratica than in salt-sensitive poplar species (Chen et al., 2001). This may be advantageous to allow activation of defense responses. But final foliar salt concentrations were as high as or even higher than in sensitive species (Chen et al., 2002). Our data on the subcellular Na+ distribution clearly demonstrate that the mesophyll cells are salt resistant because of avoidance of excess salt accumulation.
It is intriguing that the apoplast was the primary site of sodium accumulation (Fig. 6). Depending on plant species and measuring technique, apoplastic Na+ concentrations found after salt treatment differed significantly. Mühling and Läuchli (2002) found very little Na+ analyzing apoplastic wash fluids of salt-stressed maize (Zea mays) and cotton (Gossypium hirsutum). Using x-ray microanalysis, Flowers et al. (1991) found Na+ concentrations of about 600 mmol dm−3 in the apoplast of rice (Oryza sativa) leaves. Our data revealed even steeper increases in apoplastic Na+ concentrations of P. euphratica after prolonged salt exposure (Fig. 6). The actual in planta concentrations were probably lower since sample preparation leads to shrinkage of the material in the range of 30% (Winter, 1993). Still, we measured extremely high Na+ concentrations in the apoplast. One could argue that such high concentrations of Na+ may be caused by relocation of mobile cations during sample preparation. If this was the case we would have expected similar distribution patterns for K+, which were not observed (Fig. 7). In fact, we have evidence from Ce2+-binding studies that salt stress changes cell wall properties leading to higher binding capacities for cations (S. Chen and A. Polle, unpublished data). We therefore propose that in P. euphratica a significant fraction of Na+ is not free but replaces cell wall-localized cations. In accordance with this data, which clearly show that leaf cells remain turgescent (Fig. 8), this Na+ fraction would not be active as an osmolyte and thus not lead to cell dehydration as suggested earlier by Oertli (1968).
Potassium is an essential macronutrient and the most abundant cation in plants (Mäser et al., 2002). Salt tolerance is generally correlated with the capacity of plants to keep their K+ level in a defined range (Blumwald et al., 2000). Under salinity, a decrease in leaf K+ concentrations is often found leading to a suboptimal K+ supply (Marschner, 1995). However, in P. euphratica, the K+ concentrations of leaves decreased only moderately, even under persisting salt stress (Fig. 2). This is due to the fact that P. euphratica maintains higher K+ uptake and K+ concentrations in the xylem sap in the presence of high external sodium than salt-sensitive poplars (Populus spp.; Chen et al., 2003). In addition, our data show that P. euphratica redistributed intracellular K+ because the cytosolic K+ concentrations were maintained, whereas the vacuolar concentrations decreased (Fig. 7). This suggests that unlike salt-sensitive plants, P. euphratica keeps the balance of essential nutritional elements against competing Na+ ions by shifting, for example, K+ from the vacuoles to the cytoplasm, thus compensating for losses.
The Role of Calcium and Organic Metabolites in Osmotic Adjustment to High Salinity
When plants are exposed to salt stress, an essential function of Ca2+ is that of a second messenger in stress signaling (Knight and Knight, 2001). Its crucial role in triggering a signaling cascade to activate Na+/H+ antiporters has been demonstrated (Liu and Zhu, 1998; Quintero et al., 2002). To fulfill this function, the cytosolic Ca2+ concentrations, which are normally in the submicromolar range, increase transiently (Knight et al., 1997). Exposure to salt initially also increased Ca2+ uptake in P. euphratica (Chen et al., 2001). It is known that calcium ameliorates salt tolerance (Tester and Davenport, 2003). Our data do not contradict this aspect, despite the significant decreases in calcium found after exposure to high NaCl (Fig. 2). First, it is important to distinguish between the small changes in Ca concentration required for signaling and those of bulk Ca. Secondly, we show here that an increase in Ca2+ occurred after exposure to low sodium concentrations (25 mm, Fig. 2), which are subtoxic, even in salt-sensitive poplar species (Bolu and Polle, 2004). In accordance with previous studies, this might imply that Ca2+ is important in mediating salt adaptation and that this process occurs independently of osmotic stress or the necessity of acute defense against Na toxicity.
In addition to this role of Ca2+, we show that changes in bulk Ca2+ concentrations and cellular redistribution are involved in mediating long-term salt adaptation of P. euphratica. Plants grown under increasing osmotic pressure in the nutrient solution were able to keep their osmotic pressure of the cell sap equal to or just above the increases caused by sodium in the nutrient solution. This is remarkable since the accumulation of sodium in leaves was about 3-fold higher than necessary for osmotic adjustment (Figs. 1 and 2) and implies that P. euphratica is capable of regulating ion redistribution and physiological adjustment in a very precise and coordinated manner. Overaccumulation of ionic solutes was prevented by corresponding decreases in Ca2+ (Fig. 2). This ability to compensate excess sodium by decreases in Ca2+ is apparently also an important mechanism under field conditions (Fig. 5). We show that the cell wall is a major site where Na+ accumulated and partially replaced Ca2+ as well as K+ (Fig. 6). Although cell wall deposition of Na+ seems to be the key mechanism for salt resistance in P. euphratica, it is probably not important for osmotic pressure adjustment as outlined above.
Long-term Na exposure resulted in Na+ accumulation in both the cytosol and the vacuole (Fig. 6). The vacuolar Na+ accumulation was initially mainly counterbalanced by decreases in Ca2+, and, when no Ca2+ was detected any more, additional decreases in K+ occurred (Fig. 6). We can infer from these results that salt tolerance in P. euphratica requires a complex time-dependent regulation of different ion transporters. Our data would suggest that vacuolar Ca2+ transport needs to be down-regulated when Na+ increases in this compartment. This would require contrasting regulation of vacuolar transport systems for Ca2+ and Na+ such as CAX1 and NHX1 and might also explain the observed increased salt sensitivity when CAX1 was overexpressed (Cheng et al., 2004). In plant cells calcium sensing is achieved by a family of CBLs interacting with protein kinases (Luan et al., 2002). We have analyzed only one member of this family, CBL-10, because it was previously found in a stress-induced cDNA library of P. euphratica (Brosché et al., 2006). The observation that its transcription was decreased under salt shock as well as after long-term acclimation (Table II) suggests a role as negative regulator of salt adaptation in P. euphratica. Differential regulation of multiple stress pathways has, for example, been demonstrated for CBL1, which increased salt and drought tolerance of Arabidopsis (Arabidopsis thaliana) but reduced its freezing tolerance (Cheong et al., 2003). It has also been shown that a specific CaM isoform mediated salt-induced Ca2+ signaling through the activation of an MYB transcriptional activator, which up-regulated transcription of the Pro-synthesizing enzyme P5CS1 (Δ1-pyrroline-5-carboxylate synthetase-1; Yoo et al., 2005). However, the CaM clone analyzed here was not salt induced; neither was a putative P5CS1 gene (Table II). Gu et al. (2004) showed that CaM was activated in P. euphratica during recovery from salt stress and not during short-term salt exposure.
An intriguing result of our study is that osmotic pressure regulation in P. euphratica can be modeled precisely on the basis of abundant ions; simple, primary carbohydrates; and free-amino compounds (Fig. 1). It is known that P. euphratica increases Pro under salt stress (Watanabe et al., 2001; see Supplemental Fig. 1), which is the typical response of plants to osmotic stress (Taji et al., 2004; Yoo et al., 2005). But the overall concentrations of this osmolyte were too small to contribute significantly to osmotic adjustment. Our data further imply that compatible solutes such as mannitol, raffinose, galactinol, etc, which have also been shown to improve plant osmotic stress tolerance (Karakas et al., 1997; Garg et al., 2002; Taji et al., 2002, 2004; Yoo et al., 2005) play no role in osmoregulation of P. euphratica. In previous studies these compounds, which constituted only a marginal fraction to carbohydrate-based metabolite (<5%), were not increased in drought or field stress-exposed P. euphratica trees (Brosché et al., 2006; M. Bogeat-Triboulot, E. Dreyer, and A. Polle, unpublished data). This does not preclude the importance of compatible solutes for stress adaptation. It has been suggested that they are required for the stabilization of membranes and proteins (Vinocur and Altman, 2005). In fact, we found by far the highest induction under both salt shock and salt adaptation for galactinol synthase (GolS-1), a member of the raffinose oligosaccharide family (ROF). Galactinol synthase transcription was also up-regulated in Arabidopsis under cold, drought, and salt stress (Taji et al., 2002). It was one of the few genes responding to salt exposure in salt cress (Taji et al., 2004) and is involved in adaptation to heat (Panikulangara et al., 2004). Taken together all these observation point to essential roles of ROFs in abiotic stress adaptation, but their mechanistic role is still elusive.
It has also been suggested that compatible solutes may function as scavengers of reactive oxygen species (ROS). Salt stress, as with most adverse environmental conditions, can induce increased production of ROS in plants (Hasegawa et al., 2000; Zhu, 2001). For example, overexpression of SOD in rice chloroplasts protected against salt stress (Tanaka et al., 1999). Transcriptional analysis of P. euphratica indicated that typical ROS-detoxifying systems (chloroplastic SOD, glutathione peroxidase, and polyamine oxidase) were only activated during salt shock (Table II), suggesting that they are required to protect during acute stress scenarios. Extended exposure to salinity seems to influence mainly mitochondria. These organelles may have an increased necessity to detoxify ROS since alternative oxidase was elevated after salt shock as well as after salt adaptation (Table II).
CONCLUSION
P. euphratica relies on multiple adaptation mechanisms to cope with high salinity. Under extended periods of salt stress, leaves develop succulence resulting in sodium dilution. High sodium concentrations are tolerated in leaves because of apoplastic accumulation, probably leading to changes in cell wall properties allowing enhanced cation binding. Osmotic adjustment was attained by uptake of sodium, moderate increases in amino compounds, and decreases in calcium, Glc, and Fru. Since changes in cell osmotic pressure could be predicted on the basis of these compounds, we conclude that compatible solutes like Pro or polyols play no role in osmoregulation of P. euphratica. They may have other functions, for example stabilization of protein folding, thus contributing to osmoprotection. The strategy to employ mainly ions for osmotic adjustment is energetically favorable since the synthesis of compatible solutes is metabolically expensive, whereas Na is a cheap and abundant osmoticum.
MATERIALS AND METHODS
Plant Growth and Salt Stress Treatments
Populus euphratica plantlets were multiplied by micropropagation from stock cultures clone B2 obtained from trees grown in the Ein Avdat region (Israel). Plants were grown in hydroponics in Long Ashton nutrient solution (Hewitt and Smith, 1975) with photoperiod of 16 h, 250 μmol m−2 s−1 photosynthetically active radiation, and 22°C, and exposed in weekly steps of 25, 50, 100, 200, and 400 mm to increasing NaCl in the medium. Leaves were harvested after 7-d exposure to the respective NaCl concentration and kept frozen at −80°C for further analyses. To induce salt shock, control seedlings were exposed to 200 mm NaCl and harvested after 24 h.
For long-term experiments, seedlings (10 months old) were grown from seeds (Xinjiang Uygur Autonomous Region, Northwest China) in soil (Fruhstorfer Erde N) in a climate chamber (Weiss) at 12 h light with 250 μmol m−2 s−2 photosynthetically active radiation, 20°C, and a relative air humidity of 64%. Seedlings were watered daily with Long Ashton macronutrient solution (Hewitt and Smith, 1975) until drip off. Plants with similar growth phenologies were selected and stepwise acclimated to salinity by increasing the NaCl concentration from 50 mm in the first week to a final concentration of 150 mm NaCl in the third week. Leaves were harvested after additional 3, 5, and 9 weeks of 150 mm NaCl treatment for energy dispersive x-ray transmission electron microscopy (EDX-TEM) analyses.
RNA Extraction and Dot-Blot Expression Analyses
RNA was extracted after the method of Chang et al. (1993) and treated with DNAfree (Ambion). Total RNA was labeled with SuperScript III cDNA kit (Invitrogen) using [α-32P] dCTP (Amersham Biosciences) and purified with QIAquick nucleotide removal kit (Qiagen). The activity was determined by counting the Cerenkov radiation. The labeled samples (6 kBq/mL) were used as probes for dot-blot analysis.
cDNA clones of selected genes obtained from an expressed sequence tag sequencing project (ESTABLISH) of P. euphratica (Brosché et al., 2006) were cloned into T-easy vector (Promega) or pSport vector (Invitrogen) and transformed into Escherichia coli DH5α or DH12S. Plasmids were isolated after overnight culture using Mini Prep kit (Qiagen) following the instructions of the manufacturer. Inserts were amplified by PCR using vector-specific primers M13-20 and M13PR. Products were purified by the PCR purification kit (Qiagen), and the concentration of DNA was determined spectrophotometrically. Filter arrays were prepared by printing 300 ng DNA per spot on nylon membranes (Hyobond N+, Amersham Bioscience) using a Bio-Dot microfiltration apparatus (Bio-Rad Laboratories) according to the instructions of the supplier. Loading controls were performed by hybridizing against the transformation vector. Dot blots were hybridized with labeled samples overnight at 42°C (UVP Laboratory Products HB 100 Hybridiser) in Ultrahyb buffer (Ambion) and washed in saline sodium citrate (2-times 30 min at 60°C in 2× SSC, 0.5% SDS and 2-times 30 min at 60°C in 0.2× SSC, 0.5% SDS). The filters were exposed on a Fuji imaging plate (BAS 1500, Fujifilm) and analyzed with a phosphor imager using AIDA software (Image Analyzer). Each cDNA was spotted four times per filter. Four independent biological replicates were analyzed. Signals were expressed relative to actin (AJ775312). The filters also contained tubulin (AJ775509) and 18SrRNA (AJ775618) leading to similar results.
Analysis of Elements in Plant Tissues
Plant tissues were dried at 70°C and subsequently digested by using the nitric acid pressure system according to Heinrichs et al. (1986). Elemental quantification of was carried out by inductively coupled plasma-optical emission spectrometry (Spectro Analytical Instruments) at λ = 559 nm.
Analysis of Metabolites
Plant tissues were freeze dried and used for sugar analysis by ion chromatography (4500i Dionex) and amino acid determination with a LC501 amino acid analyzer (Biotronic) as described previously (Stoimenova et al., 2003).
Measurements and Calculations of Osmotic Pressure
The change in osmotic pressure in the nutrient solution caused by increasing concentrations of NaCl was calculated according to Heyrovska (1996). The osmotic pressure of leaves was determined by freezing point depression with an osmometer (Knauer, Halbmikro ML) according to the instructions of the manufacturer. To calculate changes in the osmotic pressure of P. euphratica leaves under different salt regimes, the concentrations of cations, soluble carbohydrates, and amino compounds were added (Csum) and used to calculate the osmotic pressure using the van't Hoff equation:
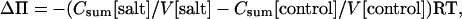 |
with R = 0.00832 L MPa K−1, T = 298.15 K, and V = water content of leaves calculated as the difference between a fresh (FM) and dry (DM) sample V = 1 − DM/FM (L). Van't Hoff equation applies strictly only to dilute solutions since strong electrolytes are not completely dissociated. Therefore, the predicted results tend to overestimate Π slightly.
Element Analysis at the Subcellular Level by EDX-TEM
Freshly harvested leaves from plants were placed in bags made of fine aluminum mesh and rapidly frozen in a mixture of propane:isopentane (2:1, v/v) at the temperature of liquid nitrogen. The leaves were freeze dried under vacuum at −48°C for 72 h (P4K, Piatkowski). Samples were infiltrated using the water-free method by Fritz (1989), avoiding ion redistribution during sample preparation. The plastic consisted of a mixture of styrene and butyl methacrylate (1:1, v/v) containing 2% of dibenzoylperoxid stabilized with 50% phthalate. Infiltrated samples were transferred into gelatin capsules and polymerized at 60°C for 24 h followed by 35°C until they were completely solid. After polymerization, the samples were cut into 1-μm-thick sections using a dry glass knife with an ultramicrotome (ULTRACUT E, Reichert-Jung). The cross sections were transferred onto adhesive-coated fine-bar copper grids (mesh 50; Fritz, 1991) and coated with carbon.
X-ray microanalyses were performed using a transmission electron microscope (Philips EM 420) with the energy dispersive system EDAX DX-4 (EDAX). The accelerating voltage was 120 kV, the take-off angle 25°, and the collection time for x-radiation was 60 life seconds. The diameter of the electron beam was smaller than 200 nm, whereas cell walls, the smallest compartment analyzed, had thicknesses of >250 nm. Each compartment (cell walls, cytoplasm, and vacuole) was examined five to 10 times per location and sample. Magnification differed from 3,300× for vacuoles to up to 8,200× for cytoplasm and cell walls. Quantification was carried out by the software EDAX mDX (EDAX) using manual background correction and comparing peak areas with those of the standards obtained from analytical calibrations in 1-μm-thick gel embeddings (Fritz and Jentschke, 1994).
Leaf Anatomy and Morphometry
Leaves were fixed in 2% formaldehyde, 5% acetic acid, 63% ethanol, and dehydrated in a series of ethanol/acetone steps, followed by the transfer into plastic (styrene methacrylate). Using an ultramicrotome (ULTRACUT E), 1-μm-thick leaf cross sections were made. Cuttings were stained at 60°C for 6 min with toluidine blue (0.1% toluidine [w/v] in 0.1% [w/v] BORAX, sodium tetra borate) and mounted on gelatin-coated glass slides with Euparal (Roth). Slices were viewed under a light microscope (Axioskop, Zeiss) using magnifications of 25× and 200×, respectively.
Morphometric measurements (leaf thickness, leaf width, cell volume, cell number, and total area of single cells) were carried out on leaf cross sections using the software analySIS (Soft Imaging System). Photographs were taken with a digital camera (Nikon CoolPix 990, Nikon).
Statistical Analyses
If not reported otherwise, five to 10 individuals were analyzed. Data are indicated as means ± sd. Statistical analyses were performed with STATGRAPHICS Plus using ANOVA (Statistical Graphics), followed by a multiple range test.
Sequence data from this article can be found in the National Center for Biotechnology Information gene bank under accession numbers AJ769227, AJ771714, AJ778382, AJ775763, AJ774827, AJ771712, AJ774576, AJ776277, AJ777563, AJ773118, AJ767460, AJ778007, AJ772704, AJ769651, AJ778881, AJ770289, AJ780241, and AJ770898.
Supplementary Material
Acknowledgments
We thank T. Klein and C. Kettner for excellent technical assistance, Dr. N. Lamersdorf and Dr. N. Loftfield for their helpful discussions and inductively coupled plasma analyses, and M. Reichel for help during field work. We acknowledge the support by the Finnish Centre of Excellence (Program 2000–2005) and by the Xinjiang Forestry Administration Bureau for their assistance in China.
This work was supported by the German Science Foundation through funding of the Poplar Research Group in Germany and by the Bundesministerium für Verbraucherschutz, Ernährung und Landwirtschaft (travel grant to A.P.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Andrea Polle (apolle@gwdg.de).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.069971.
References
- Apse MP, Aharon GS, Sneddon WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285: 1256–1258 [DOI] [PubMed] [Google Scholar]
- Blum A, Munns R, Passioura JB, Turner NC (1996) Genetically engineered plants resistant to soil drying and salt stress: how to interpret osmotic relations? Plant Physiol 110: 1051–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465: 140–151 [DOI] [PubMed] [Google Scholar]
- Bolu WH, Polle A (2004) Growth and stress reactions in roots and shoots of a salt-sensitive poplar species (Populus x canescens). Trop Ecol 45: 161–171 [Google Scholar]
- Brosché M, Vinocur B, Alatalo ER, Lamminmäki A, Teichmann T, Ottow EA, Djilianov D, Afif D, Bogeat-Triboulot MB, Altman A, et al (2006) Gene expression and metabolite profiling of Populus euphratica growing in the Negav desert. Genome Biol (in press) [DOI] [PMC free article] [PubMed]
- Browicz K (1977) Chorology of Populus euphratica Olivier. Arbor Kórnickie 22: 5–27 [Google Scholar]
- Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11: 113–116 [Google Scholar]
- Chen S, Li J, Fritz E, Wang S, Hüttermann A (2002) Sodium and chloride distribution in roots and transport in three poplar genotypes under increasing NaCl stress. For Ecol Manage 168: 217–230 [Google Scholar]
- Chen S, Li J, Wang S, Fritz E, Hüttermann A, Altman A (2003) Effects of NaCl on shoot growth, transpiration, ion compartmentation, and transport in regenerated plants of Populus euphratica and Populus tomentosa. Can J For Res 33: 967–975 [Google Scholar]
- Chen S, Li J, Wang S, Hüttermann A, Altman A (2001) Salt, nutrient uptake and transport, and ABA of Populus euphratica: a hybrid in response to increasing salt. Trees (Berl) 15: 186–194 [Google Scholar]
- Cheng NH, Pittman JK, Zhu JK, Hirschi KD (2004) The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J Biol Chem 279: 2922–2926 [DOI] [PubMed] [Google Scholar]
- Cheong YH, Kim KN, Pandey GK, Gupta R, Grant JJ, Luan S (2003) CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 15: 1833–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Schumaker K, Zhu JK (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot 55: 225–236 [DOI] [PubMed] [Google Scholar]
- Epstein E (1998) How calcium enhances plant salt tolerance. Science 280: 1906–1907 [DOI] [PubMed] [Google Scholar]
- Flowers TJ (2003) Improving crop salt tolerance. J Exp Bot 55: 307–319 [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Hajibagheri MA, Yeo AR (1991) Ion accumulation in the cell walls of rice plants growing under saline conditions: evidence for the Oertli hypothesis. Plant Cell Environ 14: 319–325 [Google Scholar]
- Fritz E (1989) X-ray microanalysis of diffusible elements in plant cells after freeze-drying, pressure-infiltration with ether and embedding in plastic. Scanning Microsc 3: 517–526 [Google Scholar]
- Fritz E (1991) The use of adhesive-coated grids for the X-ray microanalysis of dry-cut sections in the TEM. J Microsc 161: 501–504 [DOI] [PubMed] [Google Scholar]
- Fritz E, Jentschke G (1994) Agar standards for quantitative X-ray microanalysis of resin-embedded plant tissues. J Microsc 174: 47–50 [Google Scholar]
- Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99: 15898–15903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol 31: 149–190 [Google Scholar]
- Gu R, Fonseca S, Puskas LG, Hackler L, Zvara A, Dudits D, Pais MS (2004) Transcript identification and profiling during salt stress and recovery of Populus euphratica. Tree Physiol 24: 265–276 [DOI] [PubMed] [Google Scholar]
- Hamada A, Hibino T, Nakamura T, Takabe T (2001) Na+/H+ antiporter from Synechocystis species PCC 6803, homologous to SOS1, contains an aspartic residue and long C-terminal tail important for the carrier activity. Plant Physiol 125: 437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51: 463–499 [DOI] [PubMed] [Google Scholar]
- Heinrichs H, Brumsack HJ, Loftfield N, König N (1986) Verbessertes Druckaufschlussystem für biologische und anorganische Materialien. Z Pflanzenernaehr Bodenkd 149: 350–353 [Google Scholar]
- Hewitt EJ, Smith TA (1975) Plant Mineral Nutrition. English University Press, London
- Heyrovska R (1996) Physical electrochemistry of strong electrolytes based on partial dissociation and hydration: quantitative interpretation of the thermodynamic properties of NaCl(aq) from “zero to saturation”. J Electrochem Soc 143: 1789–1793 [Google Scholar]
- Kang JM, Kojima K, Ide Y, Sasaki S (1996) Growth response to the stress of low osmotic potential, salinity and high pH in cultured shoot of Chinese poplars. J For Res (Harbin) 1: 27–29 [Google Scholar]
- Karakas B, Ozias-Akins P, Stushnoff C, Suefferheld M, Rieger M (1997) Salinity and drought tolerance in mannitol-accumulating transgenic tobacco. Plant Cell Environ 20: 609–616 [Google Scholar]
- Kishor PBK, Hong Z, Miao GH, Hu CAA, Verma DPS (1995) Overexpression of Δ-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108: 1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Knight MR (2001) Abiotic stress signaling pathways: specificity and cross-talk. Trends Plant Sci 6: 262–267 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1997) Calicum signaling in Arabidopsis thaliana responding to drought and salinity. Plant J 12: 1067–1078 [DOI] [PubMed] [Google Scholar]
- Liu JP, Zhu JK (1998) A calcium sensor homolog required for plant salt tolerance. Science 280: 1943–1945 [DOI] [PubMed] [Google Scholar]
- Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W (2002) Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 14: 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HC, Fung L, Wang SS, Altman A, Hüttermann A (1997) Photosynthetic response of Populus euphratica to salt stress. For Ecol Manage 93: 55–61 [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants, Ed 2. Academic Press, London
- Mäser P, Gierth M, Schroeder JI (2002) Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil 247: 43–54 [Google Scholar]
- Mühling KH, Läuchli A (2002) Effect of salt stress on growth and cation compartmentation in leaves of two plant species differing in salt tolerance. J Plant Physiol 159: 137–146 [Google Scholar]
- Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167: 645–663 [DOI] [PubMed] [Google Scholar]
- Oertli JJ (1968) Extracellular salt accumulation, a possible mechanism of salt injury in plants. Agrochimica 12: 461–469 [Google Scholar]
- Ottow EA, Polle A, Brosché M, Kangasjärvi J, Dibrov P, Zörb C, Teichmann T (2005) Molecular characterization of PeNhaD1: the first member of the NhaD Na+/H+ antiporter family of plant origin. Plant Mol Biol 58: 73–86 [DOI] [PubMed] [Google Scholar]
- Panikulangara RJ, Eggers-Schumacher G, Wunderlich M, Stransky H, Schöffl F (2004) Galactinol synthase1: a novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant Physiol 136: 3148–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero EJ, Ohta M, Shi H, Zhu K-J, Pardo JM (2002) Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci USA 99: 9061–9066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Pellouc J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14: 401–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Ishitani M, Kim C, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97: 6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Lee BH, Wu SJ, Zhu JK (2003) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21: 81–85 [DOI] [PubMed] [Google Scholar]
- Stoimenova M, Hänsch R, Mendel R, Gimmler H, Kaiser WM (2003) The role of nitrate reduction in the anoxic metabolism of roots: characterization of root morphology and normoxic metabolism of wild type tobacco and a transformant lacking root nitrate reductase. Plant Soil 253: 145–153 [Google Scholar]
- Szabolcs I (1994) Soils and salinization. In M Pessarakli, ed, Handbook of Plant and Crop Stress. Marcel Dekker, New York, pp 3–11
- Taji T, Oshumi C, Luchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 29: 417–426 [DOI] [PubMed] [Google Scholar]
- Taji T, Seki M, Satou M, Sakurai T, Kobayashi M, Ishiyama K, Narusaka Y, Narusaka M, Zhu J-K, Shinozaki K (2004) Comparative genomics in salt tolerance between Arabidopsis and Arabidopsis-related halophyte salt cress using Arabidopsis microarrays. Plant Physiol 135: 1697–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Hibino T, Hayashi Y, Tanaka A, Kishitani S, Yokota S, Takabe T (1999) Salt tolerance of transgenic rice overexpressing yeast mitochondrial Mn-SOD in chloroplasts. Plant Sci 148: 131–138 [Google Scholar]
- Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot (Lond) 91: 503–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16: 1–10 [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218: 1–14 [DOI] [PubMed] [Google Scholar]
- Watanabe S, Katsumi K, Yuji I, Sasaki S (2001) Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Organ Cult 63: 199–206 [Google Scholar]
- Wei QJ (1993) Euphratica Poplar (in Chinese). Chinese Forestry Press, Beijing, pp 3
- Winter H (1993) Untersuchung zur akkumulation und translokation von assimilaten: subzelluläre volumina und metabolitkonzentrationen in blättern von gerste und spinat. PhD thesis. Georg-August-Universität Göttingen, Göttingen, Germany
- Xu WH (1988) Poplar (in Chinese). Heilongjiang People's Press, Harbin, China, pp 167
- Yoo RH, Park CY, Kim JC, Heo WD, Cheong MS, Park HC, Kim MC, Moon BC, Choi MS, Kang YH, et al (2005) Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem 280: 3697–3706 [DOI] [PubMed] [Google Scholar]
- Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6: 66–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










