Abstract
Lactofen belongs to the diphenylether class of herbicides, which targets protoporphyrinogen oxidase, which in turn causes singlet oxygen generation. In tolerant plants like soybean (Glycine max), the chemical nonetheless causes necrotic patches called “bronzing” in contact areas. Here it is shown that such bronzing is accompanied by cell death, which was quantified from digital microscopic images using Assess Software. Cellular autofluorescence accompanied cell death, and a homolog of the cell death marker gene, Hsr203j, was induced by lactofen in treated soybean tissues. Thus, this form of chemically induced cell death shares some hallmarks of certain types of programmed cell death. In addition to the cell death phenotype, lactofen caused enhanced expressions of chalcone synthase and chalcone reductase genes, mainly in the exposed and immediately adjacent (proximal) cells. Furthermore, isoflavone synthase genes, which are wound inducible in soybean, were up-regulated by lactofen in both proximal and distal cell zones in minimally wounded cotyledons and further enhanced in wounded tissues. Moreover, if the wall glucan elicitor from Phytophthora sojae was present during lactofen treatment, the induction of isoflavone synthase was even more rapid. These results are consistent with the fact that lactofen triggers massive isoflavone accumulations and activates the capacity for glyceollin elicitation competency. In addition, lactofen induces late expression of a selective set of pathogenesis-related (PR) protein genes, including PR-1a, PR-5, and PR-10, mainly in treated proximal tissues. These various results are discussed in the context of singlet oxygen-induced responses and lactofen's potential as a disease resistance-inducing agent.
Lactofen is a newer member of the diphenylether (DPE) class of herbicides. The major target of this class of herbicides is protoporphyrinogen oxidase (Protox) in the porphyrin biosynthetic pathway (Matringe et al., 1989; Witkowski and Halling, 1989). This enzyme is the last common step before the branching of the pathway for chlorophyll and heme synthesis. Protox from various organelles (chloroplasts, etioplasts, and mitochondria) from plants and animals is inhibited by DPE herbicides (Matringe et al., 1989). Inhibition of Protox in these organelles leads to the accumulation of protoporphyrin IX (Lydon and Duke, 1988; Matringe and Scalla, 1988; Sandman and Böger, 1988; Witkowski and Halling, 1988; Krijt et al., 1992), which is thought to result from the translocation of the Protox substrate protoporphyrinogen IX outside the organelle (Lehnen et al., 1990) followed with oxidation by nonenzymatic reactions or by herbicide-insensitive oxidases mainly in the plasma membrane (Jacobs et al., 1991; Hess, 2000, and refs. therein). In the presence of light, the accumulated protoporphyrin IX, which is now improperly compartmentalized, is unable to be utilized for further synthesis and thus reacts with oxygen to give rise to singlet oxygen (Haworth and Hess, 1988). It is thought that singlet oxygen, a form of reactive oxygen species (ROS), in turn causes lipid peroxidation, especially on membranes (Orr and Hess, 1982), damage of other cellular constituents, and eventually cell death and, thus, the herbicidal action of these chemicals (Hess, 2000).
Lactofen is used as a postemergence herbicide for the control of broadleaf weeds in soybean (Glycine max) fields. In the past few years, it has been observed that application of lactofen to soybean can reduce the severity of Sclerotinia White Mold damage in the field when the disease pressure is moderate to high (Dann et al., 1999). Subsequently, the soybean isoflavonoid phytoalexin glyceollin was reported to accumulate in lactofen-treated leaves in the field (Nelson et al., 2002). This potential for disease protection invoked our interest, and subsequent work in our laboratory using metabolite profiling showed that lactofen induces multiple and dramatic effects on overall isoflavonoid metabolism in soybean (Landini et al., 2002). First, it induces massive accumulations of several isoflavones, including daidzein and genistein, both of which have been implicated in multiple roles in the deployment of soybean phenylpropanoid defenses (Graham and Graham, 1999). In addition, while the induction of glyceollin accumulation per se is not a major effect under laboratory conditions, lactofen activates elicitation competency, i.e. the tissue's capacity for glyceollin accumulation, when challenged with the wall glucan elicitor (WGE) from Phytophthora sojae (Landini et al., 2002). Interestingly, lactofen is unusual in that it predominantly induces the accumulation of free isoflavone aglycones rather than their conjugates (Landini et al., 2002). This fact may greatly amplify the shunting of newly formed daidzein, the first committed precursor of glyceollin, into this pterocarpan phytoalexin when challenged with elicitor.
In this article, to further investigate lactofen's perturbation of isoflavone metabolism, its effect on expression of some of the genes for key phenylpropanoid enzymes was examined in the well-characterized soybean cotyledon system. In this system, the biochemistry of the temporal and spatial aspects of the phenylpropanoid responses toward P. sojae infection and WGE treatment has been particularly well delineated (Graham and Graham, 1996, 1999). Recently, we have also employed this system to investigate the up-regulation of expression of soybean pathogenesis-related (PR) protein genes by WGE (Graham et al., 2003). Since lactofen induces disease resistance in soybean in the field, the effect of lactofen on PR protein gene expression was also investigated. Finally, since lactofen causes ROS-mediated necrosis in soybean, some aspects of the nature of lactofen-induced cell death were also characterized.
RESULTS
Lactofen-Induced Cell Death in Soybean
As described in the introduction, lactofen treatment activates singlet oxygen generation, which has been thought in turn to cause cell death. As a result, certain plants, including many weeds, are eventually killed. Although soybean plants survive treatment with lactofen, it causes a so-called “bronzing” effect, which is characterized by maroon-red-colored patches on treated leaves or other tissues (Wichert and Talbert, 1993).
Cotyledons of other plants have been used in several earlier studies of DPE mode of action (e.g. Orr and Hess, 1982; Matringe and Scalla, 1988; Lehnen et al., 1990). The snapped soybean cotyledon protocol we have been using offers a minimal wound procedure that has been very useful in cellular and biochemical studies on soybean defense (Graham and Graham, 1996). It allows the treatment of freshly exposed mesophyll parenchyma cells to various factors in a highly controlled manner. As shown in Figure1B and the insets in Figure 2, the bronzing effect of lactofen is readily reproduced in this protocol. As described in “Materials and Methods,” the cell death that accompanies bronzing can be conveniently quantified microscopically using the image analysis software Assess, which was originally developed for quantitative assessment of macroscopic disease symptoms. Such analyses led to the finding that dying cells initially stain with Evan's Blue (Fig. 1A), followed by bronzing, collapse, and desiccation (Fig. 1B). The Evan's Blue-staining cells also displayed the typical yellow autofluorescence (Fig. 1, D–F), which has been shown to be associated with certain cell death phenomena, such as the hypersensitive response (Koga et al., 1988; Vanacker et al., 2000; Wäspi et al., 2001), or with spontaneous cell death mutants (Gray et al., 2002). Thus, although lactofen does not kill the soybean plant, it causes cell death at contact sites that can be readily measured in the snapped cotyledon protocol.
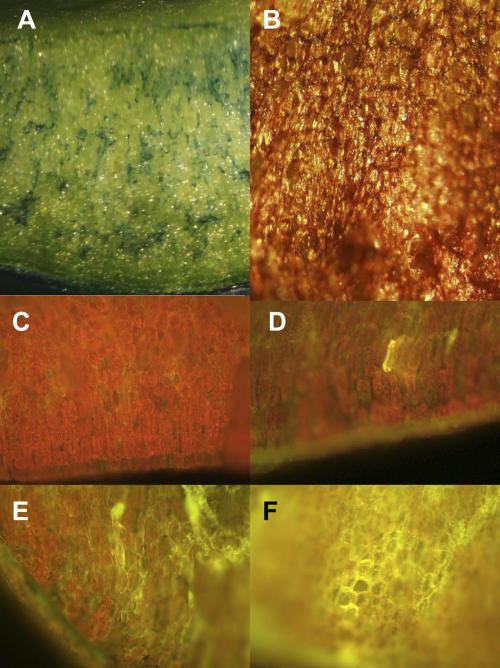
Figure 1.
Lactofen-mediated cell death. Cell death was measured using Assess Software by measuring the percent of the treated surface showing Evan's Blue vital staining (see, e.g. A, taken 24 h after treatment with 100 μm lactofen) or bronzing (see, e.g. B, taken 72 h after treatment with 300 μm lactofen). Clusters of cells that stained with Evan's Blue (A) also showed yellow autofluorescence. The red chlorophyll fluorescence of water control treated tissues is shown in C. The degree of yellow autofluorescence developed over time. Typical responses to 100 μm lactofen included fluorescence of individual cells at 12 h (D), clusters of cells at 24 h (E), and more expansive areas of cells at 48 h (F). Magnifications are 30× (A and B) and 40× (C–F).
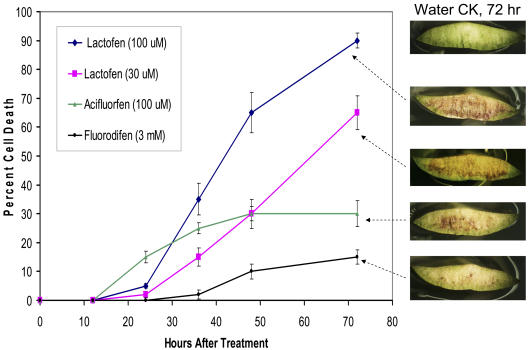
Figure 2.
Cell death induced by lactofen and other DPE herbicides. The percentage of cells showing either Evan's Blue or bronzing (see Fig. 1) were added and plotted over time for various treatments as noted. Each data point is the average of analyses using Assess Software on 15 individual cotyledons (±sd). Typical responses at 72 h are shown in the accompanying pictures.
Figure 2 shows a summary of the progression of cell death as induced by lactofen. Two other DPEs, acifluorfen and fluorodifen, were used for comparison. Acifluorfen is missing a major side chain present in lactofen and has been more commonly used in laboratory studies. It is likely that acifluorfen is generated from lactofen by deesterification in planta. Fluorodifen was chosen as a negative control because in preliminary studies it was determined that it does not cause extensive bronzing (possibly due to the fact that it is a particularly good substrate for a detoxifying soybean glutathione transferase; Skipsey et al., 1997). Unlike lactofen and acifluorfen, fluorodifen also does not induce the accumulation of isoflavones in soybean (Landini et al., 2002). Initially a dose response was done with each chemical over the range 10 μm to 3 mm. For comparative purposes, the level of lactofen used in the field was approximately 1 mm. Very clear differences in response were seen among these three DPE chemicals. In each case, cell death was dose responsive, but the lowest concentration needed to initiate effective cell death was approximately 33 μm for lactofen, 100 μm for acifluorfen, and 3 mm for fluorodifen. These three concentrations were chosen to illustrate their comparative effects in Figure 2. The effects of 100 μm lactofen are also included. In addition to the differences in dose response, clear differences in the extent and timing of response were seen. In the discussion that follows, we compare the effects of the other treatments to that of 33 μm lactofen. By 12 to 16 h, the acifluorfen-treated cotyledons began to show clusters of cells staining with Evan's Blue (approximately 5% of the total surface; data not shown), while the other treatments showed no response. At 24 h, the acifluorfen treatments showed a net cell death of 15%, of which approximately 5% were cells staining with Evan's Blue and 10% was attributable to bronzing. Once cells bronze they no longer take up the Evan's Blue stain. In contrast, the responses of lactofen-treated cells were delayed, and only very minimal Evan's Blue staining (<2%) and no bronzing was seen at 24 h. However, by 48 h, extensive and nearly equal bronzing (approximately 30%) was seen with both the acifluorfen and 33 μm lactofen treatments. At this time, Evan's Blue staining cells made up 4% of the lactofen-treated surface, but only 1% of the acifluorfen-treated surface. The very high levels of fluorodifen applied eventually resulted in very limited Evan's Blue-staining cell clusters (approximately 12% by 72 h) but never showed a significant bronzing effect.
Induced Expression of Cell Death-Related Genes in Lactofen-Treated Tissue
We explored lactofen's effects on the expression of two cell death-related genes, Hsr203j and Hir. Hsr203j was originally isolated from tobacco (Nicotiana tabacum) as a hypersensitive cell death (hypersensitive reaction [HR])-associated gene (Pontier et al., 1994). Homologs have been found in other species (for example, Pontier et al., 1998; Mould et al., 2003), and it is generally considered a marker for HR or related types of programmed cell death (PCD). It has also been shown to have certain regulatory functions for cell death in tobacco (Tronchet et al., 2001). Hypersensitive induced reaction (HIR) genes were characterized systematically in maize (Zea mays; Nadimpalli et al., 2000). They belong to the prohibitin/stomatin family of genes, many of which are involved in cell proliferation, ion channel activities, and cell death in animals. The functions of HIR in plants are less studied. Soybean homologs for both Hsr203j and Hir were identified, and it was thus of interest to investigate whether they are responsive during lactofen-induced cell death as well. Preliminary northern results with probes against the two genes were negative for Hir but positive for Hsr203j; thus, further studies were focused on Hsr203j.
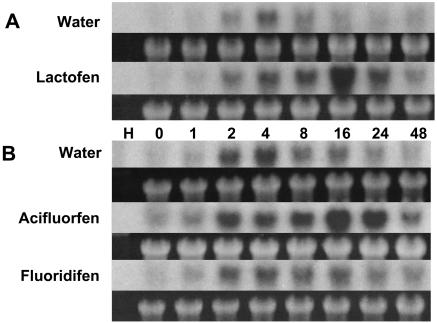
As shown in Figure 3, even the very minimal wounding associated with the snapped cotyledon assay (less than 1% cell damage is typical; Graham and Graham, 1996) causes slight and transient (2–4 h) early up-regulation of the soybean Hsr203j homolog. More dramatic induction of expression for this gene is seen somewhat later in response to lactofen in the treated surface cells (8–24 h) but is also transient and no longer strong by 48 h. Treatment with acifluorfen gave similar results to those with lactofen. Consistent with the lack of induced cell death, fluorodifen does not induce Hsr203j above background levels. Maximum induction (16 h) coincides with the early phases of cell death as measured by Evan's Blue (or autofluorescence) and precedes bronzing and cell collapse. Taken together, then, our results suggest that lactofen-induced necrosis may have some resemblance to certain aspects of other forms of PCD (see “Discussion”), and Hsr203j may provide a useful marker for this response.
Figure 3.
Lactofen induces expression of the soybean homolog of the cell death marker gene Hsr203j. Water or lactofen (300 μm) was applied to the surfaces of snapped soybean cotyledons (A) as described in “Materials and Methods.” Thin sections representing the treated and immediately adjacent (proximal) cells were collected at the indicated time (hours) after treatment, pooled, and prepared for northern blots. Ethidium bromide staining for large ribosomal RNA before blotting is shown below each autoradiogram. Alternatively (B) cotyledons were treated and analyzed in the same manner after treatment with water or either acifluorfen or fluorodifen (each at 300 μm).
The Effect of Lactofen on mRNA for Phenylpropanoid Metabolic Enzymes
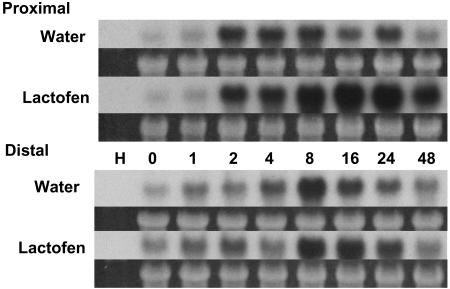
Because lactofen induces massive accumulations of a number of isoflavones in soybean (Landini et al., 2002), a few key phenylpropanoid genes were selected for northern analyses. These include genes for chalcone synthase (CHS), the first committed step for biosynthesis of the flavonoids (including the isoflavones); isoflavone synthase (IFS), the key enzyme for the biosynthesis of the isoflavonoids; and chalcone reductase (CHR), which leads to the production of the 5-doxyisoflavones, such as daidzein and glyceollin. Each of these enzymes represents a group of enzymes that is under different functional coordination, which we referred to as metabolic cassettes in the earlier article (Landini et al., 2002). Experiments were done in both a spatial and temporal framework, and a representative set of northern blots using snapped cotyledon is shown in Figures 4 and 5.
Figure 4.
Lactofen enhances expression of CHS and CHR genes. Water or lactofen treatments and tissue harvests were as in Figure 3A except that sections both proximal and distal to the point of treatment were analyzed. Hybridizations were with a CHS (A) or CHR (B) probe as indicated. Similar results were obtained with a different CHS probe.
Figure 5.
IFS genes are up-regulated by lactofen. Treatments and tissue harvests were as in Figure 3A except that sections both proximal and distal to the point of treatment were analyzed. Hybridizations were with an IFS probe.
Once again, even the simple manipulations associated with the snapped cotyledon protocol are enough to activate some expression of the CHS and IFS (and to a lesser extent CHR) genes in treated (proximal) tissues. Although each gene has somewhat different temporal and spatial patterns, by and large lactofen greatly enhanced and/or prolonged the expression of each.
In control tissues, CHS genes were turned on early and transiently at 2 to 8 h, and expression was restricted to the immediate area freshly exposed after snapping (Fig. 4A). Lactofen has little to no effect on this early expression, but induces a very strong response from 8 to 24 h and some weak expression at 16 to 24 h even in distal cells.
The patterns of expression for CHR (Fig. 4B) are somewhat similar to CHS. However, in the controls expression was weak and peaked late in proximal cells. The very strong enhancing effects of lactofen in proximal cells were quite apparent over the period 8 to 48 h. In cells distal to the point of treatment, expression was weak in both treated and control tissues.
As with CHS, expression of the IFS genes (Fig. 5) was also transiently induced in control tissues, but over a more prolonged period in proximal cells (2–24 h). Again, lactofen had little effect on early expression, but strongly up-regulated expression in these proximal cells over the period 8 to 48 h. Uniquely, expression of IFS in distal cells was also quite strong in the controls, and lactofen again gave some enhancement at later times (16–24 h).
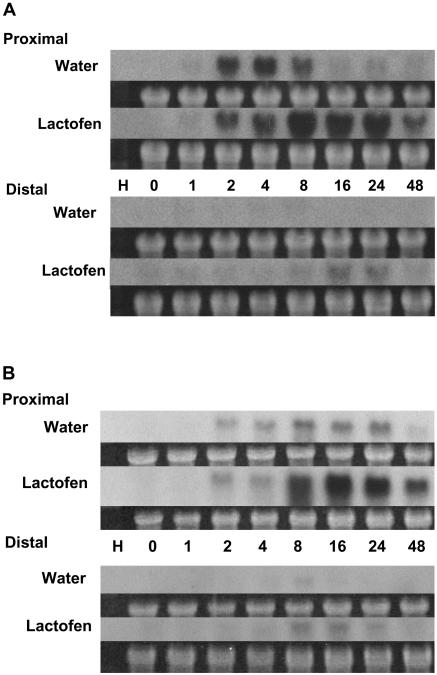
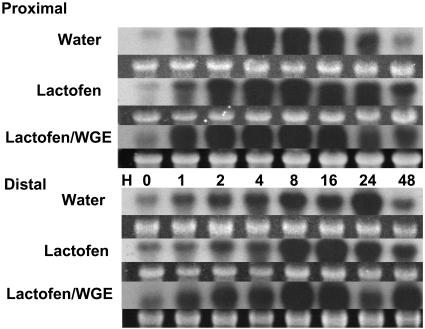
The classical cut cotyledon protocol for phytoalexin accumulation has been used to examine composite effects of elicitors or other treatments with wounding on metabolic responses and gene expressions (Graham and Graham 1991, 1996; Graham et al., 2003). When the effects of wounding are superimposed on treatment with lactofen, further enhancements or synergy can be seen. Just for an example, a northern blot with cut cotyledons using an IFS probe is shown in Figure 6. Wounding alone induces IFS in proximal sections. Lactofen prolongs induction over the period 24 to 48 h. In distal cells lactofen enhances the early phase of induction over the period 8 to 16 h. In the presence of the pathogen elicitor WGE, this enhanced expression was even more rapid, with strong induction as early as 1 h in both proximal and distal cells.
Figure 6.
Further enhanced expression of IFS genes by lactofen was seen in wounded and WGE elicitor-treated tissues. Water, lactofen (300 μm), or lactofen (300 μm) with 30 μg/mL WGE was applied to a small cut area on the abaxial surface of soybean cotyledons. In this protocol, the treated and immediately adjacent cells were harvested as proximal cells, and a transverse section 4 mm away from the edge of the wounded surface was harvested as the distal cell population. Details are described in “Materials and Methods.” RNA extraction and northern blots were as described in Figure 3A. The probe was the same IFS probe used in Figure 5.
Lactofen Induces the Expression of Some PR Protein Genes
We have identified soybean homologs for most of the 16 families of PR proteins (Van Loon and Van Strien, 1999) except the true thionins and some of the specialized chitinases. Some were characterized in our previous studies for their response to wounding and WGE treatments (Graham et al., 2003). Since the original field observations on lactofen related to its potential for disease protection of soybean (Dann et al., 1999; Nelson et al., 2002), lactofen's effects on these potentially important defense genes were also investigated.
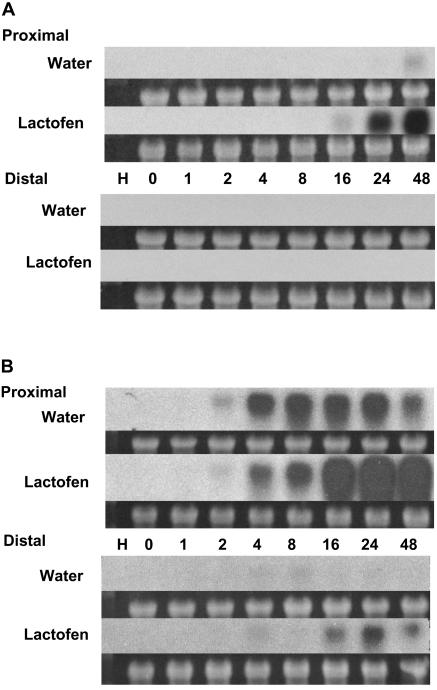
In earlier work, we reported that the PR-1a and PR-10 genes are both induced by WGE in treated (proximal) and immediately adjacent cell zones, but not in distal cells (Graham et al., 2003). As shown in Figure 7, these genes are also up-regulated strongly by lactofen in proximal but not in distal cells. The expression of the PR-1a genes is minimal in control tissues (Fig. 7A) and strongly up-regulated by lactofen only in proximal cells. PR-10 genes respond to general stress, including wounding (Crowell et al., 1992; Graham et al., 2003). As shown in Figure 7B, even the minimal wounding associated with the snapped cotyledon protocol induces expression in proximal tissues, but the expression of these genes is very strongly enhanced by lactofen over the period 16 to 48 h, with some expression even in distal tissues.
Figure 7.
PR-1a and PR-10 protein gene expression were induced by lactofen in cells proximal to treatment. Treatments and tissue harvests were as in Figure 3A except that sections both proximal and distal to the point of treatment were analyzed. Hybridizations were with a PR-1a (A) or PR-10 (B) probe as indicated.
Expression of members of three other classes of PR protein genes (PR-2, PR-4, and PR-6) were induced by wounding and enhanced by WGE. Their expression was quite different from genes for PR-1a and PR-10 in that they were often expressed more strongly in distal cells, as were the enhancements by WGE (Graham et al., 2003). Lactofen's effects on this group of PR protein genes were minimal (data not shown). This is in contrast to lactofen's pronounced up-regulation of other wound-induced genes (e.g. IFS and PR-10).
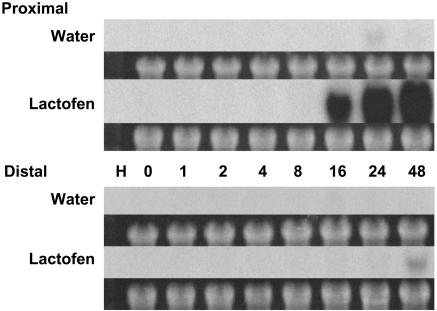
Finally, in preliminary reverse-northern blots, it was observed that a given family member of PR-5 (thaumatin/osmotin-like protein) was strongly activated by lactofen. This observation was followed up with regular northern blots, which demonstrate this very dramatic activation of expression over the period 16 to 48 h mostly only in proximal tissue (Fig. 8). In contrast, this same PR-5 gene family member was only weakly responsive to WGE elicitor treatment (M.Y. Graham, unpublished data). So this member of the PR-5 family was not only selectively up-regulated by lactofen, but its induction was among the strongest of the PR protein genes seen following lactofen treatment.
Figure 8.
PR-5 protein gene expression is markedly induced by lactofen in cells proximal to treatment. Treatments and tissue harvests were as in Figure 3A except that sections both proximal and distal to the point of treatment were analyzed. Hybridizations were with a PR-5 probe.
DISCUSSION
Lactofen-Induced Cell Death
We have demonstrated that the disease resistance-inducing herbicide lactofen induces cell death in soybean. In its earlier phases, cell death can be followed by Evan's Blue staining and autofluorescence. Later, cells become bronzed and very severely desiccated, and collapse completely. Expression of soybean homolog(s) of Hsr203j was induced over a similar time frame as the onset of cell death. Hsr203j has usually been seen to be turned on during hypersensitive cell death (Pontier et al., 1994, 1998; Mould et al., 2003) and is considered a marker for PCD (Pontier et al., 1998). Thus, lactofen-induced cell death in soybean may share some characteristics with other programmed forms of cell death, including the hypersensitive response. Consistent with this, it has been shown (in tobacco leaf discs) that three herbicides, including acifluorfen, induce internucleosomal DNA fragmentation as shown by DNA laddering and positive TUNEL staining in nuclei, two other more universal hallmarks of PCD (Chen and Dickman, 2004).
PCD has actively been studied in plants for the last decade. There are developmentally regulated and environmentally triggered programs (Dangl et al., 2000; Gray, 2004); the HR is a type of the latter. Due to its importance to disease resistance, the HR has been a focus in many systems (Jones and Dangl, 1996; Heath, 2000). Many studies have indicated that ROS are involved in the HR (Baker and Orlandi, 1995; Heath, 2000). Since lactofen causes singlet oxygen generation, ROS may provide some sort of a common thread.
To aid in understanding the mechanisms of PCD, many spontaneous cell death mutants have been characterized in various plants. Some have light-dependent phenotypes and affect the porphyrin pathway or chlorophyll catabolism. In some ways, perhaps lactofen-treated plants can be considered a phenocopy for some of these mutants. Of particular pertinence is the lesion mimic mutant, Les22, in maize, which has a defective uroporphyrinogen decarboxylase (Hu et al., 1998), which is just upstream in the same pathway as Protox, the target of lactofen. Transgenic Arabidopsis (Arabidopsis thaliana) expressing antisense to Protox, the target of the DPE herbicides, also displays a lesion mimic phenotype (Molina et al., 1999). The Arabidopsis fluorescent (Flu) mutant (Meskauskiene et al., 2001) and the lethal leaf spot (Lls1) mutant in maize (Gray et al., 1997, 2002, 2004) may also have some relevance to the effects of lactofen because of their relationships to singlet oxygen generation in chloroplasts and chlorophyll degradation, respectively.
Lactofen and Defense Gene Expression
Inasmuch as lactofen causes the generation of singlet oxygen, the induction of some PR protein genes is not surprising. Other ROS species such as superoxide or hydrogen peroxide have also been correlated with the activation of certain PR protein genes in other plant species (see, e.g. Chen et al., 1993; Bi et al., 1995; Mur et al., 2004). However, singlet oxygen has been shown to induce a somewhat selective set of genes in Arabidopsis that are different from those induced by superoxide or hydrogen peroxide (op den Camp et al., 2003). In soybean, it is interesting that lactofen causes up-regulation of selective PR protein genes, some of which are in common with, but some of which are different from those, enhanced by the WGE elicitor, which is a pathogen-derived general defense elicitor that does not induce cell death per se. Both lactofen and WGE induce a subclass of the PR protein genes for which induction is restricted mainly to proximal cells (PR-1a and PR-10). However, those PR protein genes that are enhanced in both proximal and distal cells by WGE (PR-2, PR-4, and PR-6; Graham et al., 2003) did not seem to be affected by lactofen in either cell zone.
Thaumatin/osmotin and related proteins are often induced during osmotic stress such as that induced by high salt or dehydration (Hurkman, 1992; Raghothama et al., 1993; Anžlovar and Dermastia, 2003). The fact that this class of genes was strongly induced by lactofen may reflect the dramatic desiccation and cell collapse that was observed in the later bronzing phase of lactofen-induced cell death. Their prolonged induction over the period 16 to 48 h is consistent with this observation. In this context it is interesting that one of the genes specifically activated in Arabidopsis by singlet oxygen (op den Camp et al., 2003) is a drought induced-like protein, although there is no significant homology to thaumatin.
Thaumatin-like proteins have also been shown to have antifungal activity (Vigers et al., 1992). Members of this family are also induced during pathogen infections and are classified as the PR-5 class of pathogenesis-related proteins (Van Loon and Van Strien, 1999). Thaumatin-related mRNAs measured with this particular probe were not effectively elevated in response to WGE (M.Y. Graham, unpublished data), which does not induce cell death. Consistent with this, no expressed sequence tags (ESTs) homologous to this sequence are present in a cDNA library of wounded cotyledons treated with a low level of the WGE elicitor (Gm-c1076). However, 59 closely homologous ESTs (0.85% of the library) were present in a Pseudomonas syringae pv glycinea (Psg)-incompatible infected leaf library (Gm-c1074), suggesting that a very high level of expression of this particular PR-5 member may also be associated with hypersensitive cell death.
In contrast to its effects on PR protein induction, the effects of ROS on secondary product metabolism are not as widely established. However, notably it has been demonstrated that elicitor Pep 13-induced ROS is required for up-regulation of the phenylpropanoid pathway and coumarin phytoalexin accumulation in parsley (Petroselinum crispum), although cell death is not induced by Pep-13 in this system (Jabs et al., 1997). Moreover, in Arabidopsis camalexin has also been shown to be induced by a variety of stresses, including oxidative stress induced by the DPE acifluorfen (Zhao et al., 1998). Here it was shown that the CHS, CHR, and IFS genes were significantly up-regulated by lactofen when compared to water controls, which are all consistent with the large accumulations of the isoflavones daidzein and genistein in the presence of lactofen observed earlier (Landini et al., 2002).
Lactofen-Induced Responses and Disease Resistance
All together, it is shown here that lactofen is associated with localized cell death even in the herbicide-tolerant plant soybean, and it causes up-regulations of several classes of defense-related genes. It is interesting that while some of these PR protein gene inductions are similar to those caused by WGE, others are clearly different. Thus, it is quite possible that lactofen can both complement and synergize with pathogen-derived elicitors such as WGE. Perhaps these factors may work together in the overall protection against pathogen infections in the field (Dann et al., 1999; Nelson et al., 2002).
Indeed in preliminary studies protection by lactofen against P. sojae and Psg has been observed (Larue, 2003), which is in some ways akin to broad-spectrum systemic induced resistance observed in other plant species, such as systemic acquired resistance induced in response to salicylic acid and its chemical mimics. However, despite many years of effort, the typical salicylic acid-induced systemic acquired resistance has not been convincingly demonstrated in soybean (T.L. Graham, personal communication). Thus, lactofen may belong to a new class of defense-inducing chemicals for soybean. Further studies are needed to more clearly characterize lactofen's induction of disease resistance and defense responses at the whole-plant level and how this may relate to other forms of systemic induced resistance. It is hoped that the molecular assays/markers characterized here can be used to help to dissect such processes and the factors regulating them.
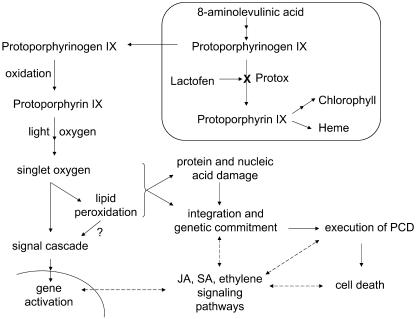
In Figure 9 an overall working model is presented to show singlet oxygen generation by lactofen and its possible connections to cell death and defense gene induction. This model is based on earlier knowledge and some new perspectives on cell death from studies of other systems. Among these is the emerging thought that it is not the ROS per se that kills the cell, but the imbalance in redox homeostasis that triggers the cell death program (Foyer and Noctor, 2005). In the case of singlet oxygen, the execution of the program appears to be genetically determined (Wagner et al., 2004). Secondly, recent evidence suggests that the effects of singlet oxygen per se may be somewhat different than other forms of ROS. As shown by op den Camp et al. (2003), singlet oxygen generation leads to very specific effects on lipid peroxidation and gene activation not shared by superoxide or hydrogen peroxide. Thus, as they hypothesize, lipid peroxidation may serve as a signaling event for downstream responses. Finally, phytohormone and defense-signaling pathways could positively or negatively influence the outcome of singlet oxygen-triggered cell death; some of their biosynthetic genes are up-regulated by singlet oxygen as well (Danon et al., 2005). While these defense-signaling pathways may modulate (or contribute to secondary) defense gene induction, the differential nature of the genes induced by singlet oxygen in soybean described here as compared to wounding or elicitor treatment (Graham et al., 2003) makes direct participation by these molecules in the singlet oxygen-induced responses less likely.
Figure 9.
Working model for lactofen-induced cell death and defense gene activation. Lactofen initiates singlet oxygen generation by inhibition of Protox within chloroplasts/etioplasts/mitochondria (described in detail in the introduction). Singlet oxygen initiates lipid peroxidation (Hess, 2000) and possible signaling cascades that stimulate gene activation (op den Camp, 2003) and commitment to the execution of PCD (Wagner et al., 2004; Foyer and Noctor, 2005). Possible associations and positive/negative influences on cell death by various defense-signaling pathways, such as those for jasmonic acid (JA), salicylic acid (SA), and ethylene (Danon et al., 2005), are shown with dotted arrows. Since the lactofen-induced defense reactions discussed here are predominantly local, for simplicity distal or systemic effects have not been included.
More work is needed to further delineate the relationship of lactofen-induced cell death to other forms of PCD and how cell death relates to the other responses examined in this article. From this study, the progression of these responses is isoflavone enzyme gene induction, followed by Hsr203j, followed by PR protein gene expression, and the final phases of cell death. We will incorporate the newly developed gene-silencing protocol in soybean (Subramanian et al., 2005) to help further define how the earlier responses are interrelated and which, if any, are causally involved in this form of chemically induced cell death.
MATERIALS AND METHODS
Plant Materials
Soybean (Glycine max L. Merr., cv Williams) seedlings were grown and cotyledons harvested from 7- to 8-d-old seedlings as described earlier. At this seedling age, the cotyledons are expanded and photosynthetically active. The cut and snapped (minimal wound) cotyledon assays were also performed as described previously (Graham and Graham, 1991, 1996, respectively) with slight modifications (Graham et al., 2003) to accommodate RNA analysis. In all the studies reported here, the detached cotyledons were incubated after treatment at room temperature under constant light (250 μmol m−2 s−1) until harvest. An advantage of the soybean cotyledon tissue is that it is made up of tightly aligned and uniform columns of mesophyll parenchyma cells, thus allowing precise spatial characterization of responses. In the snapped cotyledon protocol used in most experiments, three consecutive transverse cross sections from the point of treatment (0.6, 1.2, and 0.6 mm, respectively) were harvested to represent the proximal, near-proximal, and distal sections. For cut cotyledons, three sections of tissue were also harvested. The first two sections were cross sections from a column of cells harvested from the treated cotyledons with a number 2 cork borer as described before (Graham et al., 2003). The first cross section (0.6 mm), constituting the proximal cell section, contained the treated surface and immediately underlying cells. The second cross section, constituting the near-proximal cell section, included the remaining 1.2 mm of tissue. A third section, constituting the distal cell section, was a 0.6-mm transverse section through the original cotyledon harvested 3 to 4 mm from the edge of the wound at the point of treatment. In all cases, a minimum of 200 mg of tissue was harvested for mRNA isolation. Because of the relatively small sections taken, this normally required the pooling of samples from approximately 20 cotyledons. Tissues from individual cotyledons were pooled, flash frozen in liquid nitrogen, and stored at −80°C until use. Lactofen was applied at 300 μm for all northern blots, at 15 μL for snapped cotyledons and 20 μL for cut cotyledons. This concentration was chosen based on dose-response studies showing that it gave a saturating response.
Chemicals
Unless otherwise noted, all chemicals were purchased from Sigma Chemical Company. Lactofen (99% pure) was obtained from Valent Technologies. Acifluorfen (98% pure) and fluorodifen (99.5% pure) were obtained from Chem Service. For application to plant tissues, these DPE chemicals were dissolved in a minimal amount of isopropanol and rapidly diluted with water to a final concentration of 0.5% isopropanol. The WGE from Phytophthora sojae was purified as described previously (Graham and Graham, 1991).
Cell Death Assays
The minimal wound snapped cotyledon protocol (Graham and Graham, 1996) was used to examine the induction of cell death. In this protocol, a surface of freshly exposed, but minimally wounded, mesophyll parenchyma cells can be exposed to various treatments or conditions. In our experiments, lactofen, fluorodifen, and acifluorfen were each applied as a 10-μL droplet to 15 cotyledons per treatment. A range of concentrations (3 mm, 1 mm, 333 μm, 111 μm, 37 μm, 12 μm) were examined and water was used as a control. At 24, 36, 48, and 72 h, the 15 individual cotyledons for each treatment were stained (without removing them from the supporting water agar) with 0.05% Evan's Blue for 20 min, after which the majority of stain was drawn off with the corner of a Kimwipe and the surface blotted by pressing each cotyledon surface gently against a four-layer-thick Kimwipe pad. With this procedure, no destaining is needed. Each cotyledon was then individually photographed under identical conditions with an Olympus DP10 digital camera through an Olympus SZH zoom stereo dissection scope with an Olympus Flexilux 90HLU ring light mounted on an Olympus DF Plan 0.5× lens. The lens-mounted ring light provided very even illumination of 300 μE m−2 s−1. The zoom setting of the scope was set at 40×, so all photographs were taken at an overall magnification of 20×. At this magnification, approximately two-thirds of the total area of the cotyledon was included. The two narrow ends of the cotyledons were purposely excluded because treatments are not always uniformly spread over these surface areas.
Quantification of Cell Death
Images of each individual cotyledon were analyzed using Assess Software (American Phytopathological Society Press). It was found that the earliest signs of cell death (12–24 h depending on the herbicide) showed up as clusters of dead cells staining with Evan's Blue. Bronzing of dying cell clusters occurred later (24–72 h). Close microscopic examination revealed that bronzed cell surfaces were characterized by complete cellular collapse and desiccation. After bronzing, cells no longer stained with Evan's Blue. Thus, in order to quantify cell death over time, Assess was used to quantify the percent of the treated surface (total cotyledon surface in the images captured above), which was stained blue and that which was bronzed, and added these values together (the areas did not overlap). Assess allows one to very precisely define the color attributes for quantification. While the color thresholds used to define the blue and bronze colors will depend on the light source and microscope used, the hue-saturation-intensity color space was typically used with thresholds of 75 to 88 for bronze and 95 to 137 for blue. Assess allows the export of data directly into Excel files, where it was averaged, statistically treated, and plotted for each treatment.
Autofluorescence
The same cell clusters that stained with Evan's Blue showed yellow autofluorescence typical of induced cell death (Koga et al., 1988; Vanacker et al., 2000; Wäspi et al., 2001; Gray et al., 2002), while surrounding healthy cells did not fluoresce or take up the stain. Autofluorescence was observed using a Nikon Eclipse 6600 microscope equipped with a 100 W mercury lamp (CHIU Technical Corporation). Epifluorescence was observed using the following filter set: a blue excitation filter (450–490 nm), a 500-nm dichroic mirror, and a 515-nm barrier filter. Under these conditions, cells showed a bright yellow fluorescence. Images were collected digitally with a Spotcam CCD camera (Diagnostic).
RNA Isolation and Northern Blots
The RNA preparations, northern blots, and hybridization were carried out using standard procedures as previously described (Graham et al., 2003). Total RNA was quantified by A260 to guide in loading of gels. Except in the experiment in Figure 7B, in which 5 μg was used, 10 μg was loaded per lane. Ethidium bromide staining of ribosomal RNA was used as loading control. Filters were usually stripped according to the manufacturer's instructions (Schleicher and Schuell) for hybridizations to more than one probe. The experiments have been performed over a period of several years. Some experiments were repeated in minor variations, and only representative data are shown.
Derivation of Probes
Primer pairs of 20 nucleotides in length were designed using Primer Designer software (version 3.0, Scientific and Educational Software) or Primer-3 software (1996, 1997, Steve Rozen, Helen J. Skaletsky; http://www-genome.wi.mit.edu/genome_software/other/primer3.html). Oligo primer sets were custom synthesized at Integrated DNA Technologies. Unless otherwise specified, PCR was used to prime products from Williams soybean genomic DNA. Products of correct size were gel purified by electroelution or using the Qia Quick kit (Qiagen). Confirmation sequencing of final PCR products was carried out at the Ohio State University Plant-Microbe Genomics Facility. Probes were labeled with α-32P-dCTP (Amersham Bioscience) using Random Priming kits (Life Technologies/Invitrogen). Individual probes are described below. Because they are not gene specific, the observed up-regulations represent a collective outcome for related genes. Because autoradiograms are subject to saturation, the up-regulations observed are relative and not quantitative.
CHS
Several primer pairs were originally designed against the Chs 1 gene (Wingender et al., 1989). One pair (5-CATGGCACCTTCGTTGGATG-3; 5-AACAATGACAGCGGCTGCAC-3) gave a 475-bp product. The results reported here were using this probe. Another pair of RNA-specific primers spanning an intron generated a probe (251 bp) that gave similar results (data not shown). Complete sequences of seven copies of CHS genes in soybean have been well characterized. They are highly homologous to one another including flanking regions (Akada and Dube, 1995). The above probe does not discriminate these different members. Resequencing of the primed product(s) showed 99% identity to Chs 1, 3, 4, and 5 in the primed region; less homology was seen to Chs2 (96%), 6 (93%), and 7 (84%).
CHR
For this gene family, earlier we designed primer pairs from three to four contigs (including a singleton) we assembled ourselves from soybean ESTs using the CAP3 program. The pair (5-TTCGACACTGCTGCTGCTTA-3; 5-CTTCTGTTGCCATGCAAGGT-3) was used to prime a 445-bp product from RNA (isolated from soybean tissue 3 h after elicitor treatment) with reverse transcription-PCR. This probe gave the best hybridization in a preliminary reverse-northern screen and thus was used to follow up with regular northerns as reported here. This contig of ours corresponds to the current The Institute for Genomic Research (TIGR) TC 215169, which encompasses a cDNA clone reported earlier (Welle and Grisebach, 1989). There are four copies of CHR genes in soybean (O. Yu, personal communication). This probe most likely hybridizes to all of them.
IFS
The primers for this probe (5-GACGCCTCACCTATGATAGC-3; 5-TCTCCTCCTCACGATCTCAC-3) were originally designed against the sequence of a soybean cDNA clone when it was first reported (Steele et al., 1999). It corresponds to IFS 2, one of two genes cloned from soybean nearly concurrently by another group (Jung et al., 2000). Although the sequence of the 443-bp product is identical to IFS 2, this probe hybridizes to two bands in genomic Southerns (M.Y. Graham, unpublished data) and thus does not differentiate between the two genes. ISF 1 (AF 195798) and 2 (AF 195799) correspond to current TIGR TC 175454 and TC 175143, respectively. Although the two genes have somewhat different tissue abundance, they have similar expression patterns (Subramanian et al., 2004).
Hsr203j Homolog
The primer pair for this probe was originally designed against soybean TIGR TC 133073 (currently TC204890), which is annotated as an Hsr203j homolog. Although there are several other related sequences, this one is relatively abundant in EST libraries for both incompatible infections by P. sojae (nine hits or 0.2% in Gm-c1084) and Psg (four hits or 0.06% in Gm-c1074). The primer pair (5-TATGACGACGGTTCAGTGGA-3; 5-TTGAAGTCACCGTGTTGCTC-3) was used to prime a 446-bp product.
PR Protein Genes
Primers for the thaumatin-like protein genes (PR-5) were designed against soybean TC204396 (originally TC120222), expression of which is very abundant (59 ESTs or 0.85%) in the library Gm-c1074, which is derived from Psg-infected soybean Williams 82 leaves. Forward primer (5-ATACACGGTATGGCCAGGAA-3) and reverse primer (5-GCGGTGTTGTTAGGTCCAGT-3) gave a 498-bp product. Other probes for PR protein genes were the same as those reported earlier (Graham et al., 2003). These include genes for PR-1a, PR-2, PR-4, PR-6, and PR-10. Although all were examined, only the data for PR-1a and PR-10 are shown here.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers DQ267257 to DQ267260, DQ269446, and DQ269447.
Acknowledgments
The author acknowledges Serena Landini for expert preparation of some of the initial plant materials, and Melanie Pelow and Jerome Weidner for assistance in preparing RNA blots. The author also greatly appreciates the help of Dr. Terry Graham on cell death assays, preparation of the manuscript, and for providing laboratory space. Sincere thanks are due to Dr. Biao-Ding for the use of his fluorescence microscope and Mike Zianni for DNA sequencing. Lastly, the author thanks Valent Technology for providing the pure chemical form of lactofen and scientists at Valent, especially Ted Bean, for helping us initiate the lactofen project in our laboratory. The author also gratefully acknowledges the Ohio Plant Biotechnology Consortium for funding used in the cataloging and development of probes for defense-related genes and disease resistance markers in soybean.
This work was supported by the Ohio Plant Biotechnology Consortium (competitive grant to M.Y.G.), and by state and federal funds appropriated to the Ohio Agricultural Research and Development Center.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Madge Y. Graham (graham.19@osu.edu).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.068676.
References
- Akada S, Dube SK (1995) Organization of soybean chalcone synthase gene clusters and characterization of a new member of the family. Plant Mol Biol 29: 189–199 [DOI] [PubMed] [Google Scholar]
- Anžlovar S, Dermastia M (2003) The comparative analysis of osmotins and osmotin-like PR-5 proteins. Plant Biol (Stuttg) 5: 116–124 [Google Scholar]
- Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu Rev Phytopathol 33: 299–321 [DOI] [PubMed] [Google Scholar]
- Bi Y-M, Kenton P, Mur L, Darby R, Draper J (1995) Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. Plant J 8: 235–246 [DOI] [PubMed] [Google Scholar]
- Chen S, Dickman MB (2004) Bcl-2 family members localize to tobacco chloroplasts and inhibit programmed cell death induced by chloroplast-targeted herbicides. J Exp Bot 55: 2617–2623 [DOI] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262: 1883–1886 [DOI] [PubMed] [Google Scholar]
- Crowell DN, John ME, Russell D, Amasino RM (1992) Characterization of a stress-induced, developmentally regulated gene family from soybean. Plant Mol Biol 18: 459–466 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Dietrich RA, Thomas H (2000) Senescence and programmed cell death. In B Buchanan, W Gruissem, R Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 1044–1100
- Dann E, Diers B, Hammerschmidt R (1999) Suppression of Sclerotinia stem rot of soybean by lactofen herbicide treatment. Phytopathology 89: 598–602 [DOI] [PubMed] [Google Scholar]
- Danon A, Miersch O, Felix G, Camp RG, Apel K (2005) Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J 41: 68–80 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MY, Weidner J, Wheeler K, Pelow ML, Graham TL (2003) Induced expression of pathogenesis-related protein genes in soybean by wounding and the Phytophthora sojae cell wall glucan elicitor. Physiol Mol Plant Pathol 63: 141–149 [Google Scholar]
- Graham TL, Graham MY (1991) Glyceollin elicitors induce major but distinctly different shifts in isoflavonoid metabolism in proximal and distal soybean cell populations. Mol Plant Microbe Interact 4: 60–68 [Google Scholar]
- Graham TL, Graham MY (1996) Signaling in soybean phenylpropanoid responses: dissection of primary, secondary, and conditioning effects of light, wounding, and elicitor treatments. Plant Physiol 110: 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TL, Graham MY (1999) Role of hypersensitive cell death in conditioning elicitation competency and defense potentiation. Physiol Mol Plant Pathol 55: 13–20 [Google Scholar]
- Gray J, editor (2004) Programmed Cell Death in Plants. Blackwell Publishing, London
- Gray J, Close PS, Briggs SP, Johal GS (1997) A novel suppressor of cell death in plants encoded by the Lls1 gene of maize. Cell 89: 25–31 [DOI] [PubMed] [Google Scholar]
- Gray J, Janick-Buckner D, Buckner B, Close PS, Johal GS (2002) Light-dependent death of maize lls1 cells is mediated by mature chloroplasts. Plant Physiol 130: 1894–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Wardzala E, Yang M, Reinbothe S, Haller S, Pauli F (2004) A small family of LLS1-related non-heme oxygenases in plants with an origin amongst oxygenic photosynthesizers. Plant Mol Biol 54: 39–54 [DOI] [PubMed] [Google Scholar]
- Haworth P, Hess FD (1988) The generation of singlet oxygen by the nitrodiphenyl ether herbicide oxyfluorfen is independent of photosynthesis. Plant Physiol 86: 672–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44: 321–334 [DOI] [PubMed] [Google Scholar]
- Hess FD (2000) Light-dependent herbicides: an overview. Weed Sci 48: 160–170 [Google Scholar]
- Hu G, Yalpani N, Briggs SP, Johal GS (1998) A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize. Plant Cell 10: 1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman WJ (1992) Effect of salt stress on plant gene expression: a review. Plant Soil 146: 145–151 [Google Scholar]
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D (1997) Elicitor-stimulated ion fluxes and O2 from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA 94: 4800–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JM, Jacobs NJ, Sherman TD, Duke SO (1991) Effect of diphenyl ether herbicides on oxidation of protoporphyrinogen to protoporphyrin in organellar and plasma membrane enriched fractions of barley. Plant Physiol 97: 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Dangl JL (1996) Logjam at Styx: programmed cell death in plants. Trends Plant Sci 1: 114–119 [Google Scholar]
- Jung W, Yu O, Lau S-MC, O'Keefe DP, Odell J, Fader G, McGonigle B (2000) Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat Biotechnol 18: 208–212 [DOI] [PubMed] [Google Scholar]
- Koga H, Zeyen RJ, Bushnell WR, Ahlstrand GG (1988) Hypersensitive cell death, autofluorescence, and insoluble silicon accumulation in barley leaf epidermal cells under attack by Erysiphe graminis f. sp. hordei. Physiol Mol Plant Pathol 32: 395–409 [Google Scholar]
- Krijt J, Pleskot R, Sanitrak J, Janousek V (1992) Experimental hepatic porphyria induced by oxadiazon in male mice and rats. Pestic Biochem Physiol 42: 180–187 [Google Scholar]
- Landini S, Graham MY, Graham TL (2002) Lactofen induces isoflavone accumulation and glyceollin elicitation competency in soybean. Phytochemistry 62: 865–874 [DOI] [PubMed] [Google Scholar]
- Larue CT (2003) Lactofen-mediated accumulations of isoflavonoids in soybean leaf tissue and induction of a systemic protection response towards pathogens in lactofen treated soybeans. MS thesis. Ohio State University, Columbus, OH
- Lehnen LP, Sherman TD, Becerril JM, Duke SO (1990) Tissue and cellular localization of acifluorfen-induced porphyrins in cucumber cotyledons. Pestic Biochem Physiol 37: 239–248 [Google Scholar]
- Lydon J, Duke SO (1988) Porphyrin synthesis is required for photobleaching activity of the p-nitrosubstituted diphenyl ether herbicides. Pestic Biochem Physiol 31: 74–83 [Google Scholar]
- Matringe M, Camadro JM, Labbe P, Scalla R (1989) Protoporphyrinogen oxidase as a molecular target for diphenyl ether herbicides. Biochem J 260: 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matringe M, Scalla R (1988) Effects of acifluorfen-methyl on cucumber cotyledons: porphyrin accumulation. Pestic Biochem Physiol 32: 164–172 [Google Scholar]
- Meskauskiene R, Nater M, Goslings D, Kessler F, den Camp RO, Apel K (2001) FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 98: 12826–12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina A, Volrath S, Guyer D, Maleck K, Ryals J, Ward E (1999) Inhibition of protoporphyrinogen oxidase expression in Arabidopsis causes a lesion-mimic phenotype that induces systemic acquired resistance. Plant J 17: 667–678 [DOI] [PubMed] [Google Scholar]
- Mould MJ, Xu T, Barbara M, Iscove NN, Heath MC (2003) cDNAs generated from individual epidermal cells reveal that differential gene expression predicting subsequent resistance or susceptibility to rust fungal infection occurs prior to the fungus entering the cell lumen. Mol Plant Microbe Interact 16: 835–845 [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Sturgess FJ, Farrell GG, Draper J (2004) The AoPR10 promoter and certain endogenous PR10 genes respond to oxidative signals in Arabidopsis. Mol Plant Pathol 5: 435–451 [DOI] [PubMed] [Google Scholar]
- Nadimpalli R, Yalpani N, Johal GS, Simmons CR (2000) Prohibitins, stomatins, and plant disease response genes compose a protein superfamily that controls cell proliferation, ion channel regulation, and death. J Biol Chem 275: 29579–29586 [DOI] [PubMed] [Google Scholar]
- Nelson KA, Renner KA, Hammerschmidt R (2002) Effects of protoporphyrinogen oxidase inhibitors on soybean (Glycine max L.) response, Sclerotinia sclerotiorum disease development, and phytoalexin production by soybean. Weed Technol 16: 353–359 [Google Scholar]
- op den Camp RGL, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Göbel C, Feussner I, et al (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15: 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr GL, Hess FD (1982) Mechanism of action of the diphenyl ether herbicide acifluorfen-methyl in excised cucumber (Cucumis sativus L.) cotyledons light activation and the subsequent formation of lipophilic free radicals. Plant Physiol 69: 502–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier D, Godiard L, Marco Y, Roby D (1994) Hsr203j, a tobacco gene whose activation is rapid, highly localized and specific for incompatible plant/pathogen interactions. Plant J 5: 507–521 [DOI] [PubMed] [Google Scholar]
- Pontier D, Tronchet M, Rogowsky P, Lam E, Roby D (1998) Activation of Hsr203, a plant gene expressed during incompatible plant-pathogen interactions, is correlated with programmed cell death. Mol Plant Microbe Interact 11: 544–554 [DOI] [PubMed] [Google Scholar]
- Raghothama KG, Liu D, Nelson DE, Hasegawa PM, Bressan RA (1993) Analysis of an osmotically-regulated pathogenesis-related osmotin gene promoter. Plant Mol Biol 23: 1117–1128 [DOI] [PubMed] [Google Scholar]
- Sandmann G, Böger P (1988) Accumulation of protoporphyrin IX in the presence of peroxiding herbicides. Z Naturforsch 43c: 699–704 [Google Scholar]
- Skipsey M, Andrews CJ, Townson JK, Jepson I, Edwards R (1997) Substrate and thiol specificity of a stress-inducible glutathione transferase from soybean. FEBS Lett 409: 370–374 [DOI] [PubMed] [Google Scholar]
- Steele CL, Gijzen M, Qutob D, Dixon RA (1999) Molecular characterization of the enzyme catalyzing the aryl migration reaction of isoflavonoid biosynthesis in soybean. Arch Biochem Biophys 367: 146–150 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Graham MY, Yu O, Graham TL (2005) RNA interference of soybean isoflavone synthase genes leads to silencing in tissues distal to the transformation site and to enhanced susceptibility to Phytophthora sojae. Plant Physiol 137: 1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Hu X, Lu G, Odell JT, Yu O (2004) The promoters of two isoflavone synthase genes respond differentially to nodulation and defense signals in transgenic soybean roots. Plant Mol Biol 54: 623–639 [DOI] [PubMed] [Google Scholar]
- Tronchet M, Ranty B, Marco Y, Roby D (2001) HSR203 antisense suppression in tobacco accelerates development of hypersensitive cell death. Plant J 27: 115–127 [DOI] [PubMed] [Google Scholar]
- Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55: 85–97 [Google Scholar]
- Vanacker H, Carver TLW, Foyer CH (2000) Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper-sensitive response in the barley-powdery mildew interaction. Plant Physiol 123: 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers AJ, Weidemann S, Roberts WF, Legrand M, Selitrennikoff CP, Fritig B (1992) Thaumatin-like pathogenesis-related proteins are antifungal. Plant Sci 83: 155–161 [Google Scholar]
- Wagner D, Przybyla D, op den Camp RO, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, et al (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306: 1183–1185 [DOI] [PubMed] [Google Scholar]
- Wäspi U, Schweizer P, Dudler R (2001) Syringolin reprograms wheat to undergo hypersensitive cell death in a compatible interaction with powdery mildew. Plant Cell 13: 153–161 [PMC free article] [PubMed] [Google Scholar]
- Welle R, Grisebach H (1989) Phytoalexin synthesis in soybean cells: elicitor induction of reductase involved in biosynthesis of 6′-deoxychalcone. Arch Biochem Biophys 272: 97–102 [DOI] [PubMed] [Google Scholar]
- Wichert RA, Talbert RE (1993) Soybean [Glycine max (L.)] response to lactofen. Weed Sci 41: 23–27 [Google Scholar]
- Wingender R, Rohrig H, Horicke C, Wing D, Schell J (1989) Differential regulation of soybean chalcone synthase genes in plant defence, symbiosis and upon environmental stimuli. Mol Gen Genet 218: 315–322 [DOI] [PubMed] [Google Scholar]
- Witkowski DA, Halling BP (1988) Accumulation of photodynamic tetrapyrroles induced by acifluorfen-methyl. Plant Physiol 87: 637–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski DA, Halling BP (1989) Inhibition of plant protoporphyrinogen oxidase by the herbicide acifluorfen-methyl. Plant Physiol 90: 1239–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Williams CC, Last RL (1998) Induction of Arabidopsis tryptophan pathway enzymes and camalexin by amino acid starvation, oxidative stress, and an abiotic elicitor. Plant Cell 10: 359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]