Abstract
The refolding of the prototypic fusogenic protein hemagglutinin (HA) at the pH of fusion is considered to be a concerted and irreversible discharge of a loaded spring, with no distinct intermediates between the initial and final conformations. Here, we show that HA refolding involves reversible conformations with a lifetime of minutes. After reneutralization, low pH-activated HA returns from the conformations wherein both the fusion peptide and the kinked loop of the HA2 subunit are exposed, but the HA1 subunits have not yet dissociated, to a structure indistinguishable from the initial one in functional, biochemical and immunological characteristics. The rate of the transition from reversible conformations to irreversible refolding depends on the pH and on the presence of target membrane. Importantly, recovery of the initial conformation is blocked by the interactions between adjacent HA trimers. The existence of the identified reversible stage of refolding can be crucial for allowing multiple copies of HA to synchronize their release of conformational energy, as required for fusion.
Keywords: influenza hemagglutinin/membrane rearrangements/protein interactions/reversible change/viral fusion
Introduction
Influenza virus hemagglutinin (HA) is the prototypical fusogenic protein. Each monomer of the trimeric glycoprotein HA is composed of two subunits: HA1, responsible for binding to the target cell surface receptor; and membrane-anchored HA2, crucial for fusion. Endosomal acidification triggers refolding of HA and the fusion of viral and endosomal membranes, the critical entry step in viral infection. The pathway of HA refolding between the well-characterized initial and final conformations, and the mechanisms of HA-mediated fusion, remain unclear.
Refolding of HA at low pH involves relocation of HA1 from its initial place at the top of HA (White and Wilson, 1987; Wiley and Skehel, 1987; Godley et al., 1992) and a major refolding of HA2. This refolding results in the release of the N-terminal peptide of HA2 (the ‘fusion peptide’, FP) from the hydrophobic cavity of the protein and, thus, allows FP insertion into viral and target membranes (for a review, see Gaudin et al., 1995). In HA’s final conformation, the FP is connected to an extended coiled-coil followed by a reverse turn. The remaining C-terminal half of each HA2 monomer lies antiparallel against the N-terminal coiled-coil (Skehel and Wiley, 2000). In the final acidic conformation of HA2, as in many other fusogenic proteins, two membrane-inserting peptides of each monomer, the transmembrane domain and the FP, are now at the same end of the rod-shaped bundle. The refolding of HA also makes the protein susceptible to proteolysis by thermolysin, exposes the S–S bond between HA1 and HA2 to reducing agents such as dithiothreitol (DTT) (Wiley and Skehel, 1987) and tilts the molecule from the normal orientation towards the membrane (Tatulian et al., 1995).
Is HA refolding a single-step process or a multi-step process with distinct intermediate conformations? If the latter, are early steps reversible? While refolding of HA is generally thought to be irreversible, reversible changes have been reported.
Specifically, a low-pH-induced decrease in tryptophan fluorescence of HA (Krumbiegel et al., 1994) and an increase in liposome binding to the reconstituted HA (Tatulian and Tamm, 1996), both reflecting refolding of the protein, are reversible. Refolding of HA, detected by polarized infrared spectroscopy as acid-induced tilting of HA, was also reversible in the absence of target membrane (Tatulian and Tamm, 1996). Later stages of refolding of membrane-free HA are also partially reversible (Korte et al., 1997).
To explain the positive cooperativity of HA activation at low pH, we hypothesized that individual HA trimers first form a reversible state (Markovic et al., 2001). Interaction between adjacent trimers promotes their transition from this hypothetical early state to the irreversible lowest energy state. As a result, activation spreads among adjacent HAs, leading to the synchronized release of HA conformational energy by neighboring trimers assembled around the fusion site. The important assumption of this hypothesis, namely the existence of an early reversible conformation of low-pH-activated HA, requires experimental verification.
In this study, we show that refolding of membrane-anchored HA does indeed involve a distinct intermediate conformation, which upon reneutralization reverts to a conformation indistinguishable from the initial neutral pH form. We propose that the existence of this relatively long-lived intermediate state before the major refolding of HA is of importance for coupling between HA refolding and membrane fusion.
Results
Experimental approach
Low pH initiates the transition of HA from its initial form towards its lowest energy form. Hereafter, all HA conformations different from the initial one, in terms of either binding of any of the conformation-specific antibodies used, sensitivity to proteases and DTT, or ability to mediate fusion at low pH, will be referred to as the ‘low-pH-activated conformations’. Here, we tested whether any of the low-pH forms of HA can revert to the initial form at neutral pH. To address this question, we evaluated the changes in the number of low-pH-activated HAs with time after a short low-pH pulse.
The experimental approaches we used to follow the recovery of the initial conformation of HA have different sets of advantages and limitations. The accessibility of different epitopes and cleavage sites yields structural information about protein conformation and gives an estimate for the percentage of protein molecules having this accessibility. On the other hand, proteolysis and interactions between an antibody and its epitope can each modify the stability of some particular HA conformation and thus shift the distribution between different conformations. The fusion inactivation assay, in which recovery of the initial conformation of HA is assayed by restoration of its ability to support low-pH-triggered fusion, yields neither structural information nor quantification of conformational distributions. However, this assay does characterize the functional properties of membrane-anchored HA under relevant conditions and in the absence of alien modifiers such as antibodies and enzymes.
To evaluate whether the observed reversibility is specific for HA of a particular strain of influenza virus or reflects the general properties of HA refolding, we have used cells expressing HA (HA-cells) of two influenza strains, X31 and Japan, which differ in their patterns of activation and inactivation (Puri et al., 1990; Markovic et al., 2001).
Functional recovery of low-pH-activated HA
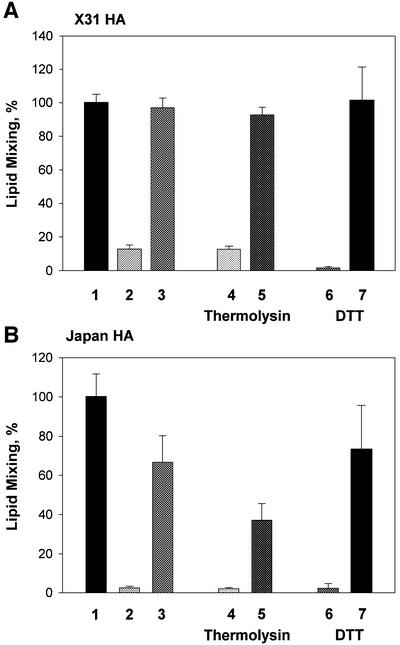
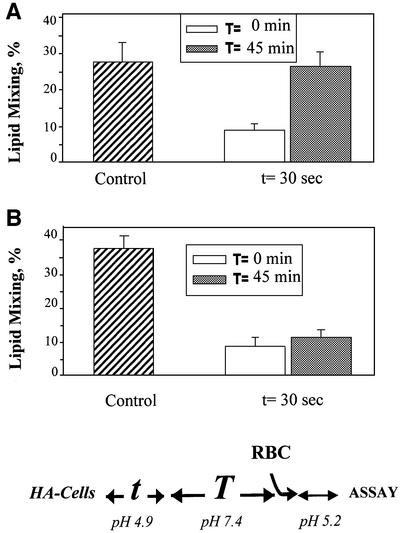
HA that is activated and then inactivated in the absence of target membrane is incapable of mediating fusion upon subsequent establishment of membrane contacts (Puri et al., 1990; Korte et al., 1997, 1999; Markovic et al., 2001). In our fusion inactivation assay, X31 and Japan HA-cells (bars 1–3 in Figure 1A and B, respectively) were first treated with an activating low-pH pulse (the ‘activating pulse’, AP) in the absence of target membrane. The cells were reneutralized and incubated at pH 7.4 for different time intervals. Finally, to evaluate remaining fusogenic activity, we incubated these cells with red blood cells (RBCs) for 15 min and, after removal of unbound RBCs, triggered HA-cell–RBC fusion by applying the second (‘fusion-triggering’) low-pH pulse, FTP. Since the rate of activation of Japan HA is rather low, the pH applied in the FTP for Japan HA-cells was usually less acidic than that applied during the AP, i.e. pH 5.2 versus 4.9 (Markovic et al., 2001). Because of an excess of fusion-competent HAs, robust fusion at pH 4.9 is insensitive to small changes in the numbers of HAs. In contrast, the number of activated HAs available for fusion at the suboptimal pH of 5.2 is significantly lower and, as a result, fusion induced by a pH 5.2 pulse is more sensitive to a small loss of fusion-competent HAs due to prior inactivation than is fusion induced by a pH 4.9 pulse.
Fig. 1. Reversible conformations of low-pH-activated HA identified by means of a fusion inactivation assay. X31 (A) or Japan HA-cells (B) at 22°C were first treated with a 5 min pH 4.9 pulse (‘AP’) in the absence of target membrane. Then, the cells were incubated at neutral pH for different times. Finally, the cells were incubated with RBCs for 15 min and, after removal of unbound RBCs, the second (‘fusion-triggering’) low-pH pulse, FTP [a 2 min pH 4.9 pulse in (A) and a 5 min pH 5.2 pulse in (B)] was applied. Here and in the experiments reported in the following figures, the final extents of lipid mixing were measured by fluorescence microscopy. The total time intervals between the activating and fusion-triggering pulses were 15 min (bars 2) and 45 min (bars 3–7). Bars 4 and 5, thermolysin (25 µg/ml), and bars 6 and 7, DTT (10 mM) were applied for either the first (bars 4 and 6, respectively) or the last (bars 5 and 7, respectively) 5 min of incubation between the activating and fusion-triggering pulses. Fusion extents were normalized to those in the control experiments, in which the FTP [a 2 min pH 4.9 pulse in (A) and a 5 min pH 5.2 pulse in (B)] was applied to the HA-cells with bound RBCs untreated with an AP. Fusion extents of 83.9 and 63.5% were taken as 100% in bars A1 and B1, respectively.
Any decrease in the number of low-pH HA forms after the AP would increase the number of HAs that can be activated by the subsequent FTP and would thus increase the fusion extent. Indeed, we found that the fusion extent increased for longer time intervals between the activating and triggering pulses (Figure 1).
This fusion increase cannot be explained by a change in RBC–HA-cell binding, as adding RBCs to Japan HA-cells either immediately after the AP or 60 min later yields the same average number of bound RBCs per HA-cell (data not shown). Nor could it be explained by the appearance of newly expressed, low-pH-untreated HA: additional HA0 molecules delivered to the cell surface during the time interval between the activating and triggering pulses would not be trypsin cleaved into the activation- and fusion-competent HA1–HA2 form. Thus, the partial recovery of fusogenic properties of HAs upon reneutralization indicated that some low-pH forms of both Japan and X31 HAs could still return to a form functionally indistinguishable from the initial one.
The place of the reversible stage in the pathway of HA refolding
We then characterized the place of the reversible stage of the low-pH-induced refolding of HA relative to some known features of this process. To test whether the reversible activation stage precedes or follows the FP exposure, an early sign of activation (White and Wilson, 1987; White, 1995), we took advantage of the fact that FP exposed in low-pH forms of HA is susceptible to thermolysin digestion (Wiley and Skehel, 1987). If low-pH forms of HA with exposed FP still revert to their initial conformation, thermolysin application immediately after the end of the AP should cleave these and more advanced low-pH forms, and thus should prevent the recovery of fusion activity observed after the FTP. We did in fact find that thermolysin treatment after the AP inhibited fusion recovery. The same thermolysin treatment applied 45 min after the AP did not block fusion (Figure 1A and B, bar 5 versus bar 4), indicating that at this stage all reversible low-pH forms of HA had reverted to the initial, thermolysin-insensitive form.
Similarly, low-pH-induced exposure of the S–S bond between the HA1 and HA2 subunits, detected as DTT-induced fusion inhibition, can be reversed by incubation at pH 7.4 (Figure 1A and B, bar 7 versus bar 6). The decrease in the number of activated HAs with time after the low-pH pulse was confirmed biochemically in experiments in which the percentage of HAs with the DTT-accessible S–S intersubunit bond was assayed, by western blotting, as the loss of HA1. Within 30 min after the 10 min pulse of pH 4.9 applied at 22°C, the amounts of activated HA decreased by 31 and 16% for X31 HA-cells and Japan HA-cells, respectively. Since internalization of neutral and acidic forms of HA is very slow (Roth et al., 1986), this disappearance of DTT-cleavable HA, along with the results of the functional experiments presented above, is strong experimental evidence for our thesis that activated HA can revert to the initial, neutral-pH conformation of HA. One might suggest that loss of HA sensitivity to thermolysin and DTT reflects the gradual masking of the cleavage sites due to HA aggregation. However, a monotonic increase in HA susceptibility to thermolysin and DTT with time at low pH (I.Markovic, unpublished data) argues against this possibility.
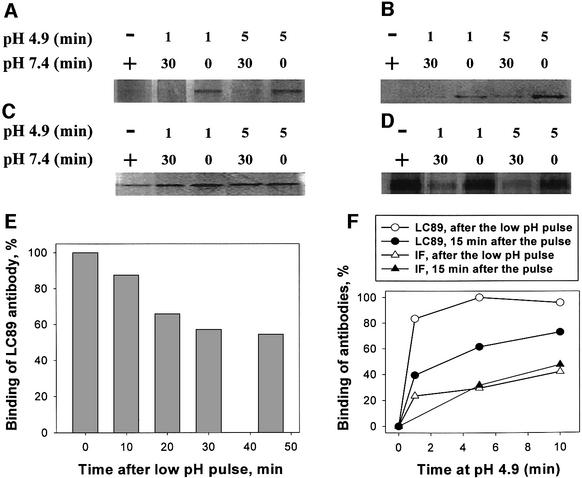
To obtain more structural data on the nature and pathway of the reversible conformations of HA, we studied the changes in accessibility of different X31 HA epitopes to conformation-specific antibodies. Two of these antibodies recognize the HA1 subunit of HA. HC67 antibody with an epitope at residue HA1 193 binds only to the neutral-pH form of HA (Daniels et al., 1983). In contrast, antibodies whose epitope is buried in the HA1 trimer interface (IF) of the neutral-pH form of HA preferentially bind to low-pH forms of HA (White and Wilson, 1987). The antibodies against HA2 (antiserum against FP and monoclonal LC89 antibody) preferentially bind to the acidic form of HA. The binding site of LC89 antibody includes residue HA2 107 (Daniels et al., 1983; Wharton et al., 1995) located in the region of HA2, which, at low pH, undergoes a helix to loop transition (‘kinked loop region’; Epand et al., 1999).
As expected, in our immunoprecipitation experiments a low-pH application decreased the reactivity for HC67 antibody and increased it for all other antibodies (Figure 2A–D). Upon reneutralization, we observed a loss of reactivity for LC89 and anti-FP antibodies seen as a loss of the HA band. For these antibodies, the intensity of the band observed immediately after the pH 4.9 pulse was restored when, after 30 min at pH 7.4, we applied the additional pH 4.9 pulse (not shown). In contrast, in the case of HC67 and IF antibodies, the amount of immunoprecipitated HA did not change towards the levels characteristic of the neutral-pH form. In fact, there was even a significant loss of the HC67 epitope already after reneutralization, indicating that this epitope disappears or becomes inaccessible only at the late stages of HA refolding. Thus, while exposure of two epitopes on HA2 was reversed upon reneutralization, the changes in the accessibility of HA1 epitopes appear to be irreversible.
Fig. 2. Changes in the accessibility of different HA epitopes at different stages of HA activation and recovery. X31 HA-cells were incubated at pH 4.9 for different times. (A–D) HA conformation was analyzed by immunoprecipitation with different primary antibodies. LC89, antiserum against fusion peptide (FP), interfacial antibody (IF) or HC67 antibody (A–D, respectively) was applied following the low-pH pulse, either after an additional 30 min incubation at pH 7.4 or immediately after the pulse (marked in the pH 7.4 line under the panel as ‘30 ’or ‘–’, respectively). The notations ‘–’ and ‘+’, for pH 4.9 and 7.4, respectively, under the panels mark the control experiments, in which HA-cells were not treated with a low-pH pulse. (E) X31 HA-cells were treated with a 5 min pH 4.9 pulse at 22°C. Accessibility of the kinked loop epitope was assayed by CELISA, with LC89 antibodies applied immediately after the pulse (taken as 100%) or after an additional 10, 20, 30 or 45 min incubation at neutral pH following the pulse. (F) X31 HA-cells were incubated at pH 4.9 (28°C) for different times. Binding of LC89 (circles) and IF (triangles) antibodies was assayed by CELISA either immediately after the low-pH pulse (open symbols) or after an additional 15 min incubation at neutral pH following the pulse (closed symbols). After subtraction of non-specific binding, the results were normalized to the levels of the binding of the corresponding antibody, LC89 and IF, detected immediately after the 5 min pH 4.9 pulse.
The above conclusion was substantiated further by using a cell surface enzyme-linked immunosorbent assay (CELISA). We treated X31 HA-cells with pulses of pH 4.9 at 22°C and, after reneutralization, assayed LC89 antibody binding with CELISA (Figure 2E). Under these conditions, if antibodies were applied immediately after the low-pH pulse, their binding was very close to the maximum LC89 binding observed when the same low-pH pulse was applied at 37°C. Incubation of HA-cells at pH 7.4 after the low-pH pulse but prior to the antibody application decreased LC89 binding. In the control experiment, we followed up the first low-pH pulse with a second one 60 min later. The level of LC89 binding measured immediately after application of the second pulse was close to that measured immediately after the first pulse (data not shown). These results indicate that the decrease in LC89 binding with time after the low-pH pulse reflects the reversion of low-pH forms of HA with an exposed kinked loop to their initial HA conformation rather than the developing inaccessibility of the kinked loop at the later stages of HA inactivation.
CELISA experiments confirmed that the irreversible stage of refolding starts prior to the relatively slow dissociation of the HA1 trimer detected as exposure of the HA1 IF epitope. To detect a measurable increase in the binding of the IF antibodies after the low-pH pulse, we had to increase the temperature to 28°C. As reported earlier (White and Wilson, 1987; Stegmann et al., 1990), the time course of exposure of the HA1 IF was very much slowed down at suboptimal temperatures. In agreement with the results of the immunoprecipitation experiments, following up a low-pH pulse with a long incubation at pH 7.4 did not result in any decrease in the binding of IF antibodies (Figure 2F).
To summarize, exposure of the HA1–HA2 disulfide bond, FP and kinked loop of HA2 takes place prior to the onset of irreversible changes. At the end of the low-pH pulse, HAs at different stages of refolding either revert to their initial conformation or proceed to later stages of major refolding, involving dissociation of the HA1 trimer.
Temperature dependence of the time course of restoration of the initial HA conformation
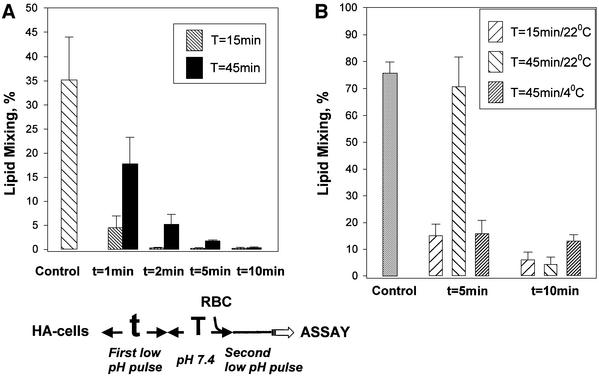
The rate at which activated HA reverts from reversible stages of refolding to the initial conformation upon reneutralization, and the time it takes for low-pH-activated HA to reach irreversible stages of refolding, both depend on the temperature. At higher temperatures, HA refolding rapidly achieves irreversible stages. At 37°C, there was no recovery of the initial form of HA after pH 4.9 pulses longer than 5 min (Figure 3A).
Fig. 3. Recovery of low-pH-activated HA molecules at 37 and 4°C evaluated by fusion inactivation assay. (A) Rapid loss of reversibility at 37°C. X31 HA-cells at 37°C were first treated with an AP (pH 4.9 for 1, 2, 5 or 10 min) applied in the absence of target membrane. The cells were incubated at pH 7.4 for 0 or 30 min, incubated with RBCs for 15 min and treated with an FTP (1 min, pH 4.9). The time interval between the end of the AP and the beginning of the FTP was either 15 or 45 min. The ‘control’ bar presents the results of the control experiment, in which only the FTP was applied to the HA-cells, with bound RBCs untreated with an AP. The final extents of lipid mixing were measured by fluorescence microscopy. (B) HA recovery is blocked at 4°C. X31 HA-cells at 22°C were first treated with an AP (pH 4.9 for 5 or 10 min) applied in the absence of target membrane. The cells were incubated at pH 7.4 for 0 or 30 min, incubated with RBCs for 15 min and treated with an FTP (2 min, pH 4.9, 22°C). During the total time interval, either 15 or 45 min, between the end of the AP and the beginning of the FTP, cells were kept at either 22 or 4°C. In the experiment represented in the ‘control’ bar, the FTP (2 min, pH 4.9, 22°C) was applied to the HA-cells, with bound RBCs untreated with an AP.
Recovery of low-pH-activated HA was inhibited at 4°C. After treating HA-expressing cells with an AP at 22°C, recovery of the initial conformation of HA at pH 7.4 proceeded much more slowly at 4°C than at 22°C (Figure 3B). Importantly, with longer low-pH pulses, refolding of HA was irreversible even when low pH was applied at 4°C (Sato et al., 1983; Ramalho-Santos et al., 1993). The irreversible stage of HA refolding, detected here by functional, fusion inactivation assay and by CELISA with IF antibodies, starts prior to the dissociation of HA1 subunits, as evidenced by the lack of detectable exposure of the IF epitope at 4°C (Stegmann et al., 1990; E.Leikina, unpublished results).
The inactivation of X31 HA at 4°C observed in our work differs from earlier findings of very slow or no X31 HA inactivation at 4°C (Stegmann et al., 1987, 1990, 1995; Ramalho-Santos et al., 1993). It is possible that this discrepancy reflects differences between the experimental models (HA-cells versus viral particles and RBCs versus liposomes).
Effects of the target membrane and HA surface density on the recovery of the initial HA conformation
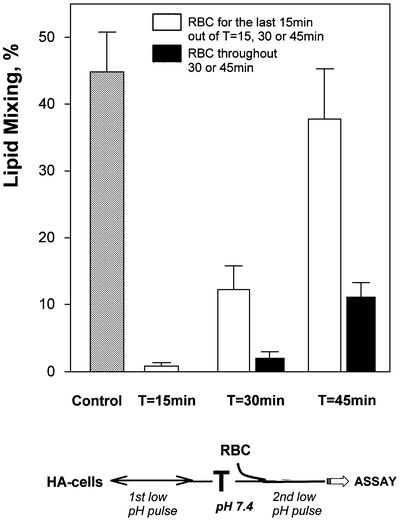
While some HAs present at the membrane after a low pH pulse are in reversible conformations, little fusion was observed if RBCs and an FTP were applied too soon after the AP (Figure 1). This can be explained either by fusion incompetence of the reversible forms of activated HA or by an accelerated transition from reversible to irreversible conformations upon RBC addition. The latter interpretation is substantiated by the finding that the rate at which HA irreversibly restructures indeed depends on the presence of a target membrane. When, after the AP, HA-cells were incubated for different times at pH 7.4 either with or without bound RBCs, the recovery of fusogenic activity as assayed after the FTP was inhibited in the presence of RBCs (Figure 4).
Fig. 4. Inhibition of the recovery of low-pH-activated HA molecules in the presence of target membrane. X31 HA-cells at 22°C were first treated with an AP (1 min at pH 4.9) applied in the absence of target membrane. The cells were then incubated at pH 7.4 for 15 or 30 min, incubated with RBCs for 15 min and treated with an FTP (2 min pH 4.9). Alternatively, RBCs were added immediately after the end of the AP and were present throughout a 15, 30 or 45 min incubation between the activating and the fusion-triggering pulses. In the experiment shown in the ‘control’ bar, the FTP (2 min at pH 4.9) was applied to the HA-cells, with bound RBCs untreated with an AP.
The rate of recovery from low-pH-activated states to the initial state also depends on the surface density of HA. We found no recovery of the initial HA conformation for Japan HA-cells with HA expression boosted from the initial level of ∼2500 trimers/µm2 to ∼12 000 trimers/µm2 by growing the cells with 5 mM sodium butyrate (NaBut; Danieli et al., 1996; Markovic et al., 2001). A 45 min incubation of HA-cells at pH 7.4 after the AP significantly increased the extent of fusion after the FTP for control cells (without NaBut), but did not restore fusion for NaBut-treated cells (Figure 5A and B, respectively). The lack of recovery for HAs at high density is not dependent artifactually upon a particular way of boosting surface density (pre-incubation with NaBut) and/or on a particular assay for recovery (functional inactivation assay). As shown in Figure 6, we found no decrease in the number of low-pH forms of HA (and thus no recovery of the initial HA conformation) with time after the low-pH pulse for viral particles known to have a very high surface density of HA (≥15 000 trimers/µm2; Taylor et al., 1987). These results suggest that when adjacent HAs are very close to each other, their interaction at acidic pH rapidly shifts the refolding from reversible conformations, which are too short lived to be resolved by our assays, towards irreversible forms.
Fig. 5. Effects of HA surface density on the recovery of the initial HA conformation. Japan HA-cells pre-incubated without (A) or with 5 mM NaBut (B) were first treated with an AP (30 s at pH 4.9, 22°C) in the absence of RBCs. Cells were then incubated at pH 7.4 for 0 or 30 min, incubated with RBCs for 15 min and treated with an FTP (2 min at pH 5.2). The time interval T between the end of the AP and the beginning of the FTP was either 15 or 45 min. In the experiment shown in the ‘control’ bar, the FTP (2 min at pH 5.2) was applied to the HA-cells, with bound RBCs untreated with an AP.
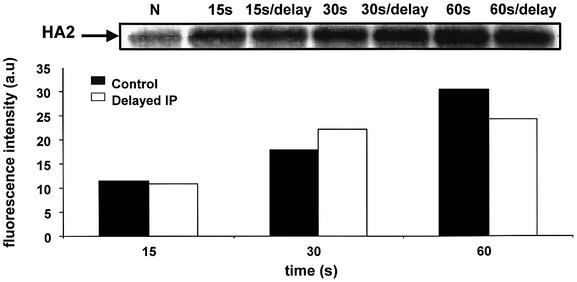
Fig. 6. Irreversibility of a low-pH-activated HA conformation with an exposed FP at influenza virus particles. Virus was treated with pH 4.9 at 22°C for 0 (marked as N), 15, 30 or 60 s. FP exposure was assayed by immunoprecipitation. The antiserum was added either immediately after reneutralization or after an additional 60 min incubation at pH 7.4. The intensity of the HA2 band in the presented gel was quantified and plotted in the bar chart, with the subtracted intensity of the band observed in the experiment without low-pH application. The total protein concentration loaded, determined by volume, is comparable in each lane, since we started the experiment with the same amount of virus for each sample.
Discussion
Acidification of HA ultimately triggers both its irreversible refolding and membrane fusion, but the specific coupling between these two processes remains unclear. In particular, little is known about the pathway of HA refolding. Here, we report that the low-pH-triggered refolding of HA, for both the fast activating X31 strain and the slow activating Japan strain (influenza subtypes H3 and H2, respectively), starts with an early reversible stage. After recovery from these reversible conformations, the conformation of reneutralized HA is indistinguishable from the initial neutral-pH conformation in low pH-dependent functional activity, accessibility of several epitopes and insusceptibility to thermolysin and DTT. This similarity strongly suggests that HA recovery from the reversible stage of activation is complete, i.e. yields the initial HA conformation.
The reversible conformation of HA is downstream of exposure of the FP, kinked loop and S–S bond between the HA1 and HA2 subunits. In the initial, neutral-pH conformation, the FP and the kinked loop stabilize each other in the cavity buried in the HA interface, and the HA1–HA2 S–S bond is located in immediate proximity to this cavity (Wiley and Skehel, 1987; Steinhauer et al., 1996; Chen et al., 1998). All these sites are close to each other and can probably be exposed in a concerted way without major refolding of the protein. In contrast, the subsequent changes in the accessibility of the two epitopes located at the HA1 require irreversible and thus, most probably, major HA refolding.
Below, we discuss the mechanisms underlying the shift from reversible to irreversible conformations of HA and the role of the reversible HA forms in fusion.
The triggering event in the transition from reversible to irreversible stages
Intertrimer interaction. The transition from reversible to irreversible stages of HA refolding is accelerated for higher densities of HA. These results indicate that the transition to the irreversible stages involves interactions between activated HA trimers that may include FP–FP (Ruigrok et al., 1988) and/or kinked loop interactions (Kim et al., 1998; Leikina et al., 2001). Facilitation of intertrimer interactions can also explain inhibition in recovery of the initial form of HA after a low-pH pulse in the presence of target membrane. Interactions of HA with receptors at the target membrane can concentrate HAs in the contact zone, increasing the local density of HA (Markovic et al., 2001; Mittal and Bentz, 2001). The hypothesis that irreversible refolding and inactivation of HA involves trimer–trimer interactions is consistent with an earlier finding that there is no inactivation of HA (of a PR/8 strain rather than the Japan and X31 strains studied here) under conditions suppressing mobility and thus lateral aggregation of HA (Tatulian and Tamm, 1996).
Along with interactions between HA trimers, the transition from reversible to irreversible refolding of HA apparently involves the dissociation of the HA1 trimer and the insertion of the HA FP into membranes.
Dissociation of the HA1 trimer. Refolding of the HA from its initial conformation to the final low-energy conformation is thought to involve extension of the coiled-coil core of HA2 and formation of an antiparallel outer layer around it (Bullough et al., 1994; Chen et al., 1999). This refolding of HA2 probably requires dissociation of the HA1 trimer. Major refolding of both subunits of HA should release most of the conformational energy of HA and might well represent the irreversible stage of HA refolding. Indeed, dissociation of HA1 trimer is found here to be irreversible.
Fusion peptide–membrane interaction. Insertion of the amphiphilic FP into membranes releases a very significant amount of energy. It is unlikely that the membrane-inserted peptide can be pulled back to its initial position within the HA cavity. Fusion intermediates such as the lysophosphatidylcholine-arrested stage (Chernomordik et al., 1997) and restricted and unrestricted hemifusion (Chernomordik et al., 1998; Leikina and Chernomordik, 2000; Melikyan et al., 2000; Markosyan et al., 2001) are downstream of the FP insertion into the membranes. Thus, HAs in these intermediates might already be in irreversible conformations.
While membrane insertion of the FP is probably irreversible, the high stability of the low-pH conformation of the membrane-free HA ectodomain (Skehel and Wiley, 2000) indicates that at later stages of the refolding HA would not recover even in the absence of the membranes.
In general, while the earliest stages of HA activation such as exposure of the FP are likely to be independent of HA–HA and HA–membrane interactions, the conditions that make fusion possible or promote it (target membrane and high surface density of activated HAs) promote transition towards irreversible refolding of HA.
The reversible stage of HA refolding and membrane fusion
On the basis of the cooperativity of HA activation, we hypothesized that fusion involves synchronized discharge of the conformational energy of the multiple adjacent HA trimers (Markovic et al., 2001). An increase in the percentage of inactivated HAs for higher surface densities of trimers (with only activatable trimers promoting the refolding of one another) indicated that there should be an early reversible form of low-pH-activated HA. It has been hypothesized that HA in this form could still revert to the initial conformation, if there are no adjacent trimers to interact with. The existence of a reversible stage of HA activation, a crucial prediction of the hypothesis, is directly documented here. Several features of the newly identified reversible conformation correspond to those predicted.
To begin with, development of the characteristic features of the reversible stage of HA refolding apparently precedes fusion, as evidenced by the inhibition of fusion when a low-pH pulse was followed immediately by application of antibodies against the FP and kinked loop (Leikina et al., 2001), or by DTT treatment (E.Leikina, unpublished results). Conditions known to promote fusion (lower pH, higher temperature and surface density of HA) also promote the transition from early reversible to late irreversible conformations of HA. This transition, which probably releases most of the conformational energy of the HA trimer, is promoted by HA–HA and HA–target membrane interactions. The target membrane accelerates this transition either by concentrating HA trimers in the contact zone (see above) or by somehow directing HA refolding toward irreversible conformations. Target membrane has been reported to promote an acid-induced irreversible tilting of HA reconstituted in supported phospholipid bilayers (Tatulian et al., 1995; Gray and Tamm, 1997).
FPs are exposed at the reversible stage prior to the onset of the major conformational change in HA that is thought to deliver the exposed FP to the target membrane. A delay in coiled-coil formation might allow the FP to become trapped in the viral membrane, which may be important for fusion (Kozlov and Chernomordik, 1998; Mittal and Bentz, 2001). Interaction between FPs of reversible HA conformations can bring adjacent HAs together. The specific interactions between activated HAs and the mechanisms by which these interactions promote fusion remain to be understood (for the hypothetical mechanism, see Kozlov and Chernomordik, 2002).
Further work is also needed to verify the hypothesis that the reversible stage of HA refolding is indeed a part of the fusion pathway rather than an alternative pathway leading to HA inactivation. If the latter, even the earliest features of HA refolding including exposure of the FP must be dependent on the presence of adjacent trimers and the target membrane. For instance, if HA refolding at high and low densities of HA proceeds via entirely different pathways, at high density, destabilization of the neutral-pH HA should be affected somehow by interactions with adjacent neutral-pH forms. To the best of our knowledge, interactions between neutral-pH forms of influenza HA, in contrast to the interactions between low-pH forms of HA, have never been documented or even hypothesized in the literature. Similarly, it appears unlikely that HA interaction with the receptors at the target membrane affects the initial destabilization of the neutral pH form of HA at acidic pH.
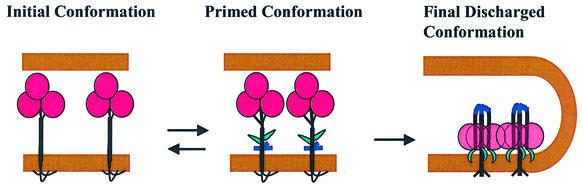
To conclude, we have identified here an early reversible stage of HA activation. At this stage, activated HAs might either revert to their initial state or proceed toward fusion-competent conformations and irreversible refolding. These results substantiate earlier work indicating that HA refolding is a multistep reaction (White and Wilson, 1987) rather than a single-step irreversible discharge of a loaded spring. We hypothesize that at low pH, HA starts to flicker between its initial conformation and an early reversible state (‘primed conformation’, Figure 7), with most of the time spent in the initial conformation. Note that this ‘primed’ conformation at acidic pH is not necessarily identical to a reversible conformation of HA characterized at neutral pH.
Fig. 7. Schematic diagram depicting refolding of acidified HA trimers in the proposed pathway from the initial neutral-pH form to a primed reversible form, followed by the irreversible transition to a final low-pH form. For the sake of simplicity, the receptors in the target membrane are not shown. The structure of the primed form is shown here as characterized at the neutral pH, and might be somewhat different at low pH. The membranes, HA1, the exposed FP and the kinked loop of HA2 are shown in brown, pink, green and blue, respectively. Exposure of the FP and kinked loop is reversible. In contrast, the dissociation of the HA1 trimer occurs already at the irreversible stage of HA refolding. The transition from primed to final form is promoted by interactions between adjacent trimers.
The delay before the discharge of most of the HA conformational energy gives the adjacent activated trimers in the contact region time to interact and to synchronize their discharge. The irreversible stage of HA refolding might involve HA refolding from a metastable reversible conformation toward the lowest energy state, the ‘final conformation’ shown in Figure 7 (discharge of the coiled-coil spring; Carr and Kim, 1993; Weissenhorn et al., 1997). Alternatively, at neutral pH, low-pH forms of individual HA trimers are less energetically favorable than their initial neutral form (Tatulian and Tamm, 1996). In this scenario, low-pH conformations are stabilized only by intertrimer interactions.
While pathways of diverse membrane fusion reactions appear to have common membrane intermediates, the structures of the specialized FPs can be rather dissimilar. For instance, the FP of flaviviruses, E protein, is strikingly different from that of influenza HA in a number of structural features (Heinz and Allison, 2001). Interestingly, as in the case of HA, refolding in E protein upon its activation starts with a reversible stage. Reversible dissociation of the protein dimer with transient exposure of the FP is followed by an irreversible reassembly of the monomers into trimers. Reversible stages of refolding have also been discussed for the FPs of rabies virus (Gaudin et al., 1999) and HIV (Doranz et al., 1999; Kliger et al., 2000). By analogy with HA-mediated fusion, we hypothesize that different viral fusion reactions and intracellular fusion involve a distinct reversible stage of refolding of fusogenic proteins that allows adjacent trigger-activated proteins to assemble at the contact site. Subsequent concerted discharge of most of the conformational energy of these proteins drives membrane fusion.
Materials and methods
Materials
Rabbit polyclonal sera directed towards the C-terminal portion of X31 HA1 and the FP of Japan HA were prepared by Covance Laboratories, Inc. (Vienna, VA). The high degree of homology between FP of X31 and Japan HA allowed the use of the same anti-FP serum on both strains of HA. Antibody LC89 was kindly provided by Drs Stephen Wharton and John J.Skehel, National Institute for Medical Research, London, UK. Two monoclonal antibodies, H26D08 antibody (Wilson, 1984) (a kind gift of Dr Judith White, University of Virginia at Charlottesville, VA) and the commercially available antibody HA.11 (Cat. no. MMS-101P, Covance Research Products, Cumberland, VA), indistinguishable in our experiments, react with an epitope HA1 98–106. Since this epitope is located at the trimer IF that appears only after low-pH-induced dissociation of the tops of HA1 subunits (Wilson, 1984), each of these antibodies is referred to as an IF. Monoclonal antibody HC67 was kindly provided by Dr John J.Skehel. Goat anti-rabbit IgG conjugated with alkaline phosphatase was purchased from Pierce, Rockford, IL. Complete protease inhibitor cocktail in tablets was purchased from Roche (Mannheim, Germany). Enhanced chemifluorescence substrates were obtained from Amersham, Buckinghamshire, UK. The neuraminidase from Clostridium perfringens, trypsin from bovine pancreas, thermolysin (Type X, from Bacillus thermoproteolyticus rokko) and PKH26 were purchased from Sigma, St Louis, MO. Immobilon-P filters were obtained from Millipore, Bedford, MA. DTT was purchased from ICN Biomedicals, Inc., Auron, OH. Influenza virus (Japan strain) was purchased from Charles River (Preston, CT), where it was propagated in the allantoic cavity of specific pathogen-free eggs and subsequently purified on a sucrose gradient.
Preparation of cells
X31 HA-cells [HA300a cells expressing X:31 HA (Kemble et al., 1993)] and Japan HA-cells (HAb2 cells expressing A/Japan/305/57 HA (Doxsey et al., 1985)] were cultured as described. Human RBCs, freshly isolated from whole blood, were labeled with lipid-soluble probe PKH26.
Unless stated otherwise, HAb2 cells were treated with 10 µg/ml trypsin (10 min, 22°C) to cleave HA0 into its fusion-competent HA1-S–S-HA2 form. For HA300a cells, trypsin was supplemented with neuraminidase (0.5 U/ml) to improve the binding of RBCs. The enzymes were applied together for 10 min at 22°C. To terminate the reaction, HA-cells were washed twice with complete medium containing 10% fetal bovine serum (FBS). After two washes with phosphate-buffered saline (PBS), cells were incubated for 10 min with a 1 ml suspension of RBCs (0.01% hematocrit). After HA-cells were washed three times with PBS to remove unbound RBC, they had 0–2 bound RBCs per cell. In the measurement of RBC binding to cells, several areas from each dish were selected. We screened at least 200 cells to find the average number of RBCs bound to each HA-cell.
Fusion inactivation assay
We evaluated changes in the numbers of activation and fusion-competent HA at the surfaces of the cells by using a fusion assay. To trigger fusion, HA-cells with bound RBCs were incubated in PBS titrated by citrate to a low pH. We ended the low-pH pulse by replacing the acidic solution with PBS. The final extent of lipid and content mixing was quantified by fluorescence microscopy 20 min after the low-pH pulse as the ratio of dye-redistributed bound RBCs to the total number of bound RBCs (Chernomordik et al., 1998).
In the absence of target membrane, low-pH-activated refolding of HA triggers an irreversible loss of fusogenic activity (Puri et al., 1990; Korte et al., 1997, 1999; Markovic et al., 2001). The more HAs undergoing this cycle of activation and inactivation, the lower the extent of fusion after a second low-pH pulse applied in the presence of RBCs (the FTP). In our experiments, we varied the time interval between the AP and the addition of RBCs. After a 15 min incubation of HA-cells with RBCs, unbound RBCs were washed out, and cells with bound RBCs were treated with an FTP.
In some experiments, HA-cells were treated with thermolysin (0.05 mg/ml for 5 min at 22°C). Washing cells twice with complete medium terminated the reaction.
Measuring HA activation/inactivation by SDS–PAGE and western blotting
HA activation was assayed exactly as in Markovic et al. (2001) by reduction of the HA1–HA2 S–S bond accessible only in low-pH HA conformations. In brief, after a low-pH pulse, HA-cells were incubated with 20 mM DTT (20 min at 27°C) to release HA1 from the membrane-anchored HA2 of the low-pH HA form. Release of HA1 was detected by quantitative western blotting.
Immunochemical characterization of HA conformations
We also characterized the conformation of HA by accessibility of different epitopes of X31 HA. HA-cells were treated with low pH as specified. Then, changes in the binding of different antibodies were assayed by either CELISA or immunoprecipitation.
CELISA was performed as in Leikina and Chernomordik (2000). For immunoprecipitation, cells treated with trypsin and neuraminidase were incubated at low pH at 37°C for the specified time. After reneutralization, cells were washed at 22°C with Ca- and Mg-free PBS supplemented with 5% FBS. Primary antibodies were applied for 60 min at 22°C immediately after the wash or after incubation at neutral pH for a specified time. After four washes with Ca- and Mg-free PBS, the cells were lysed at 22°C with buffer A (150 mM NaCl, 0.1 mM EDTA, 50 mM Tris pH 7.4) supplemented with 0.2% SDS, 1% Triton X-100 and protein inhibitor cocktail. After removal of insoluble debris by centrifugation for 10 min at 15 000 g, the lysate was incubated with protein A–Sepharose (Cat. no. 17-0780-01, Amersham, Piscataway, NJ) for 1 h at 22°C. Bound material was pelleted (10 min, 15 000 g) and washed twice with buffer A supplemented with 0.2% SDS and 1% Triton X-100, and once with plain buffer A. Samples were resuspended in SDS protein gel loading solution (Quality Biological Inc., Gaithersburg, MD) with 20 mM DTT. After 5 min of boiling, samples were analyzed with western blotting using antibodies against the C-terminus of HA1.
Immunoprecipitation experiments with virus
To search for reversible conformations of HA expressed on the viral particles, we have chosen the Japan strain of virus, which activates more slowly than the X31 strain (Markovic et al., 2001). Virus (0.2 mg of the total viral protein in buffer A) was acidified to pH 4.9 with citric acid, neutralized with NaOH and then either immediately treated with the anti-FP antiserum (1 h at 4°C) or first incubated for 1 h at 22°C and after that treated with the antiserum. The virus was lysed and processed for immunoprecipitation as described above for HA-cells. Samples were analyzed with western blotting using anti-FP antiserum.
Because the efficiency of HA activation and the extent of fusion varied somewhat from day to day, possibly because of variation in the level of HA expression, we routinely started our experiments by choosing the precise conditions of the low-pH treatment to use. Each experiment presented here was repeated at least three times, and all functional dependencies reported were observed in each experiment. The data in figures correspond either to the same representative experiment or, if shown with error bars, to results averaged from the same set of experiments.
Acknowledgments
Acknowledgements
We are indebted to Drs Michael Kozlov, Gregory Melikyan and Kamran Melikov for very useful discussions, and we thank Drs John J.Skehel, Stephen A.Wharton and Judith White for generous gifts of antibodies HC67, LC89 and H26D08.
References
- Bullough P.A., Hughson,F.M., Skehel,J.J. and Wiley,D.C. (1994) Structure of influenza haemagglutinin at the pH of membrane fusion. Nature, 371, 37–43. [DOI] [PubMed] [Google Scholar]
- Carr C.M. and Kim,P.S. (1993) A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell, 73, 823–832. [DOI] [PubMed] [Google Scholar]
- Chen J., Lee,K.H., Steinhauer,D.A., Stevens,D.J., Skehel,J.J. and Wiley,D.C. (1998) Structure of the hemagglutinin precursor cleavage site, a determinant of influenza pathogenicity and the origin of the labile conformation. Cell, 95, 409–417. [DOI] [PubMed] [Google Scholar]
- Chen J., Skehel,J.J. and Wiley,D.C. (1999) N- and C-terminal residues combine in the fusion-pH influenza hemagglutinin HA(2) subunit to form an N cap that terminates the triple-stranded coiled coil. Proc. Natl Acad. Sci. USA, 96, 8967–8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L.V., Leikina,E., Frolov,V., Bronk,P. and Zimmerberg,J. (1997) An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids. J. Cell Biol., 136, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L.V., Frolov,V.A., Leikina,E., Bronk,P. and Zimmerberg,J. (1998) The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol., 140, 1369–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli T., Pelletier,S.L., Henis,Y.I. and White,J.M. (1996) Membrane fusion mediated by the influenza virus hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J. Cell Biol., 133, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R.S., Douglas,A.R., Skehel,J.J. and Wiley,D.C. (1983) Analyses of the antigenicity of influenza haemagglutinin at the pH optimum for virus-mediated membrane fusion. J. Gen. Virol., 64, 1657–1662. [DOI] [PubMed] [Google Scholar]
- Doranz B.J., Baik,S.S. and Doms,R.W. (1999) Use of a gp120 binding assay to dissect the requirements and kinetics of human immunodeficiency virus fusion events. J. Virol., 73, 10346–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S.J., Sambrook,J., Helenius,A. and White,J. (1985) An efficient method for introducing macromolecules into living cells. J. Cell Biol., 101, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand R.F., Macosko,J.C., Russell,C.J., Shin,Y.K. and Epand,R.M. (1999) The ectodomain of HA2 of influenza virus promotes rapid pH dependent membrane fusion. J. Mol. Biol., 286, 489–503. [DOI] [PubMed] [Google Scholar]
- Gaudin Y., Ruigrok,R.W.H. and Brunner,J. (1995) Low-pH induced conformational changes in viral fusion proteins: implications for the fusion mechanism. J. Gen. Virol., 76, 1541–1556. [DOI] [PubMed] [Google Scholar]
- Gaudin Y., Tuffereau,C., Durrer,P., Brunner,J., Flamand,A. and Ruigrok,R. (1999) Rabies virus-induced membrane fusion. Mol. Membr. Biol., 16, 21–31. [DOI] [PubMed] [Google Scholar]
- Godley L. et al. (1992) Introduction of intersubunit disulfide bonds in the membrane-distal region of the influenza hemagglutinin abolishes membrane fusion activity. Cell, 68, 635–645. [DOI] [PubMed] [Google Scholar]
- Gray C. and Tamm,L.K. (1997) Structural studies on membrane-embedded influenza hemagglutinin and its fragments. Protein Sci., 6, 1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz F.X. and Allison,S.L. (2001) The machinery for flavivirus fusion with host cell membranes. Curr. Opin. Microbiol., 4, 450–455. [DOI] [PubMed] [Google Scholar]
- Kemble G.W., Henis,Y.I. and White,J.M. (1993) GPI- and transmembrane-anchored influenza hemagglutinin differ in structure and receptor binding activity. J. Cell Biol., 122, 1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Macosko,J.C. and Shin,Y.K. (1998) The mechanism for low-pH-induced clustering of phospholipid vesicles carrying the HA2 ectodomain of influenza hemagglutinin. Biochemistry, 37, 137–144. [DOI] [PubMed] [Google Scholar]
- Kliger Y., Peisajovich,S.G., Blumenthal,R. and Shai,Y. (2000) Membrane-induced conformational change during the activation of HIV-1 gp41. J. Mol. Biol., 301, 905–914. [DOI] [PubMed] [Google Scholar]
- Korte T., Ludwig,K., Krumbiegel,M., Zirwer,D., Damaschun,G. and Herrmann,A. (1997) Transient changes of the conformation of hemagglutinin of influenza virus at low pH detected by time-resolved circular dichroism spectroscopy. J. Biol. Chem., 272, 9764–9770. [DOI] [PubMed] [Google Scholar]
- Korte T., Ludwig,K., Booy,F.P., Blumenthal,R. and Herrmann,A. (1999) Conformational intermediates and fusion activity of influenza virus hemagglutinin. J. Virol., 73, 4567–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M.M. and Chernomordik,L.V. (1998) A mechanism of protein-mediated fusion: coupling between refolding of the influenza hemagglutinin and lipid rearrangements. Biophys. J., 75, 1384–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M.M. and Chernomordik,L.V. (2002) The protein coat in membrane fusion: lessons from fission. Traffic, 3, 256–267. [DOI] [PubMed] [Google Scholar]
- Krumbiegel M., Herrmann,A. and Blumenthal,R. (1994) Kinetics of the low pH-induced conformational changes and fusogenic activity of influenza hemagglutinin. Biophys. J., 67, 2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E. and Chernomordik,L.V. (2000) Reversible merger of membranes at the early stage of influenza hemagglutinin-mediated fusion. Mol. Biol. Cell, 11, 2359–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E., LeDuc,D.L., Macosko,J.C., Epand,R., Shin,Y.K. and Chernomordik,L.V. (2001) The 1–127 HA2 construct of influenza virus hemagglutinin induces cell–cell hemifusion. Biochemistry, 40, 8378–8386. [DOI] [PubMed] [Google Scholar]
- Markosyan R.M., Melikyan,G.B. and Cohen,F.S. (2001) Evolution of intermediates of influenza virus hemagglutinin-mediated fusion revealed by kinetic measurements of pore formation. Biophys. J., 80, 812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic I., Leikina,E., Zhukovsky,M., Zimmerberg,J. and Chernomordik,L.V. (2001) Synchronized activation and refolding of influenza hemagglutinin in multimeric fusion machines. J. Cell Biol., 155, 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan G.B., Markosyan,R.M., Roth,M.G. and Cohen,F.S. (2000) A point mutation in the transmembrane domain of the hemagglutinin of influenza virus stabilizes a hemifusion intermediate that can transit to fusion. Mol. Biol. Cell, 11, 3765–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A. and Bentz,J. (2001) Comprehensive kinetic analysis of influenza hemagglutinin-mediated membrane fusion: role of sialate binding. Biophys. J., 81, 1521–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri A., Booy,F.P., Doms,R.W., White,J.M. and Blumenthal,R. (1990) Conformational changes and fusion activity of influenza virus hemagglutinin of the H2 and H3 subtypes: effects of acid pretreatment. J. Virol., 64, 3824–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos J., Nir,S., Duzgunes,N., de Carvalho,A.P. and de Lima,M.d.C. (1993) A common mechanism for influenza virus fusion activity and inactivation. Biochemistry, 32, 2771–2779. [DOI] [PubMed] [Google Scholar]
- Roth M.G., Doyle,C., Sambrook,J. and Gething,M.J. (1986) Heterologous transmembrane and cytoplasmic domains direct functional chimeric influenza virus hemagglutinins into the endocytic pathway. J. Cell Biol., 102, 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok R.W., Aitken,A., Calder,L.J., Martin,S.R., Skehel,J.J., Wharton,S.A., Weis,W. and Wiley,D.C. (1988) Studies on the structure of the influenza virus haemagglutinin at the pH of membrane fusion. J. Gen. Virol., 69, 2785–2795. [DOI] [PubMed] [Google Scholar]
- Sato S.B., Kawasaki,K. and Ohnishi,S. (1983) Hemolytic activity of influenza virus hemagglutinin glycoproteins activated in mildly acidic environments. Proc. Natl Acad. Sci. USA, 80, 3153–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J.J. and Wiley,D.C. (2000) Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem., 69, 531–569. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Booy,F.P. and Wilschut,J. (1987) Effects of low pH on influenza virus. Activation and inactivation of the membrane fusion capacity of the hemagglutinin. J. Biol. Chem., 262, 17744–17749. [PubMed] [Google Scholar]
- Stegmann T., White,J.M. and Helenius,A. (1990) Intermediates in influenza induced membrane fusion. EMBO J., 9, 4231–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T., Bartoldus,I. and Zumbrunn,J. (1995) Influenza hemagglutinin-mediated membrane fusion: influence of receptor binding on the lag phase preceding fusion. Biochemistry, 34, 1825–1832. [DOI] [PubMed] [Google Scholar]
- Steinhauer D.A., Martin,J., Lin,Y.P., Wharton,S.A., Oldstone,M.B., Skehel,J.J. and Wiley,D.C. (1996) Studies using double mutants of the conformational transitions in influenza hemagglutinin required for its membrane fusion activity. Proc. Natl Acad. Sci. USA, 93, 12873–12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian S.A. and Tamm,L.K. (1996) Reversible pH-dependent conformational change of reconstituted influenza hemagglutinin. J. Mol. Biol., 260, 312–316. [DOI] [PubMed] [Google Scholar]
- Tatulian S.A., Hinterdorfer,P., Baber,G. and Tamm,L.K. (1995) Influenza hemagglutinin assumes a tilted conformation during membrane fusion as determined by attenuated total reflection FTIR spectroscopy. EMBO J., 14, 5514–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H.P., Armstrong,S.J. and Dimmock,N.J. (1987) Quantitative relationships between an influenza virus and neutralizing antibody. Virology, 159, 288–298. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W., Dessen,A., Harrison,S.C., Skehel,J.J. and Wiley,D.C. (1997) Atomic structure of the ectodomain from HIV-1 gp41. Nature, 387, 426–430. [DOI] [PubMed] [Google Scholar]
- Wharton S.A., Calder,L.J., Ruigrok,R.W., Skehel,J.J., Steinhauer,D.A. and Wiley,D.C. (1995) Electron microscopy of antibody complexes of influenza virus haemagglutinin in the fusion pH conformation. EMBO J., 14, 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. (1995) Membrane fusion: the influenza paradigm. Cold Spring Harb. Symp. Quant. Biol., 60, 581–588. [DOI] [PubMed] [Google Scholar]
- White J.M. and Wilson,I.A. (1987) Anti-peptide antibodies detect steps in a protein conformational change: low-pH activation of the influenza virus hemagglutinin. J. Cell Biol., 105, 2887–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D.C. and Skehel,J.J. (1987) The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem., 56, 365–394. [DOI] [PubMed] [Google Scholar]
- Wilson I.A. (1984) The structure of an antigenic determinant in a protein. Cell, 37, 767–778. [DOI] [PubMed] [Google Scholar]