Abstract
Using protein sequence data obtained from a calcium- and phospholipid-regulated protein kinase purified from maize (Zea mays), we isolated a cDNA encoding a calcium-dependent protein kinase (CDPK), which we designated ZmCPK11. The deduced amino acid sequence of ZmCPK11 includes the sequences of all the peptides obtained from the native protein. The ZmCPK11 sequence contains the kinase, autoregulatory, and calmodulin-like domains typical of CDPKs. Transcripts for ZmCPK11 were present in every tested organ of the plant, relatively high in seeds and seedlings and lower in stems, roots, and leaves. In leaves, kinase activity and ZmCPK11 mRNA accumulation were stimulated by wounding. The level of ZmCPK11 is also increased in noninjured neighboring leaves. The results suggest that the maize protein kinase is involved in a systemic response to wounding. Bacterially expressed glutathione S-transferase (GST)-ZmCPK11 was catalytically active in a calcium-dependent manner. Like the native enzyme, GST-ZmCPK11 was able to phosphorylate histone III-S and Syntide 2. Phosphorylation of histone was stimulated by phosphatidylserine, phosphatidylinositol, and phosphatidic acid, whereas phosphatidylcholine, lysophosphatidylcholine, phosphatidylethanolamine, diolein, and cardiolipin did not increase the enzymatic activity. Autophosphorylation of GST-ZmCPK11 was stimulated by calcium and by phosphatidic acid and, to a lesser extent, by phosphatidylserine. Phosphatidylcholine did not affect autophosphorylation. These data unequivocally identify the maize phospholipid- and calcium-regulated protein kinase, which has protein kinase C-like activity, as a CDPK, and emphasize the potential that other CDPKs are regulated by phospholipids in addition to calcium.
Calcium is an important secondary messenger in signaling pathways that respond to hormonal and environmental stimuli (Evans et al., 2001; Sanders et al., 2002). An external signal that increases the cytoplasmic calcium concentration can initiate many cellular regulatory processes. The molecular decoders of calcium signals are the calcium-binding proteins, which include protein kinases regulated by calcium. One class of these kinases in plants is the calcium-dependent protein kinases (CDPKs), also known as the calmodulin-like domain protein kinases (CPKs). The CDPKs are widespread in plants, present in parasites (Plasmodium falciparum), but absent in yeast (Saccharomyces cerevisiae), nematodes (Caenorhabditis elegans), insects (Drosophila melanogaster), and mammals (Homo sapiens; Harmon et al., 2000; Harper et al., 2004).
CDPK protein sequences include five domains: N-terminal variable domain, catalytic domain, junction domain (JD), calmodulin-like domain (CLD), and C-terminal domain. The catalytic domain of CDPKs is characteristic of Ser/Thr protein kinases. The JD is an autoinhibitory domain, which is a basic region composed of 31 amino acids that functions as a pseudosubstrate. The CLDs of typical CDPKs are composed of four EF-hand calcium-binding motifs and share 30% to 40% amino acid sequence identity with plant and animal calmodulins. Binding of calcium ions to the CLD causes conformational changes in the protein, leading to removal of the pseudosubstrate sequence from the active site and activation of the enzyme. The N- and C-terminal domains are variable, differing in their length and amino acid composition among various CDPK isoenzymes. It has been suggested that these variable domains determine the specific functions of individual CDPKs (Harmon et al., 1994; Harper et al., 1994).
Activity of some CDPKs can be stimulated by lipids. Activities of a CDPK bound to the cell membrane of oat (Avena sativa) and of AtCPK1 (formerly AK1) from Arabidopsis (Arabidopsis thaliana) are stimulated by phosphatidylinositol (PI), lysophosphatidylcholine (LysoPC), and a crude lipid fraction (Schaller et al., 1992; Harper et al., 1993). A CDPK purified from maize (Zea mays) seedlings is stimulated by PI and phosphatidylserine (PS; Szczegielniak et al., 2000), and a recombinant CDPK from Daucus carota (DcCPK1) is stimulated by PS and phosphatidic acid (PA; Farmer and Choi, 1999). The stimulation of CDPK activity by membrane lipids suggests that membrane association is a factor that contributes to CDPK activity. Phospholipids that are involved in plant cell signaling pathways are rapidly produced in response to stress. Subsequently, the newly synthesized lipids can activate enzymes by recruiting them to membrane sites or by increasing the local enzyme concentrations such that regulatory interactions are promoted (for review, see Meijer and Munnik, 2003). N-myristoylation and -palmitoylation of defined amino acid residues at the N terminus of some CDPKs are necessary for targeting to the membrane and promote protein-membrane association (Ellard-Ivey et al., 1999; Martin and Busconi, 2000). Of the 34 isoforms of CDPK in Arabidopsis, 27 are predicted to have N-myristoylation motifs (for review, see Hrabak et al., 2003), and membrane localization for many CDPKs has been demonstrated (Martin and Busconi, 2000; Romeis et al., 2001; Lu and Hrabak, 2002; Dammann et al., 2003).
In addition to a role in regulation of the activity of metabolic enzymes (for review, see Cheng et al., 2002; Harper et al., 2004), CDPKs could play essential roles in both abiotic and biotic stress signal transduction pathways (for review, see Ludwig et al., 2004). In different plant species, abiotic stresses activate transcription of various CDPKs (Monroy and Dhindsa, 1995; Yoon et al., 1999; Patharkar and Cushman, 2000; Saijo et al., 2000; Chico et al., 2002). Cold, salinity, and drought stresses cause an increase in the transcripts encoding AtCDPK10 and AtCDPK11 of Arabidopsis (Urao et al., 1994). Expression of genes encoding CDPKs is induced in Vigna radiata after mechanical stress (Botella et al., 1996) and in the moss Funaria hygrometrica during nutrient deficiency of nitrogen, sulfur, or phosphate (Mitra and Johri, 2000). Apart from an increase in the level of transcripts, various stresses have been found to directly activate CDPKs. For example, in rice (Oryza sativa), low temperatures activate a CDPK bound to membranes. This kinase is activated 10 to 20 h after the start of cold treatment, which suggests a role for this enzyme in adaptation to low temperatures (Martin and Busconi, 2001). In transgenic rice, overexpression of another CDPK, OsCDPK7, increases the tolerance of cold, salinity, and drought (Saijo et al., 2000, 2001). Using a transient expression assay in maize leaf protoplasts, Sheen demonstrated that CDPKs are involved in plant responses to abiotic stresses and abscisic acid (ABA) treatment. It was shown that two isoforms of CDPK, AtCDPK1 (also known as AtCPK10) and AtCDPK1a (also known as AtCPK30), are involved in responses to ABA, cold, darkness, and salinity signals (Sheen, 1996).
CDPKs also participate in signaling during the early stages of pathogen recognition leading to activation of plant defense mechanisms. Pathogen response pathways are often activated by interaction between a pathogen-encoded elicitor (such as the Cladosporium fulvum Avr 9 peptide) and a plant-encoded receptor (such as the tomato [Lycopersicon esculentum] Cf-9 resistance protein). This interaction in Cf-9 transgenic tobacco (Nicotiana tabacum) leads to activation by phosphorylation of CDPK (NtCDPK2), suggesting that CDPKs play an essential role in a plant defense response (Romeis et al., 2000, 2001; Ludwig et al., 2004). Although several potential pathogen-related CDPK targets have been discussed in the literature, including H+-ATPase (Schaller and Oecking, 1999), ion channels, and NADPH oxidase, direct evidence for the involvement of NtCDPK2 in the regulation of these enzymes is lacking.
We identified a 54-kD CDPK in maize seedlings (previously called ZmCPKp54). This kinase is stimulated by PS and PI in addition to calcium ions (Szczegielniak et al., 2000). Stimulation of the activity by calcium and PS, a phospholipid that is essential for the activity of protein kinase C (PKC) in animal cells, suggests that the maize protein kinase is involved in signal transduction in plant cells. Therefore, studies concerning its molecular characterization and biological role were undertaken.
RESULTS
Purification and Microsequencing of a Calcium- and Phospholipid-Activated Protein Kinase
Purification of the protein kinase with a relative molecular mass of 54 kD was based on the previously elaborated procedure (Szczegielniak et al., 2000) with some modifications. This procedure included hydrophobic-interaction chromatography (octyl-Sepharose), ion-exchange chromatography (DEAE-52 and Mono Q), and affinity chromatography on immobilized substrates (Histone H1-Sepharose and myelin basic protein [MBP]-Sepharose; Table I). The protein kinase was purified approximately 800-fold with 1% recovery of enzymatic activity. The specific activity of the purified enzyme was 917 nmol min−1 mg−1. Three-fold stimulation of the enzyme activity by PS was observed. After purification on MBP-Sepharose, the enzyme preparation was separated by preparative SDS-PAGE. Silver staining revealed only one protein band in the range 50 to 60 kD. This protein band was excised from the gel for microsequencing. Amino acid sequences of four internal tryptic peptides (I–IV) were obtained. Peptide I did not exhibit any homology to the sequence of known CDPKs, whereas peptides II to IV were localized in the conserved part of CDPKs (Fig. 1). Peptides II and III shared 100% identity with the sequence in the third and eighth subdomains of the kinase domain of known CDPKs (AtCPK11, accession no. D21806 and maize CDPK, accession no. D87044, respectively). Peptide IV was 77% identical with the sequence from the regulatory, calmodulin-like domain of soybean (Glycine max) CDPKα (SK5; accession no. P28583).
Table I.
Purification of calcium and phospholipid-regulated protein kinase from maize seedlings
The activity was measured in the presence of Ca2+ with or without phospholipids. The total and specific activities for histone III-S phosphorylation were calculated in the presence of Ca2+ and PS, and fold stimulation is relative to activity in the presence of calcium ions alone.
| Step of Purification | Protein | Total Activity | Specific Activity | Stimulation by Lipids | Fold Purification | Recovery |
|---|---|---|---|---|---|---|
| mg | nmol min−1 | nmol min−1 mg−1 | fold | % | ||
| Homogenate | 4,627 | 5,090 | 1 | 1.0 | 1 | 100 |
| Octyl-Sepharose | 60 | 1920 | 32 | 1.2 | 29 | 37 |
| DEAE-52-Cellulose | 36 | 1,440 | 40 | 2.0 | 36 | 28 |
| Histone H1-Sepharose | 3 | 360 | 120 | 3.0 | 109 | 7 |
| Mono Q on FPLC | 0.15 | 80 | 530 | 3.0 | 482 | 1 |
| MBP-Sepharose | 0.06 | 55 | 917 | 3.0 | 834 | 1 |
Figure 1.
The deduced amino acid sequence of ZmCPK11 is aligned with OsCDPK (accession no. AY144497) from rice, SK5 (CDPKα) from soybean (Harper et al., 1991), AtCPK1 from Arabidopsis (Harper et al., 1993), NtCDPK2 from tobacco (Romeis et al., 2001), ZmCPK10 from maize (Murillo et al., 2001), OsCDPK7 from rice (Saijo et al., 2000), and LeCDPK1 from tomato (Chico et al., 2002). Sequences were aligned with the PileUp program from the Genetics Computer Group suite of programs. Numbers indicate amino acid residues in the sequences. Dots indicate amino acids identical to those corresponding to the ZmCPK11 sequence. Shaded backgrounds indicate amino acids identical throughout. Dashes indicate gaps introduced to maximize the alignment. The boundaries of the variable, kinase, autoregulatory, and calmodulin-like domains are shown by arrows. The four Ca2+-binding EF-hand motifs in the calmodulin-like domain are overlined. Peptide (I–IV) data obtained from microsequencing are shown above the deduced amino acid sequence of ZmCPK11.
Cloning and Sequence Analysis of the cDNA Encoding ZmCPK11
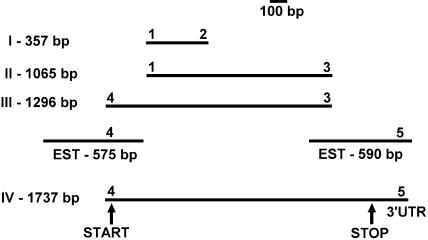
The strategy for cloning is illustrated in Figure 2. In the reverse transcription (RT)-PCR reactions using total RNA from maize seedlings as the template and appropriate primers (1 and 2, or 1 and 3; primer sequences are provided in Table II), products of 357 and 1,065 bp were obtained. The sequences of these two clones are similar to known CDPKs, but also include the sequence of a new CDPK. Using primers 3 and 4 or 4 and 5, DNA fragments of 1,296 and 1,737 bp, respectively, were obtained. Sequence analysis revealed that the cloned DNA was derived from one mRNA species. The 1,737-bp clone encodes a protein of 510 amino acids with a deduced molecular mass of 56.5 kD. Analysis of the nucleotide sequence shows that this clone contains an uninterrupted open reading frame (ORF), which begins at nucleotide position 4 and terminates at position 1,536. In an overlapping expressed sequence tag (EST; accession no. AI770867), there is an in-frame stop codon 30 nucleotides upstream from the ORF, indicating that our clone contains the full-length coding sequence. The clone was designated ZmCPK11 (and assigned accession no. AY301062). The predicted protein includes structural features of CDPKs. It additionally contains a 3′ noncoding sequence. Sequences of all four microsequenced peptides are included in the predicted sequence of ZmCPK11 (Fig. 1). Peptide I comes from the N-terminal, variable domain, whereas the rest of the peptides are from conserved parts of ZmCPK11. The protein sequence of ZmCPK11 shows similarity to other CDPKs, including OsCDPK (AY144497), 91% identity; CDPKα (SK5; Harper et al., 1991) from soybean, 78% identity; ZmCPK10 (Murillo et al., 2001), 71% identity; AtCPK1 (AK1; Harper et al., 1993), 70% identity; NtCDPK2 (Romeis et al., 2001), 69% identity; OsCDPK7 (Saijo et al., 2000), 68% identity; and LeCDPK1 (Chico et al., 2002), 60% identity. Sequence similarity analysis of ZmCPK11 and the 34 Arabidopsis CDPKs using ClustalW shows that ZmCPK11 is most closely related to AtCPKs 4, 11, and 12 (data not shown).
Figure 2.
Schematic presentation of strategy of cloning fragments and full reading frame of ZmCPK11. Primers (1–3) were designed from amino acid sequences of peptides obtained from microsequencing. Primers (4–5) were derived from EST sequences.
Table II.
Nucleotide sequences of the primers used for cloning and expression of ZmCPK11
In primers 6, 7, and 8, the restriction sites are underlined.
| No. | Sequence |
|---|---|
| 1 | 5′-GAGATCCAGATAATGCACCAC-3′ |
| 2 | 5′-AAGTACCTCTGGTGCAACATAATAAGG-3′ |
| 3 | 5′-TTTGTCAAAAAA(AT)GCGAATGCTGAAACCAA-3′ |
| 4 | 5′-CCAATGCAGCCGGACCCGAG-3′ |
| 5 | 5′-AAGCAGTTGGCGATAGTAGCCACATTGT-3′ |
| 6 | 5′-TAACCCGGGATGCAGCCGGACCCGAGC-3′ |
| 7 | 5′-GCCCTGCAGCTAGGTTTTGCTGGGATTCAA-3′ |
| 8 | 5″-GTCGACATGTACCGCATCGGCAAG-3′ |
| 9 | 5′-CTAATCGTCAACAATCCATGCG-3′ |
| 10 | 5′-GATCATATCGTCAACTGGTCCGGC-3′ |
| 11 | 5′-TATCAGCCGATGTGGGGCGTCTGG-3′ |
| 12 | 5′-GGCAGCTCGTAGCTCTTCTC-3′ |
| 13 | 5′-AACAGGGAGAAGATGACCCA-3′ |
In all CDPKs, the kinase catalytic domain, CLD, and JD are highly conserved. The kinase domain of ZmCPK11 comprises 264 amino acid residues and contains all 11 conserved subdomains and invariant amino acid residues of eukaryotic Ser/Thr protein kinases. A CLD composed of 145 amino acid residues contains four putative Ca2+-binding EF hands. Between the kinase and CLD lies the JD consisting of 31 amino acid residues. The variable domains of ZmCPK11 are different from those of known CDPKs in sequence and length. The N-terminal domain of ZmCPK11 contains 43 amino acid residues and, contrary to many known CDPKs (Hrabak et al., 2003), does not contain a myristoylation motif. The C-terminal domain contains 27 amino acid residues.
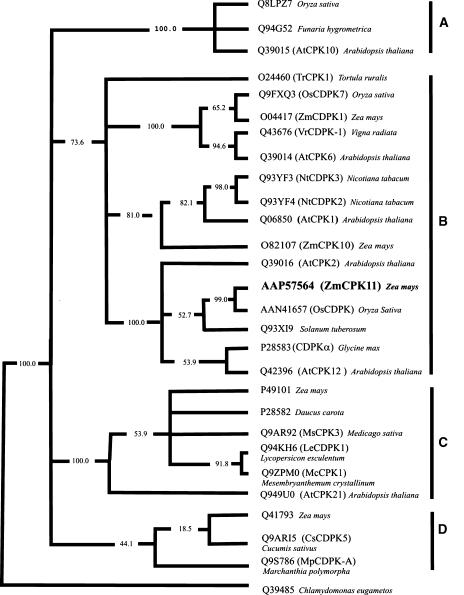
To investigate the evolutionary relationship of ZmCPK11 to other CDPKs that are stimulated by phospholipids, a phylogenetic analysis was performed using the kinase catalytic domains. To simplify the analysis, catalytic domains without deletions and insertions (Supplemental Table I) were used. Figure 3 shows a consensus parsimony tree constructed with a subset of sequences from Supplemental Table I and rooted by designating the algal sequence (Chlamydomonas eugametos) as the outlier. The CDPKs included in the tree are either activated by phospholipids, induced by stress, or expressed in male tissues. In this simplified analysis, AtCPK1 and AtCPK2 are located on separate branches, whereas in the tree for the Arabidopsis CDPK/SnRK superfamily (Hrabak et al., 2003) both of these kinases are on the same branch. Two of the three CDPKs known to be activated by phospholipids AtCPK1 (Harper et al., 1993) and ZmCPK11 are in subgroup B, whereas the third, DcCDPK (Farmer and Choi, 1999), is in subgroup C.
Figure 3.
The consensus maximal parsimony sequence similarity tree of plant CDPKs. The tree was constructed using multiple alignment of representative sets of canonical domains. Because sequences of clones for this analysis do not contain deletions or insertions, the multiple alignment contains no gaps and editing was not required. Numbers indicate the percent of bootstrap replicates.
Characterization of GST-ZmCPK11
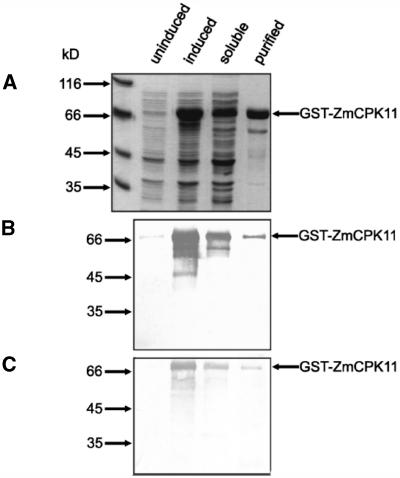
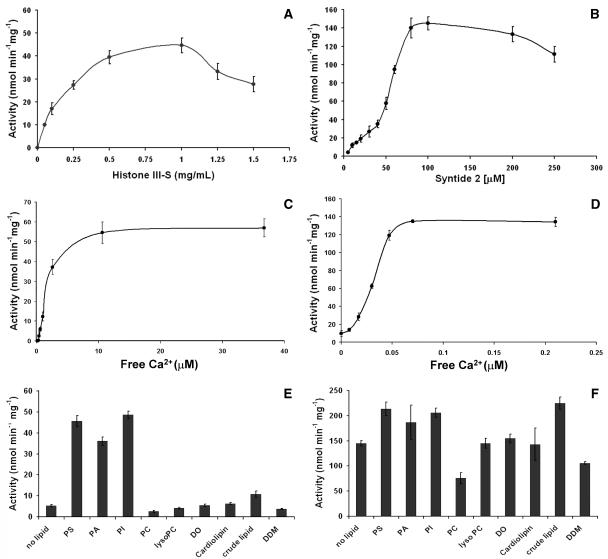
To assess the properties of ZmCPK11, the full-length ORF was expressed in Escherichia coli as a glutathione S-transferase (GST)-fusion protein. The purified recombinant protein migrated at the expected molecular mass in a denaturing electrophoretic gel. The GST-ZmCPK11 protein was the predominant band eluted from the affinity matrix with 10 mm glutathione (Fig. 4A). The identity of GST-ZmCPK11 was confirmed by western blots using antibodies against CLD and GST (Fig. 4, B and C, respectively). The recombinant kinase was able to phosphorylate both histone III-S and Syntide 2 (Fig. 5, A and B). Phosphorylation of histone III-S was maximal in the range 0.5 to 1.0 mg mL−1, whereas higher concentrations were inhibitory. The rate of Syntide 2 phosphorylation increased proportionally up to 100 μm. The Vmax for histone III-S phosphorylation was 57 nmol min−1 mg−1, which is about 3 times lower than that for Syntide 2 phosphorylation. The activity of recombinant ZmCPK11 was tested in the presence of <10 pm to 36 μm free Ca2+. The calcium requirement depended upon the nature of the substrate. Histone III-S was not phosphorylated in the presence of approximately 0.5 μm free Ca2+. At approximately 1.6 μm free Ca2+, 50% of the full activity was observed (Fig. 5C). In contrast, Syntide 2 phosphorylation was not completely inhibited by EGTA; about 10% of full activity was observed in the presence of EGTA without the addition of calcium. Half-maximal activity was observed at 30 nm of free calcium (Fig. 5D).
Figure 4.
Purification and characterization of recombinant ZmCPK11. The full-length ZmCPK11 reading frame was expressed in E. coli as a GST-fusion protein. Purification was monitored by SDS-PAGE followed by Coomassie Brilliant Blue staining (A), and western-blot analysis with antibodies against the CLD of CDPK (B) or GST (C).
Figure 5.
Biochemical properties of GST-ZmCPK11. Effect of substrates (A and B), calcium (C and D), and phospholipids (E and F) on activity of ZmCPK11. Activity was assayed at a constant 110 μm (A, B, E, and F), or different (0–170 μm; C and D) concentrations of (total) Ca2+ and constant level (100 μm) of EGTA (A–F). Calculated values of free Ca2+ are indicated in C and D. Concentration of lipids in milligrams per milliliter was PS (0.12), PA (0.25), PI (0.75), PC (0.5), LysoPC (0.5), CL (0.1), DO (0.005), and crude lipid (2.0). Concentration of DDM was 0.1 mm.
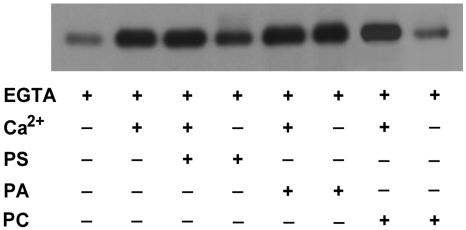
Apart from calcium, the influence of phospholipids at the concentration 0.1 to 2.0 mg mL−1 on activity of recombinant ZmCPK11 was tested. The concentrations of phospholipids that exhibit the strongest effect on enzyme activity are shown in Figure 5E. Phosphorylation of histone III-S by GST-ZmCPK11 was stimulated 6- to 9-fold by PS, PA, and PI, and about 2-fold by a crude lipid from maize. Histone phosphorylation was stimulated by PA alone to the same level as stimulation by Ca2+, whereas stimulation of activity by PS was totally calcium dependent (data not shown). The stimulation by PS and PI was comparable with activity of the native enzyme purified from maize seedlings (Szczegielniak et al., 2000). The other tested lipids—lysophosphatidylcholine (LysoPC), cardiolipin (CL), diolein (DO), phosphatidylethanolamine (PE; data not shown), and the detergent n-dodecyl-β-d-maltoside (DDM; at a concentration in the range of 0.1–2.0 mm)—did not affect the activity of the enzyme, whereas phosphatidylcholine (PC) had an inhibitory effect. The cationic headgroup of PC might act as the negative effector of the phosphorylation of the histone by ZmCPK11. When Syntide 2 was used as the substrate, the stimulation of activity by lipids was small (up to about 50%; Fig. 5F).
The autophosphorylation of ZmCPK11 was stimulated effectively by Ca2+ and PA. Stimulation of autophosphorylation by PS was increased upon addition of calcium, whereas Ca2+ did not affect the stimulation by PA. By itself, PA stimulated autophosphorylation of ZmCPK11 to the same extent as Ca2+ in the presence of PS. Autophosphorylation of ZmCPK11 was not affected by PC either in the presence or absence of calcium (Fig. 6).
Figure 6.
Effect of Ca2+, PS, PA, and PC on the autophosphorylation of ZmCPK11. Autophosphorylation was estimated in the presence of 0.1 mm EGTA and 0.11 mm Ca2+, 0.12 mg mL−1 PS or 0.25 mg mL−1 PA, or 0.50 mg mL−1 PC.
Expression Patterns of ZmCPK11 in Different Organs
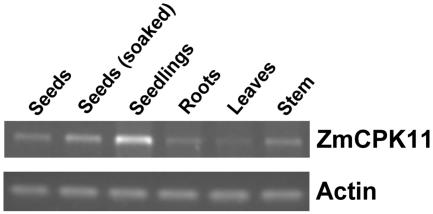
Expression of ZmCPK11 in different organs was analyzed by RT-PCR with gene-specific primers from the coding region of the protein kinase (primers 3 and 4). The ZmCPK11 transcript (1,296 bp) was expressed in each of the tested organs with the highest expression being in seedlings (Fig. 7). The transcript level increased after 24 h of imbibition of seeds.
Figure 7.
Expression of the ZmCPK11 transcript in different organs of maize: dry seeds, seeds after 24 h of soaking, 72-h seedlings, primary roots, 14-d-old leaves, and 2-month-old stem. The transcript level of ZmCPK11 was determined by RT-PCR using specific primers (3 and 4), and total RNA as the template. The data represent three independent experiments showing similar results. Controls were based on amplification of the constitutively expressed actin gene.
Effect of Wounding on Expression of ZmCPK11
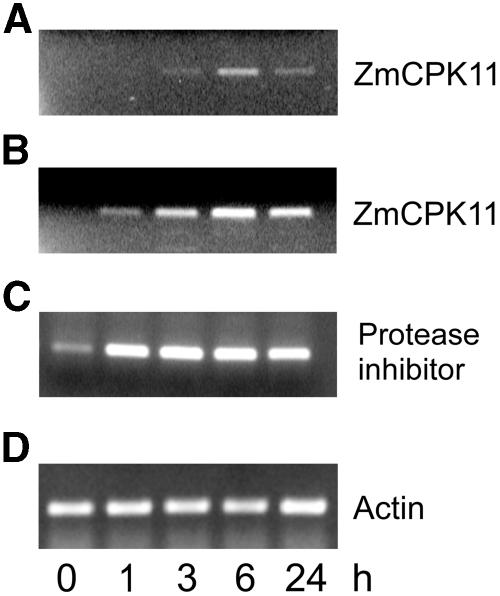
The effect of various types of abiotic stresses (high and low temperature, salinity, H2O2, drought, wounding) and ABA on expression was examined by RT-PCR. Extreme temperatures (4°C and 40°C), 500 μm H2O2, 300 mm NaCl, desiccation, and 100 μm ABA had no significant effect on transcript levels (data not shown). Only wounding of leaves induced accumulation of the ZmCPK11 transcript. Two sets of ZmCPK11 primers were used. The levels of transcripts encoding the 1,296-bp fragment (Fig. 8A), as well as the catalytic domain of ZmCPK11 (792 bp), increased gradually to the maximal level at 6 h and declined 24 h after wounding. The amount of ZmCPK11 transcript amplified with the two primers varied, most probably reflecting the dependence of the rate of amplification upon the length of synthesized fragments. The wound-inducible maize proteinase inhibitor (MPI) gene was used as a positive control (Cordero et al., 1994). The MPI transcript increased earlier than that of ZmCPK11, reaching a maximum after 1 h, and remained at the same level for 24 h after wounding (Fig. 8C). The level of constitutively expressed actin remained constant (Fig. 8D).
Figure 8.
Induction of the ZmCPK11 transcript by wounding. Total RNA from 2-week-old leaves was isolated at time zero (0 min) and at various times after wounding. A, ZmCPK11 (primers 3 and 4). B, Catalytic domain of ZmCPK11 (primers 8 and 9). C, For amplification of the MPI, primers were constructed based on the sequence published by Cordero et al. (1994), primer 10 from the upstream untranslated sequence and primer 11 from the translated C-terminal fragment of the protein. D, For amplification of actin, primers 12 and 13 were from conserved regions of the maize actin gene (Chang et al., 1999). The data represent six independent experiments showing similar results.
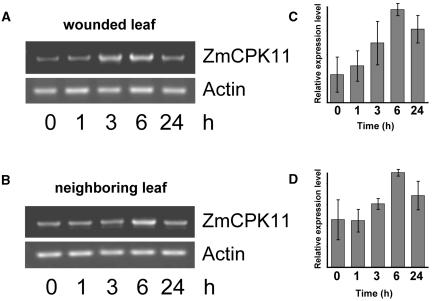
Transcript levels of ZmCPK11 were determined separately in wounded and nonwounded leaves at 0, 1, 3, 6, and 24 h (Fig. 9, A and B, respectively). Similar to the results in Figure 8, the amount of the transcript in the wounded leaf increased after 3 h, reaching a maximal level 6 h after wounding. In the noninjured neighboring leaf, no change in transcript level occurred at 3 h, but significantly increased 6 h after wounding and declined 18 h later (Fig. 9B). It should be noted that the basal level of expression of ZmCPK11 in leaves varied to some extent, probably due to handling of the plants during the experiment. The amount of ZmCPK11 transcript increased after touching of leaves to a level lower than that caused by wounding, but with a similar time course (data not shown). Experiments in which the relative expression levels of ZmCPK11 transcript in wounded (Fig. 9C) and neighboring (Fig. 9D) leaves were determined showed that, in both cases, a maximal level of expression was observed 6 h after wounding.
Figure 9.
Time-course induction of a ZmCPK11 transcript in response to wounding. Total RNA was extracted from a 2-week-old wounded leaf (A) and a neighboring leaf (B). Primers 3 and 4 and 12 and 13 were used for amplification of ZmCPK11 and actin, respectively. Relative expression levels of ZmCPK11 (average from three independent experiments) in a wounded leaf (C) and a neighboring leaf (D) are plotted on the right.
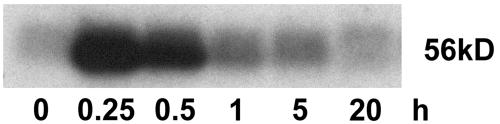
Wounding of leaves affected the enzymatic activity of CDPK of Mr 56,000. Kinase activity increased 15 min after cutting the leaves (Fig. 10) and slowly declined. Twenty hours after wounding, kinase activity had returned to the basal level and was nearly undetectable. In-gel kinase assays performed in the presence of EGTA demonstrated that the kinase activity is Ca2+ regulated (data not shown). Only one protein band in the range of Mr 54,000 to 57,000 was detected by reaction with the antibody against the CLD domain of soybean CDPK (data not shown). The observations that the deduced molecular mass of ZmCPK11 is 56.5 kD and that the expression of ZmCPK11 is regulated in response to wounding suggests that ZmCPK11 was responsible for the changes in CDPK enzymatic activity.
Figure 10.
Effect of wounding on protein kinase activity. Two-week-old leaves of maize were subjected to wounding, and extracts were prepared at the indicated time points. Protein kinase activity was monitored in leaf extracts by the in-gel kinase assay.
DISCUSSION
Using amino acid sequence data from a CDPK purified from maize seedlings, which was activated by phospholipids (Szczegielniak et al., 2000), we isolated the corresponding cDNA clone and named it ZmCPK11. The sequence of the deduced N-terminal domain of ZmCPK11 was found in translated sequences of ESTs of two monocots, sorghum (BI074799) and barley (BG368286), and also in the OsCDPK cDNA (AY144497). These monocots are of closest proximity to maize in the phylogenetic tree constructed by Volker Brendel Research Group (Plant Genome Database [http://www.plantgdb.org]). Similar N-terminal sequences were not found in any CDPKs present in dicot species. These observations suggest that similarities at the N-terminal domains of some CDPKs might be limited to the narrow groups of closely related plants. The CDPK amino acid sequences show greater conservation between orthologs in different plants than among the isologs present within a given plant species (Frattini et al., 1999; Harmon et al., 2000; Davletova et al., 2001; Cheng et al., 2002). This suggests conserved functions in different species and indicates that the evolution of new CDPK isoforms predated the divergence of monocots and eudicots (Soltis and Soltis, 2003). Phylogenetic analysis has shown that, except for subgroup C, there are representatives of CDPKs from angiosperms and seedless plants in each subgroup, which suggests that common ancestors of plant CDPKs predate even the origin of the vascular system of seed plants (Spermatophyta).
A number of known CDPKs are involved in stress signaling in plants (see introduction). One of the most important abiotic stresses is wounding, which activates signal transduction pathways directed to healing and further defense. These signaling pathways mostly include reversible protein phosphorylation, elevation of the intracellular level of calcium, and transcriptional activation of specific genes (Leon et al., 2001). Most of the induced responses occur within a few minutes to several hours after wounding. The events that occur within the first minutes after wounding, comprising the production and perception of primary signals via regulation of ion channels at the plasma membrane, are activated by reversible protein phosphorylation. In many cases, wounding represents the first stage of pathogen infection (Cheong et al., 2002). It has been documented that responses activated by wounding or mechanical injury, and by gene-for-gene plant pathogen interactions, are interlinked at the level of mitogen-activated protein kinases (MAPKs; Seo et al., 1995; Zhang and Klessig, 1997; Romeis et al., 1999). It seems that integration at the level of MAPKs of wound- and pathogen-activated responses is widely functional in plants (Leon et al., 2001).
Since wounding, but not other stresses, induced expression of ZmCPK11 in maize leaves, this suggests that ZmCPK11 is specifically involved in the wound-signaling pathway. Similar to our results with ZmCPK11, the expression of tomato CDPK (LeCDPK1) was enhanced in a rapid and transient manner in wounded leaves, peaking within 4 h of the damage; the mRNA remained high for 4 h longer and then declined (Chico et al., 2002). According to the classification of Moura et al. (2001), LeCDPK1 and ZmCPK11 are members of the late wound-inducible genes whose mRNAs increase 4 to 12 h after wounding, in contrast to several early wound-inducible genes that are transiently induced within 30 min (Heitz et al., 1997). The pathogen (Fusarium moniliforme) infection induces rapid accumulation of the transcript of another maize CDPK, ZmCPK10. The activation of ZmCPK10 is extremely rapid; transcripts could be detected 5 min after elicitation and reached the maximal level at 30 min after treatment (Murillo et al., 2001).
Plant defense responses occur not only in the attacked organ, but also in systemic organs of model dicot plants. There are indications that elicitors are involved in the initial steps of LeCDPK1 induction in wounded and distal leaves. Moreover, correlation between the time of induction of LeCDPK1 mRNA distant from the site of injury suggests a direct delivery of presynthesized LeCDPK1 mRNA via phloem (Chico et al., 2002). Phloem has recently been considered as a system for long-distance signal traffic used by plants to integrate whole-plant physiological processes. The existence of systemic responses in monocot plants, especially cereals, is still a matter of debate. Schweizer et al. (1998) demonstrated that wound-induced resistance in rice was preceded by both local and systemic accumulation of nonconjugated jasmonates. Among the RNA molecules detected in rice phloem sap, a sequence identical to a known rice CDPK was found (Hanaoka et al., 1999). These data allow speculation on the systemic traffic in monocots of mRNA upon wounding. Our results indicate that wounding also induces both local and systemic responses in maize, as demonstrated by the accumulation of the ZmCPK11 transcript in both wounded and neighboring leaves.
Present data suggest that ZmCPK11 may not only play roles in wound response. The ZmCPK11 transcript was detected in all tested organs: leaves, roots, stem, seeds, and seedlings. The transcript in seeds increased after imbibition, reaching the highest level in seedlings, which are in an intensive elongation phase; this suggests a role for this kinase in postgerminative growth. Two rice CDPK isoforms have different functions in seedling development (Frattini et al., 1999). Two other isoforms, specifically expressed in a temporal manner during seed development, are probably involved in the synthesis of storage materials (Kawasaki et al., 1993; Asano et al., 2002). Thus, CDPKs are involved in development and response to stresses.
Apart from de novo synthesis, preexisting CDPK could be activated upon wounding. Present results indicate that activity of a 56-kD CDPK increases within minutes after the wounding and later slowly returns to the basal level. Rapid and transient activation of this CDPK indicates its involvement in the early stages of stress signal transduction. Also, a tomato membrane-bound CDPK is activated 20 min after wounding (Chico et al., 2002). Faster activation of CDPKs has been observed in response to pathogen attack. After 7.5 min of elicitor treatment in transgenic tobacco cells containing the resistance gene CF-9, NtCDPK2 showed transient interconversion to an activated form. This transition was due to phosphorylation of the CDPK. The in vivo phosphorylation and interconversion of NtCDPK2 from the nonelicited into the elicited form was accompanied by a sustained 10- to 200-fold increase in enzymatic activity (Romeis et al., 2001). The results of Romeis and coworkers (2000, 2001) indicate that transient activation of CDPK after the stress is due to phosphorylation.
In addition to phosphorylation, CDPKs can be regulated by phospholipids. In the wound-signaling process, membrane lipids could provide secondary messengers affecting protein kinase activity. Many plant species demonstrate a systemic increase in PA levels after wounding (Lee et al., 2001). In castor leaves, wounding triggered a rapid hydrolysis of PC to PA and choline, which was mediated by phospholipase D (PLD). It has been proposed that wounding increases the concentration of cytoplasmic calcium, which promotes translocation within a few minutes of PLD to bind membranes (Ryu and Wang, 1996). Then PLD influences transmission of signal(s) by regulating protein phosphorylation. Inhibition of PLD by n-butanol suppressed the wound-induced activation of MAPK in suspension-cultured soybean cells, indicating that an elevation of PA levels is essential for its activation. However, MAPK is not activated by PA directly, but via upstream protein kinase(s) (Lee et al., 2001). In this regard, it was interesting that ZmCPK11 is activated by phospholipids (Szczegielniak et al., 2000), including PA (these results). This observation suggests that ZmCPK11 is involved in rapid wound-induced signaling mediated by PA, which is becoming accepted as a plant signaling molecule (Munnik, 2001; Meijer and Munnik, 2003).
For biochemical characterization of ZmCPK11, the kinase was expressed in E. coli as a GST-fusion that was soluble and could be purified by affinity chromatography on glutathione-agarose beads. The purified protein displays Ca2+-dependent phosphorylation of histone III-S and Syntide 2. The effect of calcium on histone III-S phosphorylation by recombinant ZmCPK11 is similar to that of the native enzyme (Szczegielniak et al., 2000). It is worthy of mention that the shape of the curves for calcium dependence suggests that ZmCPK11 might have two binding sites for calcium. It was previously demonstrated that recombinant CDPK from tomato (GST-LeCPK1) has two classes (high and low affinity) of Ca2+-binding sites, with dissociation constants of 0.6 and 55 μm, respectively (Rutschmann et al., 2002). The Kd value of 0.6 μm for the high-affinity binding sites in LeCPK1 is in the range of Ca2+ concentrations sufficient to induce the conformational change required for activation of many CDPKs (Lee et al., 1998; Rutschmann et al., 2002). The CDPKα protein binds 4 mol Ca2+/mol protein with high affinity (Lee et al., 1998). For the stress-inducible CDPK from ice plant (Mesembryanthemum crystallinum), McCPK1, expressed in E. coli as a GST-fusion protein, the K0.5 for calcium was estimated as 0.15 μm with histone III-S as the substrate. Such a low value for K0.5 suggests that McCPK1 could sense relatively small changes in cytoplasmic Ca2+ concentration (Chehab et al., 2004). The cytoplasmic free Ca2+ concentration under resting conditions is maintained at very low levels (10–100 nm), ensuring low CDPK activity. An increase in cytoplasmic calcium concentration activates CDPKs. Therefore, individual CDPK isoforms with different Ca2+-binding affinities could decode a subset of calcium signals (Harmon et al., 2000; Harmon, 2003).
The affinity for calcium binding varies between isoforms of CDPKs and is influenced by the type of substrate. For phosphorylation of Syntide 2 by GST-ZmCPK11, a lower concentration of Ca2+ is required than for phosphorylation of histone III-S. Moreover, in contrast to histone phosphorylation, activity of ZmCPK11 with Syntide 2 without added calcium, and in the presence of EGTA, is significantly greater than zero. These results support the previous observation (Lee et al., 1998) that peptide substrates are able to bind to the active site of CDPK in the absence of calcium. Considering that calcium ions are essential for the effect of phospholipids on ZmCPK11 (Szczegielniak et al., 2000), it is important to note that when histone III-S (not Syntide 2) was used as the substrate, activity of GST-ZmCPK11 was efficiently (6- to 9-fold) stimulated by PA, PS, and PI. The other tested phospholipids (PC, LysoPC, CL, DO, PE) and detergent (DDM) did not stimulate ZmCPK11 activity. Thus, the stimulation of activity seems to be lipid specific; however, not all negatively charged phospholipids bind to protein to the same extent and with the same affinity. The stimulation of activity is not only dependent upon the phospholipids, but also on the protein substrate. In addition to differential stimulation of phosphorylation of exogenous substrates, ZmCPK11 autophosphorylation is stimulated by calcium and phospholipids. Autophosphorylation of some CDPKs occurs by an intramolecular mechanism at submicromolar concentration of Ca2+ (for review, see Harper et al., 2004). Interestingly, the stimulation of ZmCPK11 autophosphorylation by PA is not calcium dependent, whereas autophosphorylation caused by PS is increased by Ca2+. The model study of the binding of PA and PS to the C2 domain of PKCɛ revealed the preferential affinity of C2 for PA. C2-PA interaction is Ca2+ independent and has the stabilizing effect on the protein (Corbalán-Garcia et al., 2003). As in the case PKCɛ, PA may create a better binding site with ZmCPK11 than PS. This binding may promote intramolecular rearrangements leading to autophosphorylation of ZmCPK11 and stimulation of histone phosphorylation. The nature of the lipid-binding site of ZmCPK11 is not yet known. The calcium- and phospholipid-binding domains (C2, FYVE, PX, and PH), which are potential regulatory motifs of many plant proteins (Kopka et al., 1998; van Leeuwen et al., 2004), are not present in CDPKs. Binder et al. (1994) have proposed that phosphoinositides interact with basic consensus motifs. The basic motif (K-X6-K-X-K-K) is present in the JD of ZmCPK11. Several PA-binding proteins have been identified, but a universal PA-binding domain has not yet been recognized (for review, see van Leeuwen et al., 2004) and the activating effect of certain phospholipids still awaits explanation.
Unlike AtCPK1 and DcCPK1, which are also stimulated by phospholipids, ZmCPK11 does not have a myristoylation/palmitoylation site. The ZmCPK11 protein is likely located predominantly in the cytoplasm, as is AtCPK4, which also lacks a myristoylation/palmitoylation site. Our data showing that ZmCPK11 interacts with phospholipids support the idea that this protein kinase could also associate with membranes. There is a possibility that, in stress conditions, ZmCPK11 may be translocated to the membranes, where it can be activated by phospholipids in addition to calcium. The binding of ZmCPK11 to membranes could be relatively weaker than other CDPKs, which undergo myristoylation/palmitoylation. Such a loose association would allow ZmCPK11 to partition between membranes and the cytoplasm. This hypothesis will be investigated in future work. In conclusion, our results demonstrate that some CDPK isoforms can, in a substrate-dependent manner, respond to Ca2+ and lipid signals in response to stress.
MATERIALS AND METHODS
Plant Material and Stress Treatments
Maize seeds (Zea mays cv Mona), after soaking in water at room temperature overnight, were grown at 26°C on wetted paper for 72 h in the dark. The etiolated apical parts of the seedlings were harvested, immediately frozen in liquid nitrogen, and stored at −80°C. For stress treatments, maize plants were cultivated hydroponically for 2 weeks in a growth chamber with a daily cycle of 14 h light (70–80 W/m2) at 25°C and 10 h dark at 20°C. Maize leaves (second and third leaf) were detached, preincubated for 2 h in water, and subjected to one of the following treatments: 4°C or 40°C, laid on Whatman 3MM paper to dry (drought), treated with 300 mm NaCl, 100 μm ABA, or 500 μm H2O2. Mechanical wounding was performed by cutting the lamina of the leaves with a razor blade. The wounded (local) and the upper undamaged (systemic) leaves were harvested at indicated time points. Samples were frozen in liquid nitrogen immediately after harvesting and stored at −80°C until used for RNA or protein extraction.
Purification of the Protein Kinase
The initial steps of purification starting with 20 g of maize seedlings included ammonium sulfate precipitation and octyl-Sepharose and DEAE-52 chromatography, and were performed as described previously (Szczegielniak et al., 2000). Samples from two preparations were combined and further purified by affinity chromatography on immobilized histone H1 (histone H1 was coupled to cyanogen bromide [CNBr]-activated Sepharose 4B, according to the manufacturer's instructions; Amersham-Pharmacia Biotech). After loading the enzymatic preparation, the 1-mL column was washed with buffer A (20 mm Tris-HCl, pH 7, containing 5.2 mm EDTA, 0.5 mm dithiothreitol [DTT], 0.2 mm phenylmethylsulfonyl fluoride [PMSF]) until the A280 was below 0.05. Then the protein kinase was eluted with a 6-mL gradient of 0 to 0.6 m NaCl in buffer A. The fractions exhibiting calcium- and phospholipid-dependent protein kinase activity were collected and dialyzed against 500 mL of buffer A until the conductivity was 7 μS cm−1. Four preparations of enzyme (from a total of 160 g of maize seedlings) were combined and applied to a Mono Q H5/5 column equilibrated with buffer A. The column was washed with 45 mL of buffer A and proteins were eluted with a linear gradient of 0 to 0.3 m NaCl. One-milliliter fractions were collected at a flow rate of 30 mL h−1. The protein kinase activity eluted as a single symmetrical peak at a NaCl concentration of 0.25 m. The pooled active fractions were dialyzed for 2 h against buffer A and purified by affinity chromatography on immobilized MBP. The MBP was coupled to CNBr-activated Sepharose 4B, according to the manufacturer's instructions (Amersham-Pharmacia Biotech). The enzymatic preparation was loaded onto an MBP-Sepharose column (0.5 mL), previously equilibrated with buffer A. The column was washed with buffer A until the A280 decreased below 0.05, and the protein kinase was eluted with a gradient of 0 to 0.6 m NaCl in buffer A. All purification steps were performed at 4°C. Protein concentrations were determined by the Bradford dye-binding assay (Bradford, 1979) using bovine serum albumin as the standard, or by A280.
Protein Kinase Assay
Determination of activity of the maize protein kinase was described previously (Szczegielniak et al., 2000). The standard reaction mixture (75 μL) contained the following: histone III-S (0.5 mg mL−1) or Syntide 2 (0.05 mm), Tris-HCl, pH 7.5 (20 mm), Ca(OAc)2 (0.25 mm), EGTA (0.10 mm), MgCl2 (5 mm), γ-[32P]ATP (66 μm; 130–200 cpm pmol−1), and GST-ZmCPK11 (0.5–1.0 μg). To study the effect of lipids, 0.09 to 0.15 mm Ca(OAc)2 and indicated amounts of lipids were used. Values of free calcium were calculated using the MaxChelator program (http://www.stanford.edu/∼cpatton/webmaxc/webmaxcS.htm). After 8-min incubation at 30°C, 50 μL of the assay mixture was spotted onto a square (2 cm × 2 cm) of Whatman 3MM paper (when histone III-S was used as substrate), which was immediately immersed in cold 5% (w/v) trichloroacetic acid containing 0.3% O-phosphoric acid (10 mL per paper square), and washed four times for 10 min. Then the squares were washed in 96% ethanol and allowed to dry. The radioactivity was quantified using a liquid scintillation spectrometer. When Syntide 2 was used, the reaction was stopped by applying 50 μL of assay mixture onto a square (2 cm × 2 cm) of Whatman P81 paper, which was immersed in 0.3% O-phosphoric acid, washed 5 times for 5 min, then washed in ethanol, dried, and the radioactivity measured.
Handling of Lipids and Detergent
Lipids were as follows (purchased from Sigma): PA (1,2-diacyl-sn-glycero-3-P sodium salt) from egg yolk lecithin, PI [1,2-diacyl-sn-glycero-3-phospho-(1-d-myoinositol)] from soybeans (Glycine max), PS (1,2-diacyl-sn-glycero-3-phospho-l-Ser) from bovine brain, PC (1,2-diacyl-sn-glycero-3-phosphocholine, type XVI-E) from egg yolk, LysoPC from soybeans, CL sodium salt from bovine heart, PE (type III) from egg yolk, DO [1,3-Di(cis-9-octadecenoyl)glycerol], and the detergent DDM.
PA, PI, PC, LysoPC, and PE were dissolved in chloroform (10 mg mL−1), PS and DO were dissolved in chloroform:methanol (95:5, v/v), and CL was dissolved in methanol.
Crude lipids were isolated from maize seedlings in chloroform:methanol (2:1, v/v). Then, after adding an aqueous solution of 0.88% KCl, lipids were extracted to the organic phase, concentrated by rotary evaporation, redissolved in chloroform:methanol (2:1, v/v), and stored at −20°C. Before each experiment, the appropriate amounts of each lipid were dried under a stream of nitrogen, and then 20 mm Tris-HCl, pH 7.5, was added and sonicated for 5 min on ice.
Detergent (DDM) was dissolved in water.
In-Gel Kinase Activity Assay
Nonstressed leaves and stressed leaves were separately ground in liquid nitrogen, and proteins were isolated with 200 μL of extraction buffer (50 mm Tris-HCl, pH 7.5, 250 mm Suc, 2 mm EDTA, 10 mm EGTA, 0.5 μm Na3VO4, 1 mm DTT, 200 μm PMSF, 0.5 μg mL−1 leupeptin, 1 μg mL−1 aprotinin, 0.7 μg mL−1 pepstatin). The suspensions were sonicated three times, each time for 10 s, and centrifuged at 20,000g for 10 min at 4°C, and the pellets were discarded. The supernatants were centrifuged again for 30 min at 4°C. Proteins from supernatant (26 μg/lane) were separated on 8% SDS-polyacrylamide gel containing histone III-S as a substrate (0.5 mg mL−1). The rest of the procedure was performed as described previously (Szczegielniak et al., 2000).
Autophosphorylation
GST-ZmCPK11 (2 μg) was incubated at 30°C for 8 min using the standard reaction mixture without exogenous substrate.
Microsequencing
Active fractions from the final MBP-Sepharose chromatography step were pooled and concentrated with a Centricon 30 concentrator (Amicon). Proteins were separated on preparative 12% SDS-polyacrylamide gels. After Coomassie Blue staining, the gels were washed in water for 1.5 h. The protein band in the range of 53 to 56 kD was excised, lyophilized, and sent to the Protein and Nucleic Acid Facility (Beckman Center, Stanford University Medical Center, Palo Alto, CA) for microsequencing.
RNA Isolation
Total RNA was prepared from the roots, seedlings, and nonstressed and stressed leaves using Tri Reagent (Molecular Research), following the manufacturer's directions. The quality of RNA was checked by visualizing the ethidium bromide (EtBr)-stained ribosomal RNA in agarose gels containing 1.2% formaldehyde.
Isolation of the ZmCPK11 cDNA
One microgram of total RNA from maize seedlings was reverse transcribed for 60 min at 42°C in a 20-μL reaction volume containing 1 unit of enhanced avian reverse transcriptase, 500 μm each dNTP, 1 unit of RNase inhibitor (Kit HSRT 100; Sigma), and 1.25 μm primer 3. Two microliters of the RT reaction were used for PCR in a 50-μL volume containing 1 unit JumpStart AccuTag LA polymerase, 200 μm each dNTP, and 625 nm of primers 1 and 2, or 1 and 3. The PCR conditions were 3 min, 94°C (first cycle); 45 s, 94°C; 1 min, 55°C; 2 min, 68°C (30 cycles); and 10 min, 68°C (final cycle). The sequences of tryptic peptides, derived from the microsequencing data, were used to design primers 1, 2, and 3 (Table II). The forward primer 1 corresponds to the sequence EIQIMHH located in the third subdomain of the catalytic kinase domain. The reverse primer 2 corresponds to the sequence PYYVAPEVL located in the eighth subdomain of the catalytic domain. Reverse primer 3 corresponds to the sequence LVSAFAFFDK located in the regulatory CLD of CDPK. Two resulting DNA fragments of 357 and 1,065 bp were cloned into a pGEM-T Easy vector (Promega) and sequenced. For cloning the full-length ORF, two additional specific primers were synthesized, based on maize EST sequences. The forward primer 4 was based on the sequence of a 575-bp EST clone (AI770867). In this clone, the peptide TKLPQLVTAPAPSSGRPASVLPYK, obtained from microsequencing of the purified protein kinase, was present. The sequence of this EST is similar to the end of the N-variable domain and the part of the catalytic domain of CDPKs. This indicated that the 575-bp EST clone is a fragment of ZmCPK11, containing the 5′ noncoding region and sequence encoding the N terminus. The reverse primer 5 is based on a second 590-bp EST sequence (accession no. AI745945), where the 5′ end overlapped by 102 bp with the 3′ end of our 1,065-bp clone. The 590-bp EST clone includes a 3′ noncoding region and the sequence encoding the C terminus and part of the CLD. The reaction conditions for the synthesis of the first-strand cDNA with the reverse transcriptase and total RNA from maize seedlings as a template were as before, except that primer 5 was used. For PCR, 2 μL of reverse transcriptase reaction and the pair of primers 4 and 3 or 4 and 5 were used. In the remaining PCR conditions, the reactions were as before. The two DNA fragments obtained were cloned into pGEM-T Easy and sequenced.
Expression of GST-ZmCPK11 in Escherichia coli
The full-length ZmCPK11 cDNA was amplified by PCR using primers 6 and 7 (Table I) and cloned into pGEM-T Easy. The sequence was confirmed by DNA sequencing. For expression of the GST-ZmCPK11 in bacteria, the ZmCPK11 ORF was cut from pGEM-T Easy using XmaI/NotI restriction sites and cloned to pGEX4T-1 (Amersham-Pharmacia Biotech). The ZmCPK11 construct was transformed to E. coli BL21 (DE3). Expression was induced with 0.5 mm IPTG for 4 h at 18°C. The fusion protein was expressed and purified using gluthathione-agarose beads, according to the manufacturer's instructions (Amersham-Pharmacia Biotech AB).
Immunoblot Analysis
Polyclonal antibodies against the CLD of soybean CDPKα were prepared according to the procedure previously described (Bachmann et al., 1996). The antibodies against GST were purchased from Santa Cruz Biotechnology. Proteins from clarified homogenate, after solubilization, and affinity chromatography, were separated on 10% SDS-polyacrylamide gels, and then transferred to polyvinylidenedifluoride membranes by electroblotting. The membranes were blocked for 2 h at room temp in Tris-buffered saline plus Tween (TBST; 10 mm Tris-HCl, pH 7.5, 100 mm NaCl, 0.1% Tween 20) containing 5% dry milk, and then incubated for 1.5 h in TBST buffer with primary antibody at dilution 1:2,000. After removing unbound antibodies by extensive washing, the blots were incubated with alkaline phosphatase-conjugated secondary antibodies and visualized using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-P.
Determination of Transcript Levels by RT-PCR
Total RNA was isolated from different maize organs: dry and soaked seeds, seedlings, roots, stem, and leaves (stressed or nonstressed). One microgram of RNA was reverse transcribed for 60 min at 47°C in 20 μL of reaction mixture containing 1 unit of enhanced avian reverse transcriptase, 500 μm each dNTP, 3.5 μm anchored oligo(dT) primer, 1 unit RNase inhibitor (kit, HSRT 100; Sigma). One microliter of the RT reaction was used for PCR in 20 μL of volume containing 0.4 units of Taq DNA polymerase (Fermentas), 200 μm each dNTP, 1.5 mm MgCl2, and 625 nm of the appropriate primers. Routine PCR conditions were 3 min, 94°C (first cycle); 30 s, 94°C; 30 s, 55°C; 1 min, 72°C (25 cycles for actin and MPI and 30 cycles for ZmCPK11); and 10 min, 72°C (final cycle). The PCR products were separated on 0.8% agarose gels and visualized by EtBr staining. ZmCPK11 mRNA was quantified relative to RNA loading using Gel Doc (Bio-Rad). The absence of genomic DNA in RNA samples was checked by PCR reaction, using RNA as a template instead of cDNA (without RT). No product was detected, indicating that the RNA samples were free of DNA.
Phylogenetic Analysis
Sequences of CDPKs were found using SMART (http://smart.embl-heidelberg.de; Schultz et al., 1998). The multiple sequence alignment was generated using ClustalW (Thompson et al., 1994) and analyzed and edited using the GeneDoc sequence editor (Nicholas et al., 1997). The consensus parsimony tree and consensus neighbor-joining tree were constructed using the programs from the Phylip package (Felsenstein, 1989). The 134 sequences containing the EF-hand calcium-binding motif (SMART ID EFh) typical of calmodulin-like proteins and catalytic domains of Ser/Thr kinases (SMART ID S_TKc) were found in the SP-TREMBL database. Excluded from this set were hypothetical proteins having deletions or insertions in the catalytic domains. These atypical sequences might be the result of annotation errors.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY301062 and AAP57564.
Supplementary Material
Acknowledgments
We are very grateful to Professor Jan A. Miernyk for patient correction of the manuscript and critical comments. We thank Ms. Katarzyna Róg for assistance in manuscript preparation.
This work was supported by grants from the State Committee for Scientific Research (KBN), Poland (grant nos. 3 P06A 00825 and PBZ–KBN–110/PO4/21 to G.M. and grant no. PBZ–KBN–110/PO4/20 to G.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Grażyna Muszyńska (muszynsk@ibb.waw.pl).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.066472.
References
- Asano T, Kunieda N, Omura Y, Ibe H, Kawasaki T, Takano M, Sato M, Furuhashi H, Mujin T, Takaiwa F, et al (2002) Rice SPK, a calmodulin-like domain protein kinase, is required for storage product accumulation during seed development: phosphorylation of sucrose synthase is a possible factor. Plant Cell 14: 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M, Shirashi N, Campbell WH, Yoo B-C, Harmon AC, Huber SC (1996) Identification of Ser-543 as the major regulatory phosphorylation site in spinach leaf nitrate reductase. Plant Cell 8: 505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Harper JF, Sussman MR (1994) Characterization of an Arabidopsis calmodulin-like domain protein kinase purified from Escherichia coli using an affinity sandwich technique. Biochemistry 33: 2033–2041 [DOI] [PubMed] [Google Scholar]
- Botella JR, Arteca JM, Somodevilla M, Arteca RN (1996) Calcium-dependent protein kinase gene expression in response to physical and chemical stimuli in mungbean (Vigna radiata). Plant Mol Biol 30: 1129–1137 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1979) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Chang CC, Sheen J, Bligny M, Niwa Y, Lerbs-Mache S, Stern DB (1999) Functional analysis of two maize cDNAs encoding T7-like RNA polymerases. Plant Cell 11: 911–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab EW, Patharkar OR, Hegeman AD, Taybi T, Cushman JC (2004) Autophosphorylation and subcellular localization dynamics of a salt- and water deficit-induced calcium-dependent protein kinase from ice plant. Plant Physiol 135: 1430–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SH, Willmann MR, Chen HC, Sheen J (2002) Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol 129: 469–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol 129: 661–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chico JM, Raŕces M, Téllez-Iňón MT, Ulloa RM (2002) A calcium-dependent protein kinase is systemically induced upon wounding in tomato plants. Plant Physiol 128: 256–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbalán-Garcia S, Sánchez-Carrillo S, García-García J, Gómez-Fernández JC (2003) Characterization of the membrane binding mode of the C2 domain of PKCɛ. Biochemistry 42: 11661–11668 [DOI] [PubMed] [Google Scholar]
- Cordero MJ, Raventos D, San Segundo B (1994) Expression of a maize proteinase inhibitor gene is induced in response to wounding and fungal infection: systemic wound-response of a monocot gene. Plant J 6: 141–150 [DOI] [PubMed] [Google Scholar]
- Dammann C, Ichida A, Hong B, Romanowsky SM, Hrabak EM, Harmon AC, Pickard BG, Harper JF (2003) Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiol 132: 1840–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Mészáros T, Miskolczi P, Oberschall A, Török K, Magyar Z, Dudits D, Deák M (2001) Auxin and heat shock activation of a novel member of the calmodulin like domain protein kinase gene family in cultured alfalfa cells. J Exp Bot 52: 215–221 [PubMed] [Google Scholar]
- Ellard-Ivey M, Hopkins RB, White TJ, Lomax TL (1999) Cloning, expression and N-terminal myristoylation of CpCPK1, a calcium-dependent protein kinase from zucchini (Cucurbita pepo L.). Plant Mol Biol 39: 199–208 [DOI] [PubMed] [Google Scholar]
- Evans NH, McAinsh MR, Hetherington AM (2001) Calcium oscillations in higher plants. Curr Opin Plant Biol 4: 415–420 [DOI] [PubMed] [Google Scholar]
- Farmer PK, Choi JH (1999) Calcium and phospholipid activation of a recombinant calcium-dependent protein kinase (DcCPK1) from carrot (Daucus carota L.). Biochim Biophys Acta 1434: 6–17 [DOI] [PubMed] [Google Scholar]
- Felsenstein J (1989) PHYLIP—phylogeny inference package (version 3.2). Cladistics 5: 164–166 [Google Scholar]
- Frattini M, Morello L, Brevario D (1999) Rice calcium-dependent protein kinase isoforms OsCDPK2 and OsCDPK11 show different responses to light and different expression patterns during seed development. Plant Mol Biol 41: 753–764 [DOI] [PubMed] [Google Scholar]
- Hanaoka H, Fukuda A, Sasaki T, Nemoto K, Fujiwara T, Hayashi H (1999) CDPK in rice phloem sap (abstract no. 408). In Interactions and Intersections in Plant Signaling Pathways. Keystone Symposia, February 8–14, 1999, Coeur D'Alene, ID
- Harmon AC (2003) Calcium-regulated protein kinases of plants. Gravit Space Biol Bull 16: 83–90 [PubMed] [Google Scholar]
- Harmon AC, Gribskov M, Harper JF (2000) CDPKs—a kinase for every Ca2+ signal? Trends Plant Sci 5: 154–159 [DOI] [PubMed] [Google Scholar]
- Harmon AC, Yoo BC, McCaffery C (1994) Pseudosubstrate inhibition of CDPK, a protein kinase with a calmodulin-like domain. Biochemistry 33: 7278–7287 [DOI] [PubMed] [Google Scholar]
- Harper JF, Binder BM, Sussman MR (1993) Calcium and lipid regulation of an Arabidopsis protein kinase expressed in Escherichia coli. Biochemistry 32: 3282–3290 [DOI] [PubMed] [Google Scholar]
- Harper JF, Breton G, Harmon A (2004) Decoding Ca2+ signals through plant protein kinases. Annu Rev Plant Biol 55: 263–288 [DOI] [PubMed] [Google Scholar]
- Harper JF, Huang JF, Lloyd SJ (1994) Genetic identification of an autoinhibitor in CDPK, a protein kinase with a calmodulin-like domain. Biochemistry 33: 7267–7277 [DOI] [PubMed] [Google Scholar]
- Harper JF, Sussman MR, Schaller GE, Putnam-Evans C, Charbonneau H, Harmon AC (1991) A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science 252: 951–954 [DOI] [PubMed] [Google Scholar]
- Heitz T, Bergey DR, Ryan CA (1997) A gene encoding a chloroplast-targeted lipoxygenase in tomato leaves is transiently induced by wounding, systemin, and methyl jasmonate. Plant Physiol 114: 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak EM, Chan CWM, Gribskov M, Harper JF, Choi JH, Halford N, Kudla J, Luan S, Nimmo HG, Sussman MR, et al (2003) The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol 132: 666–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Hayashida N, Baba T, Shinozaki K, Shimada H (1993) The gene encoding a calcium-dependent protein kinase located near the sbe1 gene encoding starch branching enzyme I is specifically expressed in developing rice seeds. Gene 129: 183–189 [DOI] [PubMed] [Google Scholar]
- Kopka J, Picall Ch, Hetherington AM, Müller-Röber B (1998) Ca2+/phospholipid-binding (C2) domain in multiple plant proteins: novel components of the calcium-sensing apparatus. Plant Mol Biol 36: 627–637 [DOI] [PubMed] [Google Scholar]
- Lee JY, Yoo BC, Harmon AC (1998) Kinetic and calcium-binding properties of three calcium-dependent protein kinase isoenzymes from soybean. Biochemistry 37: 6801–6809 [DOI] [PubMed] [Google Scholar]
- Lee S, Hirt H, Lee Y (2001) Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J 26: 479–486 [DOI] [PubMed] [Google Scholar]
- Leon J, Rojo E, Sánchez-Serrano JJ (2001) Wound signalling in plants. J Exp Bot 52: 1–9 [DOI] [PubMed] [Google Scholar]
- Lu SX, Hrabak EM (2002) An Arabidopsis calcium-dependent protein kinase is associated with the endoplasmic reticulum. Plant Physiol 128: 1008–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig AA, Romeis T, Jones JDG (2004) CDPK-mediated signalling pathways: specificity and cross-talk. J Exp Bot 55: 181–188 [DOI] [PubMed] [Google Scholar]
- Martin ML, Busconi L (2000) Membrane localized of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J 24: 429–435 [DOI] [PubMed] [Google Scholar]
- Martin ML, Busconi L (2001) A rice membrane-bound calcium-dependent protein kinase is activated in response to low temperature. Plant Physiol 125: 1442–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer HJG, Munnik T (2003) Phospholipid-based signaling in plants. Annu Rev Plant Biol 54: 265–306 [DOI] [PubMed] [Google Scholar]
- Mitra D, Johri MM (2000) Enhanced expression of a calcium-dependent protein kinase from the moss Funaria hygrometrica under nutritional starvation. J Biosci 25: 331–338 [DOI] [PubMed] [Google Scholar]
- Monroy AF, Dhindsa RS (1995) Low-temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25°C. Plant Cell 7: 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura DS, Bergey DR, Ryan CA (2001) Characterization and localization of a wound-inducible type I serine-carboxypeptidase from leaves of tomato plants (Lycopersicon esculentum Mill.). Planta 212: 222–230 [DOI] [PubMed] [Google Scholar]
- Munnik T (2001) Phosphatidic acid: an emerging plant lipid second messenger. Trends Plant Sci 6: 227–233 [DOI] [PubMed] [Google Scholar]
- Murillo I, Jaeck E, Cordero J, San Segundo B (2001) Transcriptional activation of a maize calcium-dependent protein kinase gene in response to fungal elicitors and infection. Plant Mol Biol 45: 145–158 [DOI] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB Jr, Deerfield DW (1997) GeneDoc: analysis and visualization of genetic variation. EMBNEW NEWS 4: 14 [Google Scholar]
- Patharkar OR, Cushman JC (2000) A stress-induced calcium-dependent protein kinase from Mesembryanthemum crystallinum phosphorylates a two-component pseudo-response regulator. Plant J 24: 679–691 [DOI] [PubMed] [Google Scholar]
- Romeis T, Ludwig AA, Martin R, Jones JDG (2001) Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J 20: 5556–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Jones JDG (2000) Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell 12: 803–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JDG (1999) Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11: 273–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutschmann F, Stalder U, Piotrowski M, Oecking C, Schaller A (2002) LeCPK1, a calcium-dependent protein kinase from tomato. Plasma membrane targeting and biochemical characterization. Plant Physiol 129: 156–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SB, Wang X (1996) Activation of phospholipase D and the possible mechanism of activation in wound-induced lipid hydrolysis in castor bean leaves. Biochim Biophys Acta 1303: 243–250 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Kinoshita N, Ishiyama K, Hata S, Kyozuka J, Hayakawa T, Nakamura T, Shimamoto K, Yamaya T, Izui K (2001) A Ca2+-dependent protein kinase that endows rice plants with cold- and salt-stress tolerance functions in vascular bundles. Plant Cell Physiol 42: 1228–1233 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K (2000) Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J 23: 319–327 [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller A, Oecking C (1999) Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell 11: 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Harmon AC, Sussman MR (1992) Characterization of a calcium- and lipid-dependent protein kinase associated with the plasma membrane of oat. Biochemistry 31: 1721–1727 [DOI] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA 95: 5857–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer P, Buchala A, Dudler R, Métraux JP (1998) Induced systemic resistance in wounded rice plants. Plant J 14: 475–481 [Google Scholar]
- Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y (1995) Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science 270: 1988–1992 [DOI] [PubMed] [Google Scholar]
- Sheen J (1996) Ca2+-dependent protein kinases and stress signal transduction in plants. Science 274: 1900–1902 [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS (2003) The role of phylogenetics in comparative genetics. Plant Physiol 132: 1790–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczegielniak J, Liwosz A, Jurkowski I, Loog M, Dobrowolska G, Ek P, Harmon AC, Muszyńska G (2000) Calcium-dependent protein kinase from maize seedlings activated by phospholipids. Eur J Biochem 267: 3818–3827 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Katagiri T, Mizoguchi T, Yamaguchi-Shinozaki K, Hayashida N, Shinozaki K (1994) Two genes that encode Ca2+-dependent protein kinases are induced by drought and high-salt stresses in Arabidopsis thaliana. Mol Gen Genet 244: 331–340 [DOI] [PubMed] [Google Scholar]
- van Leeuwen W, Ökrész L, Bögre L, Munnik T (2004) Learning the lipid language of plant signalling. Trends Plant Sci 9: 378–384 [DOI] [PubMed] [Google Scholar]
- Yoon GM, Cho HS, Ha HJ, Liu JR, Lee HP (1999) Characterization of NtCDPK1, a calcium-dependent protein kinase gene in Nicotiana tabacum, and the activity of its encoded protein. Plant Mol Biol 39: 991–1001 [DOI] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (1997) Salicylic acid activates as 48-kD MAP kinase in tobacco. Plant Cell 9: 809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.