Abstract
Reduced inorganic sulfur compounds are utilized by many bacteria as electron donors to photosynthetic or respiratory electron transport chains. This metabolism is a key component of the biogeochemical sulfur cycle. The SoxAX protein is a heterodimeric c-type cytochrome involved in thiosulfate oxidation. The crystal structures of SoxAX from the photosynthetic bacterium Rhodovulum sulfidophilum have been solved at 1.75 Å resolution in the oxidized state and at 1.5 Å resolution in the dithionite-reduced state, providing the first structural insights into the enzymatic oxidation of thiosulfate. The SoxAX active site contains a haem with unprecedented cysteine persulfide (cysteine sulfane) coordination. This unusual post-translational modification is also seen in sulfurtransferases such as rhodanese. Intriguingly, this enzyme shares further active site characteristics with SoxAX such as an adjacent conserved arginine residue and a strongly positive electrostatic potential. These similarities have allowed us to suggest a catalytic mechanism for enzymatic thiosulfate oxidation. The atomic coordinates and experimental structure factors have been deposited in the PDB with the accession codes 1H31, 1H32 and 1H33.
Keywords: cysteine persulfide haem ligand/SoxAX complex/sulfur cycle/thiosulfate oxidation/X-ray crystal structure
Introduction
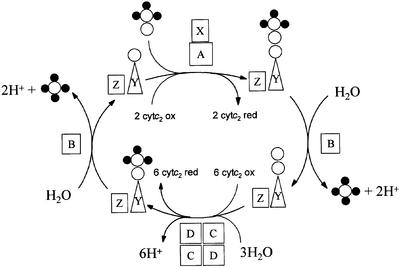
Sulfide and thiosulfate are the most abundant reduced inorganic sulfur species in the environment and are converted to sulfate, primarily by bacterial action, in the oxidative half of the sulfur cycle. Photosynthetic sulfur bacteria use the sulfur compounds as a source of electrons for reductive carbon dioxide fixation during anoxic autotrophic growth (Brune, 1989), while chemolithoautotrophic sulfur bacteria use the electrons derived from oxidation of inorganic sulfur species both in carbon dioxide fixation and as respiratory electron donors (Wood, 1988). The Sox mechanism, also referred to as TOMES (thiosulfate-oxidizing multienzyme system), is the best studied and most widely distributed pathway for the oxidation of thiosulfate to sulfate (Kelly et al., 1997; Friedrich et al., 2001). In some organisms, the Sox pathway is also employed for the oxidation of sulfide, sulfur, sulfite and tetrathionate (Mukhopadhyaya et al., 2000; Appia-Ayme et al., 2001; Rother et al., 2001). The enzymes of the Sox pathway are located in the bacterial periplasm and use a small, monohaem, c-type cytochrome as the direct electron acceptor. The current model for the Sox mechanism as it applies to thiosulfate oxidation is depicted in Figure 1. The central player is the SoxYZ complex which carries the pathway intermediates covalently bound to a cysteine residue located in a conserved Gly-Gly-Cys-Gly-Gly motif at the extreme C-terminus of the SoxY protein (Lu et al., 1985; Appia-Ayme et al., 2001; Friedrich et al., 2001; Quentmeier and Friedrich, 2001). The initial reaction in the pathway is the oxidative formation of a disulfide linkage between the sulfane sulfur of thiosulfate and the SoxY cysteine. This reaction is catalysed by the haemoprotein SoxAX complex. The terminal sulfone group of the resultant adduct is released as sulfate by SoxB in a hydrolytic reaction. The sulfane atom is then oxidized to a sulfone by the SoxCD complex and released from SoxY as sulfate by the activity of SoxB.
Fig. 1. The Friedrich–Kelly model for the Sox mechanism of thiosulfate oxidation in R.sulfidophilum. SoxAX catalyses a two-electron oxidation in which the sulfane sulfur of thiosulfate is linked to the antepenultimate cysteine residue of SoxY to produce SoxY–thiocysteine- S-sulfate. The figure is modified from Friedrich et al. (2001). Open circles represent sulfur atoms, whilst oxygen atoms are shown as filled circles.
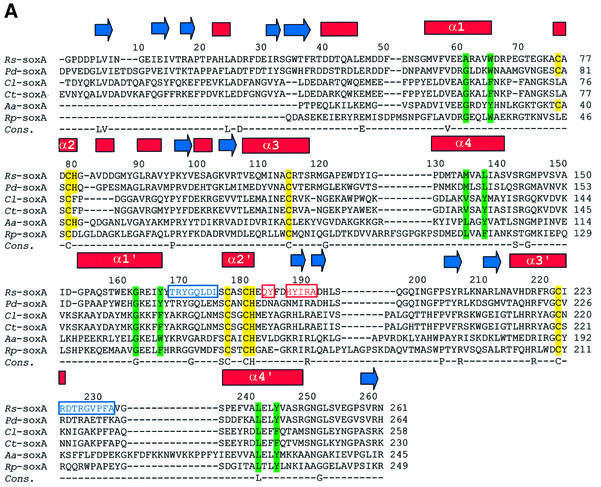
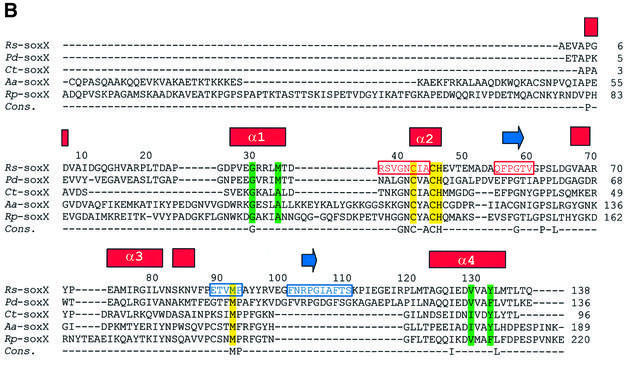
The SoxAX complex isolated from the photosynthetic marine bacterium Rhodovulum sulfidophilum is a heterodimeric c-type cytochrome in which the SoxA subunit contains two, and the SoxX subunit one, covalently bound haem (Appia-Ayme et al., 2001; Cheesman et al., 2001). The electron acceptor from R.sulfidophilum SoxAX is cytochrome c2. Sequence alignments of the R.sulfido philum SoxA and SoxX sequences with those of other sulfur-oxidizing bacteria are shown in Figure 2. Here we report the X-ray crystal structures of the oxidized form of the R.sulfidophilum SoxAX complex refined at 1.75 Å resolution and the reduced form of the enzyme refined at 1.5 Å resolution. These high resolution structures combined with spectroscopic data and chemical analysis reveal the novel arrangement of a cysteine persulfide residue co-ordinated to the catalytic c-haem of the SoxA subunit and provide insights into the mechanism of this key oxidative enzyme of the global sulfur cycle.
Fig. 2. Amino acid sequences of SoxA and SoxX subunits from different sulfur-oxidizing bacteria. (A and B) Primary sequence alignments and haem coordination in the R.sulfidophilum SoxA and SoxX subunits, respectively. Sequences are those of the SoxAX proteins from R.sulfidophilum (Appia-Ayme et al., 2001) (Rs), Paracoccus denitrificans (Friedrich et al., 2000) (Pd), Chlorobium limicola (Klarskov et al., 1998) (Cl), Chlorobium tepidum (The Institute for Genomic Research. URL http://www.tigr.org) (Ct), Aquifex aeolicus (Deckert et al., 1998) (Aa) and Rhodopseudomonas palustris (DOE Joint Genome Institute. URL http://www.jgi.doe.gov/JGI_microbial/rhodo_homepage.html) (Rp). The alignments were generated with the program ClustalW using default parameters (see http://www.ebi.ac.uk/clustalw/). Secondary structural elements (β-sheet shown by blue arrows and α-helices by red bars) are indicated above the R.sulfidophilum sequence. The four residues indicated by Ptitsyn (1998) to be diagnostic for formation of each cytochrome c fold are highlighted in green, and the four conserved helices of the folds of the two domains (α1–α4 and α1′–α4′) are labelled. CXXCH haem-binding motifs and second axial ligands are indicated in yellow. Polypeptides contributing to the interface of the SoxAX complex and forming part of interface region A (see text) are boxed in blue; those forming part of interface region B are boxed in red. A consensus sequence showing identical residues is also given.
Results and discussion
The architecture of the SoxAX complex
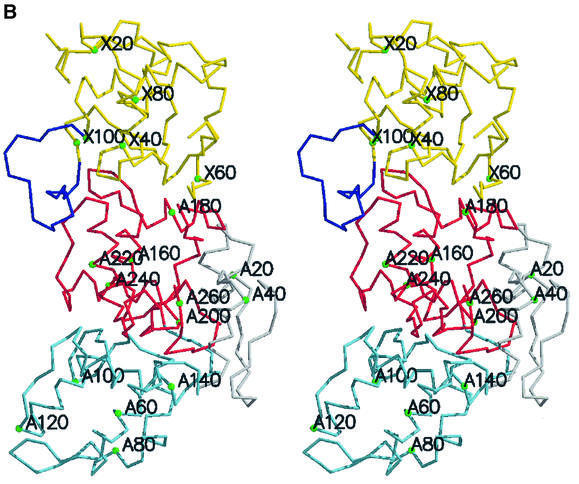
The crystal structure of the SoxAX heterodimer with four molecules in the asymmetric unit of a P21 cell was solved at 2.5 Å resolution by Fe-multiple wavelength anomalous dispersion (MAD). The structure subsequently was refined at 1.75 Å resolution using data collected from a crystal of space group P21212 with a single molecular copy in the asymmetric unit (Table I). The heterodimer seen in the crystal corresponds to the oligomeric state observed in solution by analytical ultracentrifugation studies (Appia-Ayme et al., 2001). SoxAX is an elongated molecule of approximate dimensions 34 × 36 × 75 Å3 (Figure 3A and B). The SoxA subunit is organized into two domains each binding a c-type haem with histidine–cysteine axial coordination. These are designated haems 1 and 2, labelled in the order in which the corresponding CXXCH ligand motifs appear in the SoxA sequence. Post-translational modification of the cysteine ligand to haem 2 is apparent and is discussed further below. The single domain SoxX subunit binds a c-type haem with histidine–methionine axial ligands (haem 3) and forms the interface with the C-terminal domain of SoxA. The haem ligands observed in the structure are in agreement with those inferred from previous spectroscopic characterization of oxidized protein samples (Cheesman et al., 2001). The geometrical arrangement of the haem groups is presented in Table II. The use of cysteinate as a haem iron ligand is unusual, with only cytochromes P450, the CO sensor protein CooA and cystathionine β-synthase previously having been reported to have such a ligand (Ojha et al., 2000). Indeed, SoxA is the first c-type cytochrome with cysteine haem iron coordination and only the second structurally characterized haemoprotein containing a haem with a cysteine– histidine ligand set, cystathionine β-synthase being the other (Meier et al., 2001).
Table I. Data collection and refinement statistics.
| Data collection | MAD data collection space group P21 a = 81.2 Å b = 102.9 Å c = 114.4 Å β = 110.5° |
|
|
Oxidized, space group P21212 a = 66.4 Å b = 87.3 Å c = 71.7Å | Reduced, space group P21212 a = 65.8 Å b = 87.2 Å c = 71.5 Å |
| |
λ1 = 1.729 Å |
λ2 = 1.741 Å |
λ3 = 1.000 Å |
λ = 0.933 Å |
λ = 0.98 Å |
| Resolution (Å) | 2.5 | 2.5 | 2.55 | 1.75 | 1.5 |
| Completeness (%) | 95.0 (92.2) | 94.8 (89.6) | 98.2 (88.7) | 93.4 (91.4) | 99.3 (98.4) |
| Rsyma (%) | 4.9 (9.1) | 5.1 (10.4) | 3.1 (5.0) | 7.0 (16.8) | 4.1 (28.4) |
| Ranomb (%) | 4.4 (7.1) | 3.5 (8.7) | 1.8 (5.4) | – | – |
| (<I>/<σI>) >3 (%) | 92.9 (82.4) | 90.6 (73.9) | 96.6 (89.2) | 78.7 (44.2) | 78.6 (48.4) |
| Independent reflections | 61 072 | 61 950 | 58 706 | 42 647 | 66 141 |
| Total reflections | 903 590 | 901 411 | 101 074 | 262 534 | 670 264 |
| Overall temperature factor (Å2) | 34.7 | 36.7 | 36.7 | 17.8 | 13.2 |
| SOLVE mean FOMc |
0.74 |
|
|
|
|
| Refinement statistics |
P21 |
P21212 oxidized |
P21212 reduced |
|
|
| Wavelength (Å) | 1.000 | 0.933 | 0.98 | ||
| Resolution range (Å) | 20–2.55 | 20–1.75 | 25–1.5 | ||
| Refined structured | SoxA 1–261, SoxX 1–138, + 691 water | SoxA 1–261, SoxX 1–51, 55–137, + 353 water | SoxA 1–261, SoxX 1–51, 55–137, + 667 water, + 2 ethylene glycol | ||
| Rcryste | 21.0 (29.6) | 17.7 (21.3) | 19.9 (23.5) | ||
| Rfreef | 26.4 (36.8) | 21.4 (23.0) | 23.0 (27.4) | ||
| R.m.s.ds | |||||
| Bonds (Å) | |||||
| C–N | 0.005 | 0.009 | 0.005 | ||
| N–Cα | 0.009 | 0.014 | 0.008 | ||
| Cα–C | 0.012 | 0.016 | 0.009 | ||
| Angles (°) | |||||
| N–Cα–C | 0.88 | 1.08 | 1.36 | ||
| C–N – Cα | 1.30 | 1.77 | 2.16 | ||
| Mean temperature factors (Å2) | |||||
| Main chain atoms | 18.5 | 16.5 | 14.4 | ||
| Side chain atoms | 19.1 | 21.9 | 17.3 |
Figures in parentheses refer to data in the highest resolution bin.
aRsym = ΣΣ|Ii – <I>|/Ii, where <I> is the average of symmetry equivalent reflections and the summation extends over all observations for all unique reflections.
bRanom = Σ|<I+> – <I–>|/Σ|<I+> + <I–>|.
cAverage figure of merit (FOM) calculated by SOLVE (Terwilliger and Berendzen, 1999).
dThe final structural model for the P21 crystal form contained four copies of the indicated residues of SoxA and SoxX in the asymmetric unit
eRcryst = Σ||F| – |Fc||/Σ|F|.
fFor Rfree, the summations extends over a subset (5%) of reflections excluded from all stages of refinement.
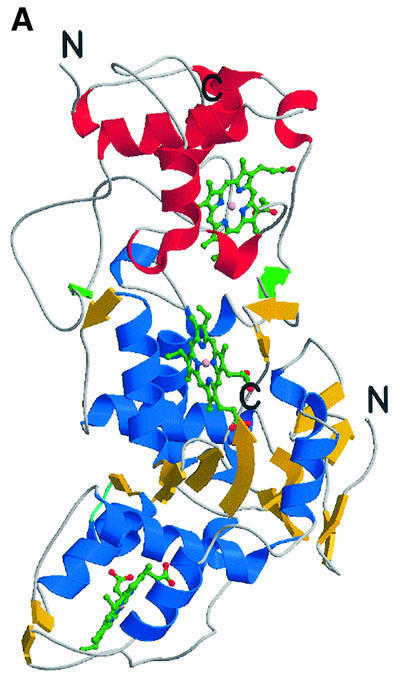
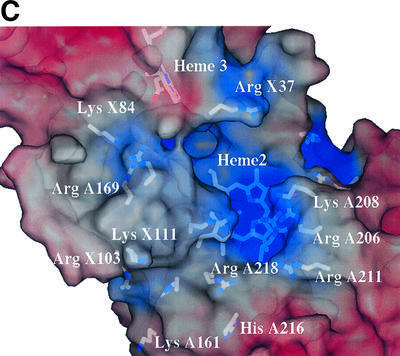
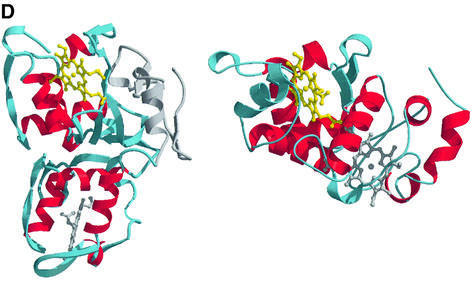
Fig. 3. The overall fold of the SoxAX complex. (A) The architecture of the complex. α-helices are shown in blue, and β-strands in yellow for SoxA, whilst for SoxX they are shown in red and green, respectively. The SoxA subunit is composed of two cytochrome c domains related by a pseudo 2-fold axis and contains two c-type haems with histidine–cysteine axial ligands. SoxX consists of a single cytochrome c fold with a histidine– methionine co-ordinated haem. (B) A stereo view of the Cα trace of the SoxAX molecule with every twentieth residue labelled. The SoxX subunit is coloured yellow, whilst for SoxA, the N-terminal 50 amino acids are coloured grey and the SoxAN and SoxAC cytochrome c domains are coloured blue and red, respectively. An insertion in the SoxX sequence from R.sulfidophilum relative to other cytochromes c (shown in purple) forms a major component of the subunit interface. (C) A close-up view of the electrostatic potential at the solvent-accessible molecular surface showing the substrate access channel and the binding groove for the C-terminal peptide of SoxYZ. The positions of arginine, lysine and histidine residues contributing to a basic substrate access funnel are shown. (D) Comparison of SoxA (left) with the two-domain cytochrome c fold of cytochrome c4 from Pseudomonas stutzeri (right). The C-terminal domains of SoxA and cytochrome c4 (yellow haems) are presented in the same orientation as determined by DALI (Holm and Sander, 1993). The four conserved helices (α1–α4) of the cytochrome c fold are shown for each domain in red. The N-terminal 50 amino acids of SoxA (shown in grey) pack against the face of the SoxAC domain, which in the previously characterized two-domain cytochromes c interacts with the N-terminal domains.
Table II. Haem geometric parameters and solvent accessibility.
| |
Haems 1–2 |
Haems 2–3 |
|
| Distance (Fe–Fe) (Å) | 31.4 (31.0) | 19.0 (18.8) | |
| Distance (closest approach) (Å) | 23.9 (23.4) | 10.7 (10.6) | |
| Interplanar angle (°) |
56.1 (55.1) |
80.95 (78.3) |
|
| |
Haem 1 |
Haem 2 |
Haem 3 |
| Ligand distances (Å) | |||
| Fe–S | 2.45 (2.37) | 2.41 (2.35) | 2.34 (2.43) |
| Fe–N | 2.08 (2.11) | 2.08 (2.08) | 2.05 (2.07) |
| Haem solvent accessibility (Å2) | 179.2 (179.7) | 56.7 (53.3) | 80.8 (82.9) |
The figures in parentheses refer to the average calculated over the four independent molecules of the P21 cell.
The haem 2–haem 3 interplanar angle and distance of closest approach together with the haem solvent-accessible areas are consistent with haem 3 being the route for electron transfer away from the complex (Table II). The solvent-exposed edge of haem 3, the presumed locus for cytochrome c2 binding, lies on the opposite face of SoxX from haem 2. The edge–edge separation of haems 1 and 2, in contrast to that between haems 2 and 3, is sufficiently long to preclude efficient electron transfer in the absence of a chain of cofactors (Page et al., 1999). An extended groove runs across the molecule at the subunit interface. This leads to a solvent-filled channel lined with basic residues, at the bottom of which the haem 2 iron atom and modified cysteine ligand lie exposed to solvent (Figure 3C). These observations, together with the post-translational modification to haem 2, led us to identify this haem as the catalytic active site.
The folds of the SoxA and SoxX subunits
The SoxX subunit has a c-type cytochrome fold as defined in the SCOP database (Holm and Sander, 1993). Yeast mitochondrial cytochrome c (PDB entry 1ycc, 108 residues) was found to be most similar, with 82 structurally aligned residues and an r.m.s.d. of 2.3 Å. The SoxX subunit can thus be classified as a member of the mono-domain cytochrome c family (Murzin et al., 1995). Inspection of the SoxA subunit reveals a pronounced internal pseudo-dyad encompassing residues 51–150 and 151–250 for domains that we term SoxAN and SoxAC, respectively. Alignment of these domains gave an overall r.m.s.d. of 1.8 Å for 93 structurally conserved residues for which the percentage residue identity was 18%. The haem groups in each domain are also well conserved, having an r.m.s.d. of 1.2 Å in this alignment (excluding propionate groups). The folds of these domains are clearly highly homologous and, as was the case for SoxX, each of these A-subunit domains has a c-type cytochrome fold. Again taking the mitochondrial cytochrome c for comparison, the r.m.s.ds are 2.7 and 3.1 Å for the SoxAN and SoxAC domains (both 69 aligned residues), respectively. The structure, in conjunction with sequence alignment, suggests that SoxA, apart from the N-terminal 50 amino acids, has evolved from duplication of a cytochrome c gene.
The di-haem SoxA subunit
Like SoxA, proteins of the two-domain cytochrome c family possess a pseudo 2-fold symmetry axis relating the two haem domains (Murzin et al., 1995). However, fold homology searches failed to align the SoxA subunit with members of this family (Chen et al., 1994; Fülöp et al., 1995; Kadziola and Larsen, 1997). As such, SoxA represents the first example of a new family of two-domain cytochromes c in which the packing of the two domains differs radically from the previously characterized examples (Figure 3D). The novel domain arrangement seen in SoxA may arise because a combination of the C-terminal (residues 251–261) and N-terminal polypeptides (residues 1–50) of SoxA block the face of the C-terminal domain that forms the domain interface in the other two-domain cytochrome c proteins. The result is that in SoxA the two domains pack with their C-terminal α4 helices arranged about the local pseudo 2-fold axis relating the SoxAN and SoxAC domains. A major consequence of this unusual packing is to give a distance of closest approach of the haems in SoxA (24 Å) that is much greater than that seen in the other two-domain cytochrome c proteins (<14 Å). The result is that effective electron transfer between the SoxA haems is precluded.
It is notable that the haem 1 consensus Cys-Xaa-Xaa-Cys-His haem-binding motif is absent from the SoxA proteins of Rhodopseudomonas palustris and of Chlorobium species (Figure 2A). In place of haem 1, an intradomain disulfide bridge is found between the remaining (second) cysteine of the haem-binding motif and the conserved cysteine residue that is a ligand to that haem in R.sulfidophilum SoxA (Klarskov et al., 1998). However, the α-carbon atoms of these cysteines in the SoxAN domain are >10 Å apart, a distance greater than the maximum for a disulfide bridge (Sowdhamini et al., 1989). The question then naturally arises as to whether, nevertheless, the AN domain of the R.palustris and Chlorobium sp. SoxA proteins can adopt the cytochrome c fold but substitute a disulfide bridge for the c haem to form a different superfamily. An important observation in this respect is that the haem-less SoxA domains preserve the cluster of hydrophobic residues at positions A61 (6), A65 (10), A134 (94) and A137 (97) (tuna cytochrome c residue numbering in parentheses; see Figure 2A) in the N- and C-terminal helices of the fold. These four residues are not involved in haem binding and are conserved in otherwise highly divergent cytochromes c. This, together with the absence of an apparent functional role for these residues, has led to the suggestion that they are involved in a common folding nucleus of all subfamilies of c-type cytochromes (Ptitsyn, 1998). It therefore appears reasonable to conclude that the haem-less SoxAN domain in these proteins may adopt a modified cytochrome c fold.
The nature of the subunit interface
The SoxAX complex is purified readily from the periplasm of R.sulfidophilum, suggesting a subunit interaction that is both specific and of relatively high affinity (Appia-Ayme et al., 2001). At present, the complex of yeast cytochrome bc1 with cytochrome c (Lange and Hunte, 2002) is the sole example of a crystal structure of a productive electron transfer complex involving two cytochrome c proteins. Furthermore, there are few examples of stable, structurally characterized homo-oligomeric proteins with the cytochrome c fold. Under these circumstances, the nature of the subunit interface in the SoxAX complex is of some interest. In an attempt to determine whether the SoxAC: SoxX packing is structurally unique, the PQS server (Henrick and Thornton, 1998) was used to generate an independent set of stable cytochrome c dimer packings. Adding to these the domain packings observed in the two-domain cytochromes c, a total of 11 independent cytochrome c:cytochrome c domain packings were found. Comparison reveals that the SoxAC:SoxX domain packing bears a passing resemblance to that observed between monomers of the cytochrome c6 from the green alga Chlamydomonas reinhardtii although the relative orientation of the haems is significantly different. It is appropriate to note that there is some evidence that the dimerization of the latter has functional significance (Kerfeld et al., 1995). Notably, there is no similarity between the packing of cytochrome c domains at the SoxAC:SoxX interface and that observed in the yeast cytochrome bc1:cytochrome c complex (Lange and Hunte, 2002). Thus, the packings of the c-type cytochrome domains both within SoxA and at the subunit interface are essentially unique. Cytochrome c domains are limited in their packing arrangements if the haem groups are to fall within suitable minimum edge–edge distances for electron transfer without the requirement for other cofactors. This requirement is clearly illustrated in the packing of domains within the SoxA subunit.
Analysis of the high resolution structure reveals a subunit interface that is well packed. This is evidenced by the shape complementarity statistic (Sc) (Lawrence and Colman, 1993), which has a value of 0.74 and is consistent with previously compiled statistics on permanent heterodimeric complexes (Jones and Thornton, 1996). Despite being well packed, the interface is also apparently fairly plastic. The largest r.m.s.d. between pairs of high and low resolution structures of the complex is ∼0.6 Å (based on all α-carbon atoms), and this difference can be accounted for in the most part by a rigid body screw domain displacement of SoxX relative to SoxAC of 4° and a translation of 0.2 Å. As is often the case, the root cause of this difference in the SoxAX structures may be attributable to crystal lattice packing contacts. However, whilst the haem edge–edge distances and interplanar angles are not affected greatly by the excursions of the SoxX subunit relative to SoxA (Table II), the observed plasticity at least suggests that conformational changes may occur on binding of SoxYZ. The increased mobility of SoxX is also reflected in the average atomic temperature factor for atoms in this subunit (25.5 Å2) being greater than that for the SoxA subunit (15.1 Å2).
The interface of the complex is predominantly hydrophobic in nature, in keeping with that observed in other stable electron transfer complexes (Mathews and White, 1993). Approximately 2500 Å2 is buried on complex formation (6.8% of the total accessible surface area), of which apolar and aromatic residues contribute 42%. This buried area is consistent with that observed in other heterodimeric complexes (Jones and Thornton, 1996). The interface is composed of two distinct, non-overlapping contact regions which we label A and B. Four short polypeptide stretches in SoxA form the majority of the interaction surface with SoxX (Figure 2A). Those contributing to region A are 183–184 and 187–191, whilst 168–175 and 224–232 contribute to region B. In SoxX, a number of short polypeptides are involved in forming the interface: residues 37–44 and 55–60 (region A), and residues 89–93 (region B) (Figure 2B). These three polypeptides contribute ∼50% of the total buried surface area of the SoxX subunit in the complex. The remainder comes from residues 101–110 (region B) which form part of a loop extending from the core domain structure to form an intimate association with residues of SoxA. Notably, this interface loop (residues 99–118) constitutes a major insertion in SoxX relative to other members of the cytochrome c superfamily. Interestingly, this loop is also missing in the SoxX proteins from Chlorobium tepidum, Aquifex aeolicus and R.palustris (Figure 2B). In addition, the overall sequence homology of the other contact peptides in SoxX relative to the corresponding regions in the loop-less proteins appears to be low (Figure 2B) and this may have important consequences in terms of the stability of their complexes. It should be noted that the absence of a permanent SoxAX complex in these organisms may imply generation of a means of stabilizing one-electron-oxidized intermediates.
A single midpoint potential using NADH as reductant has been observed for R.sulfidophilum SoxAX with a value of +180 mV that has been assigned to two redox-linked haems (Little, 2000). The packing of SoxAC to SoxX at the subunit interface provides a haem–haem distance of closest approach of 10.7 Å. This distance is marginally shorter than that seen in the di-haem cytochrome c peroxidase (14.5 Å) and the cytochrome c4 protein (11.2 Å) and involves transversing a region of the interface predicted to be solvent accessible. However, electron transfer should be rapid and essentially independent of the environment between the haems for a separation of 14 Å or less (Page et al., 1999). We therefore assume that haems 2 and 3 constitute the redox-linked pair. The haem groups arranged closest by the SoxAC:SoxX packing are the C-pyrrole rings. Several lines of evidence have implicated the exposed edge of the haem pyrrole ring C in electron transfer in c-type cytochromes (Pelletier and Kraut, 1992).
Post-translational modification to catalytic haem ligand
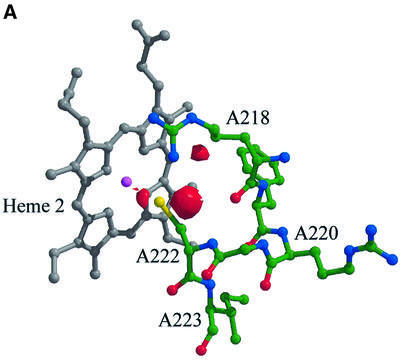
Fourier maps calculated using a fully refined structural model based on the predicted amino acid sequence of SoxA reveal regions of unexplained difference electron density adjacent to the cysteine axial haem ligand to haem 2 in the vicinity of its terminal sulfur atom. The |Fo – Fc| difference electron density peaks are at >5.2σ and 11.5σ from the mean electron density calculated over the volume of the molecule (where σ is the standard deviation of the distribution), indicating the presence of at least one additional atom (Figure 4A). The refined cysteine sulfur to ferric haem iron distance is 2.57 Å in this model. Given the likely geometry of the modified amino acid as indicated by the electron density, cysteine persulfide and cysteine sulfenic acid are the most probable derivatives. There is precedent for both modifications in other protein crystal structures (Claiborne et al., 1999; Bordo et al., 2000). We attempted to refine both possibilities against our X-ray data with the result that both the cysteine persulfide sulfane sulfur to haem iron distance and the alternative cysteine sulfenic acid terminal oxygen to haem iron distance refined to 2.41 Å. However, whilst the cysteine persulfide form showed only minor residual difference electron density after the modification, the cysteine sulfenic acid residue showed a +7.0σ difference electron density peak in the vicinity of the sulfenic oxygen atom. This strongly suggests that cysteine persulfide is the post-translational modification observed for CysA222. Unfortunately, anomalous difference Fourier maps calculated using the iron K-edge wavelength data set (λ1 in Table I), which could positively identify a terminal sulfane sulfur atom on this residue, were inconclusive due to the proximity of the additional atom to the iron centre. So, in the absence of an unambiguous sulfur anomalous scattering signal, further supportive evidence was sought for this interpretation.
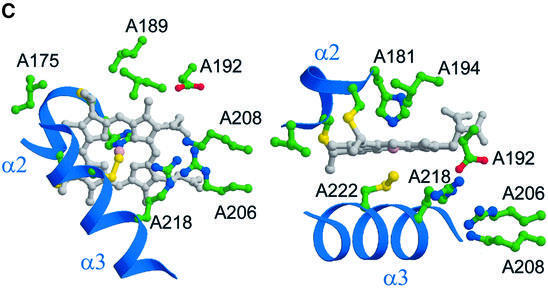
Fig. 4. The active site of SoxAX. (A and B) Post-translational modification of the haem 2 axial ligand. (A) The (Fo – Fc) Fourier map contoured at the 3.5σ level (shown in red), where σ represents the r.m.s. electron density for the unit cell. This map has been calculated using structure factor amplitudes and phases calculated from a refined model with a cysteine at residue A222. Note the small region of difference electron density adjacent to the side chain of ArgA218. This feature is also found in (Fo – Fc) maps calculated using the final refined structural model containing a cysteine persulfide haem ligand. The atoms and chemical bonds of haem 2 are coloured grey, except the haem iron which is magenta. (B) Stereo view of the (2Fo – Fc) electron density map around haem 2 calculated at 1.75 Å resolution and contoured at the 1.2σ level. This map has been calculated from a refined structural model with a cysteine persulfide at residue A222. (C) Orthogonal views of the active site region. CysA222 and HisA181 provide the axial ligands to the iron atom of haem 2. The basic residues A218, A206 and A208 form a possible proton-transfer relay from the active site.
While the additional density associated with the haem 2 cysteine ligand does not give an unambiguous sulfur anomalous scattering signal, additional spectroscopic and biochemical evidence strongly supports the assignment of a sulfur atom to this density. A combined EPR and MCD analysis of oxidized SoxAX indicates that haems 1 and 2 both have thiolate haem iron ligands (Cheesman et al., 2001). Haem 2, therefore, should be directly co-ordinated by a sulfur atom. To confirm the assignment of this atom as sulfur, we undertook a chemical analysis of the purified SoxAX protein and determined that SoxAX contains 1.0 mol of labile sulfur per three haems. Thus, there is chemical evidence for one atom of labile sulfur in each SoxAX molecule. Parallel quantitation of the low spin ferrocytochrome EPR signals indicates that there is 1.0 mol labile sulfur per pair of thiolate-ligated haems (data not shown). The modification to CysA222 was therefore taken to be the cysteine persulfide, and a view of the final electron density map is shown in Figure 4B.
Persulfide groups are generated in the catalytic cycles of various enzymes, for example, rhodanese (thiosulfate sulfurtransferase) (Gliubich et al., 1996; Bordo et al., 2000) and the biosynthetic enzyme, ThiI (Palenchar et al., 2000). In all cases, the persulfide groups are stabilized by the influence of their protein environment. This is certainly also the case in the SoxAX complex where co-ordination of the cysteine persulfide sulfane sulfur to haem ferric iron will stabilize the anionic form of the modified residue.
The enzyme active site
Amongst other indicators, the unprecedented post-translational modification to the CysA222 ligand to haem 2 and its accessibility to substrates suggest this haem to be the most likely site of thiosulfate oxidation (Figure 4C). A number of conserved arginine residues (A190, A206 and A218) plus other less well-conserved basic residues (LysA208 and ArgA224) can be found in the vicinity. This cluster of residues provides a highly basic electrostatic potential at the molecular surface in this region lining a channel which provides access to the active site for anionic substrates (Figure 3C). Electrostatic field calculations carried out with SPOCK (Christopher, 1997) show that in the vicinity of the presumed thiosulfate-binding site adjacent to the cysteine persulfide ligand to haem 2, the field rises to a value of ∼20 kT/e (ionic strength 0.15 M; relative dielectric constants of protein and solvent taken to be 4 and 80, respectively). The guanidinyl groups of arginines at positions A218 and A206 are found in close proximity (the distance of closest approach is 3.4 Å) and that of ArgA218 approaches to within 3.5 Å of the haem 2 plane. This suggests a low pKa for one (or both) of these residues and neutral charge at the pH of the crystal. The guanidinyl group of ArgA218 also lies within 3.8 Å of the persulfide sulfane sulfur of the side chain of CysA222 and the side chain populates a minor conformer of arginine (χ1, χ2) space (Kleywegt and Jones, 1998). It is certainly the case that ArgA218 is found in an unusual environment, and circumstantial evidence from the final Fourier maps also suggests that this residue may adopt alternative conformation(s) (Figure 4A). Our inference is that the side chain of this residue may be conformationally flexible, although the side chain average atomic B-value for this residue is not unusual, having a value of 16.4 Å2 compared with a molecule average of ∼20 Å2. Also evident from inspection of a molecular surface representation of the active site region is a groove running across the face of the complex, which may serve as part of the docking site for the SoxYZ co-substrate (Figure 3C). Preliminary docking studies using a Gly-Gly-Cys-Gly-Gly pentapeptide as a model for the flexible C-terminal region of SoxY demonstrate that there is sufficient space to locate the acceptor cysteine sulfur adjacent to haem 2 but insufficient for it to approach closely enough to act as an alternative haem iron axial ligand (data not shown).
The structure of dithionite-reduced SoxAX
Upon reduction of the SoxAX complex with dithionite, a change in the ligands of haem 2 is detectable spectroscopically (Cheesman et al., 2001). The suggested interpretation is that the thiolate ligand has become either protonated or replaced by an unidentified ligand. In the latter case, replacement of the thiolate haem ligand with one provided by the protein is envisaged. Redox-linked switches of amino acid ligands have been observed in other haemoproteins (Williams et al., 1997; Lanzilotta et al., 2000). In the absence of a major conformational change in SoxAX, ArgA218 is the only residue of the active site that could provide an alternative ligand for haem 2. To gain insight into this phenomenon, we wished to compare the structures of the enzyme in the oxidized and reduced states. Crystals of the enzyme were therefore treated with dithionite and the enzyme verified to be in the haem reduced state by single crystal microspectrophotometry. The reduced enzyme structure was solved by molecular replacement and refined to convergence at 1.5 Å resolution. The refined oxidized and reduced structures are very similar, with an r.m.s.d. of 0.17 Å over all 394 Cα atoms, rising to 0.35 Å when calculated over all atoms (including the haem groups). When the superposition was performed over all atoms within a 12 Å radius of the terminal sulfane sulfur of A222, the r.m.s.d. drops to 0.13 Å. Thus, the active site is essentially unchanged. In particular, the ArgA218 side chain does not move on reduction, being almost superimposible on that of the oxidized protein. The average B-value of its side chain atoms (10.5 Å2 compared with a molecule average of ∼16 Å2) drops slightly from that of the oxidized state, but no other systematic change in this residue was detected. The peak in the Fo – Fc Fourier map observed for the oxidized enzyme adjacent to the side chain Cγ atom is still present, albeit diminished, having a maximum value of 3.5σ (the corresponding value in the oxidized enzyme map was ∼5σ). Thus, at least in the crystalline state, ligand switching is not observed in the reduced SoxAX heterodimer and, by elimination, protonation of the thiolate ligand becomes the likely explanation for the observed spectroscopic changes. However, the physiological relevance of this observation made on a chemically reduced enzyme in the absence of substrate remains unclear.
A model for the mechanism of SoxAX
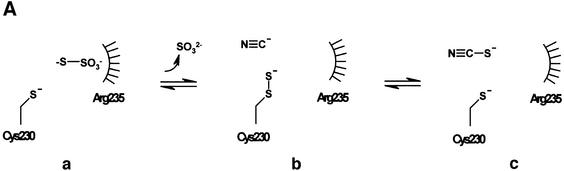
Friedrich et al. (2001) have ascribed the role of the SoxAX complex to the two-electron oxidation of substrates such as thiosulfate and sulfite during transfer to SoxY, yet the mechanism for enzymatic oxidation of reduced sulfur compounds is not well characterized. (Note that SoxY forms part of a complex with SoxZ, and subsequent reference to it in the text should be taken to imply involvement of the SoxYZ complex.) What insights then can the crystal structure of the enzyme provide to help explain the molecular basis for thiosulfate oxidation? A useful paradigm is found in the sulfurtransferases, such as rhodanese, which catalyse the transfer of a sulfane sulfur atom from an anionic donor substrate (such as thiosulfate) to a thiophilic acceptor via a sulfur-substituted enzyme covalent intermediate (Westley, 1973). The intermediate form (Figure 5Ab) is characterized by formation of a persulfide linkage to a catalytic cysteine residue, readily cyanolysed to restore the cysteinate (Figure 5Ac). Crystal structures are available for both forms of the enzyme from mammalian (Gliubich et al., 1996) and bacterial (Bordo et al., 2000) sources. These reveal an active site which is characterized by an unusually strong positive electrostatic field. This is generated by a combination of the unique alignment of peptide dipoles from an active site hexapeptide loop and from five basic residues which lie within 7 Å of the catalytic cysteine. One of these, a mobile arginine residue (Arg235 in the Azotobacter vinelandii enzyme), lies within 3.3 Å of the catalytic cysteine persulfide sulfane sulfur. The result of this field is to produce a highly stabilized persulfide form of the catalytic cysteine residue and also to provide a strong anion-binding site suitable for the substrate, thiosulfate. The electrostatic field in this binding site calculated for the bacterial enzyme (18 kT/e) is similar in magnitude to that calculated for the corresponding site in SoxAX.
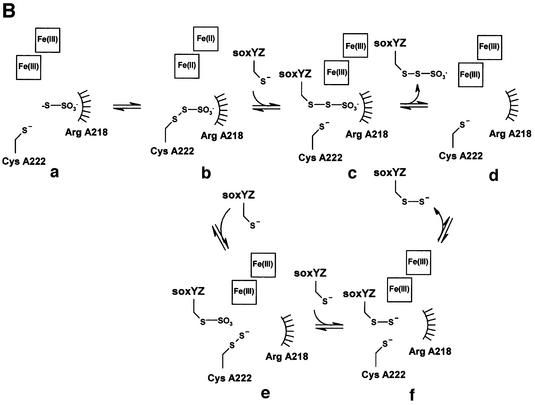
Fig. 5. Insights into the mechanism of thiosulfate oxidation. (A) The mechanism of the sulfurtransferase, rhodanese. (B) The proposed mechanisms for thiosulfate oxidation by SoxAX. Note that the point at which reoxidation of haems 2 and 3 occurs is not known.
The SoxAX and rhodanese structures have different folds and use different methods to stabilize the anionic form of a catalytic cysteine residue, yet arguably may share common features in their mechanisms. We have drawn on these parallels to suggest two possible catalytic mechanisms for reduced sulfur compound oxidation by SoxAX (Figure 5B). Whilst rhodanese transfers a sulfur atom from donor to acceptor groups without loss of electrons (consistent with the absence of a redox active group on the enzyme), SoxAX must perform the two-electron oxidative transfer of thiosulfate to the carrier protein SoxY. This is achieved by means of haems 2 and 3, which accommodate the two electrons liberated by the reaction. The enzyme must also provide a means for stabilization of the anionic forms of the catalytic cysteine residue and its persulfide derivative, and this is achieved by using this residue as axial ligand to an oxidized ferric c-type haem. Note that although cysteine persulfide is the crystallographically observed form for this residue in SoxAX, minor structural adjustments in this region would allow the alternative cysteinate form to bind to the haem 2 iron. Thus, as with rhodanese, it appears that both forms of the enzyme can be stabilized. The array of basic residues in the vicinity of the active site provides a strong electropositive potential which may also contribute to stabilization of the cysteinate form. The proximal arginine, A218, in the SoxAX structure shares features in common with Arg235 of rhodanese, lying immediately adjacent to the catalytic cysteine. We suggest a similar primary role for ArgA218, that is to provide an anionic-binding site suitable for the substrate sulfonyl group, orienting the substrate sulfane sulfur relative to the terminal catalytic cysteinate sulfur (Figure 5Ba). Transfer of the thiosulfate moiety to CysA222 now takes place, generating a two-electron-oxidized SoxA–thiocysteine-S-sulfate intermediate. The electrophilic character of ArgA218 polarizes the terminal sulfur–sulfur bond leaving the outer sulfane sulfur as the preferred axial ligand to ferrous haem iron. We presume that this results in a conformational change in the side chain of this residue leading to rearrangement of the ferrous haem iron axial ligand (Figure 5Bb).
One of two possibilities now occurs. In the first case, docking of SoxY results in formation of the SoxY– thiocysteine-S-sulfate product (Figure 5Bc). Diffusion of modified SoxY from the active site and reoxidation of haems 2 and 3 will restore the active resting state of SoxAX (Figure 5Bd). However, in this case, the crystallographically observed cysteine persulfide is not represented. In a second scenario, binding of SoxY results in generation of the alternative SoxY–cysteine-S-sulfate derivative through transfer of sulfite. The cysteine persulfide-modified enzyme (Figure 5Be) now represents a direct intermediate in the oxidative pathway. Import antly, it should also be possible to transfer the terminal enzyme-bound sulfane sulfur from this enzyme form to SoxY. In this case, SoxAX is acting as a sulfurtransferase, i.e. as a rhodanese-like enzyme. However, it may be possible that in the absence of SoxY and cytochrome c2, the intermediate (Figure 5Bb) can break down by a two-electron reduction process to give the crystallographically observed CysA222 persulfide with release of sulfite (not shown). We note that this is likely to occur during enzyme purification since thiosulfate is present in the cell lysate while the acceptor forms of SoxY and cytochrome c2 can no longer be regenerated. Note also that sulfite is predicted to be a free intermediate in this scheme as is observed during oxidation of thiosulfate by R.sulfidophilum (Appia-Ayme et al., 2001). Without further experimentation, it is difficult to conclude which of these pathways is more likely to be operative in vivo.
Conclusions
The crystal structure of the haemoprotein SoxAX complex presented here gives the first insights into the molecular basis for oxidation of reduced sulfur compounds by enzymes of the sulfur cycle. The analysis reveals a unique cysteine persulfide–histidine ligand pair to the presumed catalytic c-haem. Structural analysis of the dithionite-reduced enzyme reveals no significant differences compared with the crystal structure of the oxidized enzyme. Similarities between the active sites of the SoxAX complex and the sulfurtransferase, rhodanese, have allowed us to propose plausible mechanistic schemes that can now be tested by a combination of biochemical, structural and genetic approaches.
Materials and methods
Crystallization and crystal characterization
The SoxAX complex from R.sulfidophilum DSM1374T was purified as described previously (Appia-Ayme et al., 2001) from cultures grown photolithotrophically in the presence of thiosulfate. Crystals of typical dimensions 200 × 50 × 5 µm3 grew from vapour diffusion experiments using 15% (w/v) PEG 8K and 0.2 M magnesium acetate in 100 mM sodium MES buffer at pH 6.5. The crystals could be cryoprotected by transferring them to stabilization solution containing 25% (v/v) ethylene glycol. Initial X-ray data collection indicated that the crystals belong to space group P21 with cell parameters of a = 81.2 Å, b = 102.9 Å and c = 114.4 Å, with β = 110.5° at 100 K, and contain four copies of SoxAX per asymmetric unit. Subsequent data collection indicated the presence of a second crystal form growing under the same condition. These crystals belong to space group P21212 with cell parameters of a = 66.4 Å, b = 87.3 Å and c = 71.7 Å, and contain a single copy of SoxAX per asymmetric unit.
MAD phasing and structure solution of the P21 crystal form
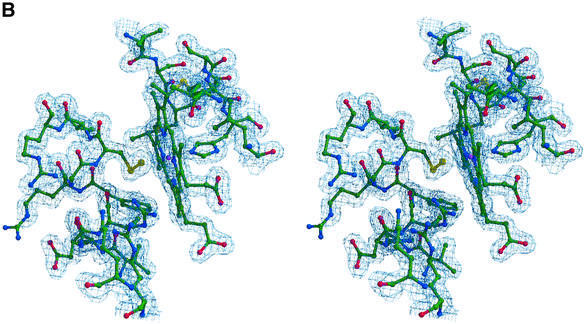
Data for the MAD phasing were collected from a single crystal at three different wavelengths (λ1–λ3) around the iron K-edge on station BM14 at ESRF, Grenoble, and processed using DENZO (Otwinowski and Minor, 1997) to 2.55 Å resolution. Determination of heavy atom sites and initial phase estimates were obtained using the program SOLVE (Terwilliger and Berendzen, 1999) using λ1 as the reference data set, and improved by solvent flattening using DM (Cowtan, 1994) at a nominal solvent content of 49%. The mean figure of merit for acentric reflections to 2.55 Å resolution was 0.814 after solvent flattening, and Fourier maps calculated with these phases showed clear and contiguous electron density of sufficient quality to trace residues 4–261 of SoxA and residues 1–51 and 55–137 of SoxX. Model building was performed using the program O (Jones et al., 1991), and all refinement procedures performed with CNS (Brünger et al., 1998). Application of the non-crystallographic symmetry (NCS) operators followed by rigid body refinement for this model gave an Rcryst of 36.9% and Rfree (Brünger, 1992) of 36.5%. Manual rebuilding and simulated annealing refinement resulted in a model consisting of residues 1–261 of SoxA and residues 1–51 and 55–137 of SoxX, with an Rcryst of 28.6% and Rfree of 30.1%. Incorporation of cysteine persulfide and residues 52–54 of SoxX followed by a single round of conjugate gradient refinement with an NCS restraint of 30 kcal/mol/Å2 resulted in an Rcryst of 26.4% and an Rfree of 29.4%. After addition of 691 water molecules into significant residual electron density, the structure refined to give a final Rcryst of 21.0% and an Rfree of 26.4%. When analysed for stereochemical quality using PROCHECK (Laskowski et al., 1993), the final structure (1H31) has 99.1% of residues in the most favoured regions of the Ramachandran plot, and only three residues from each copy of SoxAX fall in generously allowed regions.
Structure solution of the P21212 crystal form
High resolution data were collected from a single crystal on station ID14.2 at the ESRF, Grenoble, and processed using DENZO to 1.75 Å resolution. Molecular replacement using MOLREP of the CCP4 program suite (CCP4, 1994) was performed to transform the preliminary MAD-phased model into the crystallographic cell of the 1.75 Å high resolution data. Simulated annealing refinement resulted in a model with an Rcryst of 25.3% and Rfree of 27.3%. After incorporation of the cysteine persulfide, the three N-terminal residues of SoxA and 353 water molecules, and temperature factor refinement, the model converged to give a final Rcryst of 17.7% and Rfree of 21.4%. The final structure (1H33) has 99.4% of residues in the most favoured (91.1%) and additionally allowed (8.3%) regions of the Ramachandran plot, and only two residues fall in generously allowed regions. These two residues, ArgA190 and AlaX107, both lie on the extremities of β-turns which line the periphery of the active site cleft. Searches for structural homology were performed using DALI (Holm and Sander, 1993). DynDom (Haywood and Berendsen, 1998) was used for analysis of domain motions. Analysis of the interface was carried out using programs from the CCP4 program suite (CCP4, 1994) and Sc (Lawrence and Colman, 1993). Calculations of molecular electrostatics were performed with SPOCK (Christopher, 1997). Figures were produced using a combination of the programs MOLSCRIPT (Kraulis, 1991) and RASTER3D (Merritt, 1997).
Structure solution of reduced SoxAX
Aerobically grown single crystals of SoxAX were transferred to a reducing solution containing 15% (w/v) PEG 8K, 0.2 M magnesium acetate, 20 mM sodium dithionite, 25% (v/v) ethylene glycol and 100 mM sodium MES buffer at pH 6.5, and equilibrated for 30 min at 20°C before transfer to liquid nitrogen. High resolution data were collected from a single crystal on station 14.2 at the SRS, Daresbury Laboratory, and processed using DENZO to 1.5 Å resolution. The crystal was essentially isomorphous to that used for high resolution data collection from the oxidized form, being of space group P21212 with cell parameters a = 66.4 Å, b = 87.3 Å and c = 71.7 Å at 100 K. Structure solution and refinement proceeded as for the high resolution oxidized crystal form. After incorporation of the 261 residues of SoxA, including a cysteine persulfide, and 667 water molecules, and temperature factor refinement, the model converged to give a final Rcryst of 19.9% and Rfree of 23.0%. The final structure (1H32) has 99.4% of residues in the most favoured (91.1%) and additionally allowed (8.3%) regions of the Ramachandran plot. As with the high resolution oxidized structure, only residues ArgA190 and AlaX107 fall in generously allowed regions. Absorbance spectra measured at 100 K from this crystal before and following X-ray data collection using a 4DX Systems microspectrophotometer verified that crystalline SoxAX was reduced during the X-ray data collection experiment.
Sulfur analysis
The labile sulfur content of SoxAX was determined as the sulfide released following denaturation of the protein by guanidine hydrochloride in the presence of dithiothreitol (Beinert, 1983). Haem was quantified by the alkaline pyridine ferrohaemochrome method (Bartsch, 1971). The low-spin ferric EPR signals were acquired and quantified as described (Cheesman et al., 2001), with the total content of thiolate-ligated haems being taken as the sum of the LS1a, LS1b and LS2 signals.
Acknowledgments
Acknowledgements
We thank Judith Mayne, David Clarke and Jason Southgate for technical assistance. We acknowledge access to the UEA Centre for Metalloprotein Spectroscopy and Biology. This work was funded by the BBSRC through grant 83/B15211 and by the Wellcome Trust through project grant 059531/Z/99/Z.
References
- Appia-Ayme C., Little,P.J., Matsumoto,Y., Leech,A.P. and Berks,B.C. (2001) A cytochrome complex essential for photosynthetic oxidation of both thiosulfate and sulfide in Rhodovulum sulfidophilum. J. Bacteriol., 183, 6107–6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch R.G. (1971) Cytochromes: bacterial. Methods Enzymol., 23, 344–363. [Google Scholar]
- Beinert H. (1983) Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron–sulfur proteins. Anal. Biochem., 131, 373–378. [DOI] [PubMed] [Google Scholar]
- Bordo D., Deriu,D., Colnaghi,R., Carpen,A., Pagani,S. and Bolognesi,M. (2000) The crystal structure of a sulfurtransferase from Azotobacter vinelandii highlights the evolutionary relationship between the rhodanese and phosphatase enzyme families. J. Mol. Biol., 298, 691–704. [DOI] [PubMed] [Google Scholar]
- Brune D.C. (1989) Sulfur oxidation by phototrophic bacteria. Biochim. Biophys. Acta, 975, 189–221. [DOI] [PubMed] [Google Scholar]
- Brünger A.T. (1992) Free R value—a novel statistical quantity for assessing the accuracy of crystal structures. Nature, 355, 472–475. [DOI] [PubMed] [Google Scholar]
- Brünger A.T. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- CCP4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. [DOI] [PubMed] [Google Scholar]
- Cheesman M.R., Little,P.J. and Berks,B.C. (2001) Novel heme ligation in a c-type cytochrome involved in thiosulfate oxidation: EPR and MCD of SoxAX from Rhodovulum sulfidophilum. Biochemistry, 40, 10562–10569. [DOI] [PubMed] [Google Scholar]
- Chen Z.-W., Koh,M., Van Driessche,G., Van Beeuman,J.J., Bartsch,R.G., Meyer,T.E., Cusanovich,M.A. and Mathews,F.S. (1994) The structure of flavocytochrome c sulfide dehydrogenase from a purple phototrophic bacterium. Science, 266, 430–432. [DOI] [PubMed] [Google Scholar]
- Claiborne A., Yeh,J.I., Mallett,T.C., Luba,J., Crane,E.J., Charrier,V. and Parsonage,D. (1999) Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry, 38, 15407–15416. [DOI] [PubMed] [Google Scholar]
- Christopher J.A. (1997) SPOCK: The Structure Properties and Observation Toolkit. Center for Macromolecular Design, Texas A and M University, College Station, Texas, TX.
- Cowtan K. (1994) ‘dm’: an automated procedure for phase improvement by density modification. Joint CCP4 ESF-EACBM Newsl. on Protein Crystallography, 31, 34–38. [Google Scholar]
- Deckert G. et al. (1998) The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature, 392, 353–358. [DOI] [PubMed] [Google Scholar]
- Friedrich C.G., Quentmeier,A., Bardischewsky,F., Rother,D., Kraft,R., Kostka,S. and Prinz,H. (2000) Novel genes coding for lithotrophic sulfur oxidation of Paracoccus pantotrophus GB17. J. Bacteriol., 182, 4677–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C.G., Rother,D., Bardischewsky,F., Quentmeier,A. and Fischer,J. (2001) Oxidation of reduced inorganic sulfur compounds by bacteria: emergence of a common mechanism? Appl. Environ. Microbiol., 67, 2873–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülöp V., Ridout,C.J., Grenwood,C. and Hajdu,J. (1995) Crystal structure of the di-haem cytochrome c peroxidase from Pseudomonas aeruginosa. Structure, 3, 1225–1233. [DOI] [PubMed] [Google Scholar]
- Gliubich F., Gazerro,M., Zanotti,G., Delbono,S., Bombieri,G. and Berni,R. (1996) Active site structural features for chemically modified forms of rhodanese. J. Biol. Chem., 271, 21054–21061. [DOI] [PubMed] [Google Scholar]
- Haywood S. and Berendsen,H.J.C. (1998) Systematic analysis of domain motions in proteins from conformational change: new results on citrate synthase and T4 lysozyme. Protein Struct. Funct. Genet., 30, 144–154. [PubMed] [Google Scholar]
- Henrick K. and Thornton,J.M. (1998) PQS: a protein quaternary structure file server. Trends Biochem. Sci., 23, 358–361. [DOI] [PubMed] [Google Scholar]
- Holm L. and Sander,C. (1993) Protein-structure comparison by alignment of distance matrices. J. Mol. Biol., 233, 123–138. [DOI] [PubMed] [Google Scholar]
- Jones S. and Thornton,J.M. (1996) Principles of protein–protein interactions. Proc. Natl Acad. Sci. USA, 93, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.A., Zou,J.Y., Cowan,S.W. and Kjeldgaard,M. (1991) Improved methods for building protein models in electron density maps and location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- Kadziola A. and Larsen,S. (1997) Crystal structure of the dihaem cytochrome c4 from Pseudomonas stutzeri determined at 2.2 Å resolution. Structure, 5, 203–216. [DOI] [PubMed] [Google Scholar]
- Kelly D.P., Shergill,J.K., Lu,W.-P. and Wood A.P. (1997) Oxidative metabolism of inorganic sulfur compounds by bacteria. Antonie van Leeuwenhoek, 71, 95–107. [DOI] [PubMed] [Google Scholar]
- Kerfeld C.A., Anwar,H.P., Interrante,R., Merchant,S. and Yeates,T.O. (1995) The structure of chloroplast cytochrome c6 at 1.9 Å resolution: evidence for functional oligomerization. J. Mol. Biol., 250, 627–647. [DOI] [PubMed] [Google Scholar]
- Klarskov K., Verte,F., Van Driessche,G., Meyer,T.E., Cusanovich,M.A. and Van Beeumen,J. (1998) The primary structure of soluble cytochrome c-551 from the phototrophic green sulfur bacterium Chlorobium limicola, strain Tassajara, reveals a novel c-type cytochrome. Biochemistry, 37, 10555–10562. [DOI] [PubMed] [Google Scholar]
- Kleywegt G.J. and Jones,T.A. (1998) Databases in protein crystallo graphy. Acta Crystallogr. D, 54, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Kraulis P.J. (1991) MOLSCRIPT—a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr., 24, 946–950. [Google Scholar]
- Lange C. and Hunte,C. (2002) Crystal structure of the yeast cytochrome bc1 complex with its bound substrate cytochrome c. Proc. Natl Acad. Sci. USA, 99, 2800–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzilotta W.N., Schuller,D., Thorsteinsson,M.V., Kirby,R.L., Roberts,G.P. and Poulos,T.L. (2000) Structure of the CO-sensing transcription activator CooA. Nat. Struct. Biol. 7, 876–880. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., MacArthur,M.W., Moss,D.A. and Thornton,J.M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr., 26, 283–291. [Google Scholar]
- Lawrence M.C. and Colman,P.M. (1993) Shape complementarity at protein–protein interfaces. J. Mol. Biol., 234, 946–950. [DOI] [PubMed] [Google Scholar]
- Little P.J. (2000) Photolithotrophic thiosulfate oxidation in a purple ‘non-sulfur’ bacterium. PhD Thesis, University of East Anglia, UK.
- Lu W.-P., Swoboda,B.E.P. and Kelly,D.P. (1985) Properties of the thiosulfate-oxidizing multi-enzyme system from Thiobacillus versutus. Biochim. Biophys. Acta, 828, 116–122. [Google Scholar]
- Mathews F.S. and White,S. (1993) Electron transfer proteins/enzymes. Curr. Opin. Struct. Biol., 3, 902–911. [Google Scholar]
- Meier M., Janosik,M., Kery,V., Kraus,J.P. and Burkhard,P. (2001) Structure of human cystathionine β-synthase: a unique pyridoxal 5′-phosphate-dependent heme protein. EMBO J., 20, 3910–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt E.A. and Bacon,D.J. (1997) Raster3D: photorealistic molecular graphics. Methods Enzymol., 277, 505–524 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyaya P.N., Deb,C., Lahiri,C. and Roy,P. (2000) A soxA gene, encoding a diheme cytochrome c and a sox locus, essential for sulfur oxidation in a new sulfur lithotrophic bacterium. J. Bacteriol., 182, 4278–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzin A.G., Brenner,S.E., Hubbard,T. and Chothia,C. (1995) SCOP: a structural classification of proteins database for the investigation of sequences and structures. J. Mol. Biol., 247, 536–540. [DOI] [PubMed] [Google Scholar]
- Ojha S., Hwang,J., Kabil,O., Penner-Hahn,H.E. and Banerjee,R. (2000) Characterization of the heme in human cystathionine β-synthase by X-ray absorption and electron paramagnetic resonance spectroscopies. Biochemistry, 39, 10542–10547. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z. and Minor,W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol., 276, 307–326. [DOI] [PubMed] [Google Scholar]
- Page C.C., Moser,C.C., Chen,X.X. and Dutton,P.L. (1999) Natural engineering principles of electron tunnelling in biological oxidation–reduction. Nature, 402, 47–52. [DOI] [PubMed] [Google Scholar]
- Palenchar P.M., Buck,C.J., Cheng,H., Larson,T.J. and Mueller,E.G. (2000) Evidence that ThiI, an enzyme shared between thiamine and 4-thiouridine biosynthesis, may be a sulfurtransferase that proceeds through a persulfide intermediate. J. Biol. Chem., 275, 8283–8286. [DOI] [PubMed] [Google Scholar]
- Pelletier H. and Kraut,J. (1992) Crystal structure of a complex between electron-transfer partners, cytochrome c peroxidase and cytochrome c. Science, 258, 1748–1755. [DOI] [PubMed] [Google Scholar]
- Ptitsyn O.B. (1998) Protein folding and protein evolution: common folding nucleus in different subfamilies of c-type cytochromes? J. Mol. Biol., 278, 655–666. [DOI] [PubMed] [Google Scholar]
- Quentmeier A. and Friedrich,C.G. (2001) The cysteine residue of the SoxY protein as the active site of protein-bound sulfur oxidation of Paracoccus pantotrophus GB17. FEBS Lett., 503, 168–172. [DOI] [PubMed] [Google Scholar]
- Rother D., Henrich,H.-J., Quentmeier,A., Bardischewsky,F. and Friedrich,C. (2001) Novel genes of the sox gene cluster, mutagenesis of the flavoprotein SoxF and evidence for a general sulfur-oxidising system in Paracoccus pantotrophus GB17. J. Bacteriol., 183, 4499–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowdhamini R., Srinivasan,N., Shoichet,B., Vonsanti,D., Ramakrishnan,C. and Balaram,P. (1989) Stereochemical modeling of disulfide bridges—criteria for introduction into proteins by site-directed mutagenesis. Protein Eng., 3, 95–103. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. and Berendzen,J. (1999) Automated structure solution for MIR and MAD. Acta Crystallogr. D, 55, 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westley J. (1973) Rhodanese. Adv. Enzymol., 39, 327–368. [DOI] [PubMed] [Google Scholar]
- Williams P.A., Fulop,V., Garman,E.F., Saunders,N.F., Ferguson,S.J. and Hajdu,J. (1997) Heme-ligand switching during catalysis in crystals of a nitrogen-cycle enzyme. Nature, 389, 406–412. [DOI] [PubMed] [Google Scholar]
- Wood P.M. (1988) Chemolithotrophy. In Anthony,C. (ed.), Bacterial Energy Transduction. Academic Press, London, UK, pp. 183–230.