Abstract
The low-density lipoprotein receptor-related protein (LRP) has recently been implicated in numerous intracellular signaling functions, as well as in Alzheimer’s disease pathogenesis. Studies have shown that the β-amyloid precursor protein (APP) interacts with LRP and that this association may impact the production of amyloid β-protein (Aβ). In this report, we provide evidence that LRP regulates trafficking of intracellular proteins independently of its lipoprotein receptor functions. We show that in the absence of LRP, Aβ production, APP secretion, APP internalization, turnover of full-length APP and stability of APP C-terminal fragments are affected. Importantly, these changes are not APP isoform dependent. Using deletion constructs, the critical region in LRP that modulates APP processing was mapped to a seven peptide domain around the second NPXY domain (residues 4504–4510). Therefore, we propose a model by which LRP functionally modulates APP processing, including those steps critical for Aβ production, through interactions of the cytosolic domains.
Keywords: amyloid β-peptide/β-amyloid precursor protein/low-density lipoprotein receptor-related protein/processing/trafficking
Introduction
The low-density lipoprotein (LDL) receptor-related protein (LRP) is a member of the LDL receptor family of endocytic receptors. Although structurally similar to other members of the LDL receptor gene family, LRP is considerably larger. LRP is a 600 kDa transmembrane glycoprotein that is cleaved in the trans-Golgi network by furin to generate a 515 kDa α- and an 85 kDa β-subunit (Herz et al., 1990). The subunits remain associated in a non-covalent fashion as they are routed to the cell surface (Herz et al., 1990; Willnow et al., 1996); from there, LRP recycles between endosomal locations and the cell surface (Ward et al., 1989). More than 19 ligands have been reported to bind to the large 515 kDa N-terminal fragment of LRP, which can be divided into four ligand-binding domains (Moestrup et al., 1993; Willnow et al., 1994; Holtzman et al., 1995; Bu and Rennke, 1996; Horn et al., 1997), but not to the 85 kDa C-terminal β-subunit of LRP. While the LDL receptor appears to function exclusively in lipoprotein metabolism, increasing evidence suggests that LRP and the other members of the gene family have diverse biological roles, including signal transduction (Boucher et al., 2002; Loukinova et al., 2002) and neurotransmission (reviewed by Herz, 2001).
LRP and several of its ligands: β-amyloid precursor protein (APP), apolipoprotein E (ApoE) and α2-macroglobulin (α2M), all of which are found within senile plaques, have been genetically associated with Alzheimer’s disease (AD; reviewed by Hyman et al., 2000). In addition, LRP has been shown to be a receptor for the kunitz proteinase inhibitor (KPI)-containing isoform of the amyloid precursor protein (APP). As the KPI isoform is present as a cell surface or secreted molecule, both the secreted (APPs) and cell surface APP, upon complexing with serum proteases, have been shown to interact with LRP (Kounnas et al., 1995; Knauer et al., 1996; Rebeck et al., 2001). LRP has been shown to mediate clearance of amyloid β-protein (Aβ)–α2M complexes in vitro and possibly facilitate the clearance of Aβ from brain in vivo (Narita et al., 1997; Kang et al., 2000; Shibata et al., 2000). Aβ, derived from APP, is the principal constituent of senile plaques deposited in brains of AD individuals and is believed to hold a central position in AD pathogenesis (reviewed by De Strooper and Annaert, 2000).
Although the factors governing production and deposition of Aβ are not fully understood, it has been shown that APP can undergo at least two post-translational processing pathways. In one pathway, APP is cleaved within the Aβ region by a proteinase activity known as α-secretase, which recently has been determined as a member of the ADAM family metalloproteinases (reviewed by De Strooper and Annaert, 2000). This gives rise to APPs and prevents generation of an intact Aβ polypeptide. The residual 10 kDa C-terminal APP fragment (CTF-α) remains within the plasma membrane and can be cleaved by γ-secretase activity to release a truncated Aβ peptide, termed p3. Alternatively, APP can undergo proteolytic cleavages by β-secretase (BACE) followed by γ-secretase to generate Aβ (Hussain et al., 1999; Vassar et al., 1999). This is referred to as the amyloidogenic pathway of APP processing, which can take place intracellularly in the secretory compartments or following internalization of cell surface APP in the endocytic pathway (Koo and Squazzo, 1994; Cook et al., 1997; Hartmann et al., 1997; Skovronsky et al., 1998). It has been suggested that the cytoplasmic YENPTY sequence mediates APP internalization into endosomes and, in turn, generation of the secreted pool of Aβ (Koo and Squazzo, 1994; Perez et al., 1999).
In a recent study, LRP was linked directly to Aβ production in cells overexpressing APP751. When LRP binding was blocked by the 39 kDa receptor-associated protein, RAP, or in cells deficient in LRP, Aβ levels were dramatically reduced (Ulery et al., 2000). This led the authors to propose that interaction between LRP and the KPI domain of APP at the cell surface modulates APP internalization and subsequent Aβ production. Although this attractive hypothesis is consistent with the current models of Aβ production, it implies that the neuron-specific APP695 isoform, which lacks the KPI domain, is excluded from this interaction. This prediction would, at first approximation, be surprising since neuronal APP is believed to be the major source of Aβ in brain. In this study, we tested this hypothesis and our results failed to confirm this proposed model. We show in cells deficient in LRP that a number of steps in APP processing, trafficking and turnover are indeed impaired in the absence of LRP. Surprisingly, these perturbations are present with both APP695 and APP751 isoforms. Moreover, these alterations can be restored when an artificial LRP construct, which truncates the N-terminus of the 85 kDa β-subunit but retains the entire cytoplasmic domain, is expressed in the LRP-deficient cells, indicating that interactions between the respective cytoplasmic regions play a major role in APP processing. We further narrowed the essential region in the cytoplasmic tail to a small domain that encompasses the second NPXY motif in LRP. In view of the findings that a number of cytoplasmic proteins have been shown to interact with the cytoplasmic tail of both APP and LRP, we propose a model by which APP processing is modulated by communication between the cytoplasmic domains of APP and LRP, possibly via the cytoplasmic adaptor proteins.
Results
APP processing is altered in the absence of LRP
To determine whether the processing of APP is affected in the absence of LRP, APP751 was first introduced into mouse fibroblasts deficient in LRP (LRP–/–) and corresponding control (LRP+/–) cells by retroviral-mediated infection. Single clones of comparable expression were chosen for analyses. When the cell supernatants were normalized to APP expression, the level of APPs in LRP–/– cells transfected with APP751 was 2.5-fold higher than in LRP+/– cells (Figure 1A), consistent with the recently reported results (Ulery et al., 2000).
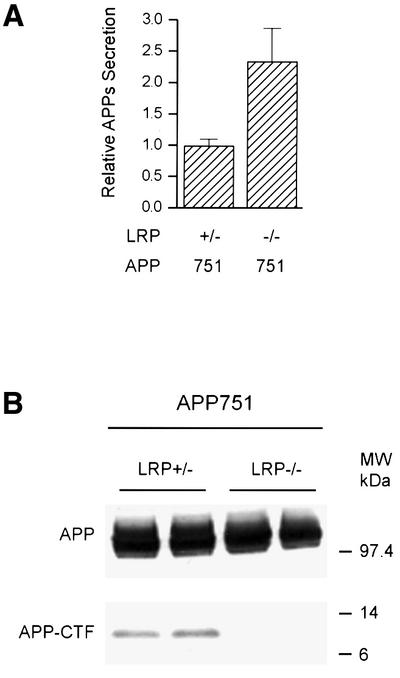
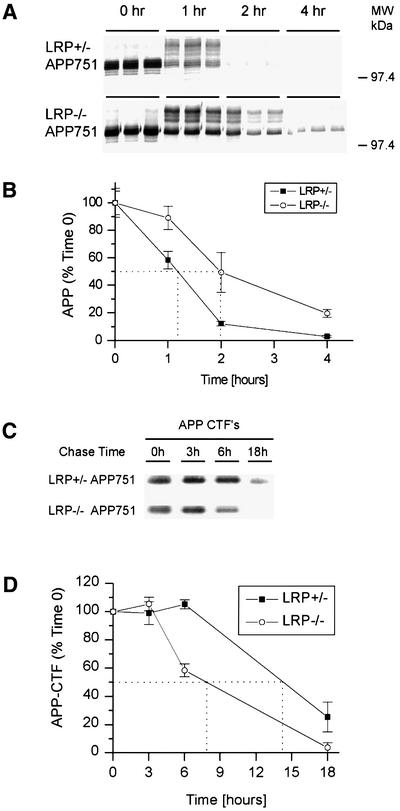
Fig. 1. APPs and APP-CTF levels in LRP–/– cells. Mouse embryonic fibroblasts lacking the LRP gene (LRP–/–) and corresponding LRP-expressing control fibroblasts (LRP+/–) were stably transfected with APP751. APPs was immunoprecipitated using the monoclonal antibody 1G7-5A3 and the samples immunoblotted with an APP polyclonal antibody (863) as described in Materials and methods. (A) LRP-deficient cells secrete ∼2.5-fold more APPs than LRP-expressing cells. Results are expressed as the average of two experiments in triplicate ± SEM. (B) APP expression in LRP+/– and LRP–/– fibroblasts was determined by immunoblotting with a polyclonal antibody (CT15) raised against the C-terminus of APP. Single clones of similar APP expression were selected (top panel). In LRP–/– cells, there is a dramatic reduction in the level of APP-αCTF (bottom panel).
We next assayed for APP C-terminal fragment (CTF) in LRP-deficient cells because APPs secretion and the generation of CTFs frequently are correlated with each other. Although the expression of APP in LRP–/– and control LRP+/– cells was comparable (Figure 1B, top), the levels of CTF, specifically α-secretase-cleaved CTF (αCTF), were significantly decreased in LRP–/– cells (between 4- and 6-fold; average 5.2 ± 1.8-fold, n = 4), as compared with controls (Figure 1B, bottom).
The marked reduction in the amount of APP-CTF in LRP-deficient cells was unexpected. Accordingly, we next examined the turnover of full-length APP by pulse–chase analysis in LRP+/– and LRP–/– fibroblasts stably transfected with APP751 to determine whether instability of APP can account for loss of CTFs. Unexpectedly, the turnover rate of APP in LRP–/– fibroblasts was dramatically slowed rather than increased, as compared with control fibroblasts, indicating that LRP normally facilitates the rapid degradation of APP. Even after a 4 h chase period, APP can still be detected in LRP–/– fibroblasts at a time when APP in control cells was virtually all degraded (Figure 2A). The normal short half-life of APP (∼70 min) was almost doubled (∼120 min) in LRP-deficient cells (Figure 2B).
Fig. 2. Turnover of full-length APP and APP-αCTF in LRP–/– cells. LRP+/– and LRP–/– fibroblasts were pulse labeled with [35S]methionine/cysteine for 15 min and chased for 0, 1, 2 and 4 h (A). At time 0, APP consists predominantly of immature N-glycosylated species. Both the N-glycosylated and mature N- and O-glycosylated species are abundant at 1 h for both cell types. After a 2 h chase period, the APP level is dramatically reduced in LRP+/– cells and hardly detectable at 4 h. In contrast, APP can still be detected in LRP–/– cells even after a 4 h chase period. (B) The half-life was determined by quantitating the results from (A) as shown. (C) APP-αCTF turnover was determined by metabolically labeling LRP+/– and LRP–/– fibroblasts with [35S]methionine/cysteine for 1 h and chasing for 3, 6 and 18 h. Similar amounts of APP-αCTFs are present after the labeling and after the 3 h chase period in LRP+/– and LRP–/– cells. Even after an 18 h chase period, APP-αCTFs can still be detected in LRP+/– fibroblasts. However, APP-αCTFs in LRP–/– fibroblasts were almost completely degraded. (D) The half-life of APP-αCTF was determined by quantitating the results from (C) as shown. The experiment in (A) was performed in triplicate and that in (C) in duplicate, and representative experiments are shown.
The reduced turnover of full-length APP in LRP-deficient cells is surprising because increased stability of APP intuitively should have resulted in more, not less, APP-CTF. Therefore, the turnover of APP-CTF itself was next analyzed by the pulse–chase paradigm. The cells were labeled for 1 h, a duration that was found to result in comparable levels of CTFs in both LRP–/– and LRP+/– cells (Figure 2C), indicating that generation of CTFs was not impaired. After the initial 3 h chase period, APP-CTF levels in LRP+/– and LRP–/– cells remained similar. At later time points, however, APP-CTFs in LRP–/– cells were considerably more unstable. After an 18 h chase period when APP-CTFs were still present in LRP+/– fibroblasts, APP-CTFs in LRP–/– fibroblasts were almost completely degraded (Figure 2C). The turnover rate of APP-CTFs in LRP–/– cells was approximately doubled (Figure 2D). These results therefore indicate that the low levels of APP-CTF in LRP–/– cells are due in part to reduced stability of the fragments in the absence of LRP.
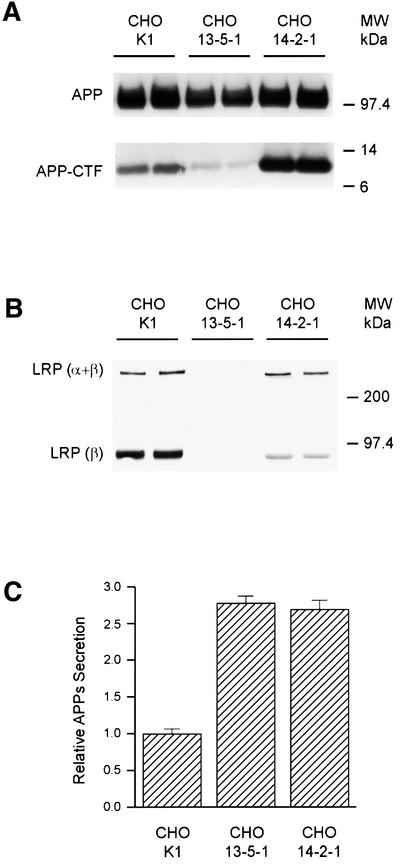
The above studies were performed in mouse fibroblasts overexpressing human APP. To exclude false-positive results caused by overexpression, we next analyzed untransfected CHO cells deficient in LRP (13-5-1) (FitzGerald et al., 1995). Confirming the results in mouse fibroblasts overexpressing APP, the levels of CTFs derived from endogenous APP, predominantly APP751, were consistently decreased in LRP-deficient CHO 13-5-1 cells as compared with controls (Figure 3A). In addition, since generation of APP-CTFs can occur both in the secretory and in the late endosomal–lysosomal compartments, a CHO cell line expressing a mutant form of LRP (14-2-1) was analyzed to determine where the turnover of APP-CTF was perturbed. Specifically, we asked whether retention of LRP in the endoplasmic reticulum (ER) and Golgi complex might restore the increased instability of APP-CTF seen in LRP-deficient cells. The CHO cell line 14-2-1 expresses a mutant LRP molecule that is defective in Golgi to plasma membrane trafficking, resulting in retention of LRP in the ER and Golgi compartments (FitzGerald et al., 1995). In these cells, the abnormal trafficking of LRP is reflected in the increased ratio of full-length (α + β subunit) LRP to light chain (β-subunit) LRP because the mutant LRP is retained in the early compartment, thus reducing the level of furin-cleaved β-subunit (Figure 3B, top). Interestingly, in CHO 14-2-1 cells, not only was the endogenous APP-CTF level not reduced as in CHO 13-5-1 LRP-deficient cells, but the level even exceeded that seen in control CHO K1 cells, while the full-length APP remained unchanged (Figure 3A). These results indicate that LRP in the early compartments specifically influences the stability of APP-CTFs. However, secretion of APPs (Figure 3C) in 14-2-1 cells remains elevated to a level similar to that of cells lacking LRP expression, indicating that post-Golgi APP trafficking steps are also highly dependent on LRP (see below). Importantly, these results show that the effects of LRP on APP-CTF and APPs are distinct properties that may be spatially segregated.
Fig. 3. APP processing in CHO cell lines. (A) Endogenous full-length APP (upper panel) and APP-CTF (lower panel) were immunoblotted with APP C-terminal antibody (CT15) from the CHO cell line 13-5-1 deficient in LRP, the CHO cell line 14-2-1 expressing a mutant LRP defective in trafficking to the plasma membrane, and the control CHO K1 cell line. Note that cells lacking LRP (13-5-1) show a significant reduction in the level of APP-αCTF (bottom panel), while the cells expressing the LRP trafficking mutant (14-2-1) show higher endogenous APP-αCTF levels. (B) LRP is detected by immunoblotting with the polyclonal antibody 1704 raised against the C-terminus of LRP. As expected, no LRP can be seen in the CHO cell line 13-5-1 (middle lanes), while the amount of β-subunit in CHO 14-2-1 cells is significantly less than in control cells, with the ratio indicating lack of furin cleavage due to retention in the early secretory compartments. Uncleaved full-length LRP (α + β) can be visualized and the levels are comparable between CHO 14-2-1 and CHO K1 control cells. (C) Secretion of endogenous APPs into medium was increased in both CHO 13-5-1 and CHO 14-2-1 cells as compared with control CHO K1 cells.
Abnormalities in APP processing are isoform independent
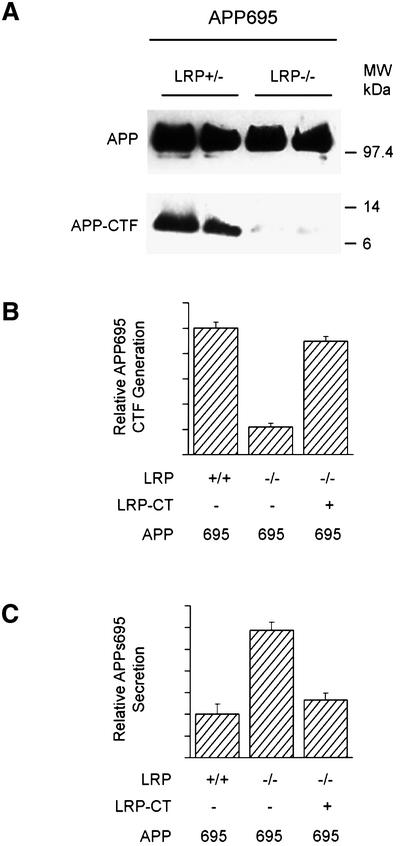
The above results are consistent with the notion that LRP influences many aspects of APP processing, indeed much more than the changes in APPs and Aβ that were reported recently (Ulery et al., 2000). We next wanted to determine whether the KPI domain in APP is solely responsible for these changes in APP processing. For this analysis, single clones of LRP–/– and LRP+/– fibroblasts expressing comparable levels of APP695 were isolated. Surprisingly, the levels of the APP-CTFs derived from APP695 were also significantly reduced in LRP–/– cells as compared with controls (Figure 4A). To confirm this finding of LRP on the processing of the APP695 isoform, CHO K1 and CHO 13-5-1 cells were transfected with APP695, and APPs secretion and APP-CTF levels were assessed. Consistent with our previous findings in LRP-deficient fibroblasts and LRP-deficient CHO cells expressing APP751, APP695 expressed in CHO cells also demonstrated both the reduction in APP-CTF level (Figure 4B) and an increase in APPs secretion (Figure 4C). The magnitude of the changes in these two parameters is virtually identical to that seen with the APP751 isoform.
Fig. 4. Alterations of APP processing in LRP–/– cells are isoform independent. (A) LRP+/– and LRP–/– cells stably transfected with APP695 were analyzed for levels of APP-αCTFs. Single clones of similar APP expression were analyzed (top panel). Note the dramatic reduction in APP-CTF expression in LRP –/– cells (bottom panel). Quantitation of APP-αCTF (B) and APPs (C) levels in LRP-deficient CHO 13-5-1 and control CHO cells transfected with APP695. Note that APP-αCTF and APPs derived from APP695 were altered similarly in LRP-deficient cells as those from APP751. Introduction of LRP-CT in the LRP- deficient cells restored the abnormalities in APP-αCTF (B) and APPs (C) back to control levels. Results are the average ± SEM (n = 2).
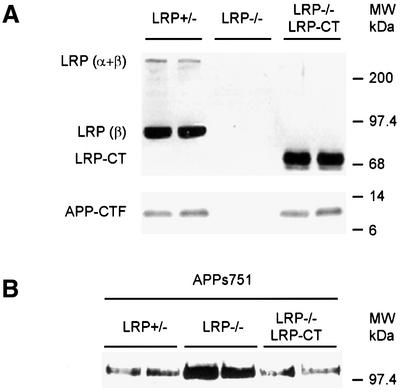
The above observations suggested that although the interaction between LRP and APP is greater with the KPI-containing APP isoform (Rebeck et al., 2001), the processing of APP is not regulated exclusively through interaction of its KPI domain with LRP, since the APP695 isoform lacking this domain also demonstrate LRP-dependent regulation of CTF and APPs levels. Rather, the results suggest that the respective C-termini of APP and LRP play functionally important roles in APP trafficking, a concept first proposed by Trommsdorff et al. (1998). To test this hypothesis, a truncated LRP construct consisting of the C-terminal 370 amino acids of LRP (LRP-CT) was engineered, and stably introduced into LRP–/– cells expressing APP751. This follows the approach taken by several laboratories that used mini-LRP receptors to delineate a number of functional ligand-binding domains of LRP (Willnow et al., 1994; Li et al., 2000). However, the LRP-CT construct used in this study is an N-terminal-deleted β-subunit, thus lacking all four functional ligand-binding domains, but this truncated construct nevertheless does reach the cell surface (data not shown). Interestingly, when the LRP-CT construct was introduced into LRP-deficient cells expressing APP751, the diminished levels of APP-CTFs were restored to levels similar to LRP+/– cells (Figure 5A). To confirm this hypothesis further, we re-examined the LRP-dependent changes in APPs secretion first noted in this study (Figure 1A). Consistent with the APP-CTF result, introduction of the LRP-CT construct into LRP–/– cells also restored the levels of APPs back to the levels seen in APP751-overexpressing cells (Figure 5B). Finally, the restoration of the abnormalities in APPs secretion and APP-CTF levels by LRP-CT were also seen in APP695-transfected cells (Figure 4B and C), confirming that the KPI domain is not essential for LRP-dependent aspects of APP processing. Taken together, these experiments indicate that the β-subunit, possibly only the cytoplasmic domain of LRP, play an important role in regulating APP processing.
Fig. 5. Processing of APP751 in LRP–/– cells can be restored by expression of an LRP-CT construct. (A) LRP–/– fibroblasts transfected with APP751 were infected with a retrovirus expressing the LRP-CT construct and immunoblotted with the LRP C-terminal antibody 1704. No signal is seen in the LRP–/– cells (middle lanes, top panel). The LRP-CT protein is a truncated β-subunit (right lanes, LRP–/– LRP-CT) and migrates faster than the authentic β-subunit (left lanes, LRP+/–) and, as expected, the full-length LRP species (α + β) is absent in the LRP–/– LRP-CT cells (right lanes, top panel). In the bottom panel, immunoblotting of APP-αCTF was carried out with the APP C-terminal antibody CT15. Note that following expression of the LRP-CT construct in LRP–/– cells, the normally low levels of APP-CTF in LRP–/– cells (middle lanes, bottom panel) are now restored to the level seen in LRP+/– cells (compare right with left lanes, bottom panel). (B) Levels of APPs in medium of LRP+/–, LRP–/– and LRP–/– LRP-CT cells were determined by immunoprecipitation/western blotting as before. As with APP-CTF levels, expression of LRP-CT in LRP–/– cells restores the abnormal levels of APPs. Similarly to APP695, APPs release derived from APP751 is decreased after introduction of LRP-CT in LRP–/– cells.
Internalization of APP in LRP+/–, LRP–/– and LRP–/– LRP-CT fibroblasts
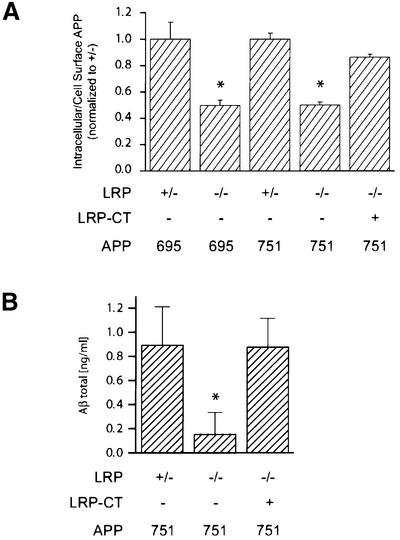
The internalization of cell surface APP is believed to represent a major pathway for generation and subsequent release of Aβ into the extracellular space. In LRP–/– and LRP+/– cells stably transfected with APP695 or APP751 constructs, APP endocytosis was measured using iodinated APP antibody. With this established assay, the internalization of APP was reduced by 50% in LRP–/– cells as compared with LRP+/– control cells. Significantly, this reduction in APP internalization was seen in both APP695- and APP751-expressing cells (Figure 6A). We then focused on APP751-expressing LRP–/– cells and asked whether this defect in APP internalization is related similarly to the absence of the LRP C-terminus. Indeed, consistent with all the changes noted above, the impairment in APP internalization was largely restored to control levels following introduction of the LRP-CT construct in LRP–/– cells (Figure 6A).
Fig. 6. APP internalization and Aβ secretion in LRP–/– cells. (A) Steady-state internalization of APP from the cell surface was measured with iodinated 1G7 antibody at 37°C (see Materials and methods). In LRP+/– control cells expressing either APP695 or APP751, ∼55% of APP is internalized. In this experiment, the ratios of internalized to cell surface APP are normalized to the LRP+/– control cells. LRP–/– cells stably transfected with APP751 or APP695 show a 50% reduction in APP endocytosis as compared with control (LRP+/–) cells. The reduction in APP internalization in LRP–/– cells is restored similarly after expression of the LRP-CT construct. (B) Reduction in Aβ release in LRP–/– cells is rescued by expression of the LRP-CT construct. Aβ was measured by ELISA from LRP–/–, LRP–/–LRP-CT and LRP+/– cells after 72 h collection. The Aβ level is reduced 5-fold in LRP–/– cells transfected with APP751 as compared with control LRP+/– cells transfected with APP751, normalized for the level of APP expression. The reduction in Aβ in LRP–/– cells is restored to control level after expression of the LRP-CT construct. All panels show the results (average ± SEM) of representative experiments performed in triplicate. Statistical analysis was performed using Student’s t-test (*P < 0.05).
To exclude the possibility that the reduced internalization of APP in LRP–/– cells is due to a more general defect in receptor-mediated endocytosis, internalization of transferrin receptor was assessed by transferrin (Tfn) uptake experiment. CHO-deficient LRP (13-5-1) and control CHO K1 cells were incubated with [125I]Tfn (3 µg/ml) for 30 min under steady-state conditions. In contrast to the reduced internalization of APP in LRP-deficient cells, the percentage of internalized Tfn remained the same regardless of whether LRP was present (average of 74.61 ± 1.17% and 74.45 ± 0.69% in CHO 13-5-1 and control cells, respectively), indicating that there was no defect in the endocytosis of the transferrin receptor.
Aβ levels in LRP+/–, LRP–/– and LRP-CT fibroblasts
As described above, it was reported recently that loss of LRP is associated with a marked reduction in Aβ levels, which was attributed to loss of the normal interaction between LRP and the KPI domain of APP (Ulery et al., 2000). In view of the changes in APP internalization that are independent of the KPI isoform, we measured the levels of Aβ in LRP-deficient fibroblasts. In LRP–/– cells transfected with APP751, the level of Aβ in the medium was reduced to ∼20% of that of control LRP+/– cells transfected with APP751 after 72 h collection. However, expression of the LRP-CT construct in LRP–/– cells resulted in a dramatic increase in the amount of Aβ in the medium (Figure 6B). Indeed, similarly to APPs, Aβ from LRP–/– cells expressing LRP-CT was restored to the level present in LRP+/– control cells. This finding is therefore consistent with the restoration by the truncated LRP-CT construct of essentially all the defects in APP processing seen in the absence of LRP.
The C-terminus of LRP modulates APP processing
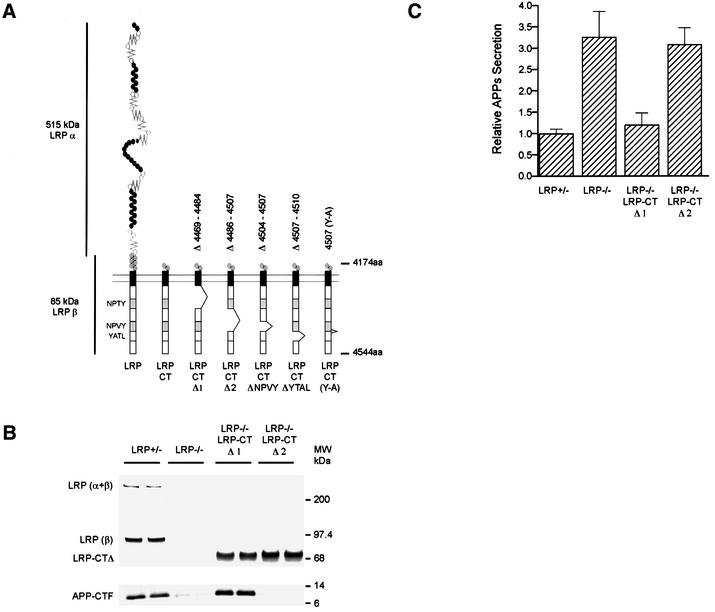
In this study, each LRP-dependent step in APP processing perturbed in the absence of LRP can be restored by the expression of the LRP-CT and, where tested, both APP695 and APP751 isoforms showed the same defects when LRP is absent. These results provided strong evidence that the LRP-CT, and potentially only the cytoplasmic domain, is important in the regulation of multiple steps in APP processing. A number of important physiological roles have been attributed to the cytoplasmic tail of LRP, among which are two NPXY motifs that have been shown to interact with a family of cytoplasmic adaptor proteins. However, the N-terminal truncated LRP-CT construct still contains ∼230 amino acids in the extracellular region where interaction with APP theoretically can take place, even though it does not contain any known ligand-binding domain. Therefore, to clarify which region in the LRP-CT is involved in the modulation of APP processing, two LRP-CT mutants, each with a deletion of the respective NPXY motif, were engineered. The two constructs, LRP-CTΔ1 and LRP-CTΔ2, with deletions of ∼20 amino acids each, were stably transfected into LRP–/– cells expressing APP751. Single clones of comparable expression were isolated for analysis (Figure 7B). As before, loss of LRP was associated with a large decrease in the levels of APP-CTFs. Interestingly, LRP-CTΔ1, but not LRP-CTΔ2, restored APP-CTFs to control levels (Figure 7B, bottom). Similarly, introduction of LRP- CTΔ1, but not the LRP-CTΔ2, construct into LRP–/– cells also restored the levels of APPs to those seen in LRP+/– control cells (Figure 7C). These results indicate that the responsible regulatory sequences are contained within LRP-CTΔ2, or between amino acids 4486 and 4507. This region contains the tetrapeptide NPVY motif, or the second of the two NPXY motifs in the cytoplasmic tail (Figure 7A). Thus, this experiment established that it is specifically the cytoplasmic domain of LRP that is critical for modulating these parameters in APP processing.
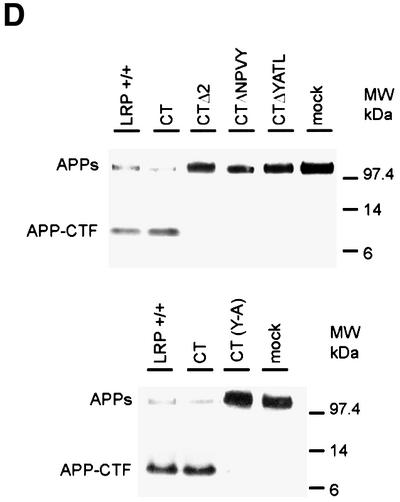
Fig. 7. The region around the second NPXY domain in LRP regulates APP processing. (A) Schematic illustration of two LRP-CT deletion constructs, LRP-CTΔ1 (lacking amino acids 4469–4484) or LRP-CTΔ2 (lacking amino acids 4486–4507). (B) Expression of LRP-CTΔ1 and LRP-CTΔ2 in LRP–/– cells is comparable to the endogenous level of LRP as seen by immunoblotting with LRP antibody 1704 (upper panel). The reduction in the levels of APP-CTFs in LRP–/– cells is restored by expression of the LRP-CTΔ1 but not the LRP-CTΔ2 construct. (C) Consistent with the results shown in (B), APPs secretion was restored to control LRP+/– levels only in the LRP-CTΔ1-transfected cell line but not in the LRP-CTΔ2 cell line. (D) LRP-CTΔNPVY (lacking amino acids 4504–4507), LRP-CTΔYATL (lacking amino acids 4507–4510) and LRP-CT (Y4507A) were expressed in LRP-deficient CHO 13-5-1 cells together with APP695 [see (A) for schematic]. Similarly to LRP-CTΔ2, expression of neither LRP-CTΔNPVY (top panel), LRP-CTΔYATL (top panel) nor LRP-CT (Y4507A) (bottom panel) was able to restore the reduction in APP-CTF levels or the increase in APPs to that seen in control K1 cells. In contrast, the expression of LRP-CT restored the deficiencies in APP processing in LRP–/– CHO 13-5-1 cells to control levels.
To narrow down the sequence within the LRP-CT responsible for these effects, we engineered two additional deletion constructs lacking either the tetrapeptide NPVY domain (LRP-CTΔNPVY) or the adjacent tetrapeptide YATL domain (LRP-CTΔYATL). Transfection of APP695 and the respective LRP-CT constructs into LRP-deficient CHO 13-5-1 cells revealed that, unlike LRP-CT, neither LRP-CTΔNPVY nor LRP-CTΔYATL was able to restore the levels of APP-CTF or APPs to those seen in wild-type LRP+/– control cells (Figure 7D). Because both deletion constructs overlap the tyrosine residue at position 4507, this suggested that this tyrosine is a critical residue. Consequently, an alanine-substituted mutant of LRP-CT at position 4507 (LRP-CT YA) was engineered and transfected together with APP695 into LRP-deficient cells. As predicted, LRP-CT YA behaved like the two LRP-CT deletion mutants (LRP-CTΔNPVY and ΔYATL) with respect to APPs and APP-CTF levels (Figure 7D), indicating that Tyr4507 is indispensable in the regulation of APP processing.
Discussion
The view that lipoprotein receptors function predominantly, if not exclusively, in lipid transport has been challenged by recent findings that several members of the family which are receptors for ApoE function in development and signaling processes (reviewed by Herz and Beffert, 2000; Boucher et al., 2002; Loukinova et al., 2002; May et al., 2002). In addition, a number of cytoplasmic adaptor and scaffold proteins bind to the cytoplasmic domain of LRP, although specific functions related to these interactions remain unclear. Therefore, the current study was designed to test the hypothesis that the regulation of APP processing by LRP occurs via the KPI domain and, if not, to test the hypothesis that this effect is mediated by the cytoplasmic domain of LRP. Our studies showed that LRP modulates multiple steps in APP processing and trafficking, including the turnover of both full-length and C-terminal fragments of APP, secretion of APPs, internalization of cell surface APP and the generation and release of Aβ peptide. Significantly, because the processing of both APP695 and APP751 was altered in the absence of LRP, these changes must be independent of the KPI isoform of APP. This then led us to demonstrate that the cytoplasmic domain of LRP, specifically the amino acid residues around the second NPXY motif, encompassing a seven amino acid region (NPVYATL), is critical for the regulation of APP processing. Taken together, we propose a model for LRP regulation of APP processing in which interaction between the cytoplasmic rather then the extracellular domains of LRP and APP are responsible for changes in APP processing and Aβ generation.
In addition to the changes in the levels of APPs and Aβ noted previously (Ulery et al., 2000), our study showed that the absence of LRP produced two unanticipated effects on the turnover of APP. Specifically, the half-life of full-length of APP was increased whereas the half-life of APP-CTFs was reduced. However, the slower turnover of full-length APP and its reduced internalization in the absence of LRP does not account for the decreased stability in APP-CTFs. We hypothesize that this change in APP-CTF stability is related to LRP’s effects in the ER and intermediate compartments. This is because the level of APP-CTF was markedly increased in the CHO 14-2-1 cell line expressing a LRP trafficking mutant. LRP is not absent in this cell line but rather does not traffic to the cell surface as it is retained intracellularly. Thus, the elevation of APP-CTFs in CHO 14-2-1 cells indicates an important interaction that influences the stability of APP-CTFs in these early compartments and prevents the CTFs from rapid degradation. This interpretation not only is consistent with the evidence that the ER and intermediate compartments are major sources of APP-CTFs (Annaert et al., 1999; Yan et al., 2001), but it is also in agreement with the finding that APP associates with endogenous full-length (α + β subunits) LRP (data not shown), thereby placing the interaction in the same intracellular compartments. Taken together, we hypothesize that the interaction between LRP and APP influences the turnover of APP-CTFs after their formation in the early secretory compartments.
In this study, we provided evidence that LRP’s effects on APP processing are mediated through the LRP cytoplasmic domain and that this interaction occurs early in the secretory compartments. By introducing various truncated LRP constructs into LRP-deficient cells, our studies showed that the distal C-terminus in the LRP cytoplasmic region is the major determinant of these effects. In particular, the LRP-CT deletion constructs (Δ1 and Δ2) implicated the second of the two NPXY motifs as important in this interaction. This notion was confirmed when two smaller deletion constructs, LRP-CTΔNPVY (lacking residues 4504–4507) and LRP-CTΔYATL (lacking residues 4507–4510), were both unable to rescue the defects in APP processing in LRP-deficient cells. Because an alanine substitution mutant of LRP-CT at Tyr4507 was also unable to rescue the abnormalities in APP processing in LRP-deficient cells, we postulate that this tyrosine residue in the C-terminus is critical for LRP’s effects on APP processing. However, as detailed alanine scanning mutagenesis has not been performed, we cannot exclude the possibility that other positions within this seven amino acid region are also required. Nevertheless, because LRP has been shown to complex with APP (Rebeck et al., 2001), although probably not by direct binding, we propose that this interaction is required to modulate APP processing. As LRP affects not only trafficking but also the stability of full-length APP and the CTFs, we conclude that these effects must be mediated in multiple intracellular compartments.
In the absence of LRP, the level of Aβ is markedly diminished, confirming an earlier report (Ulery et al., 2000). This decrease in Aβ is also correlated with a specific reduction in APP internalization from the cell surface. The impairment in APP internalization is in agreement with the hypothesis that endocytic processing is a major contributor to Aβ release. Similarly, the increase in APPs secretion is consistent with previous reports showing that loss of the APP endocytic signal elevates the levels of APPs in the medium (Koo et al., 1996). In this regard, it is interesting that the NPVYATL motif in LRP implicated in our studies overlaps with the internalization signal in LRP. Although LRP contains two NPXY and two dileucine motifs that are consensus signals for endocytosis, Li and colleagues recently reported that the -YATL-tetrapeptide domain overlapping with the second NPXY motif in the cytoplasmic tail of LRP is a major determinant in LRP internalization (Li et al., 2000). Since the region deleted in the LRP-CTΔ2, LRP-CTΔNPVY and LRP-CTΔYATL constructs includes the tyrosine in the second NPXY motif (4486–4507), internalization of the truncated LRP molecule is probably impaired. It has been proposed that the LRP-dependent changes are mediated via a cytoplasmic adaptor protein, which is capable of interacting with both APP and LRP via its multiple binding domains (Trommsdorff et al., 1998). Whether LRP and APP internalization is regulated coordinately through direct interaction or indirectly through adaptor proteins to regulate Aβ production is an intriguing possibility that awaits further study.
In summary, our results differed significantly from the proposed model in which LRP modulates APP processing via interaction in the respective extracellular domains, in particular the KPI domain of APP (Ulery et al., 2000). Previous studies have demonstrated convincingly that the interaction of the KPI isoform of APP with LRP is significantly greater than that of the non-KPI-containing APP695 isoform, especially in complexes containing proteinases (Kounnas et al., 1995; Knauer et al., 1996; Kinoshita et al., 2001; Rebeck et al., 2001). The results reported in this study do not argue against this association. Rather, our findings suggest that the functional consequences of the interaction between LRP and the KPI isoform of APP are distinct from the APP processing steps examined in this study, namely, APP turnover, APPs secretion, APP internalization and Aβ production. Lastly, as members of the LDL receptor gene family, including LRP, are implicated in neurodevelopment, it is possible that the internalization of LRP and its binding partners might be important for transducing these extracellular signals (Trommsdorff et al., 1998; Hiesberger et al., 1999). Coincidently, APP recently has been shown to play a role in gene transcription similar to that of the Notch receptor (Cao and Sudhof, 2001). In the latter situation, the cytoplasmic domain of APP together with Fe65 and Tip60 are required for a multimeric complex to translocate into the nucleus to affect transcription by a heterologous signaling system. Thus, LRP and APP, together with the cytoplasmic proteins, may indeed function beyond trafficking events and affect the transduction of cellular signals. If so, LRP may play a greater and more varied role in AD pathogenesis than has been suspected.
Materials and methods
Cell lines and cDNA constructs
Mouse fibroblasts deficient in LRP (LRP–/–; PEA 13) and corresponding LRP-expressing control cells (LRP+/–; PEA 10) were obtained from the American Type Culture Collection (Rockville, MD) and cultured as described previously (Kang et al., 2000). An LRP-deficient CHO cell line (13-5-1), a CHO cell line expressing an LRP trafficking mutant (14-2-1) and corresponding CHO K1 control cells (a kind gift of Dr S.Leppla) were grown in α-Dulbecco’s modified Eagle’s medium (αDMEM) supplemented with 10% fetal bovine serum (FitzGerald et al., 1995). Human APP695 and APP751 were inserted into the pBabepuro retroviral expression vector (Morgenstern and Land, 1990) and transferred into the GP + E86 packaging cell line. Stable transformants were selected with puromycin (5 µg/ml). After infection with recombinant viruses, mouse fibroblasts were selected with puromycin (2.5 µg/ml) and analyzed after clonal selection for equal APP expression. The last 370 amino acids of the 601 amino acid LRP β-subunit, consisting of the transmembrane domain and the entire cytoplasmic region, and its corresponding signal peptide sequence (LRP-CT) were subcloned into the pBabehygro retroviral expression vector (Morgenstern and Land, 1990) and transferred into the GP + E86 packaging cell line. Stable transformants were selected with hygromycin (2.5 µg/ml). After infection with recombinant viruses, LRP–/– mouse fibroblasts were selected with hygromycin (2.5 µg/ml) and analyzed (LRP–/–CT). Deletion substitution constructs lacking amino acids 4469–4484 (LRP-CTΔ1) and 4486–4507 (LRP-CTΔ2), designed to exclude the two NPXY motifs (residues 4470–4473 and 4504–4507, respectively), and an alanine substitution mutant at Tyr4507 (LRP- CT YA) were generated by PCR. Retroviruses expressing the two deletion constructs were used to infect LRP–/– fibroblasts as before. After hygromycin selection, individual clones were selected for analysis based on comparable expression levels of LRP-CTΔ1 and LRP-CTΔ2. LRP deletion constructs lacking amino acids 4505–4507 (LRP-CTΔNPVY) or 4507–4510 (LRP-CTΔYATL) were generated by PCR and were transiently transfected into CHO 14-2-1 cells using Fugene6 (Roche).
Antibodies
The polyclonal antiserum (1704) was generated by immunizing rabbits with a synthetic peptide corresponding to the last 15 amino acids of the cytoplasmic domain of human LRP coupled to keyhole limpet hemocyanin. Monoclonal antibodies 1G7, 5A3 and 26D6, which react with the ectodomain of APP, have been described previously (Koo et al., 1996; Kang et al., 2000). Polyclonal antibody CT15, which reacts with the cytoplasmic domain of APP, and the polyclonal antiserum 863, which was raised against the mid-region of APP, have been described previously (Sisodia et al., 1993; Marquez-Sterling et al., 1997).
Immunoprecipitation and immunoblotting
For detection of intracellular proteins, cell extracts were prepared using an NP-40 lysis buffer. To detect APPs and secreted Aβ, media conditioned by the respective cell lines for 24 or 72 h were collected for analyses. For APPs detection, conditioned media were immunoprecipitated using the monoclonal APP ectodomain antibodies 1G7 and 5A3. Extracts and immunoprecipitates were fractionated by SDS–PAGE in 4–12% Tris–glycine gels. In all cases, gel loading was normalized to total protein content in the cell extract or the corresponding cell extracts when medium samples were used. Western blotting was carried out with the indicated antibodies and detected by enhanced chemiluminescence (Pierce). Quantitation of the chemiluminescence signal was carried out with a CCD camera imaging system (GeneGnome, Syngene, Frederick, MD).
Aβ ELISA measurements
Media from LRP–/–, LRP+/– and LRP–/–CT fibroblasts were collected after an incubation period of 72 h in serum-free IS-CHO medium. Debris was removed by centrifugation (13 000 r.p.m. for 20 min) and the supernatants were subjected to Aβ40 quantification using a standard sandwich enzyme-linked immunosorbent assay (ELISA) described previously (Kang et al., 2000).
Metabolic labeling
Confluent cultures of APP751-transfected LRP+/– and LRP–/– fibroblasts were incubated in methionine-free DMEM supplemented with 150 µCi/ml [35S]methionine/cysteine for 15 min. Cells were lysed immediately (time 0) or chased for 1, 2 or 4 h to determine the turnover of APP. APP-CTF turnover was determined by incubation with 150 µCi/ml [35S]methionine/cysteine for 1 h. Cells were lysed immediately (time 0) or chased for 3, 6 or 18 h. For these studies, APP was immunoprecipitated with polyclonal antibody CT15. The immunoprecipitates were fractionated by SDS–PAGE (6% Tris–glycine gels for full-length APP and 4–12% Tris–Tricine gels for APP-CTF) and exposed either to film or to phosphoimaging for quantitation.
Internalization assay
To measure internalization of cell surface APP, iodinated 1G7 antibody was added to confluent cultures of LRP+/–, LRP–/– or LRP–/–CT fibroblasts stably transfected with APP695 or APP751 at 37°C for 30 min. After incubation, cells were rapidly chilled on ice, and the reaction was quenched by the addition of ice-cold binding medium. Chilled cells were washed extensively, and the remaining surface antibody was detached by two acid washes. The cells were then lysed and collected for analysis. Acid-labile and acid-resistant radioactivity represents the surface and internalized pools of APP, respectively. Specific binding was determined after subtraction of the radioactive counts obtained from parallel cultures of untransfected LRP+/– and LRP–/– fibroblasts. The ratio of acid-resistant to acid-labile counts therefore provided a measure of the internalized versus cell surface pools of APP. All experiments in this study were repeated 1–3 times. Results are presented as the averages either of all experiments ± SEM or of a representative experiment ± SEM. For Tfn uptake experiment, bovine holo-Tfn (Sigma) was iodinated as above and added to CHO K1 and CHO 13-5-1 cells exactly as described by Zuk and Elfrink (1999).
Acknowledgments
Acknowledgements
We thank Dr David Kang, Dr Salvador Soriano, Dr Il-Sang Yoon, Chris Brechtel and Dr Maria Kounnas for helpful discussions, Mary Turner for assistance in the Aβ assay, Dr Joachim Herz for the gift of LRP constructs, and Dr S.Leppla for the gift of CHO cells. This work is supported by NIH grant AG12376 (E.H.K.), the Allied Signal Award for Aging Research (E.H.K.), the American Health Assistance Foundation A2001-06 (C.U.P.) and the AFAR Research Grant Program (C.U.P.).
References
- Annaert W.G. et al. (1999) Presenilin 1 controls γ-secretase processing of amyloid precursor protein in pre-Golgi compartments of hippocampal neurons. J. Cell Biol., 147, 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher P., Liu,P., Gotthardt,M. Hiesberger,T., Anderson,R.G. and Herz,J. (2002) Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in caveolae. J. Biol. Chem., 277, 15507–15513. [DOI] [PubMed] [Google Scholar]
- Bu G. and Rennke,S. (1996) Receptor-associated protein is a folding chaperone for low density lipoprotein receptor-related protein. J. Biol. Chem., 271, 22218–22224. [DOI] [PubMed] [Google Scholar]
- Cao X. and Sudhof,T.C. (2001) A transcriptively active complex of APP with Fe65 and histone acetyltransferase Tip60. Science, 293, 115–120. [DOI] [PubMed] [Google Scholar]
- Cook D.G., Forman,M.S., Sung,J.C., Leight,S., Kolson,D.L., Iwatsubo,T., Lee,V.M. and Doms,R.W. (1997) Alzheimer’s A β(1–42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat. Med., 3, 1021–1023. [DOI] [PubMed] [Google Scholar]
- De Strooper B. and Annaert,W. (2000) Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci., 113, 1857–1870. [DOI] [PubMed] [Google Scholar]
- FitzGerald D.J., Fryling,C.M., Zdanovsky,A., Saelinger,C.B., Kounnas,M., Winkles,J.A., Strickland,D. and Leppla,S. (1995) Pseudomonas exotoxin-mediated selection yields cells with altered expression of low-density lipoprotein receptor-related protein [published erratum appears in J. Cell Biol., 130, 1015]. J. Cell Biol., 129, 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann T. et al. (1997) Distinct sites of intracellular production for Alzheimer’s disease A β40/42 amyloid peptides. Nat. Med., 3, 1016–1020. [DOI] [PubMed] [Google Scholar]
- Herz J. (2001) The LDL receptor gene family: (un)expected signal transducers in brain. Neuron, 29, 571–581. [DOI] [PubMed] [Google Scholar]
- Herz J. and Beffert,U. (2000) Apolipoprotein E receptors: linking brain development and Alzheimer’s disease. Nat. Rev. Neurosci., 1, 51–58. [DOI] [PubMed] [Google Scholar]
- Herz J., Kowal,R.C., Ho,Y.K., Brown,M.S. and Goldstein,J.L. (1990) Low density lipoprotein receptor-related protein mediates endocytosis of monoclonal antibodies in cultured cells and rabbit liver. J. Biol. Chem., 265, 21355–21362. [PubMed] [Google Scholar]
- Hiesberger T., Trommsdorff,M., Howell,B.W., Goffinet,A., Mumby,M.C., Cooper,J.A. and Herz,J. (1999) Direct binding of reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron, 24, 481–489. [DOI] [PubMed] [Google Scholar]
- Holtzman D.M., Pitas,R.E., Kilbridge,J., Nathan,B., Mahley,R.W., Bu,G. and Schwartz,A.L. (1995) Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc. Natl Acad. Sci. USA, 92, 9480–9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn I.R., van den Berg,B.M., van der Meijden,P.Z., Pannekoek,H. and van Zonneveld,A.J. (1997) Molecular analysis of ligand binding to the second cluster of complement-type repeats of the low density lipoprotein receptor-related protein. Evidence for an allosteric component in receptor-associated protein-mediated inhibition of ligand binding. J. Biol. Chem., 272, 13608–13613. [DOI] [PubMed] [Google Scholar]
- Hussain I. et al. (1999) Identification of a novel aspartic protease (Asp 2) as β-secretase. Mol. Cell. Neurosci., 14, 419–427. [DOI] [PubMed] [Google Scholar]
- Hyman B.T., Strickland,D. and Rebeck,G.W. (2000) Role of the low-density lipoprotein receptor-related protein in β-amyloid metabolism and Alzheimer disease. Arch. Neurol., 57, 646–650. [DOI] [PubMed] [Google Scholar]
- Kang D.E. et al. (2000) Modulation of amyloid β-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J. Clin. Invest., 106, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A., Whelan,C.M., Smith,C.J., Mikhailenko,I., Rebeck,G.W., Strickland,D.K. and Hyman,B.T. (2001) Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J. Neurosci., 21, 8354–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer M.F., Orlando,R.A. and Glabe,C.G. (1996) Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP). Brain Res., 740, 6–14. [DOI] [PubMed] [Google Scholar]
- Koo E.H. and Squazzo,S.L. (1994) Evidence that production and release of amyloid β-protein involves the endocytic pathway. J. Biol. Chem., 269, 17386–17389. [PubMed] [Google Scholar]
- Koo E.H., Squazzo,S.L., Selkoe,D.J. and Koo,C.H. (1996) Trafficking of cell-surface amyloid β-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J. Cell Sci., 109, 991–998. [DOI] [PubMed] [Google Scholar]
- Kounnas M.Z., Moir,R.D., Rebeck,G.W., Bush,A.I., Argraves,W.S., Tanzi,R.E., Hyman,B.T. and Strickland,D.K. (1995) LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell, 82, 331–340. [DOI] [PubMed] [Google Scholar]
- Li Y., Marzolo,M.P., van Kerkhof,P., Strous,G.J. and Bu,G. (2000) The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J. Biol. Chem., 275, 17187–17194. [DOI] [PubMed] [Google Scholar]
- Li Y., Cam,J. and Bu,G. (2001) Low-density lipoprotein receptor family: endocytosis and signal transduction. Mol. Neurobiol., 23, 53–67. [DOI] [PubMed] [Google Scholar]
- Loukinova E. et al. (2002) Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function between LRP and the PDGF receptor. J. Biol. Chem., 277, 15499–15506. [DOI] [PubMed] [Google Scholar]
- Marquez-Sterling N.R., Lo,A.C., Sisodia,S.S. and Koo,E.H. (1997) Trafficking of cell-surface β-amyloid precursor protein: evidence that a sorting intermediate participates in synaptic vesicle recycling. J. Neurosci., 17, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P., Reddy,Y.K. and Herz,J. (2002) Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J. Biol. Chem., 277, 18736–18743. [DOI] [PubMed] [Google Scholar]
- Moestrup S.K., Holtet,T.L., Etzerodt,M., Thogersen,H.C., Nykjaer,A., Andreasen,P.A., Rasmussen,H.H., Sottrup-Jensen,L. and Gliemann,J. (1993) α2-macroglobulin–proteinase complexes, plasminogen activator inhibitor type-1-plasminogen activator complexes and receptor-associated protein bind to a region of the α2-macroglobulin receptor containing a cluster of eight complement-type repeats. J. Biol. Chem., 268, 13691–13696. [PubMed] [Google Scholar]
- Morgenstern J.P. and Land,H. (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res., 18, 3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M., Holtzman,D.M., Schwartz,A.L. and Bu,G. (1997) α2-macroglobulin complexes with and mediates the endocytosis of β-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J. Neurochem., 69, 1904–1911. [DOI] [PubMed] [Google Scholar]
- Perez R.G., Soriano,S., Hayes,J.D., Ostaszewski,B., Xia,W., Selkoe,D.J., Chen,X., Stokin,G.B. and Koo,E.H. (1999) Mutagenesis identifies new signals for β-amyloid precursor protein endocytosis, turnover and the generation of secreted fragments, including Aβ42. J. Biol. Chem., 274, 18851–18856. [DOI] [PubMed] [Google Scholar]
- Rebeck G.W., Moir,R.D., Mui,S., Strickland,D.K., Tanzi,R.E. and Hyman,B.T. (2001) Association of membrane-bound amyloid precursor protein APP with the apolipoprotein E receptor LRP. Brain Res. Mol. Brain Res., 87, 238–245. [DOI] [PubMed] [Google Scholar]
- Shibata M. et al. (2000) Clearance of Alzheimer’s amyloid-β1–40 peptide from brain by LDL receptor-related protein-1 at the blood–brain barrier. J. Clin. Invest., 106, 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia S.S., Koo,E.H., Hoffman,P.N., Perry,G. and Price,D.L. (1993) Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J. Neurosci., 13, 3136–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovronsky D.M., Doms,R.W. and Lee,V.M. (1998) Detection of a novel intraneuronal pool of insoluble amyloid β protein that accumulates with time in culture. J. Cell Biol., 141, 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M., Borg,J.P., Margolis,B. and Herz,J. (1998) Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem., 273, 33556–33560. [DOI] [PubMed] [Google Scholar]
- Ulery P.G., Beers,J., Mikhailenko,I., Tanzi,R.E., Rebeck,G.W., Hyman,B.T. and Strickland,D.K. (2000) Modulation of β-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. J. Biol. Chem., 275, 7410–7415. [DOI] [PubMed] [Google Scholar]
- Vassar R. et al. (1999) β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science, 286, 735–741. [DOI] [PubMed] [Google Scholar]
- Ward D.M., Ajioka,R. and Kaplan,J. (1989) Cohort movement of different ligands and receptors in the intracellular endocytic pathway of alveolar macrophages. J. Biol. Chem., 264, 8164–8170. [PubMed] [Google Scholar]
- Willnow T.E., Orth,K. and Herz,J. (1994) Molecular dissection of ligand binding sites on the low density lipoprotein receptor-related protein. J. Biol. Chem., 269, 15827–15832. [PubMed] [Google Scholar]
- Willnow T.E., Rohlmann,A., Horton,J., Otani,H., Braun,J.R., Hammer,R.E. and Herz,J. (1996) RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J., 15, 2632–2639. [PMC free article] [PubMed] [Google Scholar]
- Yan R., Han,P., Miao,H., Greengard,P. and Xu,H. (2001) The transmembrane domain of the Alzheimer’s β-secretase (BACE1) determines its late Golgi localization and access to β-amyloid precursor protein (APP) substrate. J. Biol. Chem., 276, 36788–36796. [DOI] [PubMed] [Google Scholar]
- Zuk P.A. and Elferink,L.A. (1999) Rab15 mediates an early endocytic event in Chinese hamster ovary cells. J. Biol. Chem., 274, 22303–22312. [DOI] [PubMed] [Google Scholar]