Abstract
TRAF2 serves as a central regulator of the cellular response to stress and cytokines through the regulation of key stress-signaling cascades. Here we demonstrate that wild-type, but not RING mutant, Siah2 targets TRAF2 for ubiquitylation and degradation in vitro. Siah2 mediates equally efficient ubiquitylation of RING mutant TRAF2. In vivo, Siah2 primarily targets TRAF2 for degradation under stress conditions. Tumor necrosis factor-α (TNF-α) and actinomycin D treatment results in accelerated TRAF2 degradation in wild-type mouse embryo fibroblasts (MEFs), as compared with Siah2–/– cells. Similarly, TRAF2 half-life is prolonged in Siah2–/– compared with wild-type MEFs subjected to stress stimuli. Siah2 efficiently decreases TNF-α-dependent induction of JNK activity and transcriptional activation of NF-κB. Apoptosis induced by TNF-α and actinomycin D treatment is increased upon expression of Siah2, or attenuated upon expression of TRAF2 or RING mutant Siah2. Identifying Siah2 as a regulator of TRAF2 stability reveals its role in the regulation of TRAF2 signaling following exposure to stress.
Keywords: JNK/NF-κB/Siah2/stress/TRAF2
Introduction
Members of the tumor necrosis factor receptor (TNFR) superfamily play important roles in regulating the cellular response to cytokines, stress and DNA damage (Arch et al., 1998). Stimulation of a TNFR results in receptor trimerization and the recruitment of TNFR-associated factors (TRAFs) and/or TNFR-associated death domain protein (TRADD) to the cytoplasmic regions of the receptors (Arch et al., 1998). TRAF2 is a prototypical member of the TRAF family of proteins, originally purified from a protein complex associated with TNFR2 (Wajant and Scheurich, 2001). Mechanisms that underlie TRAF2 activation include its direct association with TNFR2, recruitment to TNFR1 through its interaction with the adaptor protein TRADD (Hsu et al., 1996) or its oligomerization (Baud et al., 1999). TRAF2 can also interact directly with other members of the TNFR superfamily including CD40, CD30, CD27, 4-1BB and RANK, which are primarily implicated in immune functions (Duckett and Thompson, 1997; Arch and Thompson, 1998; Nguyen et al., 1999). TRAF2 also interacts with RIP, GCK, ASK1 and NIK, and plays a critical role in the regulation of most stress kinases, including ASK1, MEKK1 and IKK (Rothe et al., 1995; Liu et al., 1996; Natoli et al., 1997; Germani et al., 1999) and their corresponding transcription factors, including p53, c-Jun and NF-κB (Shi and Kehrl, 1997; Nishitoh et al., 1998; Devin et al., 2000). TRAF2 can negatively regulate apoptotic signals from TNFR1 through interaction with IAPs and activation of NF-κB (Shu et al., 1996; Wang et al., 1998). These functions position TRAF2 as an important mediator of anti-apoptotic signals. TRAF2 activity has been shown to be regulated by multiple mechanisms including its oligomerization or binding with other TRAF family members, as shown for TRAF6 (Arch et al., 1998), and its degradation by proteins implicated in the cellular pathways activated by CD30, CD40 or Epstein–Barr virus oncoprotein latent membrane protein 1 (Duckett and Thompson, 1997; Brown et al., 2001).
TRAF2 can be targeted for proteolytic degradation and also belongs to the family of C3HC4 RING finger proteins, a motif present in several proteins with E3 ubiquitin ligase activity (Joazeiro et al., 2000); therefore, we investigated TRAF2 function as an E3 ligase and as a target of other E3 ligases in response to stress and cytokine signaling.
We have shown recently that Siah proteins share strong structural homology with the TRAF proteins (Polekhina et al., 2002). Furthermore, Siah proteins interact with the TRAF2-binding protein PW-1 (Relaix et al., 1998, 2000). Accordingly, we examined the possible effect of Siah proteins on TRAF2 ubiquitylation. Both Siah1 and Siah2 are RING finger proteins with E3 ligase activity (Tang et al., 1997; Hu and Fearon, 1999). The Drosophila homolog sina plays an important role in the phyllopod-dependent degradation of the transcriptional repressor tramtrack (Tang et al., 1997) as for the formation of the R7 photoreceptor in the developing eye of Drosophila melanogaster (Della et al., 1993). Siah1 has been implicated in the proteolytic degradation of DCC, c-myb, BOB.1/OBF.1, Kid and β-catenin (Hu et al., 1997; Hu and Fearon, 1999; Germani et al., 2000; Boehm et al., 2001; Liu et al., 2001; Matsuzawa and Reeed, 2001; Tiedt et al., 2001). Siah2 also targets the degradation of DCC, BAG-1 and nuclear receptor co-repressor (Matsuzawa et al., 1998; Zhang et al., 1998; Sourisseau et al., 2001).
In this study, we demonstrate that Siah2 targets the ubiquitylation and degradation of TRAF2, and that conditions which result in cellular apoptosis require Siah2 for degradation of TRAF2. Accordingly, through its regulation of TRAF2 stability, Siah2 emerges as a key regulator of TRAF2-dependent signaling in response to tumor necrosis factor-α (TNF-α) treatment and UV irradiation.
Results
Siah2 associates with TRAF2 and induces TRAF2 ubiquitylation in vitro
In light of the strong structural homology shared between Siah and TRAF proteins (Polekhina et al., 2002), we tested whether, like Siah proteins, TRAF2 may possess E3 ligase activity, and whether Siah proteins may target TRAF2 for ubiquitylation.
We first assessed whether TRAF2 elicits E3 ligase activity on its own. Bacterially purified TRAF2 does not possess E3 ligase activity in vitro (using E1 and UBCH5b or a mixture of Ubch5b, Ubch5c, Ubch7 or Ubc13 and Mms2; H.Habelhah, S.G.Cho and Z.Ronai, unpublished data). Yet, upon the addition of reticulocyte lysate, TRAF2 underwent efficient ubiquitylation in vitro (data not shown), suggesting that reticulocyte lysates may contain proteins that acquire TRAF2 E3 ligase activity or that target TRAF2 for efficient ubiquitylation. In vitro ubiquitylation of TRAF2 was also seen in the presence of cellular extracts (data not shown).
To investigate the possible role of Siah2 in targeting TRAF2 ubiquitylation, we used a bacterially expressed and purified form of GST–Siah2. Unlike TRAF2, bacterially expressed and purified GST–Siah2 exhibited strong E3 ligase activity in vitro (Figure 1A). In vitro ubiquitylation reactions using GST–TRAF2 as a substrate and bacterially produced and purified Siah2 (cleaved of its GST moiety) resulted in efficient ubiquitylation of TRAF2. Siah2-targeted in vitro ubiquitylation of GST–TRAF2 was not affected upon deletion of, or mutation within, the RING domain of TRAF2 (Figure 1B), suggesting that this domain is not required for ubiquitylation of TRAF2 by Siah2. Addition of bacterially expressed Siah2 to His-tagged TRAF2, in the presence of E1, UBCH5b, hemagglutinin-tagged ubiquitin (HA-Ub) and ATP reconstituted an in vitro ubiquitylation reaction that induced efficient ubiquitylation of His-TRAF2 (Figure 1C). Stringent washing conditions were used in these reactions to ensure the removal of non-covalently bound protein(s) from TRAF2 prior to its separation by SDS–PAGE and immunoblot analysis. These observations provide direct evidence for the ability of Siah2 to target TRAF2 ubiquitylation in vitro.
Fig. 1. (A) Siah2 targets the ubiquitylation of TRAF2 in vitro. Bacterially expressed and purified GST–Siah2 was subjected to an in vitro ubiquityl ation reaction in the presence of E1 and/or E2. The lower panel depicts Ponceau S staining of the blot. (B) Bacterially expressed and purified GST–TRAF2 in its wild-type, RING mutant (G-Rm-TRAF2; C74A, H76A) or RING-deleted (G-ΔN-TRAF2; Δ1–87aa) forms were subjected to an in vitro ubiquitylation reaction by a soluble form of bacterially expressed and purified Siah2. Following the reaction, GST–TRAF2 was subjected to extensive washing with PBS buffer containing empigen BB (1%) NP-40 (0.5%), LiCl (0.5 M) β-ME (0.1%) and EDTA (2 mM). Ubiquitylation of TRAF2 was detected using anti-HA antibody. The lower panel depicts Ponceau S staining of GST–TRAF2 used for the reaction. (C) Bacterially expressed and purified His-TRAF2 and Siah2 in solution were subjected to an in vitro ubiquitylation reaction followed by immunoprecipitation of TRAF2 using anti-TRAF2 antibody. After extensive washing, ubiquitylation of TRAF2 was detected using anti-HA antibody. The middle panel depicts the amount of TRAF2 immunoprecipitated in these reactions. The lower panel depicts immunoblot analysis of Siah2, which was performed on the supernatant (material that was not immunoprecipitated by TRAF2 antibodies). (D) In vitro translated and 35S-labeled TRAF2 (in reticulocyte lysates that were immunodepleted of Siah2) was subjected to an in vitro ubiquitylation/degradation reaction, which was carried out in the presence of E1, E2 and GST–Siah2 for the indicated times. The degree of degradation was assessed by direct assessment of the radioactive signal from each lane. The degree of ubiquitylation is shown as marked in the right panel. (E) In vitro translated and 35S-labeled TRAF2 was subjected to an in vitro ubiquitylation/degradation reaction as indicated in (D), except that RING mutant Siah2 was also added to the reaction as indicated. (F) In vitro translated and 35S- labeled TRAF2 or Siah2 were incubated with GST, GST–Siah2 or GST–TRAF2 on beads for 1 h at 4°C followed by four washes with buffer A or B (see Materials and methods for details), subsequent separation by SDS–PAGE and detection of bound [35S]TRAF2 or [35S]Siah2.
Addition of bacterially expressed and purified Siah2 to in vitro translated 35S-labeled TRAF2 resulted in efficient degradation of TRAF2 in a time-dependent manner (Figure 1D). To achieve this degree of degradation (60% of TRAF2 was degraded within 30 min), TRAF2 translation was carried out in reticulocyte lysates that were immunodepleted of Siah2, further pointing to the role of Siah2 in the degradation of TRAF2. The degree of in vitro degradation of TRAF2 coincides with the level of its polyubiquitylation (Figure 1D). RING mutant TRAF2 was also degraded efficiently in this reaction (Figure 1D), consistent with our findings that Siah2 can ubiquitylate this TRAF2 mutant. The lack of complete degradation under these conditions suggests that a portion of TRAF2 is not modified properly or may be subject to degradation by other mechanisms. Addition of RING mutant Siah2 to the in vitro ubiquitylation and degradation reaction attenuated the degree of TRAF2 ubiquitylation and degradation elicited by Siah2 (Figure 1E). These findings suggest that Siah2-mediated TRAF2 ubiquitylation results in TRAF2 degradation in vitro. GST ‘pull-down’ experiments using TRAF2 as bait confirmed its association with Siah2; reciprocal analysis using Siah2 as bait also identified the interaction between TRAF2 and Siah2 (Figure 1F). These data establish that interaction between Siah2 and TRAF2 enables Siah2 targeting of TRAF2 for ubiquitylation and degradation.
Expression of Siah2 decreases TRAF2 protein levels in vivo
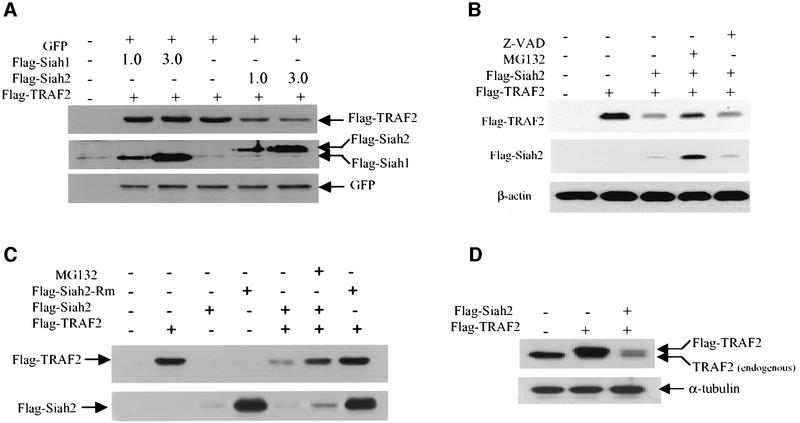
We next assessed whether Siah2 can target TRAF2 for degradation in vivo. Exogenous expression of Siah2, but not of Siah1, in HeLa cells efficiently reduced steady-state levels of Flag-tagged TRAF2 (Figure 2A). The degree of TRAF2 degradation by Siah2 varied from 50 to 80% depending on the level of Siah2 expression. The effect of Siah2 on TRAF2 stability is proteasome dependent, since degradation was blocked by incubation with the proteasome inhibitor MG132 (Figure 2B and C), but not by the caspase inhibitor Z-VAD (Figure 2B). Expression of Siah2 did not result in complete degradation of TRAF2, suggesting that a small portion of TRAF2 may either be subjected to degradation by other E3 ligases, require additional modification or reside within cellular compartments that are not available to Siah2 activities.
Fig. 2. Siah2 mediates TRAF2 degradation in vivo. (A) HeLa cells were transfected with Flag-TRAF2, green fluorescent protein (GFP) and Flag-Siah1 or Flag-Siah2. After 24 h, the cells were mock treated or treated with MG132 (40 µM) for 4 h. Cells were harvested, washed and pellets were solubilized with RIPA buffer, and steady-state levels of Flag-TRAF2, Flag-Siah2 and Flag-Siah1 were detected with anti-Flag antibody; the same membrane was reprobed with anti-GFP antibody. (B) Steady-state levels of exogenously expressed TRAF2 were monitored following expression of Siah2 in the presence of either proteasome inhibitor (MG132, 40 µM) or caspase inhibitor (Z-VAD-FMK; 50 µM). HeLa cells were transfected with Flag-TRAF2 and Flag-Siah2, and 24 h later cells were mock treated or treated with MG132 or Z-VAD for 4 h. Cells were then harvested and solubilized with RIPA buffer. Total TRAF2 and Siah2 levels were detected with anti-Flag antibodies. The membrane was reprobed with antibodies to β-actin. (C) HeLa cells were transfected with Flag-TRAF2, Flag-Siah2 and/or RING domain-mutated Flag-Siah2-Rm (H99A/C102A). After 24 h, the cells were mock treated or treated with MG132 (40 µM) for 4 h, and steady-state levels of Flag-TRAF2, Flag-Siah2 and Flag-Siah2-Rm were detected with anti-Flag antibody. (D) HeLa cells were transfected with Flag-TRAF2 and/or Flag-Siah2. After 24 h, cells were harvested, and steady-state levels of transfected (Flag-TRAF2) and endogenous TRAF2 were detected with anti-TRAF2 antibody (Santa Cruz; A20). The same membrane was probed with anti-α-tubulin antibody.
A RING mutant (H99A/C102A) form of Siah2, which was designed to abolish the E3 ligase activity of Siah2, was expressed at higher levels than wild-type Siah2 (Figure 2C). Additionally, MG132 treatment increased wild-type Siah2 protein levels (Figure 2B and C). These findings suggest that, like Siah1 (Hu and Fearon, 1999), Siah2 catalyzes self-ubiquitylation and regulates its own stability. Forced expression of RING-mutated Siah2 failed to decrease steady-state levels of transfected TRAF2, suggesting that the intact RING of Siah2 is required for its ability to reduce TRAF2 stability (Figure 2C). Forced expression of Siah2 also induced degradation of endogenous TRAF2 (Figure 2D). These results suggest that Siah2 targets the degradation of TRAF2 in vivo.
Half-life of TRAF2 is prolonged in Siah2-null cells that are subjected to stress stimuli
To assess further the role of Siah2 in degradation of TRAF2, we compared the TRAF2 half-life in mouse embryo fibroblasts (MEFs) derived from wild-type or Siah2–/– mice (D.D.L.Bowtell, unpublished data). Under normal growth conditions, there was a limited difference in the half-life of TRAF2 between wild-type and Siah2-null cells (Figure 3A). However, in response to stress in the form of actinomycin D treatment, pulse–chase labeling revealed a half-life of 6–7 h for TRAF2 in Siah2–/– MEFs, as compared with 3–4 h in wild-type MEFs (Figure 3B). In response to TNF-α and actinomycin D treatment, there was a marked decrease in the half-life of TRAF2 in wild-type MEFs (from 4.5 to 2 h) compared with Siah2–/– cells (from 7 to 4 h; Figure 3C). The difference is seen most clearly 2 h after treatment, when 50% of TRAF2 is degraded in the wild-type MEFs compared with 15% in the Siah2–/– cells (Figure 3C). These observations suggest that Siah2 primarily affects TRAF2 stability in response to stress. The fact that TNF-α and actinomycin treatment can still act to decrease the half-life of TRAF2 in Siah2-null cells, albeit to a lesser degree than the decrease induced in wild-type cells, suggests that other proteins may be capable of targeting TRAF2 for degradation in the absence of Siah2. RT–PCR analysis confirmed that Siah2–/– cells lack Siah2 mRNA transcript (Figure 3D). These observations provide direct genetic evidence for the role of Siah2 in the regulation of TRAF2 stability in cells subjected to stress conditions. The relatively long half-life of TRAF2 is in line with other Siah2 substrates reported so far, as both DCC and N-CoR are relatively long-lived proteins (Zhang et al., 1998; Sourisseau et al., 2001).
Fig. 3. TRAF2 half-life is prolonged in Siah2-null MEFs subjected to stress. (A) Analysis of TRAF2 half-life in Siah2-null MEFs that were transfected with Flag-TRAF2. After 24 h, the cells were pulse-labeled with [35S]methionine/cysteine and the half-life of Flag-TRAF2 was monitored following its immunoprecipitation at each of the indicated time points. (B) Pulse–chase labeling of exogenously expressed TRAF2 was carried out as indicated in (A), except that cells were also treated with actinomycin D, as one form of stress. The right panel depicts the corresponding quantification based on phosphoimaging analysis. (C) The half-life of TRAF2 was assessed in wild-type and Siah2-null MEFs prior to and following treatment with actinomycin D and TNF-α. (D) RT–PCR analysis to monitor the presence of Siah2 mRNA (5′ primer, CTGTTTCCCTGTAAGTATGCTACC and 3′ primer, CACTGACAGCATGTAGATATCGTG) in wild-type but not Siah2-null cells. The right panel depicts a control RT–PCR for levels of GAPDH mRNA expression.
Siah2 is required for TRAF2 degradation in response to stress conditions
To assess the importance of Siah2 in the regulation of TRAF2, we have monitored changes in the stability of endogenously expressed TRAF2 following exposure to cytokines or stress. While TNF-α treatment did not alter levels of endogenously expressed TRAF2 in HeLa cells, the combination of TNF-α and cycloheximide caused degradation of TRAF2 within 2–4 h of treatment (Figure 4A). Degradation of TRAF2 following TNF-α and cycloheximide treatment could be attenuated upon expression of RING mutant Siah2 (Figure 4B). Similarly, UV irradiation induced degradation of endogenous TRAF2 within 6 h after treatment, and this degradation could be inhibited by expression of the RING mutant form of Siah2 (Figure 4C). These data suggest that degradation of TRAF2 following exposure to selective stress conditions that induce apoptosis, including TNF-α plus cycloheximide or UV irradiation, is mediated by Siah2.
Fig. 4. Siah2-dependent degradation of TRAF2 following TNF-α and actinomycin D treatment. (A) Cycloheximide chase of endogenous TRAF2 in HeLa cells following treatment with human TNF-α (20 ng/ml). (B) Expression of the Siah2 RING mutant inhibits degradation of TRAF2 following TNF-α and cycloheximide treatment. (C) The steady-state level of endogenous TRAF2 was monitored at the indicated times after treatment of HeLa cells with UV irradiation (30 J/m2) in the presence or absence of RING mutant Siah2. (D) The steady-state levels of endogenous TRAF2, TRAF5, TRAF6, cIAP1, cIAP2, xIAP and BAG1 were monitored at the indicated times after treatment of littermate wild-type and Siah2–/– primary MEFs with mouse TNF-α (10 ng/ml) and actinomycin D (1 µg/ml). (E) Cycloheximide–chase analysis of endogenous TRAF6 half-life in Siah2 wild-type and null cells was carried out at the indicated times following treatment with IL-1β.
Importantly, treatment with either human (not shown) or mouse TNF-α and actinomycin D resulted in degradation of endogenous TRAF2, but not of TRAF5 or TRAF6, in wild-type but not in Siah2–/– primary MEFs (Figure 4D). Steady-state levels of TRAF2 were similar in the wild-type and Siah2-null MEFs, supporting the notion that Siah2 primarily serves to limit TRAF2 availability in response to stress. Other anti-apoptotic proteins (c-IAP1, c-IAP2 and X-IAP) that bind to TRAF2 and/or function to inhibit TNF-α-induced apoptosis were unaffected. Similarly, expression of the anti-apoptotic protein BAG-1, which is degraded by Siah2 during apoptosis of olfactory neurons, was also unaltered (Figure 4D). Further illustrating the specificity of Siah2 for TRAF2, treatment of wild-type or Siah2-null MEFs with interleukin (IL)-1β with or without cycloheximide, to activate signaling through TRAF6, did not alter TRAF6 protein abundance (Figure 4E). These findings provide direct genetic evidence for the role of Siah2 in the degradation of TRAF2 and demonstrate that Siah2 is necessary for degradation of TRAF2 following stress conditions that induce apoptosis.
Siah2 attenuates TRAF2-dependent activation of JNK
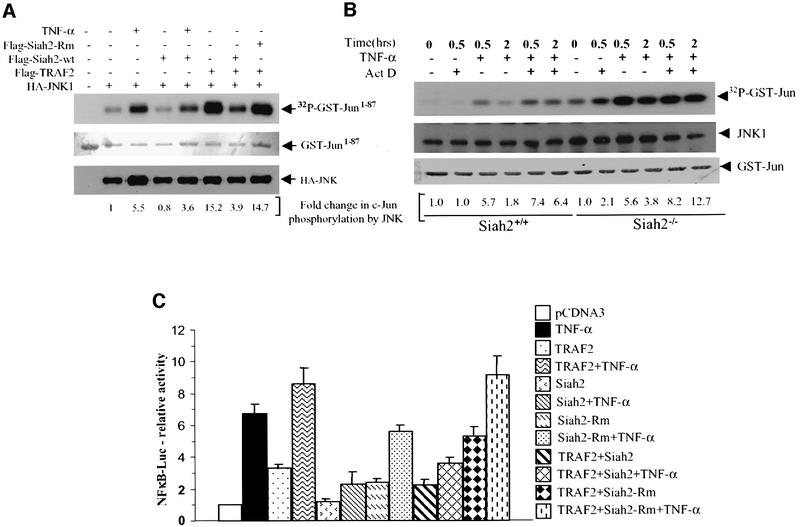
As TRAF2-null mice exhibit impaired JNK activation in response to TNF-α (Yeh et al., 1997), we assessed the effect of Siah2 on JNK activation following TNF-α treatment or overexpression of TRAF2. Expression of wild-type, but not RING mutant, Siah2 decreased TNF-α-mediated activation of JNK, as measured via immunokinase reactions using c-Jun as a substrate. Furthermore, JNK activation by TRAF2 was inhibited upon expression of wild-type but not mutant forms of Siah2 (Figure 5A). These findings suggest that Siah2 attenuates TRAF2-mediated activation of JNK activity.
Fig. 5. Siah2 expression decreases TRAF2-dependent activation of JNK- and TRAF2-dependent transcriptional activation of NF-κB. (A) In vitro immunokinase assays were carried out using GST–Jun1–87 as substrate. HA-JNK was immunopurified from HeLa cells that were treated with TNF-α or co-transfected with Flag-TRAF2, Flag-Siah2 or RING-mut Siah2, as indicated in the figure. The middle panel depicts Ponceau S staining of the membrane to control for substrate level, and the lower panel reveals immunoblotting of HA-JNK to control for the amount of immunoprecipitated JNK, using the same membrane. Fold change in c-Jun phosphorylation by JNK is shown under each lane, based on phosphoimager analysis. (B) Endogenous JNK activity was assayed via immunokinase reactions [as detailed in (A)] of wild-type and Siah2-null MEFs under normal growth (basal) conditions as well as following treatment with actinomycin D (1 µg/ml) or TNF-α (40 ng/ml) or both, as indicated in the figure. The middle panel depicts immunoblotting of JNK to control for the amount of immunoprecipitated JNK. The lower panel depicts the amount of GST–Jun used as a substrate. Values shown under each lane represent fold change in c-Jun phosphorylation by JNK based on phosphoimager analysis. (C) HeLa cells were transfected with the indicated plasmids, and 24 h later cells were treated with TNF-α (10 ng/ml) for 6 h. Luciferase activity was measured using the Luciferase assay system (Promega) and values were normalized based on β-gal activities. Data are representative of triplicate experiments.
Further assessment of JNK activation was carried out in wild-type and Siah2-null MEFs. JNK immunokinase reactions revealed that 2 h after TNF-α and actinomycin D treatment, Siah2–/– cells exhibited a greater degree of JNK activation, when compared with wild-type cells (Figure 5B). This prolonged JNK activation coincides with the increased stability of TRAF2 in Siah2-null cells, seen under these conditions. These findings provide important support for the physiological importance of Siah2 in the regulation of TRAF2 signaling.
Siah2 attenuates TRAF2-dependent activation of NF-κB
To determine the biological implications of Siah2 targeting of TRAF2 ubiquitylation and degradation, we monitored the effect of Siah2 on TRAF2-mediated activation of NF-κB. Forced expression of Siah2 efficiently decreased TNF-α- and TRAF2-mediated transcriptional activition of NF-κB (Figure 5C). Conversely, expression of RING mutant Siah2 increased TRAF2-dependent NF-κB transcriptional activity (Figure 5C). These findings establish that Siah2 can modulate TRAF2-mediated activation of NF-κB transcription. Whereas TNF-α-induced NF-κB was attenuated efficiently by Siah2 expression, NF-κB activation by IL-1β (which utilizes the TRAF6 signaling cascade) was not affected upon expression of Siah2, further demonstrating the specificity of Siah2 for TRAF2 (data not shown).
Siah2 inhibits anti-apoptotic effects of TRAF2
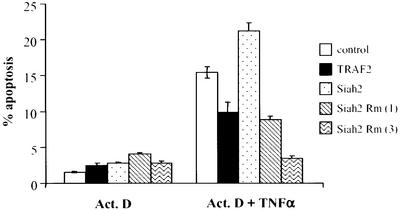
Since TRAF2-mediated activation of NF-κB is central in cellular protection from apoptosis, we tested the possibility that TRAF2’s ability to elicit anti-apoptotic signals is regulated by Siah2. Treatment of NIH 3T3 cells with TNF-α and actinomycin D causes a 5-fold increase in the degree of apoptosis within 12 h. Expression of Siah2 under these conditions further increased apoptosis (Figure 6). These data suggest that Siah2-mediated TRAF2 degradation may enhance cellular apoptosis induced by TNF-α. Indeed, expression of RING mutant Siah2 efficiently inhibited, in a dose-dependent manner, apoptosis induced by TNF-α and actinomycin D treatment. Similarly, expression of TRAF2 attenuated the degree of apoptosis seen under these conditions (Figure 6). Interestingly, cell death is initiated as early as 2 h following TRAF2 degradation in actinomycin D- and TNF-α-treated NIH 3T3 cells (data not shown). These data suggest that the anti-apoptotic activities of TRAF2 may be limited by degradation by Siah2. The latter is in agreement with the finding that TRAF2-null cells are substantially more sensitive to apoptosis induced by TNF-α (Yeh et al., 1997).
Fig. 6. Siah2 expression alters the degree of actinomycin D- and TNF-α- induced apoptosis. NIH 3T3 cells were transfected with GFP and the indicated constructs (1 or 3 µg as noted in the figure), and 24 h later cells were subjected to treatment with actinomycin D (2 µg/ml) and, as indicated, with TNF-α (20 ng/ml) for a period of 12 h. The degree of apoptosis was assessed via propidium iodide staining and flow cytometric analysis of GFP-positive cells using a FACS Calibur flow cytometer (Becton Dickinson, Mountain View, CA) and the CellQuest program. Percentage apoptosis was calculated from GFP-positive cells with sub-2N DNA content.
Discussion
TRAF2 is a central regulator of cytokine and stress signaling pathways that control cell growth and death. Availability of TRAF2 is therefore expected to play a critical role in the response of downstream signaling events to cytokine and stress conditions. Here we provide evidence for the regulation of TRAF2 stability by the potent E3 ligase Siah2. While bacterially expressed TRAF2 does not exhibit E3 ligase activity on its own, TRAF2 is ubiquitylated efficiently in vivo, suggesting that it may acquire E3 ligase activity in vivo upon modification or association with another cellular protein, or that TRAF2 is targeted in vivo by anther E3 ligase. Indeed, TRAF2 is subject to efficient ubiquitylation and subsequent degradation by Siah2 in vitro and in vivo. There are several lines of evidence for the role of Siah2 in regulation of TRAF2 ubiquitylation and degradation: (i) bacterially expressed and purified components demonstrated Siah2 association with, and ubiquitylation of, TRAF2; (ii) Siah2-dependent ubiquitylation and degradation of [35S]TRAF2 occurs in vitro; (iii) expression of Siah2 efficiently reduced levels of transfected and endogenous TRAF2; (iv) expression of Siah2 RING mutant prolonged the TRAF2 half-life and protected TRAF2 from degradation in response to UV or TNF-α and cycloheximide treatment; (v) Siah2-null MEFs exhibit a prolonged TRAF2 half-life in response to stress; (vi) endogenous TRAF2 is degraded in response to actinomycin D and TNF-α treatment in wild-type but not in Siah2-null cells; and (vii) inducible JNK and NF-κB activity is attenuated upon expression of Siah2.
Our findings suggest that Siah2 limits the half-life of TRAF2 under stress conditions. We find that inhibition of protein or RNA synthesis is required to trigger TNF-α-induced degradation of TRAF2 by Siah2. One possible explanation consistent with these findings is the presence of a short-lived protein that inhibits association of Siah2 and TRAF2, or inhibits Siah2-mediated TRAF2 ubiquitylation. Accordingly, TNF-α-induced TRAF2 degradation is observed only after the levels of such a protein are decreased by cycloheximide or actinomycin D treatment. Overexpression of Siah2 bypasses the need for removal of such an inhibitory protein and can induce TRAF2 degradation in the absence of other stimuli.
It is also possible that TNF-α or UV treatment induces certain modifications to either Siah2 or TRAF2 that permit efficient association and consequent targeting for ubiquitylation. This may explain the limited degree of association found in our GST pull-down assays. Our results do not exclude the possible existence of a cofactor that may facilitate Siah2 targeting of TRAF2 for degradation, similar to the role of Cks1 in Skp2-mediated p27 degradation (Ganoth et al., 2001) or PHYLLOPOD in SINA-mediated TRAMTRACK degradation (Tang et al., 1997; Li et al., 2002). It is of interest to note that treatment of bone marrow cells with RANKL and interferon-γ resulted in degradation of TRAF6 within the same time frame found for TRAF2 degradation by Siah2 in the present studies (Takayanagi et al., 2000).
Siah2-mediated TRAF2 degradation in MEFs was seen upon treatment with either human or mouse TNF-α in combination with actinomycin D. Since human TNF-α activates only TNFR1 in murine cells (Lewis et al., 1991), this may represent a novel pathway by which TRAF2 stability is regulated. Furthermore, activation of the IL-1 receptor via IL-1 results in activation of NF-κB, which is not affected by Siah2 (data not shown), further illustrating the specificity of Siah2 in the regulation of TNF-α-mediated TRAF2 activities.
Our preliminary results suggest that Siah2 primarily targets TRAF2 that is found in the insoluble fraction of the cell, likely to be associated with TNFR1 within the membrane in stimulated cells (data not shown), suggesting that Siah2 may function to limit the availability of TRAF2 for signal transduction. Consistent with this, overexpression of Siah2 can inhibit TNF-α- and TRAF2-mediated NF-κB activation. Importantly, Siah2 efficiently inhibits TRAF2-dependent activation of JNK, an important stress kinase which previously was shown to require TRAF2 for its activation (Yeh et al., 1997). Additionally, apoptosis induced by TNF-α and actinomycin D treatment was inhibited by RING mutant Siah2 expression and enhanced by wild-type Siah2. Thus, regulation of TRAF2 degradation by Siah2 modulates signal transduction and apoptosis induced by TNF-α.
These observations position Siah2 as a regulator of the cell’s ability to commit to apoptosis; as long as TRAF2 is available, cell commitment is delayed. Along these lines, human melanoma cells that exhibit decreased TRAF2 levels after UV treatment are more sensitive to apoptosis compared with melanoma cells in which the level of TRAF2 was not reduced by UV treatment (Ivanov et al., 2001). Elevated TRAF2 levels are also often seen in human tumors and in Hodgkin’s disease (Zapata et al., 2000; Murray et al., 2001). It will be of interest to determine whether impaired Siah2 expression or function may account for these observations.
Our findings are supported further by the strong structural homology shared between Siah and TRAF proteins. The structural analysis reveals that Siah is a dimeric protein and that the substrate-binding domain (SBD) adopts an eight-stranded β-sandwich fold that is highly similar to the TRAF-C region of TRAF2 (Polekhina et al., 2002). TRAF2 structural studies revealed that TNFR2 binds to a conserved shallow surface depression on one TRAF-C domain (Park et al., 1999), suggesting that the same interaction may take place with a corresponding domain of Siah. Indeed, expression of the Siah SBD sufficed to induce NF-κB activation (Polekhina et al., 2002). Given the trimerization of TRAF2 upon TNF-α stimulation, and the trimerization of TNFR2, one may envisage recruitment of Siah2 as part of such trimers, which, in turn, would enable targeting of TRAF2 for ubiquitylation and degradation. Alternatively, a common binding protein for TRAF-C and the Siah SBD may provide the required link to facilitate targeting of TRAF2 by Siah2. The latter also supports the notion that targeting of TRAF2 by Siah is more efficient in the insoluble fraction of the cells where TRAF-C and the Siah SBD are likely to interact with the same protein(s).
It is of interest to note that despite the growing number of proteins shown to be targeted by Siah2 for ubiquitylation and degradation, neither the Siah2-null mice nor their MEFs exhibit phenotypes that are expected upon altered half-life of the corresponding substrates (D.D.L.Bowtell, unpublished observations). Whereas Siah2-null cells exhibit marked differences in TRAF2 half-life and in JNK activities under stress conditions, corresponding changes in sensitivity to apoptosis in response to stress were not consistent (although they were observed in HeLa or 3T3 cells that were subjected to altered Siah2 expression). This may relate to an alternative pathway that may have been activated to compensate for the lack of Siah2. It is also plausible that in its absence, Siah1 may displace some of the functions of Siah2, thereby masking the lack of Siah2. The generation of mice lacking Siah1 and Siah2 will allow such questions to be addressed.
Given the multiple signals that activate TRAF2 and the diversity of TRAF2 effectors (Roperch et al., 1999), Siah2 emerges as a key component in the regulation of stress signaling cascades. Consistent with this, Siah2 has been shown to target BAG-1 for proteolytic degradation during apoptosis of olfactory neurons (Sourisseau et al., 2001). In light of the importance and complexity of TRAF signaling, Siah2 may not be the only component involved in regulation of TRAF protein’s stability. Indeed, a recent study has implicated cIAP in the regulation of TRAF2 stability (Li et al., 2002). While further work will be required to determine the possible relationship between these E3 ligases, it is possible that cIAP targets a different pool of TRAF2 (i.e. soluble) than Siah2 (insoluble). Based on our findings, it is expected that other regulatory cascades will be strongly linked to, or dependent upon, the Siah2 pathway identified in the present study.
Materials and methods
Cell culture and transfections
HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with fetal bovine serum (10%) and antibiotics. Primary and immortalized wild-type and Siah2–/– MEFs were maintained in DMEM supplemented with calf serum (10%), 0.02 mM β-mercaptoethanol (β-ME), sodium pyruvate and antibiotics. Cells were transfected using LipofectAMINE PLUS reagent (Invitrogene/Lifetech) according to the manufacturer’s protocol. In all cases, the total amount of DNA was normalized by the addition of empty control plasmids.
In vitro ubiquitylation assay
Bacterially expressed and glutathione–beads-purified GST–TRAF2 (1 µg/10 µl) and GST–Siah2 (1 µg/10 µl) bound to glutathione–beads were washed once with ubiquitylation buffer [50 mM Tris–HCl pH 8.0, 5 mM MgCl2, 0.5 mM dithiothreitol (DTT), 2 mM NaF and 3 µM okadaic acid], and then subjected to an in vitro ubiquitylation reaction in ubiquitylation buffer supplemented with purified HA-Ub (0.5 µg), 2 mM ATP, E1 (15 ng; Affinity Research, UK), and purified UbcH5b for 45 min at 37°C (Fuchs et al., 1998). GST–TRAF2 or GST–Siah2 beads were then washed extensively with phosphate-buffered saline (PBS) containing 0.5 M LiCl, 1% Empigen BB, 0.1% β-ME and 2 mM EDTA, before being eluted in Laemmli buffer, separated by 8% SDS–PAGE, transferred onto a nitrocellulose membrane, and analyzed by western blot using anti-HA antibody and Ponceau S staining.
In vitro binding assay
Bacterially expressed and purified GST, GST–TRAF2 or GST–Siah2 bound to glutathione–beads were incubated first with 1% bovine serum albumin (BSA) in PBS for 1 h followed by incubation with 35S-labeled TRAF2 or Siah2 which was in vitro translated using the TNT-coupled reticulocyte lysate system (Promega). Bead-bound material was subjected to four washes with buffer A (PBS containing 0.25% NP-40, 0.1% β-ME, 2 mM EDTA) or buffer B (buffer A supplemented with 300 mM NaCl) before being subjected to separation by SDS–PAGE.
Protein stability analysis
To monitor changes in the stability of TRAF2, pulse–chase analysis was performed using [35S]methionine/cysteine mix as previously described (Lomaga et al., 1999). Briefly, 106 immortalized wild-type and Siah2–/– MEFs (described elsewhere) were transfected with Flag TRAF2 (0.5 µg) and wild-type or RING mutant forms of Siah2 (2 µg), as indicated, using LipofectAMINE PLUS (Invitrogen/Lifetech). At 24 h post-transfection, cells were incubated with methionine/cysteine-free medium for 45 min before the addition of [35S]methionine/cysteine mix (0.2 mCi/ml) for 1 h (time 0), followed by chase (with medium containing excess of cold methionine/cysteine mix) for the times indicated in the Results (chase). Immunoprecipitation of exogenously labeled TRAF2 was carried out on an equal amount of protein extracts with the aid of anti-Flag antibodies. Cycloheximide chase experiments were performed on cells transfected as indicated above, with cycloheximide (20 µg/ml) added to the cultures 24 h after transfection (time 0). Proteins were prepared at the indicated times and equal amounts were subjected to immunoblot analysis using TRAF2 antibodies.
Protein preparations
Cells cultured on 100 mm plates were washed twice with ice-cold PBS before protein preparation. Unless indicated otherwise, proteins were prepared using RIPA buffer [50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 1 mM DTT, and 2 mM EDTA supplemented with 0.2 mM phenylmethylsulfonyl fluoride (PMSF) and a cocktail of protease inhibitors]. For the preparation of soluble versus insoluble fractions, cell pellets were first solubilized with TNE lysis buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.25% NP-40, 2 mM EDTA, 1 mM DTT, 0.5 mM PMSF and a cocktail of protease inhibitors) on ice for <10 min, followed by centrifugation (12 500 g) for 20 min at 4°C. Supernatant consisted of the soluble protein fraction. Pellets were washed with TNE buffer and subjected to treatment with RIPA buffer on ice for 30 min, which generated the insoluble protein fraction.
Luciferase assays
Cells were transiently transfected with reporter plasmids (0.2 µg) together with the expression vectors (0.2–0.6 µg) and pCMV-β-gal (0.1 µg). The reporter construct used was the NF-κB target sequence linked to a luciferase reporter plasmid. The amount of DNA for each transfection was equalized by addition of the respective empty vectors. At 24 h post-transfection, cells were treated with TNF-α (10 ng/ml) or IL-1β (20 ng/ml) and proteins were prepared 6 h later. Luciferase activity was measured in a luminometer and normalized to the β-galactosidase activity in the same cells.
JNK immunokinase assays
JNK immunokinase assays were carried out using a fusion protein (GST–Jun1–87) as substrate in 1× kinase buffer (20 mM HEPES pH 7.4, 0.5 mM EGTA, 1 mM DTT, 2 mM MgCl2, 2 mM MnCl2, 0.1 mM NaVO3, 5 mM β-glycerolphosphate, 75 mM NaCl and 0.25% NP-40), as described previously. Briefly, 2 µg of GST–Jun1–87 was incubated with HA-JNK, which was immunopurified from transfected HeLa cells, or with endogenous JNK that was immunopurified from wild-type or Siah2–/– MEFs, in the presence of 1× kinase buffer containing 1 µCi of [γ-32P]ATP and 25 µM cold ATP for 30 min at 30°C. Phosphorylated GST–Jun1–87 was separated by SDS–PAGE, transferred to a nitrocellulose membrane and the amount of phosphorylated GST–Jun1–87 was detected by autoradiography and quantified via a phosphoimager. The same membrane was used for immunobloting of JNK, to control for equal immunoprecipitations, and for Ponceau S staining of the GST–Jun1–87 level to ensure equal amounts of substrates.
Acknowledgments
Acknowledgements
We thank Michael Karin, Serge Fuchs, Z.Q.Pan and members of the Ronai laboratory for advice and discussions. This study was supported by NCI grant CA77389 (to Z.R. and D.S.) and a grant from the National Health and Medical Research Council of Australia (to D.D.L.B.).
References
- Arch R.H. and Thompson,C.B. (1998) 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)–nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor κB. Mol. Cell. Biol., 18, 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch R.H., Gedrich,R.W. and Thompson,C.B. (1998) Tumor necrosis factor receptor associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev., 12, 2821–2830. [DOI] [PubMed] [Google Scholar]
- Baud V., Liu,Z.G., Bennett,B., Suzuki,N., Xia,Y. and Karin,M. (1999) Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain Genes Dev., 13, 1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J., He,Y., Greiner,A., Staudt,L. and Wirth,T. (2001) Regulation of BOB.1/OBF.1 stability by SIAH. EMBO J., 20, 4153–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K.D., Hostager,B.S. and Bishop,G.A. (2001) Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein–Barr virus oncoprotein latent membrane protein 1 (LMP1). J. Exp. Med., 193, 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della N.G., Senior,P.V. and Bowtell,D.D.L. (1993) Isolation and characterisation of murine homologues of the Drosophila seven in absentia gene (sina). Development, 117, 1333–1343. [DOI] [PubMed] [Google Scholar]
- Devin A., Cook,A., Lin,Y., Rodriguez,Y., Kelliher,M. and Liu,Z.G. (2000) The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity, 12, 419–429. [DOI] [PubMed] [Google Scholar]
- Duckett C.S. and Thompson,C.B. (1997) CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev., 11, 2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S.Y., Adler,V., Buschmann,T., Yin,Z., Wu,X., Jones,S.N. and Ronai,Z. (1998) JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev., 12, 2658–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoth D., Bornstein,G., Ko,T.K., Larsen,B., Tyers,M., Pagano,M. and Hershko,A. (2001) The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat. Cell Biol., 3, 321–324. [DOI] [PubMed] [Google Scholar]
- Germani A., Romero,F., Houlard,M., Camonis,J., Gisselbrecht,S., Fischer,S. and Varin-Blank,N. (1999) hSiah2 is a new Vav binding protein which inhibits Vav-mediated signaling pathways. Mol. Cell. Biol., 15, 3798–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germani A., Bruzzoni-Giovanelli,H., Fellous,A., Gisselbrecht,S., Varin-Blank,N. and Calvo,F. (2000) SIAH-1 interacts with α-tubulin and degrades the kinesin Kid by the proteasome pathway during mitosis. Oncogene, 19, 5997–6006. [DOI] [PubMed] [Google Scholar]
- Hsu H., Shu,H.B., Pan,M.G. and Goeddel,D.V. (1996) TRADD–TRAF2 and TRADD–FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell, 84, 299–308. [DOI] [PubMed] [Google Scholar]
- Hu G. and Fearon,E.R. (1999) Siah-1 N-terminal RING domain is required for proteolysis function and C-terminal sequences regulate oligomerization and binding to target proteins. Mol. Cell. Biol., 19, 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Zhang,S., Vidal,M., Baer,J.L., Xu,T. and Fearon,E.R. (1997) Mammalian homologs of seven in absentia regulate DCC via the ubiquitin–proteasome pathway. Genes Dev., 11, 2701–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov V.N., Fodstad,O. and Ronai,Z. (2001) Expression of ring finger-deleted TRAF2 sensitizes metastatic melanoma cells to apoptosis via up-regulation of p38, TNFα and suppression of NF-κB activities. Oncogene, 20, 2243. [DOI] [PubMed] [Google Scholar]
- Joazeiro C.A.P. and Weissman,A.M. (2000) RING finger proteins: mediators of ubiquitin ligase activity. Cell, 102, 549–552. [DOI] [PubMed] [Google Scholar]
- Lewis M., Tartaglia,L.A., Lee,A., Bennett,G.L., Rice,G.C., Wong,G.H., Chen,E.Y. and Goeddel,D.V. (1991) Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc. Natl Acad. Sci. USA, 88, 2830–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yang,Y. and Ashwell,J.D. (2002) TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature, 416, 345–347. [DOI] [PubMed] [Google Scholar]
- Liu J., Stevens,J., Rote,C.A., Yost,H.J., Hu,Y., Neufeld,K.L., White,R.L. and Matsunami,N. (2001) Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol. Cell, 7, 927–936. [DOI] [PubMed] [Google Scholar]
- Liu Z.G., Hsu,H., Goeddel,D.V. and Karin,M. (1996) Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death Cell, 87, 565–576. [DOI] [PubMed] [Google Scholar]
- Lomaga M.A. et al. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40 and LPS signaling. Genes Dev., 13, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa S. and Reed,J.C. (2001) Siah-1, SIP and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol. Cell, 7, 915–926. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S., Takayama,S., Froesch,B.A., Zapata,J.M. and Reed,J.C. (1998) p53-inducible human homologue of Drosophila seven in absentia (Siah) inhibits cell growth: suppression by BAG-1. EMBO J., 17, 2736–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P.G., Flavell,J.R., Baumforth,K.R., Toomey,S.M., Lowe,D., Crocker,J., Ambinder,R.F. and Young,L.S. (2001) Expression of the tumour necrosis factor receptor-associated factors 1 and 2 in Hodgkin’s disease. J. Pathol., 194, 158–164. [DOI] [PubMed] [Google Scholar]
- Natoli G., Castanzo,A., Ianni,A., Templeton,D.J., Woodgett,J.R., Balsano,C. and Levrero,M. (1997) Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science, 275, 200–203. [DOI] [PubMed] [Google Scholar]
- Nishitoh H., Saitoh,M., Mochida,Y., Takeda,K., Nakano,H., Rothe,M., Miyazono,K. and Ichijo,H. (1998) ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell, 2, 389–395. [DOI] [PubMed] [Google Scholar]
- Nguyen L.T. et al. (1999) TRAF2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity, 11, 379–389. [DOI] [PubMed] [Google Scholar]
- Park Y.C., Burkitt,V., Villa,A.R., Tong,L. and Wu,H. (1999) Structural basis for self-association and receptor recognition of human TRAF2. Nature, 398, 533–538. [DOI] [PubMed] [Google Scholar]
- Polekhina G., House,C.M., Traficante,N., Mackay,J.P., Relaix,F., Sassoon,D.A., Parker,M.W. and Bowtell,D.D.L. (2002) Siah ubiquitin ligase is structurally related to TRAF and modulates TNFα signaling. Nat. Struct. Biol., 9, 68–75. [DOI] [PubMed] [Google Scholar]
- Relaix F., Wei,X.J., Wu,X. and Sassoon,D.A. (1998) Peg3/Pw1 is an imprinted gene involved in the TNF–NFκB signal transduction pathway. Nat. Genet., 18, 287–291. [DOI] [PubMed] [Google Scholar]
- Relaix F., Wei,X.J., Li,W., Pan,J., Lin,Y., Bowtell,D.D., Sassoon,D.A. and Wu,X. (2000) Pw1/Peg3 is a potential cell death mediator and cooperates with Siah1a in p53-mediated apoptosis. Proc. Natl Acad. Sci. USA, 97, 2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roperch J.P. et al. (1999) SIAH-1 promotes apoptosis and tumor suppression through a network involving the regulation of protein folding, unfolding and trafficking: identification of common effectors with p53 and p21(Waf1). Proc. Natl Acad. Sci. USA, 96, 8070–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M., Sarma,V., Dixit,V.M. and Goeddel,D.V. (1995) TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science, 269, 1424–1427. [DOI] [PubMed] [Google Scholar]
- Shi C.S. and Kehrl,J.H. (1997) Activation of stress-activated protein kinase/c-Jun N-terminal kinase, but not NF-κB, by the tumor necrosis factor (TNF) receptor 1 through a TNF receptor-associated factor 2- and germinal center kinase related-dependent pathway. J. Biol. Chem., 272, 32102–32107. [DOI] [PubMed] [Google Scholar]
- Shu H.B., Takeuchi,M. and Goeddel,D.V. (1996) The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc. Natl Acad. Sci. USA, 93, 13973–13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau T., Desbois,C., Debure,L., Bowtell.,D.D., Cato,A.C., Schneikert,J., Movse,E. and Michel,D. (2001) Alteration of the stability of Bag-1 protein in the control of olfactory neuronal apoptosis. J. Cell Sci., 114, 1409–1416. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. et al. (2000) T-cell-mediated regulation of osteoclasto genesis by signalling cross-talk between RANKL and IFN-γ. Nature, 408, 600–605. [DOI] [PubMed] [Google Scholar]
- Tang A.H., Neufeld,T.P., Kwan,E. and Rubin,G.M. (1997) PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell, 90, 459–467. [DOI] [PubMed] [Google Scholar]
- Tiedt R., Bartholdy,B.A., Matthias,G., Newell,J.W. and Matthias,P. (2001) The RING finger protein Siah-1 regulates the level of the transcriptional coactivator OBF-1. EMBO J., 20, 4143–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H. and Scheurich,P. (2001) The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Int. J. Biochem. Cell Biol., 33, 19–32. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Mayo,M.W., Korneluk,R.G., Goeddel,D.V. and Baldwin,A.S.,Jr (1998) NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science, 281, 1680–1683. [DOI] [PubMed] [Google Scholar]
- Yeh W.C. et al. (1997) Early lethality, functional NF-κB activation and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity, 7, 715–725. [DOI] [PubMed] [Google Scholar]
- Zapata J.M. et al. (2000) TNFR-associated factor family protein expression in normal tissues and lymphoid malignancies. J. Immunol., 165, 5084–5096. [DOI] [PubMed] [Google Scholar]
- Zhang J., Guenther,M.G., Carthew,R.W. and Lazar,M.A. (1998) Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev., 12, 1775–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]