Abstract
The genome of Saccharomyces cerevisiae contains 35 members of a family of transport proteins that, with a single exception, are found in the inner membranes of mitochondria. The transport functions of the 15 biochemically identified mitochondrial carriers are concerned with shuttling substrates, biosynthetic intermediates and cofactors across the inner membrane. Here the identification of the mitochondrial carrier for the essential cofactor thiamine pyrophosphate (ThPP) is described. The protein has been overexpressed in bacteria, reconstituted into phospholipid vesicles and identified by its transport properties. In confirmation of its identity, cells lacking the gene for this carrier had reduced levels of ThPP in their mitochondria, and decreased activity of acetolactate synthase, a ThPP-requiring enzyme found in the organellar matrix. They also required thiamine for growth on fermentative carbon sources.
Keywords: mitochondria/proteomics/Saccharomyces cerevisiae/thiamine pyrophosphate transporter/YGR096w
Introduction
Several cofactors (vitamins, coenzymes and prosthetic groups) are essential for the functioning of important metabolic processes occurring in mitochondria. Most of them have to be imported into the organelle, but little is known about the molecular basis of their transport.
Studies with intact mitochondria have suggested that some cofactors such as ascorbate (Ingebretsen and Normann, 1982), pyridoxine (Lui et al., 1981), pyridoxal 5′-phosphate (Lui et al., 1982), biotin (Said et al., 1992) and hydroxycobalamine (Fenton et al., 1976) diffuse into the mitochondria, whereas others such as thiamine (Barile et al., 1986), folates (Cybulski and Fisher, 1981; Horne et al., 1992), lipoate (Totskii, 1976) and coenzyme A (Neuburger et al., 1984; Tahiliani, 1991) are transported by carrier-mediated processes. The transport of thiamine and of the thiamine cofactors, thiamine pyrophosphate (ThPP) and thiamine monophosphate (ThMP), has been studied extensively (Barile et al., 1986, 1990, 1998). It has been proposed that there are separate carriers for thiamine (Barile et al., 1986), for ThPP/thiamine exchange (Barile et al., 1990), for ThPP and ThMP transport (Barile et al., 1998) and for ThMP uniport or ThMP/Pi exchange (Barile et al., 1998). However, the protein(s) responsible for these activities have never been isolated or identified hitherto.
Saccharomyces cerevisiae uses external thiamine for the production of ThPP or it can synthesize the cofactor itself (Hohmann and Meacock, 1998), and ThPP is an essential coenzyme for five enzymes in yeast, namely pyruvate decarboxylase (PDC) and transketolase, which are in the cytosol, and acetolactate synthase (ALS) and the E1 components of pyruvate dehydrogenase and oxoglutarate dehydrogenase (OGDH), which are in the mitochondria. The subcellular distribution of enzymes involved in thiamine metabolism shows that ThPP is synthesized in the cytosol and then imported into mitochondria. For example, thiamine pyrophosphokinase is found only in the cytosol of both yeast and mammalian cells (Deus and Blum, 1970; Hohmann and Meacock, 1998).
So far, three proteins have been identified as being responsible for the transport of cofactors into mitochondria. They are the carriers for FAD (Tzagoloff et al., 1996), folates (Titus and Moran, 2000) and coenzyme A (Prohl et al., 2001). They belong to the family of mitochondrial carriers that is characterized by a tripartite structure, consisting of three tandem sequence repeats containing distinctive sequence motifs, each repeat being folded into two transmembrane α-helices joined by an extensive hydrophilic loop (Saraste and Walker, 1982; Palmieri, 1994). In post-genomic studies, a number of members of this family have been identified by overexpression and reconstitution. They are the carriers for dicarboxylates, ornithine, succinate–fumarate, oxaloacetate–sulfate, oxodicarboxylates, aspartate–glutamate, deoxynucleotides, glutamate (Fiermonte et al., 2002 and references therein), the citrate carrier (Kaplan et al., 1995) and the adenine nucleotide transporter in peroxisomes (Palmieri et al., 2001a).
Here we report the identification and functional characterization of Tpc1p (encoded by YGR096w). It has the characteristic features of the mitochondrial carrier family and is the sequence in S.cerevisiae with the closest similarity to that of the human deoxynucleotide carrier (DNC) (Dolce et al., 2001). Tpc1p was overexpressed in bacteria, reconstituted into phospholipid vesicles and identified from its transport properties as the mitochondrial carrier for ThPP. Consistent with this function, tpc1Δ cells exhibited lower intramitochondrial levels of ThPP, decreased activities of ALS and OGDH, and auxotrophy for thiamine on fermentative carbon sources.
Results
Bacterial expression of Tpc1p
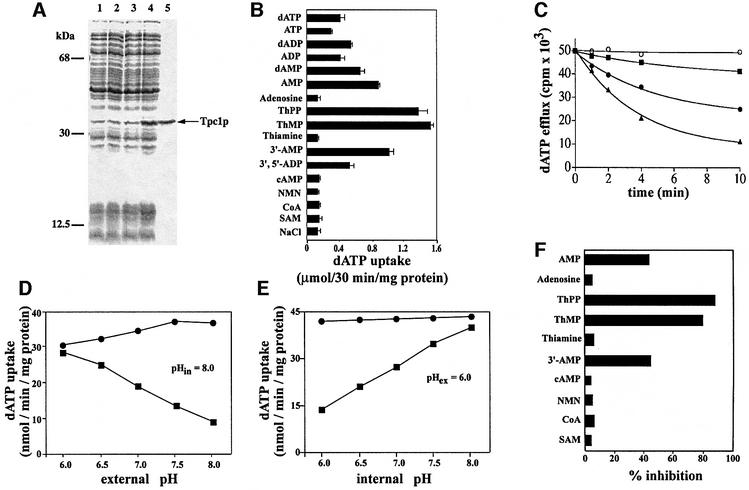
Tpc1p was expressed at high levels in Escherichia coli C0214(DE3) (Figure 1A, lane 4). It accumulated as inclusion bodies, and was purified by centrifugation and washing (Figure 1A, lane 5). The apparent molecular mass of the recombinant protein was 35.5 kDa (the calculated value with initiator methionine was 35 152 Da). The identity of the purified protein was confirmed by N-terminal sequencing. About 60–80 mg of purified protein were obtained per liter of culture. The protein was not detected in bacteria harvested immediately before induction of expression (Figure 1A, lane 2), nor in cells harvested after induction but lacking the coding sequence in the expression vector (Figure 1A, lane 3).
Fig. 1. Tpc1p catalyzes the transport of ThPP and ThMP. (A) Expression of yeast Tpc1p in E.coli. Proteins were separated by SDS–PAGE and stained with Coomassie Blue. The positions of the markers (bovine serum albumin, carbonic anhydrase and cytochrome c) are shown on the left. Lanes 1–4: E.coli CO214(DE3) containing the expression vector with (lanes 2 and 4) and without (lanes 1 and 3) the coding sequence of Tpc1p. Samples were taken at the time of induction (lanes 1 and 2) and 5 h later (lanes 3 and 4). The same number of bacteria was analyzed in each sample. Lane 5: purified Tpc1p (2 µg) originating from bacteria shown in lane 4. (B) Substrate specificity of Tpc1p. Proteoliposomes were pre-loaded internally with various substrates (concentration, 10 mM). Transport was started by external addition of 0.2 mM [α-35S]dATP and stopped after 30 min. (C) Efflux of [α-35S]dATP from proteoliposomes reconstituted with Tpc1p. Proteoliposomes were reconstituted in the presence of 1 mM dATP, 30 mM MES/30 mM HEPES (buffer A) at pH 6.0. The internal substrate pool was labeled by carrier-mediated exchange equilibration. Then the proteoliposomes were passed through Sephadex G-75 columns pre-equilibrated with 50 mM NaCl and 0.1 mM MES/0.1 mM HEPES pH 6.0. The efflux of [α-35S]dATP was started by adding buffer A at pH 6 (filled squares), buffer A at pH 8.0 (filled circles), buffer A at pH 8.0 with 20 mM bathophenanthroline and 60 µM p-CMBS (open circles) or buffer A at pH 8.0 with 10 mM ThPP (filled triangles). (D and E) Dependence on trans-membrane pH gradient of the dATP uniport by Tpc1p. The reconstitution mixture contained 10 mM dATP (dATP/dATP exchange) or 10 mM NaCl (dATP uniport), and buffer A at pH 8.0 (D) or at various pH values from 6.0 to 8.0 (E). After reconstitution of Tpc1p into liposomes, a mixture of 50 mM NaCl and 0.1 mM MES/0.1 mM HEPES at pH 8.0 (D) or various pH values from 6.0 to 8.0 (E) was used to equilibrate and to elute the Sephadex G-75 columns. Transport was started by adding 1 mM [α-35S]dATP together with buffer A at pH values from 6.0 to 8.0 (D) or at pH 6.0 (E). The reaction was terminated after 3 min: dATP uniport (filled squares), dATP/dATP exchange (filled circles). Similar results were obtained in three independent experiments. (F) Effect of externally added substrates on the uptake of [α-35S]dATP into proteoliposomes reconstituted with recombinant Tpc1p. Transport was started by adding 0.2 mM [α-35S]dATP to proteoliposomes containing 10 mM NaCl and no substrate, and stopped after 3 min. All substrates were added together with [α-35S]dATP at a final concentration of 0.8 mM. The extents of inhibition (%) from a representative experiment are reported.
Functional characterization of recombinant Tpc1p
Tpc1p was reconstituted into liposomes, and its transport properties were tested in homo-exchange (same substrate inside and outside) experiments. Using external and internal substrate concentrations of 1 and 10 mM, respectively, the reconstituted protein catalyzed an active [α-35S]dATP/dATP exchange, but not homo-exchanges for phosphate, adenine, adenosine, thymidine, pyruvate, malonate, succinate, malate, oxoglutarate, citrate, carnitine, ornithine, lysine, arginine, histidine, tyrosine and tryptophan. No [α-35S]dATP/dATP exchange activity was observed by reconstitution of sarkosyl-solubilized material from bacterial cells lacking the expression vector for Tpc1p.
The substrate specificity of reconstituted Tpc1p was examined by measuring the uptake of [α-35S]dATP into proteoliposomes that had been pre-loaded with various potential substrates (see Figure 1B). The highest activities of [α-35S]dATP uptake into proteoliposomes were with internal ThPP and ThMP. Significant activities were also observed with internal dAMP, dADP, dATP, AMP, ADP, 3′-AMP and 3′,5′-ADP. In contrast, the uptake of [α-35S]dATP was low in the presence of other nucleotides (cAMP, NMN, CoA and SAM), thiamine and adenosine. The residual activity in the presence of these substrates was virtually the same as in the absence of internal substrate (NaCl present), indicating that Tpc1p is able to catalyze a unidirectional transport (uniport) of dATP, besides the exchange reaction. Deoxynucleotides and nucleotides were transported with the following order of efficiency: NMP > NDP > NTP; those of C, T, U and G were transported with a slightly lower efficiency than those of A (activities from ∼250 to 500 nmol/30 min/mg protein) (data not shown). Nucleosides, purines, pyrimidines and dideoxynucleotides were not exchanged with [α-35S]dATP.
A substantial efflux of [α-35S]dATP from pre-labeled proteoliposomes occurred when adding only buffer to proteoliposomes (more at pH 8.0 than at pH 6.0), and the efflux was prevented by inhibitors of dATP/dATP exchange (Figure 1C). The addition of 10 mM ThPP or (not shown) ThMP at external pH 8.0 induced a greater efflux of [α-35S]dATP from proteoliposomes (Figure 1C). Similar results were obtained using [35S]dCTP or [14C]ADP, instead of dATP.
The rate of dATP uniport (measured as uptake by proteoliposomes in the absence of internal substrate) increased markedly on decreasing the external pH from 8.0 to 6.0 (at a fixed internal pH of 8.0) (Figure 1D), as well as on increasing the internal pH from 6.0 to 8.0 (at a fixed external pH of 6.0) (Figure 1E). In contrast, the rate of dATP/dATP exchange was virtually unaffected by changing either the external or the internal pH from 6.0 to 8.0, at fixed pH in the other compartment (Figure 1D and E). These experiments, showing that the proton gradient imposed across the proteoliposomal membrane stimulates markedly the net transport of dATP without influencing the dATP/dATP exchange, suggest that dATP is transported by the reconstituted Tpc1p together with H+ or in exchange for OH–.
Since radioactive ThPP is not commercially available, another approach was employed to measure the transport of this coenzyme across the membrane of liposomes reconstituted with Tpc1p and pre-loaded with ThPP. As shown in Table I, the efflux of ThPP from the vesicles increased on increasing the external pH from 6.0 to 8.0 (at a fixed internal pH of 6.0) and on adding dATP and AMP but not adenosine, cAMP and NMN. These results provide direct evidence that ThPP is transported by Tpc1p by both uniport and exchange.
Table I. Efflux of ThPP from proteoliposomes.
| External pH | External substrate | ThPP efflux (µmol/60 min/mg protein) | |
|---|---|---|---|
| Expt 1 | 6.0 | None | 0.08 |
| 6.5 | None | 0.13 | |
| 7.0 | None | 0.26 | |
| 7.5 | None | 0.47 | |
| 8.0 | None | 0.60 | |
| Expt 2 | 7.0 | None | 0.23 |
| 7.0 | dATP | 0.61 | |
| 7.0 | AMP | 0.65 | |
| 7.0 | Adenosine | 0.24 | |
| 7.0 | cAMP | 0.23 | |
| 7.0 | NMN | 0.19 |
Proteoliposomes were reconstituted with recombinant Tpc1p in the presence of 20 mM ThPP and 30 mM MES/30 mM HEPES at pH 6.0 (expt 1) or at pH 7.0 (expt 2). After reconstitution of Tpc1p into liposomes, a mixture of 50 mM NaCl and 0.1 mM MES/0.1 mM HEPES (expt 1) or 30 mM MES/30 mM HEPES (expt 2) at the same pH as that of the reconstitution mixture was used to equilibrate and to elute the Sephadex G-75 columns. Transport was started by adding 30 mM MES/30 mM HEPES at the indicated pH values (expt 1) or the indicated substrates at 10 mM (expt 2). The reaction was terminated after 60 min and the ThPP released from proteoliposomes was measured. Similar results were obtained in three independent experiments.
The uptake of 0.1 mM [α-35S]dATP by proteoliposomes containing 10 mM NaCl and no substrate (reaction time 3 min) was inhibited by 20 mM bathophenanthroline (86% inhibition), 20 mM pyridoxal 5′-phosphate (58%), 60 µM p-chloromercuribenzene sulfonate (p-CMBS; 69%) and 60 µM mersalyl (65%), which are inhibitors of many mitochondrial carriers. A specific inhibitor of the mitochondrial citrate carrier, 1,2,3-benzenetricarboxylate (2 mM), reduced the rate of dATP uniport to 42%. No significant inhibition was observed with 0.1 mM carboxyatractyloside and α-cyanocinnamate, 0.01 mM bongkrekate, 2 mM butylmalonate and phenylsuccinate, and 1 mM N-ethylmaleimide (inhibitors of other characterized mitochondrial carriers).
In addition, [α-35S]dATP uptake by unloaded proteoliposomes was inhibited strongly by external addition of ThPP or ThMP (Figure 1F). A lower inhibition was observed with AMP, 3′-AMP or (not shown) other (deoxy)nucleotides. In contrast, very low inhibition was detected with cAMP, SAM, NMN, CoA, thiamine and adenosine (Figure 1F) and (not shown) (deoxy)nucleosides, nitrogen bases and substrates of other mitochondrial carriers.
Kinetic characteristics of recombinant Tpc1p
The Km and Vmax values for dATP uptake by unloaded proteoliposomes (measured as uniport at 25°C) were 0.51 ± 0.04 mM and 38 ± 7 µmol/min per g of protein, respectively (mean values from 18 experiments). ThPP, ThMP, dADP, ADP and AMP inhibited dATP uptake competitively (not shown), and their Ki values were 0.23, 0.27, 0.61, 0.70 and 0.58 mM, respectively (means of three experiments).
tpc1Δ yeast cells are not able to grow on fermentative carbon sources in the absence of thiamine
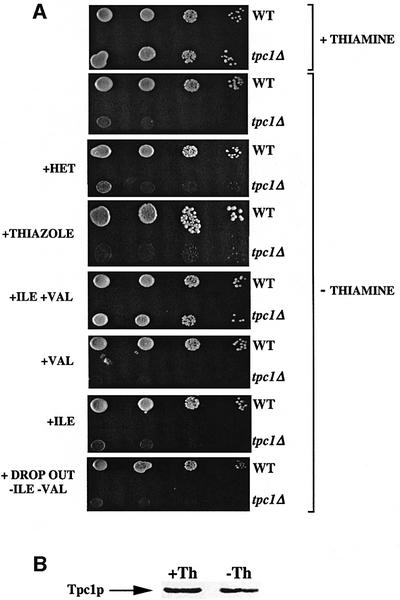
Having established the transport function of Tpc1p by in vitro assays, the effect of deleting its gene on yeast cells was investigated. The tpc1Δ strain was tested for its ability to grow in the absence of different cofactors. Yeast cells lacking TPC1 showed substantial growth on YP and synthetic complete media (SC) containing either fermentative (2% glucose and 2% galactose) or non-fermentative (3% glycerol, 2% lactate, 2% ethanol, 2% pyruvate and 10 mM oxaloacetate) carbon sources, similarly to the wild-type strain. However, on synthetic minimal medium (SM) supplemented with glucose or (not shown) galactose, tpc1Δ cells, but not wild-type cells, exhibited an auxotrophy for thiamine (Figure 2A), but not for other cofactors such as pyridoxine, pantothenate, riboflavin, biotin, niacin, folate, p-aminobenzoate and inositol (not shown). Growth of tpc1Δ cells on SM supplemented with non-fermentative carbon sources was virtually indistinguishable from that of wild-type cells. Growth on thiamine-less SM supplemented with glucose or galactose was fully restored by complementing the deletion strain with the TPC1-pRS416 plasmid (not shown), demonstrating that this difference in phenotype is the result of the absence of Tpc1p protein and not a secondary effect. In contrast, the growth phenotype of tpc1Δ cells was not restored by complementing the knock-out strain with the DNC-pYES2 plasmid (not shown).
Fig. 2. tpc1Δ mutant is auxotrophic for thiamine. (A) Growth behavior of tpc1Δ in various conditions. Four-fold serial dilutions of wild-type and tpc1Δ mutant strains were plated on solid SM supplemented with 2% glucose. Where indicated, 0.4 mg/l thiamine, 4 mg/ml HET, 4 mg/ml thiazole, 340 µg/ml valine (VAL) or 430 µg/ml isoleucine (ILE) was also present. (B) The expression level of Tpc1p is independent of the presence of thiamine in the SM medium. Equal amounts of mitochondrial lysates from wild-type cells grown on galactose-supplemented SM with or without thiamine (Th) were separated by SDS–PAGE, transferred to nitrocellulose and immunodecorated with the antiserum directed against Tpc1p.
The addition of thiazole or 4-methyl-5-(β-hydroxyethyl)thiazole (HET), two precursors of thiamine biosynthesis, did not restore the growth of the deletion strain on thiamine-less SM (Figure 2A). It should be noted that thiazole or HET, at the same concentrations, fully restored the growth of S.cerevisiae cells deleted of thi4, a mitochondrial thiamine biosynthetic gene, on thiamine-less SM (Praekelt et al., 1994). Furthermore, since the expression of the genes encoding enzymes involved in thiamine biosynthesis (thi4, thi5 and thi6) is repressed by thiamine (Nosaka et al., 1993, 1994; Praekelt et al., 1994), we investigated the expression of Tpc1p in the presence and absence of thiamine. The immunodecoration of mitochondria isolated from wild-type cells grown on galactose-supplemented SM with or without thiamine revealed that Tpc1p is present in similar amounts (Figure 2B), i.e. the expression of the Tpc1p gene is not repressed by thiamine. All these results suggest that Tpc1p is not required for the synthesis of HET or thiamine.
Interestingly, the growth of tpc1Δ on thiamine-less SM in the presence of glucose or (not shown) galactose was fully restored by the simultaneous addition of valine and isoleucine (see Figure 2A). Each of these supplements, when added alone, did not sustain growth of tpc1Δ (Figure 2A). Furthermore, the growth of the deletion strain on thiamine-less SM in the presence of fermentative carbon sources was not restored by addition of drop-out (a mixture of amino acids and nitrogen bases) less valine and isoleucine (Figure 2A). We conclude that the effect of valine plus isoleucine in restoring the deletion strain viability on thiamine-less SM is specific.
Tpc1p is required for entry of ThPP into mitochondria
The auxotrophy for isoleucine and valine of tpc1Δ cells on thiamine-less SM suggests that the deletion of Tpc1p causes a defect in the reaction catalyzed by ALS, which is the only ThPP-requiring enzyme in the biosynthetic pathway of branched chain amino acids in yeast. This enzyme is encoded by ilv2 and is located exclusively in mitochondria. It condenses two molecules of pyruvate to form 2-acetolactate and CO2 in the first step of valine and leucine synthesis, or one molecule of pyruvate and one of 2-oxobutyrate to produce 2-aceto-2-hydroxybutyrate in the first step of isoleucine synthesis.
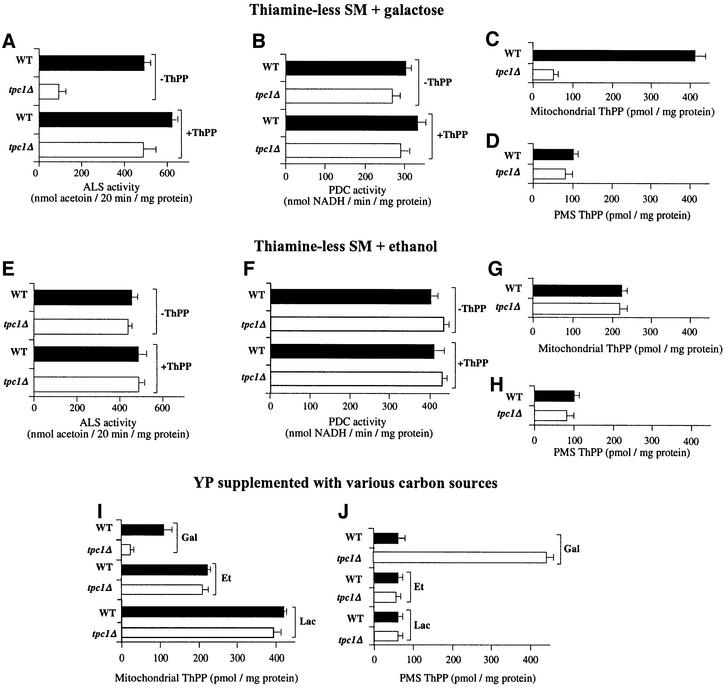
Direct assays of ALS showed that the activity of this enzyme in mitochondria (mitochondrial extracts) from the tpc1Δ cells grown on thiamine-less SM supplemented with galactose is 5-fold lower than that of mitochondria from the wild-type strain (see Figure 3A). The addition of ThPP to the assay mixture of ALS restored the activity nearly completely (Figure 3A). Under the same conditions, the activity of OGDH was 4-fold lower in the mutant than in the wild-type cells, and full restoration was observed when ThPP was added to the assay mixture (not shown). These results indicate that the low activity of ALS and OGDH in the knock-out strain may be caused by a lack of ThPP in the mitochondria of the mutant. In contrast, the activity of PDC, another ThPP-requiring enzyme, which is located in the cytosol, was not significantly different in tpc1Δ and wild-type cells, and was virtually unchanged by the addition of ThPP to the assay mixture (Figure 3B).
Fig. 3. Tpc1p is required for entry of ThPP into mitochondria. Mitochondria and post-mitochondrial supernatants (PMS) were isolated from wild-type and tpc1Δ cells grown on thiamine-less SM supplemented with galactose (A–D) or ethanol (E–H), or grown on YP (I and J) supplemented with galactose (Gal), ethanol (Et) or lactate (Lac). (A and E) Mitochondria from tpc1Δ cells grown on galactose, but not on ethanol, are defective in ALS activity. Mitochondria from wild-type and tpc1Δ cells were lysed in a mixture containing 0.05% Triton X-114 and pyruvate-less assay buffer for ALS with or without 1 mM ThPP. The extracts were centrifuged at 12 000 g for 10 min at 4°C. The ALS assay at 30°C was started by adding 50 mM pyruvate to the extracts. (B and F) tpc1Δ PMS contain wild-type levels of PDC activity. PMS from wild-type and tpc1Δ cells were pre-incubated with or without 5 mM thiamine at 30°C in a medium containing 75 mM MgCl2 and 20 mM PIPES pH 7.0. After 3 min, an aliquot of the pre-incubation mixture was added to the assay buffer for PDC. (C, D and G–J) Mitochondria, but not PMS, isolated from tpc1Δ cells grown on galactose (but not on ethanol or lactate), contain decreased levels of ThPP. Mitochondrial extracts (C, G and I) and PMS (D, H and J) from wild-type and tpc1Δ cells were assayed for ThPP content.
We then measured the content of ThPP in mitochondria and post-mitochondrial supernatant (PMS) of wild-type and mutant cells. The amount of ThPP in mitochondria from tpc1Δ was ∼8-fold lower than in the organelles from wild-type cells, whereas in PMS it was not significantly different (Figure 3C and D). In addition, the mitochondrial ThPP level of knock-out cells was enhanced nearly to the wild-type level by complementation with the TPC1-pRS416 plasmid (not shown). Therefore, in the mutant, there is a shortage of ThPP in mitochondria and not in PMS. Also, the lower mitochondrial level of ThPP is responsible for the lower activity of ALS and OGDH, supporting the view that Tpc1p is required for the entry of ThPP into mitochondria.
In striking contrast to the results of Figure 3A–D, when wild-type and mutant cells were grown on thiamine-less SM supplemented with ethanol, instead of galactose, the levels of ThPP in mitochondria and PMS, as well as the activities of ALS and PDC, were not significantly different in the two strains, in agreement with the lack of phenotype of tpc1Δ cells grown on non-fermentative carbon sources (Figure 3E–H). One explanation for these results is that another protein capable of transporting ThPP into mitochondria is expressed in the presence of ethanol and the other non-fermentative substrates.
With this hypothesis in mind, we determined the content of ThPP in mitochondria and PMS of yeast cells grown on YP medium supplemented with fermentable and non-fermentable substrates. It was observed that in the presence of galactose, also in YP medium, as observed in SM, the mitochondria of tpc1Δ cells contain less ThPP than those of wild-type cells (Figure 3I). Furthermore, on YP medium supplemented with galactose, the amount of ThPP in PMS of the mutant is markedly increased: ∼7.5-fold that in PMS of the wild-type strain (Figure 3J). On the same medium in the presence of the non-fermentable substrates ethanol and lactate, no differences in ThPP content were observed in either mitochondria or PMS between wild-type and mutant cells (Figure 3I and J).
Influence of carbon source on the expression of Tpc1p
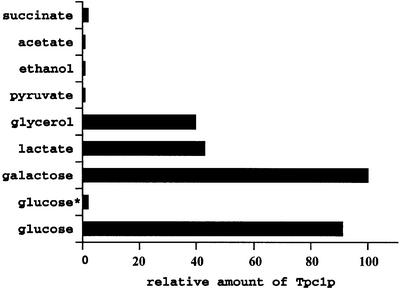
Tpc1p was expressed at higher levels on glucose and galactose media than on media supplemented with non-fermentable carbon sources (Figure 4). In particular, on succinate, acetate, ethanol and pyruvate, the amount of Tpc1p was negligible. Likewise, a very marked decrease in Tpc1p expression was observed during the diauxic shift when S.cerevisiae growing on glucose in batch culture exhausted the glucose and began to oxidize the ethanol produced by fermentation (Figure 4). In agreement with the results of Figure 4, a significantly higher transcript expression of TPC1 (YGR096w) in anaerobiosis as compared with aerobiosis has been observed previously (ter Linde et al., 1999). To quantify Tpc1p, various amounts of mitochondrial samples from yeast cells fed on galactose were loaded onto the gel and immunoblotted simultaneously with the appropriate range of bacterially expressed Tpc1p standard. In four determinations, the abundance of Tpc1p was 32 ± 7 pmol/mg of protein.
Fig. 4. Comparison of the expression of Tpc1p on various carbon sources. Cells were harvested from exponentially growing cells on YP medium supplemented with the indicated carbon sources. Glucose* indicates cells harvested after diauxic shift. The amount of Tpc1p protein was estimated by densitometry upon immunodecoration of mitochondrial protein with a specific antiserum. Similar results were obtained in four independent experiments. The amount of Tpc1p present in mitochondria from galactose-fed cells was taken as 100%.
Tpc1p is an integral protein of the inner mitochondrial membrane
The above experiments concerning the influence of Tpc1p on the physiology and biosynthetic pathways in S.cerevisiae are entirely consistent with it being a component of mitochondria. This conclusion was confirmed by comparison of mitochondrial membranes from wild-type and deletion strains. A single immunoreactive band with an apparent molecular mass of ∼35 kDa was detected in wild-type mitochondria, but not in mitochondria from the deletion strain with an antiserum against the bacterially expressed Tpc1p (Figure 5A). The contents of the ADP/ATP carrier (Figure 5A) and (not shown) the phosphate, succinate–fumarate and dicarboxylate carriers detected with specific antibodies were essentially the same in both wild-type and tpc1Δ mitochondria. Therefore, the absence of Tpc1p protein from the tpc1Δ strain does not affect the expression of other carriers.
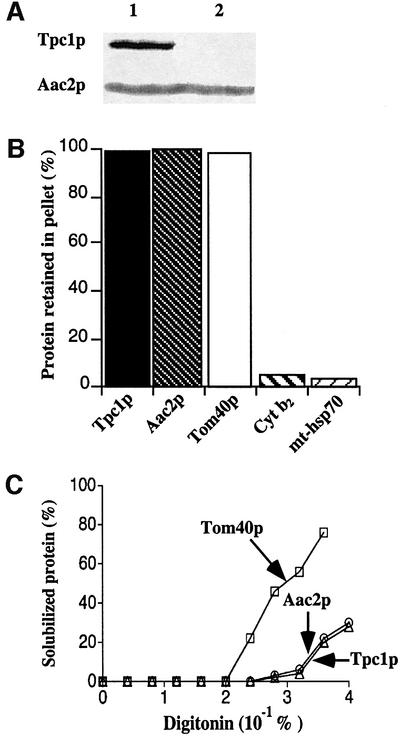
Fig. 5. Tpc1p is an integral protein of the inner mitochondrial membrane. (A) Immunoblot analysis of Tpc1p in yeast mitochondria. Equal amounts of mitochondrial lysates from wild-type (lane 1) and tpc1Δ mutant (lane 2) were separated by SDS–PAGE, transferred to nitrocellulose and immunodecorated with antibodies directed against Tpc1p and the ADP/ATP carrier (Aac2p). (B) Tpc1p is not extracted from mitochondrial membranes by carbonate treatment. Proteins of pellets were analyzed by SDS–PAGE and western blotting using antisera directed against Tpc1p, Aac2p (inner membrane component), Tom40p (outer membrane component), cytochrome b2 (Cyt b2; intermembrane space marker) and mitochondrial hsp70 (mt-hsp70; matrix protein). The total amount of protein in the pellet and supernatant was set to 100%. (C) Digitonin fractionation of mitochondria. Tpc1p, Aac2p and Tom40p were estimated by immunoblotting. Their content in the unextracted membrane fraction was taken as 100%.
The submitochondrial location of Tpc1p was examined by separation of soluble and peripheral proteins from integral membrane proteins of wild-type mitochondria by carbonate treatment. As illustrated in Figure 5B, Tpc1p remained in the membrane protein fraction, as did the ADP/ATP carrier and Tom40p (marker proteins of inner and outer mitochondrial membranes, respectively), but the intermembrane space protein cytochrome b2 and the matrix protein hsp70 were in the soluble and peripheral protein fraction. Therefore, Tpc1p is an integral mitochondrial membrane protein. Both Tpc1p and the ADP/ATP carrier were solubilized from wild-type mitochondria at the same concentration of digitonin, greater than that required to solubilize the outer membrane protein Tom40p (Figure 5C). Therefore, Tpc1p is an integral protein of the inner mitochondrial membrane.
Taken together, these experiments and the experiments on the deletion strains leave no room for doubt that Tpc1p is found in the inner membranes of the mitochondria of S.cerevisiae. At present, there is no evidence that the protein is found uniquely in that cellular location, and therefore this aspect is being investigated by the construction of strains with green fluorescent protein (GFP) fused to Tpc1p (F.M.Lasorsa and F.Palmieri, unpublished results).
Discussion
The transport characteristics and kinetic parameters of the recombinant and reconstituted Tpc1p from S.cerevisiae show that it is the mitochondrial transporter for ThPP and ThMP. Tpc1p also transports structurally related nucleotides to a lesser extent, but not thiamine, nucleosides, purines and pyrimidines. The substrate specificity of Tpc1p is distinct from that of any other previously characterized mitochondrial carrier. Tpc1p is also different from the human DNC (Dolce et al., 2001), although it is its closest yeast relative. First, the yeast Tpc1p and the human DNC have sequence identity of 25%, indicating that they are not orthologs. Secondly, overexpression of DNC does not complement the thiamine auxotrophy of the tpc1Δ strain. Thirdly, DNC catalyzes an obligatory counter-exchange whereas Tpc1p catalyzes both uniport and exchange. Fourthly, unlike DNC, Tpc1p transports ThPP and ThMP efficiently and is completely unaffected by carboxyatractyloside and bongkrekic acid.
Since ThPP is produced in the cytosol by thiamine pyrophosphokinase (Hohmann and Meacock, 1998), the primary function of Tpc1p is probably to catalyze the uniport uptake of ThPP into the mitochondria, where it is required for the activity of ALS, pyruvate dehydrogenase and OGDH. In addition, since ThPP is hydrolyzed in the mitochondrial matrix by a ThPPase activity, as shown in rat liver (Barile et al., 1998) and S.cerevisiae (F.Bisaccia, unpublished data), a further important physiological function of Tpc1p is to catalyze the exchange between cytosolic ThPP and intramitochondrial ThMP. In energized mitochondria, both the uptake of ThPP and the exchange between ThPPout and ThMPin are favored, since ThPP and ThMP are co-transported with H+ in a pH gradient-dependent manner. It should be noted that the uniport reaction is necessary in special conditions (when the intramitochondrial level of ThPP has to be increased), for example, when cells divide or when cells are transferred from a medium containing valine, leucine and isoleucine to one without these amino acids. In the absence of branched chain amino acids, it is well known that the biosynthesis of ALS is derepressed (Xiao and Rank, 1988, 1990).
The following important points in the Results support Tpc1p controlling the uptake of ThPP into mitochondria. First, mitochondria from tpc1Δ cells exhibit a lower level of ThPP and a decreased activity of the ThPP-dependent enzymes ALS and OGDH, which is restored on adding the coenzyme to the assay medium. Secondly, the lack of ThPP is restricted to the matrix of mitochondria and does not concern the PMS. Also the striking and marked increase in the PMS ThPP content in the tpc1Δ strain grown on thiamine-containing YP medium in the presence of galactose may be explained as an adaptive response of the tpc1Δ cells (probably by activation of the Thi10p plasma membrane transporter) to compensate for the mitochondrial ThPP defect. Thirdly, the tpc1Δ strain does not grow on thiamine-less SM medium supplemented with fermentable carbon sources. However, growth is restored by the addition of valine plus isoleucine because, under fermentative conditions, ThPP is required in mitochondria mainly for the synthesis of branched chain amino acids. Thus, the simplest explanation of the growth phenotype of the tpc1Δ mutant is that it is caused mainly by insufficient activity of ALS (the first enzyme of the biosynthesis of branched chain amino acids) and that it is rescued by addition of the end products of the biosynthetic pathway of these amino acids. It should be noted that leucine was always added to our media since the yeast strain employed in the present investigation is auxotrophic for this amino acid. The experimental data cannot be explained by proposing that Tpc1p is required for the transport across the mitochondrial membrane of intermediates in thiamine biosynthesis. The reaction in ThPP biosynthesis that produces HET-P from 2-pentulose-5-phosphate, glycine and cysteine occurs in the mitochondria and is catalyzed by Thi4p (Hohmann and Meacock, 1998). The main indication that Tpc1p is not involved either in the uptake of the substrates or in the export of the product of the reaction catalyzed by Thi4p is the observation that tpc1Δ cells retain thiamine auxotrophy in the presence of HET that was shown to bypass the reaction catalyzed by the mitochondrial Thi4p (Praekelt et al., 1994). Moreover, the expression of TPC1 is not repressed by the addition of thiamine, unlike that of all the other genes known to be involved in the biosynthesis of thiamine. Finally, the decrease in intramitochondrial ThPP content is also observed in tpc1Δ cells grown on thiamine-containing galactose-supplemented YP medium, i.e. under conditions in which the endogenous synthesis of thiamine is not required.
One striking observation of this investigation is that tpc1Δ cells grow normally on thiamine-less SM supplemented with non-fermentative carbon sources, in contrast to the lack of growth on fermentative substrates. This observation and the finding that Tpc1p is little or not expressed on non-fermentable substrates strongly suggest that ThPP is imported into the mitochondria by a different transporter under these conditions. The existence of a second carrier for ThPP mainly expressed on non-fermentable carbon sources is also supported by the findings (i) that the intramitochondrial level of ThPP is the same in tpc1Δ and wild-type cells in respiratory conditions and (ii) that a small amount of ThPP is present in tpc1Δ cells on glucose or galactose. These effects warrant further study. It is noteworthy that the expression of some mitochondrial carriers in S.cerevisiae increases during the diauxic shift from fermentation to respiration (DeRisi et al., 1997). It may be that one of these carriers is the ThPP transporter under respiratory conditions, whereas Tpc1p is the prevailing ThPP transporter in the presence of glucose and possibly in anaerobiosis.
Materials and methods
Yeast strains, media and preparation of mitochondria
The Tpc1p gene was deleted by homologous recombination of the auxotrophic marker HIS3 at the TPC1 locus of S.cerevisiae strain YPH499 (MATa ade2-101 his3-Δ 200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 TPC1::HIS3). The deletion of TPC1 was verified by PCR and western blot analysis. The coding sequence and the promoter of TPC1 were cloned into the plasmid pRS416 (Sikorsky and Hieter, 1989), and the coding sequence of DNC (Dolce et al., 2001) into the plasmid pYES2 (Invitrogen) behind the inducible Gal-Cyc promoter. These plasmids were introduced into the tpc1Δ yeast strain, and transformants were selected for uracil auxotrophy. Wild-type fungi and the deletion strain were grown in rich medium containing 2% bactopeptone and 1% yeast extract (YP), SC or SM (Sherman, 1991) with or without thiamine. All media were supplemented with either fermentable or non-fermentable carbon sources (2% glucose or 2% galactose, and 3% glycerol, 2% ethanol, 3% acetate, 2% lactate, 2% pyruvate or 2% succinate). The final pH was adjusted to 4.5 or, with pyruvate or acetate, to 6.5. Mitochondria were isolated by standard procedures (Daum et al., 1982). Extractions of mitochondria with sodium carbonate or with digitonin were performed as described previously (Palmieri et al., 1999). The amount of Tpc1p in wild-type mitochondria was determined by quantitative immunoblotting as described previously (Palmieri et al., 1999).
Bacterial expression of Tpc1p
The coding sequence of TPC1 (corresponding to open reading frame YGR096w) was amplified from S.cerevisiae genomic DNA by PCR. Forward and reverse oligonucleotide primers were synthesized corresponding to the extremities of the TPC1 coding sequence with additional NdeI and HindIII sites, respectively. The amplified product was cloned into the pMW7 expression vector. Transformants of E.coli DH5α cells were selected on ampicillin (100 µg/ml), and screened by direct colony PCR and by restriction digestion of purified plasmids. The sequence of the insert was verified. Tpc1p was overexpressed at 30°C in E.coli C0214(DE3) (Fiermonte et al., 1998a; Palmieri et al., 2001b). Inclusion bodies were purified on a sucrose density gradient (Fiermonte et al., 1993), washed at 4°C with TE buffer (10 mM Tris–HCl, 1 mM EDTA pH 6.5), then twice with a buffer containing Triton X-114 (3%, w/v), 1 mM EDTA and 10 mM PIPES–KOH pH 6.5, and once again with TE buffer. Proteins were separated by SDS–PAGE in 17.5% gels and either stained with Coomassie Blue or transferred to nitrocellulose membranes for immunodetection with a rabbit antiserum raised against bacterially expressed Tpc1p. The N-terminus was sequenced, and the yield of purified Tpc1p was estimated by laser densitometry of stained samples (Fiermonte et al., 1998b).
Reconstitution into liposomes and transport measurements
Tpc1p was solubilized in 1.8% sarkosyl (w/v), and a small residue was removed by centrifugation (258 000 g, 1 h). The solubilized protein was reconstituted into liposomes in the presence of 30 mM MES/30 mM HEPES (buffer A) at pH 7.0 (except where otherwise indicated) and with or without substrate (Palmieri et al., 1995). External substrate was removed from proteoliposomes on Sephadex G-75 columns, pre-equilibrated with buffer A at pH 7.0 (except where otherwise indicated). The amount of protein incorporated into liposomes was measured as described previously (Fiermonte et al., 1998b); in all cases, it was ∼18% of the protein added to the reconstitution mixture. Transport at 25°C was started by adding [α-35S]dATP (NEN Life Science Products) to substrate-loaded proteoliposomes (exchange) or to unloaded proteoliposomes (uniport), and terminated by addition of 60 µM p-CMBS and 20 mM bathophenanthroline (Palmieri et al., 1995). In controls, inhibitors were added with the labeled substrate. Entrapped radioactivity was counted (Palmieri et al., 1995). The experimental values were corrected by subtracting control values. The initial transport rate was calculated from the radioactivity taken up by proteoliposomes after 3 min (in the initial linear range of substrate uptake). Various other transport activities were also assayed by the inhibitor-stop method. For efflux measurements, proteoliposomes containing 1 mM dATP were labeled with carrier-free [α-35S]dATP by carrier-mediated exchange equilibration (Palmieri et al., 1995). After 60 min, the external radioactivity was removed by passing the proteoliposomes through Sephadex G-75. Efflux was started by adding unlabeled external substrate or buffer alone, and terminated by adding the inhibitors indicated above.
Miscellaneous
ALS (EC 4.1.3.18), OGDH (EC 1.2.4.2) and PDC (EC 4.1.1.1) activity measurements were performed according to published protocols (Magee et al., 1968; Gubler and Wittorf, 1970; Hirabayashi and Harada, 1971). ThPP was assayed essentially as described by Gubler and Wittorf (1970) by measuring holo-PDC activity, which derives from the addition of ThPP to enzymatically inactive apoenzyme. Immediately prior to assay, apo-PDC was suspended in 0.05 M PIPES pH 6.5. Holoenzyme reconstitution was performed by incubating either standard ThPP solutions or extracts with 75 mM MgCl2 and 50 mM PIPES pH 6.7 for 3 min at 30°C. The rate of NADH oxidation was determined using alcohol dehydrogenase (EC 1.1.1.1) as an ancillary enzyme.
Acknowledgments
Acknowledgements
This work was supported by grants from MURST-PRIN, MURST L.488/92 CO3 and CO4, MURST-CNR L.95/95, CEGBA, CNR target project on Biotechnology and by the European Social Fund.
References
- Barile M., Passarella,S. and Quagliariello,E. (1986) Uptake of thiamine by isolated rat liver mitochondria. Biochem. Biophys. Res. Commun., 141, 466–473. [DOI] [PubMed] [Google Scholar]
- Barile M., Passarella,S. and Quagliariello,E. (1990) Thiamine pyro phosphate uptake into isolated rat liver mitochondria. Arch. Biochem. Biophys., 280, 352–357. [DOI] [PubMed] [Google Scholar]
- Barile M., Valenti,D., Brizio,C., Quagliariello,E. and Passarella,S. (1998) Rat liver mitochondria can hydrolyse thiamine pyro phosphate to thiamine monophosphate which can cross the mitochondrial membrane in a carrier-mediated process. FEBS Lett., 435, 6–10. [DOI] [PubMed] [Google Scholar]
- Cybulski R.L. and Fisher,R.R. (1981) Uptake of oxidized folates by rat liver mitochondria. Biochim. Biophys. Acta, 646, 329–333. [DOI] [PubMed] [Google Scholar]
- Daum G., Bohni,P.C. and Schatz,G. (1982) Import of proteins into mitochondria. J. Biol. Chem., 257, 13028–13033. [PubMed] [Google Scholar]
- DeRisi J.L., Iyer,V.R. and Brown,P.O. (1997) Exploring the metabolic and genetic control of gene expression on a genomic scale. Science, 278, 680–686. [DOI] [PubMed] [Google Scholar]
- Deus B. and Blum,H. (1970) Subcellular distribution of thiamine pyrophosphokinase activity in rat liver and erythrocytes. Biochim. Biophys. Acta, 219, 489–492. [DOI] [PubMed] [Google Scholar]
- Dolce V., Fiermonte,G., Runswick,M.J., Palmieri,F. and Walker,J.E. (2001) The human mitochondrial deoxynucleotide carrier and its role in toxicity of nucleoside antivirals. Proc. Natl Acad. Sci. USA, 98, 2284–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton W.A., Ambani,L.M. and Rosenberg,L.E. (1976) Uptake of hydroxycobalamin by rat liver mitochondria. Binding to a mitochondrial protein. J. Biol. Chem., 251, 6616–6623. [PubMed] [Google Scholar]
- Fiermonte G., Walker,J.E. and Palmieri,F. (1993) Abundant bacterial expression and reconstitution of an intrinsic membrane transport protein from bovine mitochondria. Biochem. J., 294, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiermonte G., Palmieri,L., Dolce,V., Lasorsa,F.M., Palmieri,F., Runswick,M.J. and Walker,J.E. (1998a) The sequence, bacterial expression and functional reconstitution of the rat mitochondrial dicarboxylate transporter cloned via distant homologs in yeast and Caenorhabditis elegans. J. Biol. Chem., 273, 24754–24759. [DOI] [PubMed] [Google Scholar]
- Fiermonte G., Dolce,V. and Palmieri,F. (1998b) Expression in Escherichia coli, functional characterization and tissue distribution of isoforms A and B of the phosphate carrier from bovine mitochondria. J. Biol. Chem., 273, 22782–22787. [DOI] [PubMed] [Google Scholar]
- Fiermonte G., Palmieri,L., Todisco,S., Agrimi,G., Palmieri,F. and Walker,J.E. (2002) Identification of the mitochondrial glutamate transporter: bacterial expression, reconstitution, functional characterization and tissue distribution of two isoforms. J. Biol. Chem., 277, 19289–19294. [DOI] [PubMed] [Google Scholar]
- Gubler C.J. and Wittorf,J.H. (1970) Use of coenzyme analogs to study thiamine diphosphate (cocarboxylase) binding in yeast pyruvate decarboxylase. Methods Enzymol., 18, 117–120. [Google Scholar]
- Hirabayashi T. and Harada,T. (1971) Isolation and properties of ketoglutarate dehydrogenase complex from baker’s yeast (Saccharomyces cerevisiae). Biochem. Biophys. Res. Commun., 45, 1369–1375. [DOI] [PubMed] [Google Scholar]
- Hohmann S. and Meacock,P.A. (1998) Thiamine metabolism and thiamine diphosphate-dependent enzymes in the yeast Saccharomyces cerevisiae: genetic regulation. Biochim. Biophys. Acta, 1385, 201–219. [DOI] [PubMed] [Google Scholar]
- Horne D.W., Holloway,R.S. and Said,H.M. (1992) Uptake of 5- formyltetrahydrofolate in isolated rat liver mitochondria is carrier-mediated. J. Nutr., 122, 2204–2209. [DOI] [PubMed] [Google Scholar]
- Ingebretsen O.C. and Normann,P.T. (1982) Transport of ascorbate into guinea pig liver mitochondria. Biochim. Biophys. Acta, 684, 21–26. [DOI] [PubMed] [Google Scholar]
- Kaplan R.S., Mayor,J.A., Gremse,D.A. and Wood,D.O. (1995) High level expression and characterization of the mitochondrial citrate transport protein from the yeast Saccharomyces cerevisiae.J. Biol. Chem., 270, 4108–4114. [DOI] [PubMed] [Google Scholar]
- Lui A., Lumeng,L. and Li,T.K. (1981) Metabolism of vitamin B6 in rat liver mitochondria. J. Biol. Chem., 256, 6041–6046. [PubMed] [Google Scholar]
- Lui A., Lumeng,L. and Li,T.K. (1982) Transport of pyridoxine and pyridoxal 5′-phosphate in isolated rat liver mitochondria. J. Biol. Chem., 257, 14903–14906. [PubMed] [Google Scholar]
- Magee P.T. and Robichon-Szulmajster,H. (1968) The regulation of isoleucine–valine biosynthesis in Saccharomyces cerevisiae. Eur. J. Biochem., 3, 507–11. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Day,D.A. and Douce,R. (1984) Transport of coenzyme A in plant mitochondria. Arch. Biochem. Biophys., 229, 253–258. [DOI] [PubMed] [Google Scholar]
- Nosaka K., Kaneko,Y., Nishimura,H. and Iwashima,A. (1993) Isolation and characterization of a thiamine pyrophosphokinase gene, THI80, from Saccharomyces cerevisiae. J. Biol. Chem., 268, 17440–17447. [PubMed] [Google Scholar]
- Nosaka K., Nishimura,H., Kawasaki,Y., Tsujihara,T. and Iwashima,A. (1994) Isolation and characterization of the THI6 gene encoding a bifunctional thiamine-phosphate pyrophosphorylase/hydroxyethylthiazole kinase from Saccharomyces cerevisiae.J. Biol. Chem., 269, 30510–30516. [PubMed] [Google Scholar]
- Palmieri F. (1994) Mitochondrial carrier proteins. FEBS Lett., 346, 48–54. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Indiveri,C., Bisaccia,F. and Iacobazzi,V. (1995) Mitochondrial metabolite carrier proteins: purification, reconstitution and transport studies. Methods Enzymol., 260, 349–369. [DOI] [PubMed] [Google Scholar]
- Palmieri L., Vozza,A., Agrimi,G., De Marco,V., Runswick,M.J., Palmieri,F. and Walker,J.E. (1999) Identification of the yeast mitochondrial transporter for oxaloacetate and sulfate. J. Biol. Chem., 274, 22184–22190. [DOI] [PubMed] [Google Scholar]
- Palmieri L., Rottensteiner,H., Girzalsky,W., Scarcia,P., Palmieri,F. and Erdmann,R. (2001a) Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J., 20, 5049–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri L. et al. (2001b) Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J., 20, 5060–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praekelt U.M., Byrne,K.L. and Meacock,P.A. (1994) Regulation of THI4 (MOL1), a thiamine-biosynthetic gene of Saccharomyces cerevisiae. Yeast, 10, 481–490. [DOI] [PubMed] [Google Scholar]
- Prohl C., Pelzer,W., Diekert,K., Kmita,H., Bedekovics,T., Kispal,G. and Lill,R. (2001) The yeast mitochondrial carrier Leu5p and its human homologue Graves’ disease protein are required for accumulation of coenzyme A in the matrix. Mol. Cell. Biol., 21, 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said H.M., McAlister-Henn,L., Mohammadkhani,R. and Horne,D.W. (1992) Uptake of biotin by isolated rat liver mitochondria. Am. J. Physiol., 263, G81–G86. [DOI] [PubMed] [Google Scholar]
- Saraste M. and Walker,J.E. (1982) Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett., 144, 250–254. [DOI] [PubMed] [Google Scholar]
- Sherman F. (1991) Getting started with yeast. Methods Enzymol., 194, 3–21. [DOI] [PubMed] [Google Scholar]
- Sikorski R.S. and Hieter,P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae.Genetics, 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani A.G. (1991) Evidence for net uptake and efflux of mitochondrial coenzyme A. Biochim. Biophys. Acta, 1067, 29–37. [DOI] [PubMed] [Google Scholar]
- ter Linde J.J., Liang,H., Davis,R.W., Steensma,H.Y., van Dijken,J.P. and Pronk,J.T. (1999) Genome-wide transcriptional analysis of aerobic and anaerobic chemostat cultures of Saccharomyces cerevisiae.J. Bacteriol., 181, 7409–7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus S.A. and Moran,R.G. (2000) Retrovirally mediated comple mentation of the glyB phenotype. Cloning of a human gene encoding the carrier for entry of folates into mitochondria. J. Biol. Chem., 275, 36811–36817. [DOI] [PubMed] [Google Scholar]
- Totskii V.N. (1976) Mechanisms and ways of regulation of lipoic acid penetration into biological structures. Biokhimiia, 41, 1094–1105. [PubMed] [Google Scholar]
- Tzagoloff A., Jang,J., Glerum,D.M. and Wu,M. (1996) FLX1 codes for a carrier protein involved in maintaining a proper balance of flavin nucleotides in yeast mitochondria. J. Biol. Chem., 271, 7392–7397. [DOI] [PubMed] [Google Scholar]
- Xiao W. and Rank,G.H. (1988) The yeast ILV2 gene is under general amino acid control. Genome, 30, 984–986. [DOI] [PubMed] [Google Scholar]
- Xiao W. and Rank,G.H. (1990) Branched chain amino acid regulation of the ILV2 locus in Saccharomyces cerevisiae. Genome, 33, 596–603. [DOI] [PubMed] [Google Scholar]