Abstract
Post-transcriptional gene silencing (PTGS) is characterized by the accumulation of short interfering RNAs that are proposed to mediate sequence-specific degradation of cognate and secondary target mRNAs. In plants, it is unclear to what extent endogenous genes contribute to this process. Here, we address the role of the endogenous target genes in transgene-mediated PTGS of β-1,3-glucanases in tobacco. We found that mRNA sequences of the endogenous glucanase glb gene with varying degrees of homology to the Nicotiana plumbaginifolia gn1 transgene are targeted by the silencing machinery, although less efficiently than corresponding transgene regions. Importantly, we show that endogene-specific nucleotides in the glb sequence provide specificity to the silencing process. Consistent with this finding, small sense and antisense 21- to 23-nucleotide RNAs homologous to the endogenous glb gene were detected. Combined, these data demonstrate that a co-suppressed endogenous glucan ase gene is involved in signal amplification and selection of homologous targets, and show that endogenous genes can actively participate in PTGS in plants. The findings are introduced as a further sophistication of the post-transciptional silencing model.
Keywords: co-suppression/gene silencing/β-1,3-glucanase/RNAi/tobacco
Introduction
In a wide variety of organisms, the introduction of transgenes into the genome may result in co-suppression of the transgene and homologous endogenous genes (Pal-Bhadra et al., 1997; Ruiz et al., 1998; Vaucheret et al., 1998; Kooter et al., 1999; Dernburg et al., 2000; Cogoni, 2001). Many cases of co-suppression are based on sequence-specific degradation of transgene and endogenous mRNAs (Depicker and Van Montagu, 1997; Meins, 2000; Vaucheret et al., 2001). This post-transcriptional gene silencing (PTGS) mechanism, originally discovered in transgenic plants (Napoli et al., 1990; van der Krol et al., 1990), is also capable of targeting viral RNA and is thought to be a natural defense mechanism against viral infection in plants (Vance and Vaucheret, 2001; Voinnet, 2001; Waterhouse et al., 2001). Sequence-specific RNA silencing is also observed in Neurospora crassa (quelling; Romano and Macino, 1992; Cogoni et al., 1996), Caenorhabditis elegans (RNAi; Fire et al., 1998), Drosophila (Tuschl et al., 1999; Kennerdell and Carthew, 2000) and mammalian cells (Elbashir et al., 2001a).
Biochemical and genetic studies conducted in the above organisms revealed striking similarities between PTGS, RNAi and quelling (Hammond et al., 2001; Matzke et al., 2001). RNA silencing is initiated upon production of double-stranded (ds) RNA (Fire et al., 1998; Hamilton et al., 1998; Waterhouse et al., 1998) and correlates with the accumulation of small 21- to 23-nucleotide (nt) RNAs (Hamilton and Baulcombe, 1999; Parrish et al., 2000; Zamore et al., 2000). The causal relationship between dsRNA, small RNAs and target degradation was demonstrated in cell-free Drosophila systems, where the dsRNA trigger molecule is processed into the 21- to 23-nt short interfering RNAs (siRNAs) that guide target mRNA cleavage (Hammond et al., 2000; Elbashir et al., 2001b). Processing of the dsRNA requires the RNase III-related Dicer enzyme, whereas degradation of single-stranded target RNA (ssRNA) is catalyzed by the RNA-induced silencing complex (RISC) (Bernstein et al., 2001). The Dicer–RISC pathway provides a basic framework to account for passive targeting of sequences homologous to the dsRNA trigger.
Additional processes at the RNA level involving RNA-dependent RNA polymerase (RdRP) activity on silencing targets have been proposed to account for signal amplification and maintenance of silencing (Dougherty and Parks, 1995; Wassenegger and Pelissier, 1998). Proteins with homology to RdRP were shown to be required for efficient silencing in fungal (Cogoni and Macino, 1999), nematode (Smardon et al., 2000) and plant (Dalmay et al., 2000; Mourrain et al., 2000) systems. Further support for the role of RdRPs in RNA silencing comes from studies with a Drosophila embryo extract demonstrating that siRNAs serve as primers to convert the target mRNA into dsRNA that subsequently generates new siRNAs (Lipardi et al., 2001). Evidence for a mechanism of dsRNA synthesis, secondary siRNA amplification and subsequent degradation of new silencing targets was also obtained for C.elegans and referred to as transitive RNAi (Sijen et al., 2001).
For plants, the contribution of endogenous genes to the post-transcriptional silencing process is still to be clarified. Early reports revealed trends that an increased expression of endogenous genes correlated with initiation or enhancement of silencing (Smith et al., 1990; Seymour et al., 1993; Boerjan et al., 1994; Dorlhac de Borne et al., 1994), which could be interpreted as arguments supporting an active role of endogenous genes in PTGS. A number of recent investigations suggest, however, that endogenous genes, unlike transgenes, do not actively participate in the amplification and maintenance phases of the silencing process. First, the endogenous ribulose biphosphate carboxylase small subunit (rbcS) and phytoene desaturase (pds) genes seem to lack the capacity to sustain virus-induced gene silencing (VIGS) in wild-type Nicotiana benthamiana plants (Jones et al., 1999; Voinnet et al., 2000). In contrast, VIGS of a GFP transgene did progress towards autonomy after elimination of the viral trigger. Secondly, in contrast to the GFP transgene, the endogenous plant genes rbcS and pds seem to lack the capacity for secondary siRNA production upon VIGS (Vaistij et al., 2002). Thirdly, graft-induced silencing of an endogenous plant gene was not maintained upon regrafting onto wild-type stocks, in contrast to graft-induced silencing of a transgene (Palauqui and Vaucheret, 1998). Together, these observations suggest that transgenes participate in the different phases of silencing, such as signal amplification and target selection, whereas endogenous genes may be mere passive targets for silencing, at least in the absence of the original silencing trigger.
To further explore the role of endogenous plant genes in post-transcriptional gene silencing, we examined whether an endogenous glucanase gene actively participates in transgene-mediated co-suppression. Silenced plants of the transgenic tobacco line T17 display co-suppression of the Nicotiana plumbaginifolia gn1 transgene and a group of highly conserved endogenous β-1,3-glucanase genes (de Carvalho et al., 1992; de Carvalho Niebel et al., 1995; van Eldik et al., 1998). These endogenous genes have nearly identical coding sequences and are ∼81% similar to the gn1 transgene (de Carvalho Niebel et al., 1995). Here, we show that internal mRNA sequences from a member of the co-suppressed endogenous glucanase family, the glb gene, with a low homology to the gn1 transgene are still targeted for silencing, although less efficiently than corresponding transgene sequences. In contrast, artificial tester sequences sharing the consensus sequence between the transgene and the endogenous glb gene, but with different mismatching nucleotides at non-identical positions, are not targeted by the silencing mechanism. This indicates that, in addition to the transgene, the endogenous genes provide sequence specificity to the silencing process. Consistent with this, we show that small sense and antisense RNAs specific for the endogenous glucanase genes accumulate in co-suppressed T17 plants. These data provide the first direct evidence for the involvement of endogenous plant genes in siRNA production and selection of targets for RNA silencing.
Results
Endogenous glb mRNA sequences are targeted by the silencing machinery, yet less efficiently than corresponding transgenic gn1 mRNA sequences
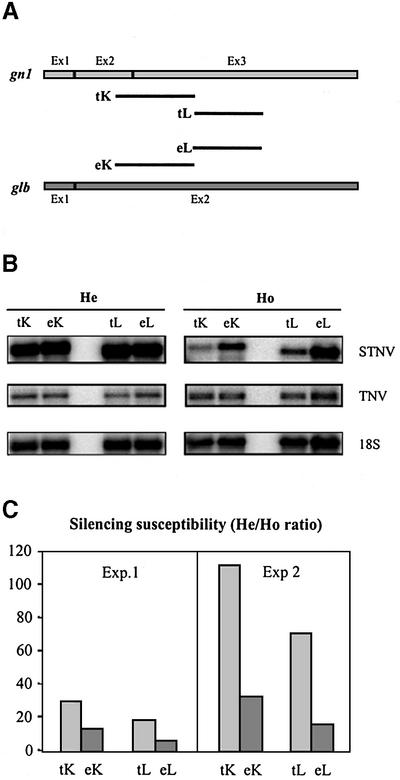
As a first step in understanding the role of the endogenous glucanase genes in co-suppression, we compared the silencing susceptibility of sequences derived from the endogenous glb gene with those of corresponding transgene regions of the same length. To enable quantitative comparisons, we used a two-component viral system consisting of an autonomous helper virus (tobacco necrosis virus; TNV) encoding the viral replicase and a separate chimeric satellite virus (satellite tobacco necrosis virus; STNV) containing the silencing test sequence. In this system, the accumulation level of chimeric STNV RNA in protoplasts of hemizygous, glucanase-expressing (He) compared with homozygous, silenced (Ho) T17 plants (He/Ho ratio) is a measure of silencing susceptibility. Using this reporter system, we previously showed that with the exception of the 5′ and 3′ gn1 mRNA termini, sequences throughout the gn1 mRNA are targets for post-transcriptional silencing. Furthermore, we showed that the silencing susceptibility of a target sequence increases with its length (Jacobs et al., 1999). Chimeric STNV RNAs containing corresponding gn1 and glb regions (K and L, 351 and 297 nt, respectively; Figure 1A) were co-delivered with TNV RNA to protoplasts of He and Ho T17 plants. In parallel, chimeric STNV RNAs containing silencing-insensitive 5′ and 3′ gn1 mRNA sequences were included in the experiment. Twenty hours after delivery, total RNA was harvested for northern blot analysis. To verify whether differences in chimeric STNV-glucanase RNA accumulation in electroporated protoplasts were caused by variation in replicase availability, we systematically measured TNV RNA accumulation in the inoculated protoplasts. In each sample, TNV accumulated to comparable levels, implying that observed differences in STNV-glucanase RNA accumulation were due to different efficiencies of silencing (Figure 1B).
Fig. 1. Endogenous glb sequences are targeted less efficiently by the silencing mechanism than corresponding transgene gn1 sequences. (A) Schematic presentation of the gn1 and glb mRNAs. Exons are indicated. The glb test regions eK and eL, and the corresponding gn1 test regions tK and tL, are represented with solid lines. The two K regions both have a length of 351 nt, and share 77% homology with a maximal stretch of 32 nt of uninterrupted homology. The two L regions both have a length of 297 nt, and share 83% homology with a maximal stretch of 36 nt of uninterrupted homology. Plasmids carrying the test regions between the STNV leader and trailer (Jacobs et al., 1999) were linearized and in vitro transcribed to produce chimeric viral RNAs for delivery into protoplasts. (B) Northern blot analysis of total RNA extracted from protoplasts of hemizygous, expressing (He) and homozygous, silenced (Ho) T17 plants 20 h after delivery of TNV RNA and chimeric STNV RNA containing the test sequences shown in (A). 32P-labeled RNA probes for detection of viral RNAs and rRNA were complementary to the (+) strand of the STNV trailer, the (+) strand of TNV and 18S rRNA sequences. For TNV and 18S rRNA probings, exposures of equal duration are shown for hemizygous and homozygous samples. For the STNV probing, a longer exposure is shown for homozygous samples as compared with hemizygous samples. (C) Relative accumulation of chimeric STNV RNAs in protoplasts of hemizygous versus homozygous plants (He/Ho ratio). In each case, the STNV signal was normalized to the 18S rRNA signal before calculation of the ratio. A higher He/Ho ratio indicates a higher silencing susceptibility. Results from two independent experiments are shown.
The chimeric STNV RNAs containing silencing-insensitive 5′ and 3′ gn1 mRNA sequences accumulated to comparable levels in protoplasts of hemizygous and homozygous plants (data not shown), showing that protoplasts of both genotypes support viral RNA accumulation similarly.
The results in Figure 1B (top panels) show that in protoplasts of expressing (He) T17 plants, each of the chimeric STNV-glucanase RNAs accumulated to relatively high levels, whereas in protoplasts of silenced (Ho) T17 plants much lower accumulation levels were measured for all STNV-glucanase RNAs. This implies that the silencing machinery targets the K and L regions of both the transgenic and the endogenous mRNA. Importantly, comparison of the silencing susceptibilities of transgenic and endogenous K and L regions (He/Ho ratios, calculated as outlined above) shows that transgenic sequences are substantially more susceptible to silencing than the corresponding endogenous sequences (Figure 1C). In two independent experiments, the He/Ho ratio for the transgenic K region was 2.2- and 3.3-fold higher than for the corresponding endogenous sequence. Similarly, the He/Ho ratio for the transgenic L region was 3.2- and 4.2-fold higher than for the corresponding endogenous sequences. These data indicate that, for both regions, transgenic mRNA sequences are more sensitive to PTGS than the corresponding endogenous glb mRNA regions.
Endogene-specific nucleotides contribute to silencing efficiency
The previous experiment showed that transgenic glucanase mRNA sequences are targeted more efficiently than corresponding endogenous glb mRNA sequences. This could mean that PTGS in the T17 line is primarily directed against the transgene and that the endogenous glucanase mRNAs are passively silenced by transgene-derived mediators of silencing-related RNA degradation, such as the 21- to 23-nt RNAs described by Hamilton and Baulcombe (1999). In this view, ‘cross’-silencing of endogenous mRNAs would be less efficient than silencing of transgene mRNAs as the co-suppressed endogenous mRNAs would not allow perfect pairing with transgene-derived mediators over most of their sequence. Alternatively, the lower silencing susceptibility of endogenous compared with transgenic mRNA sequences could relate to a difference in abundance of transgene- and endogene-specific mediators. This would imply that the co-suppressed endogenous genes acquire an active role in the silencing process through the production of endogene-specific mediators at some stage after initiation.
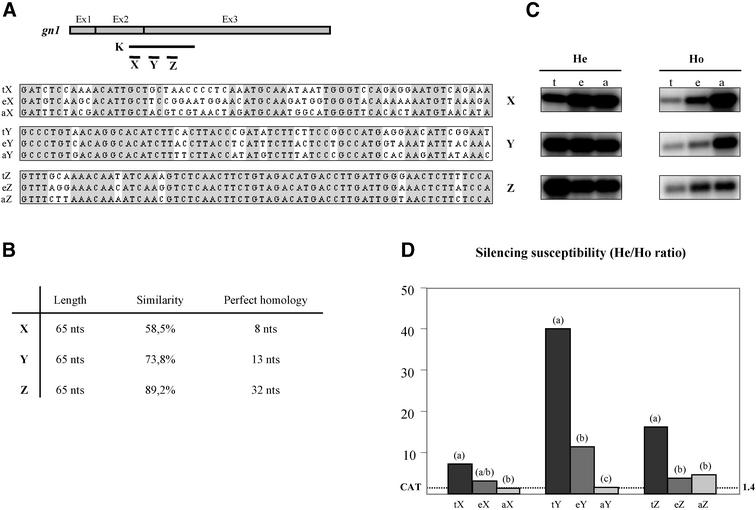
To distinguish between the above two scenarios, we set up a functional assay to examine whether the silencing susceptibility of a certain test sequence is determined exclusively by the homology level (or similarity) to the transgene or whether endogene-specific nucleotides at non-identical positions contribute to the silencing efficiency of these sequences. To verify the silencing effect of random nucleotide sequences in these non-identical positions, we determined the silencing efficiency of artificial tester sequences that correspond to the consensus sequence between the transgene and the endogenous genes but lack homology to both sequences at non-identical positions (Figure 2A).
Fig. 2. Endogene-specific nucleotides contribute to sequence specificity of the RNA degradation step of PTGS. (A) Upper part: position of the X, Y and Z test subregions within the K test region. Lower part: display of the homology between the glb, gn1 and artificial test sequences in the X, Y and Z subregions. The gray boxes show the ‘consensus sequence’ between the transgenic, endogenous and artificial test sequences. Plasmids carrying the test regions between the STNV leader and trailer (Jacobs et al., 1999) were linearized and in vitro transcribed to produce chimeric viral RNAs for delivery into protoplasts. (B) Features of the subregions X, Y and Z. The length of the X, Y and Z test sequences is shown, as well as the overall similarity between endogenous, transgenic and artificial sequences. For each subregion, the largest stretch of uninterrupted homology between the endogenous, transgenic and artifical sequences is shown. (C) Northern blot analysis of total RNA extracted from protoplasts of He and Ho T17 plants 20 h after delivery of TNV RNA and chimeric STNV RNA containing transgenic, endogenous and artificial test sequences as shown in (A). The 32P-labeled RNA probe was complementary to the (+) strand of the STNV trailer. (D) Relative accumulation of chimeric STNV RNAs in protoplasts of hemizygous versus homozygous plants (He/Ho ratio). Bars represent the average of two (Z region) or three (X and Y region) independent experiments. CAT: STNV-CAT; the He/Ho ratio for the silencing-insensitive STNV CAT RNA is indicated by the horizontal dashed line. Letter codes above columns indicate statistical significance of the differences between silencing susceptibilities within each region, as determined by repeated measures ANOVA. Columns marked a, b, c differ significantly at 95% confidence. The column marked a/b differs from those marked a and b at 88 and 80% confidence, respectively.
We selected for this purpose three 65 nt regions of the gn1 and glb mRNA (X, Y and Z; Figure 2A). In these regions, the homologies between the transgenic, the endogenous glb and the artificial tester sequences are 58, 74 and 89%, respectively (Figure 2B).
We reasoned that if the co-suppressed endogenous genes have merely a passive role in PTGS, endogenous and artificial tester sequences with similar homology to the transgene will in all instances be equally susceptible to silencing. Furthermore, in this scenario, endogenous and artificial tester sequences corresponding to the X region are unlikely to be recognized by the silencing mechanism as the overall homology to the gn1 transgene is only 58% and the largest stretch of perfect homology is only 8 nt. Alternatively, if endogene-specific mediators of RNA silencing were to exist in silenced plants, endogenous mRNA sequences should be targeted consistently more efficiently than the corresponding artificial tester sequences. Furthermore, even the low-homology endogenous X region (eX) would be expected to be susceptible to PTGS.
Chimeric STNV RNAs containing the transgenic, endogenous and artificial glucanase tester sequences were co-delivered with wild-type TNV RNA to protoplasts of expressing and silenced T17 plants, and viral RNA accumulation was determined 20 h after inoculation (Figure 2C). TNV accumulated to comparable levels in all samples (data not shown).
For each region tested, we observed the strongest silencing effect for STNV RNAs containing transgenic sequences (Figure 2D). This confirms the previous observation that transgenic mRNA regions are more susceptible to PTGS than endogenous glb sequences. The data also show that the silencing machinery recognizes the ‘high’-homology artificial tester region Z (aZ), indicating that an overall homology of 89% and/or a region of 32 nt perfect homology to the co-suppressed genes is sufficient to carry a sequence into PTGS via cross-silencing. This is not surprising taking into account that RNA molecules as short as 21–23 nt can mediate degradation of homologous target RNA in vitro and in intact plants (Elbashir et al., 2001b; Thomas et al., 2001), implying that a target size of well under 32 nt can be sufficient.
Importantly, the experiments show that chimeric STNV RNAs carrying the endogenous ‘low’- and ‘medium’-homology regions X and Y were silenced, whereas chimeric STNV RNAs containing the corresponding artificial tester regions X and Y were not. The silencing insensitivity of artificial tester sequences in regions X and Y indicates that silencing of the endogenous X and Y sequences was not due to cross-silencing via transgene-specific mediators, and thus implies that these endogenous regions are recognized by endogene-specific siRNAs. In other words, the data indicate that, in addition to the transgene, the endogenous genes provide specificity to the silencing process.
Small sense and antisense RNAs specific for the co-suppressed endogenous genes accumulate in silenced T17 plants
Following current silencing models, participation of the endogenous glucanase genes in the sequence-specific RNA degradation step of PTGS would involve the production of endogene-specific siRNAs. We therefore set out to detect such small RNAs in protoplasts of non-infected, silenced T17 plants.
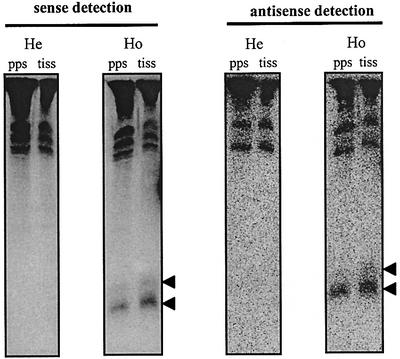
As a first step, we examined whether PTGS in the T17 line correlates with the presence of small 21- to 23-nt RNAs and whether these silencing molecules accumulate in protoplasts of silenced (Ho) T17 plants. To this end, polyethylene glycol (PEG)-enriched low-molecular-weight RNA–DNA fractions from protoplasts and leaf tissue of silenced (Ho) and expressing (He) T17 plants were separated on a 15% polyacrylamide gel, blotted onto a nylon membrane and hybridized with riboprobes corresponding to nearly the entire gn1 mRNA region (see Materials and methods). The results in Figure 3 show that small sense and antisense glucanase oligonucleotides accumulate in protoplasts and leaf tissue of silenced (Ho) T17 plants, but not in protoplasts and tissue of expressing (He) T17 plants. RNase and DNase treatment of the low-molecular-weight fraction confirmed that the small nucleotides accumulating in silenced T17 plants are ribonucleic acids (data not shown). Taken together, the results show that silencing in the T17 line is associated with the presence of 21- to –23-nt glucanase RNAs and that these molecules accumulate to similar levels in protoplasts and tissue of silenced plants.
Fig. 3. Glucanase silencing correlates with accumulation of small sense and antisense RNAs homologous to gn1. (A) Nucleic acids from protoplasts and leaf tissue of hemizygous, expressing and homozygous, silenced T17 plants were enriched by PEG. Samples of 10 µg were separated on a 15% polyacrylamide gel and blotted to membranes. Filters were hybridized with 32P-labeled RNA probes corresponding to the (+) or (–) strand of the gn1 cDNA. The arrows indicate the position of the small RNA species. Comparison to RNA size markers of 20, 22, 25 and 28 nt indicates that the small RNA species have a length of 21 and 23 nt. He, hemizygous, expressing plants; Ho, homozygous, silenced plants; pps, leaf protoplasts; tiss, leaf tissue.
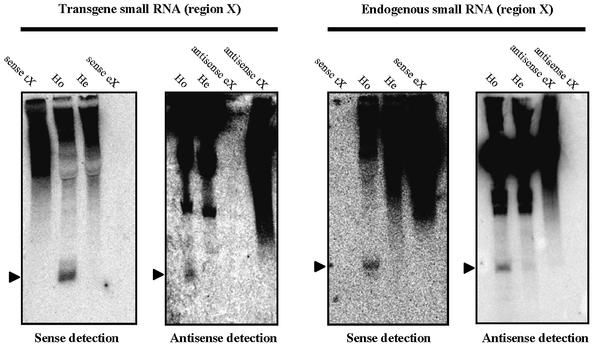
To detect endogene- and transgene-specific small RNAs in protoplasts of silenced plants, we probed for small RNAs corresponding to region X of gn1 and glb (Figure 2A). This region was chosen because the degree of homology between endogenous and transgenic X sequences is expected to be sufficiently low (58%) for specific detection of endogene- and transgene-derived small RNAs in hybridization experiments.
Equal amounts of PEG-enriched small RNA fractions from protoplasts of silenced and expressing T17 plants were run on polyacrylamide gels, blotted onto nylon membranes and hybrized with four RNA probes for detection of endogene- and transgene-specific small sense and antisense RNAs corresponding to the X region. Control hybridizations with in vitro synthesized endogenous and transgene sequences corresponding to region X demonstrated that no cross-hybridization occurred. Therefore, any signal generated by each of the four probes was specific for the endogenous or transgenic sequences in either orientation (Figure 4).
Fig. 4. Transgene- and endogene-specific small RNAs accumulate in protoplasts of silenced T17 plants. Nucleic acids from protoplasts and leaf tissue of hemizygous, expressing and homozygous, silenced T17 plants were enriched by PEG. Samples of 45 µg and in vitro synthesized control RNAs were separated on a 15% polyacrylamide gel, blotted onto membranes and hybridized with 32P-labeled RNA probes corresponding to the (+) or (–) strand of transgenic and endogenous X region. The arrowheads indicate the position of the small RNA. Control RNAs: sense tX, sense eX: in vitro synthesized sense RNAs of ∼70 nt corresponding to transgenic or endogenous region X; antisense tX, antisense eX: in vitro synthesized antisense RNAs of ∼70 nt, corresponding to transgenic and endogenous region X. Probes for eX detection do not cross-hybridize to in vitro synthesized tX RNA and vice versa.
Small RNAs were detected in protoplasts of silenced plants with all four probes. This demonstrates that small sense and antisense RNAs originating from the transgene and the endogenous glucanase genes co-exist in protoplasts of silenced T17 plants. This implies that a co-suppressed endogenous glucanase gene is involved in synthesis of the siRNA signal. Interestingly, quantification relative to in vitro synthesized control RNAs showed that endogene-specific guide RNAs corresponding to region X are ∼3- to 10-fold more abundant than the corresponding transgene-specific RNAs (data not shown). This is consistent with the observation that transgenic mRNAs are better targets for PTGS than endogenous mRNAs.
In conclusion, the siRNA detection experiments and the functional assays indicate that, in the presence of the gn1 transgene, an endogenous glucanase gene is activated for production of siRNAs, leading to a more active silencing than would occur in a scenario of mere passive involvement.
Discussion
In the present study, we have investigated whether endogenous target genes actively participate in transgene-mediated co-suppression. Several studies in plants suggest that whereas transgenes can become actively involved in setting up and maintaining a silenced state, endogenous genes may be merely passive targets for the silencing mechanism. In this paper, we provide the first direct evidence that an endogenous target gene is actively involved in the silencing process of transgene-mediated co-suppression in plants. The detection of endogene-specific small sense and antisense RNAs, and the preferential suppression of the accumulation of chimeric STNV RNAs containing these endogene-specific sequences in protoplasts of silenced T17 plants, indicate that a co-suppressed endogenous glucanase gene is a template for small RNA synthesis and thereby contributes to selection of silencing targets.
In principle, the small endogene-specific sense RNAs we observed in silenced plants could be products either of endogenous mRNA degradation or of active synthesis. However, biochemical evidence indicates that degradation of single-stranded silencing target RNA does not lead to the formation of the characteristic small siRNAs (Elbashir et al., 2001b). Furthermore, passive glucanase mRNA degradation does not explain the accumulation of small antisense RNAs. We therefore conclude that the endogene-specific small sense and antisense RNAs derive from a process that involves the synthesis of antisense RNA molecules on a sense template. Lipardi et al. (2001) have recently provided evidence for a relay-amplification mechanism in which primary siRNAs prime the synthesis of dsRNA, leading to production of secondary siRNAs. This mechanism is proposed to be responsible for the transition of virus-induced transgene silencing into a virus-independent maintenance stage in plants (Jones et al., 1999; Voinnet et al., 2000) and for spreading of the silencing target spectrum or transitive RNAi in nematodes (Sijen et al., 2001; Vaistij et al., 2002). The observation that in protoplasts of silenced T17 plants both endogene- and transgene-specific small RNAs accumulate is consistent with a process of secondary siRNA production. The viral RNA delivery experiments performed in this study demonstrate a functional consequence thereof for silencing efficiency. Therefore, we propose that in T17 plants spreading of RNA targeting (transitive PTGS) between transgenes and endogenous genes takes place and, in more general terms, that this mechanism contributes to the specificity and efficiency of gene silencing in plants.
At this stage, it is unclear how general are our findings. Several observations have suggested that the transitivity process and the underlying mechanisms do not apply to endogenous plant genes. It was shown, for example, for two endogenous plant genes that VIGS does not progress into a virus-free maintenance phase and is not associated with production of endogenous siRNAs and spreading of RNA targeting (Jones et al., 1999; Voinnet et al., 2000; Vaistij et al., 2002). This raises the question why in the T17 line a co-suppressed endogenous target gene participates in siRNA production. First, it is possible that transgenes are more effective than viral RNAs in recruiting endogenous genes into an active role in silencing. This could be the case if recruitment of endogenous genes into an active role requires a nuclear phase, which the viral inducers may be less able to provide. Secondly, it is possible that some endogenous genes are more susceptible to transitivity than others. This could relate to a range of factors, among which are the chromatin status, the RNA expression level, the capacity to form specific secondary structures or the presence of specific recognition elements, for example. More generally, this could also apply to transgenes. The observation that, in accordance to what is observed for endogenous genes, silencing of transgenes does not by default lead to spreading of RNA targeting (English et al., 1996; Elmayan et al., 1998; Wang et al., 2001) supports the idea that there is no principal distinction between transgenes and endogenous genes with respect to their ability for recruitment into an active role in RNA silencing.
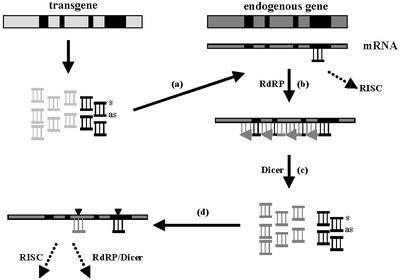
Based on our results and in line with the model for transitive RNAi (Sijen et al., 2001), we propose a model for the communication between inducing transgene and endo genous target genes in plants in which the endogenous genes adopt an active role in PTGS via a process involving four major steps (Figure 5): (a) interaction between transgene-derived siRNA and endogenous glb mRNA; (b) RdRP-mediated complementary RNA synthesis; (c) Dicer-mediated production of glb-derived siRNA; and (d) targeting of endogenous mRNA by cognate siRNA. The model presupposes that the transgene is the first to produce siRNAs, as silencing is frequently observed upon introduction of (complex) transgene sequences. The proposed steps require functional RdRP and Dicer-like activities, but do not depend on the RISC component, which is still under debate for plants.
Fig. 5. Model explaining the activation of endogenous genes in PTGS. It assumes that transgene-specific (light gray) and ‘common’ (black) siRNAs are produced from the inducing transgene. The small transgene-derived ‘common’ antisense siRNAs anneal to homologous regions (black boxes) in the endogenous mRNA (a). The resulting partial dsRNA–RNA hybrids are recognized by an RNA-dependent RNA polymerase (RdRP) for elongation (b) or by the RNA-induced silencing complex (RISC) for direct degradation (dashed arrow). The dsRNA molecules resulting from RdRP activity are processed further by a Dicer-like enzyme, leading to the accumulation of secondary, endogene-derived siRNAs, the sequence of which can be endogene specific (dark gray) or common (black) (c). The endogene-specific secondary siRNAs can directly tag secondary targets such as endogene-specific regions in the endogenous mRNA (d), which subsequently become substrates for RISC-related degradation or a second round of RdRP-mediated dsRNA production and Dicer cleavage (dashed arrows).
In another case of transgene-induced silencing, Han and Grierson (2002) detected small RNAs corresponding to the 5′ end of the co-suppressed genes, but could not distinguish whether these are of endogenous or transgenic origin. They did not detect small RNAs corresponding to the endogene-specific 3′ mRNA region of the co-suppressed endogenous gene. In this silencing case, the transgene is truncated at the 3′ end and has no homology to the 3′ part of the endogenous gene. The findings are consistent with the above model under the assumptions that (i) the 5′ small RNAs are at least partly of endogenous origin and (ii) secondary siRNA production is directional and proceeds in a 3′ to 5′ direction relative to the template mRNA.
For the T17 silencing case, we observed that gn1 and glb leader sequences are not targeted by a silencing mechanism (Jacobs et al., 1999; our unpublished data). This is surprising, as the RdRP-mediated relay-amplification reactions on mRNA templates predict the synthesis of siRNAs corresponding to 5′ mRNA termini. In view of the model, the absence of such 5′ siRNAs could mean either that the 5′-termini of glucanase mRNA are protected against RdRP-dependent synthesis of antisense RNA or that these sequences are protected from Dicer-mediated degradation.
In the model, we propose that communication between the transgene and the homologous endogenous genes occurs at the RNA level. However, this does not preclude the possibility that in this or other silencing cases additional DNA–DNA or RNA–DNA interactions may be involved. For instance, the observations that transitivity occurred following bombardment with promotorless DNA constructs in N.benthamiana (Voinnet et al., 1998) and that transitivity was associated with the activity of Polycomb type chromatin binding proteins in Drosophila (Pal-Bhadra et al., 1999) suggest the existence of additional target spreading mechanisms at the DNA level.
The importance of PTGS in natural gene regulation is not clear, although there are some indications that PTGS and RNAi are genetically linked with developmental control (Matzke et al., 2001). Such a role would (at least) require that endogenous genes are capable of producing mediators of sequence-specific RNA degradation. The observation that in silenced T17 plants the endogenous glucanase genes are involved in production of siRNAs and silencing target selection adds evidence to the idea that post-transcriptional silencing driven by and directed against endogenous genes is part of the natural repertoire of mechanisms to regulate gene expression in plants.
Materials and methods
Plant material and growth conditions
Homozygous and hemizygous T17 plants (transgenic Nicotiana tabacum cv Petit Havana SR1; de Carvalho et al., 1992) and untransformed SR1 tobacco plants were germinated and grown in vitro on solid medium in a growth chamber (25°C, 70 µmol/m2/s light intensity, 14 h light:10 h dark period). Plants were propagated via cuttings every 7–9 weeks.
Plasmid constructions
The construction of plasmids pTNV-A and pSTNV-2 containing full-length cDNA copies of the TNV-A and STNV-2 genomes, respectively, and permitting synthesis of infectious in vitro transcripts, is described elsewhere (Andriessen, 1997). The construction of pKB11 from pSTNV-2 is described in Andriessen (1997). In this construct, the distal 456 nt of the STNV CP and the first 105 nt of the STNV-2 trailer are replaced by the npt II coding region. The construction of plasmids pSTNV-tK and pSTNV-tL is described in Jacobs et al. (1999) (plasmids -tk and -tl are identical to pSTNV-K and pSTNV-L). Plasmids pSTNV-eK, pSTNV-eL and pSTNV-eM were synthesized from pKB11 by replacing a HindIII–SalI fragment containing the npt II coding region by fragments covering the selected sequences. The inserted fragments were obtained by PCR on the pglb3 plasmid. Plasmids pSTNV-tX to pSTNV-aZ were constructed from pKB11 by replacing the HindIII–SalI fragment by synthetic dsDNA oligonucleotides spanning the selected regions. The sequence of artificial tester oligonucleotides (aX, aY and aZ) was designed such that all conserved nucleotides between gn1 and glb were maintained, while at positions where the sequence diverged, a random nucleotide was introduced that was different from those present in the gn1 and glb sequence. The construction of pMA491 (pSTNV-CAT) is described in Andriessen (1997).
In vitro transcription of viral RNAs
TNV-A RNA was synthesized in vitro from plasmid pTNV-A linearized at the BsaI site, using T7 RNA polymerase. The resulting transcript of 3684 nt, differing from the natural RNA only by the substitution of the 5′-terminal A by a G residue, is capable of autonomous replication in tobacco protoplasts and supports STNV replication (Andriessen, 1997). All chimeric STNV-glucanase RNAs were synthesized in vitro from the appropriate pSTNV plasmids linearized at the BamHI site, using T7 RNA polymerase. The resulting transcripts comprise the 32 nt STNV leader (except for the first nucleotide, which is G instead of A), followed by 141 nt encoding the N-terminal part of the STNV coat protein, fused with the selected test sequences and followed by the STNV-2 trailer. In all chimeric STNV RNAs, a 3′-terminal extension of 7 nt is added to the trailer, relative to wild-type STNV-2. Similar chimeric STNV RNAs containing different test sequences were previously shown to be biologically active (Jacobs et al., 1999). All in vitro transcripts were prepared using the Megascript kit (Ambion). The final RNA concentration was determined spectrophotometrically, and the integrity and length of all transcripts were assessed by denaturing agarose gel electrophoresis of RNA samples.
Protoplast preparation and electroporation experiments
Protoplasts were prepared from the top leaves of 7- to 9-week-old plants grown from cuttings, as described by De Block et al. (1987). Protoplasts were electroporated as described by Meulewaeter et al. (1992). Per 1 × 106 protoplasts, 0.2 pmol of TNV-A RNA and 2 pmol of chimeric STNV-2 RNA were used as an inoculum. After the electroporation, the protoplasts were washed in order to remove dead cells and excess, extracellular inoculum RNA. Subsequently, the protoplasts were incubated at a concentration of 0.5–1 × 106/ml in 5 ml of incubation medium, in the dark at 24°C. Twenty hours after electroporation, dead cells were removed by centrifugation at 80 g, and RNA was extracted from the surviving (floating) protoplasts.
RNA isolation and analysis
RNA was extracted from protoplasts as described by Jones et al. (1985). Total RNA (1 µg per lane) was electrophoresed in 1.5% agarose– formaldehyde gels according to Sambrook et al. (1989). To verify RNA quality and quantity, ethidium bromide (0.25 µg per RNA sample) was added to samples before denaturing at 65°C. After electrophoresis, gels were blotted onto Hybond-N+ membranes (Amersham) by capillary transfer in 20× SSC. The RNA was fixed on the membranes by baking. Riboprobes to detect sense TNV, sense STNV tailer and 18S rRNA sequences were prepared from appropriate templates using a Promega in vitro transcription kit. Blots were hybridized overnight at 68°C in 50% formamide, 10% dextran sulfate, 5× SSC, 1% SDS, 100 µg/ml denatured sheared herring sperm DNA. Filters were washed at least four times at 65°C, with the final wash in 0.1× SSC, 0.1% SDS. Signals were quantified using a Molecular Dynamics PhosphorImager and the Imagequant software.
To detect the small 21- to 23-nt RNAs, an adapted version of the Hamilton and Baulcomb (1999) protocol was used. Total nucleic acids were extracted from protoplasts of expressing and silenced T17 plants. From the nucleic acid extract, low-molecular-weight RNA was enriched by precipitation with 10% PEG (mol. wt 8000) and 0.5 M NaCl. The low-molecular-weight fraction was separated by electrophoresis through 15% polyacrylamide–7 M urea–0.5× Tris–borate–EDTA gels, transferred onto Hybond N+ filters (Amersham) and fixed by UV cross-linking. The filters were pre-hybridized in 45% formamide, 7% SDS, 0.3 M NaCl, 0.05 M Na2HPO4/NaH2PO4 pH 7, 1× Denhardt’s solution and denatured herring sperm DNA (100 mg/ml). Hybridization was in the same solution using hydrolyzed α-32P-labeled RNA probes corresponding to the (+) or (–) strand of the gn1 cDNA or the X-region of gn1 or glb, with an average length of 50 nt. Hybridized filters were washed twice for 20 min with 2× SSC/0.2% SDS at 50°C. PCR fragments containing the relevant sequences linked to a T7 (sense transcripts) or an SP6 (antisense transcripts) promotor were used to generate the riboprobes. Control hybridizations on Southern blots showed that the riboprobes were specific for the selected region.
Acknowledgments
Acknowledgements
We thank Michael Metzlaff, Gerben van Eldik, Frank Meulewaeter and Rene Ruiter for critical reading of the manuscript. We thank Geert De Meyer for statistical analysis of the data and Fred Meins, Jr for provision of the pGLB3 plasmid. This work is supported by the Instituut voor de aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen (IWT-Vlaanderen).
Note added in proof
Recently, endogenous small RNAs with typical characteristics of siRNAs have been cloned from Arabidopsis inflorescence tissues (Llave et al., 2002). This provides further support for a potential role of sequence-specific RNA silencing in endogenous plant gene regulation.
Reference
Llave,C., Kasschau,K.D., Rector,M.A. and Carrington,J.C. (2002) Endogenous and silencing associated small RNAs in plants. Plant Cell, 14, 1–15.
References
- Andriessen M. (1997) Cis- and trans-acting elements in satellite tobacco necrosis virus RNA replication. PhD thesis, Ghent University, Belgium.
- Bernstein E., Caudy,A.A., Hammond,S.M. and Hannon,G.J. (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature, 409, 363–366. [DOI] [PubMed] [Google Scholar]
- Boerjan W., Bauw,G., Van Montagu,M. and Inze,D. (1994) Distinct phenotypes generated by overexpression and suppression of S-adenosyl-l-methionine synthetase reveal developmental patterns of gene silencing in tobacco. Plant Cell, 6, 1401–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogoni C. (2001) Homology-dependent gene silencing mechanisms in fungi. Annu. Rev. Microbiol., 55, 381–406. [DOI] [PubMed] [Google Scholar]
- Cogoni C. and Macino,G. (1999) Gene silencing in Neurospora crassa requires a protein homologous to RNA-dependent RNA polymerase. Nature, 399, 166–169. [DOI] [PubMed] [Google Scholar]
- Cogoni C., Irelan,J.T., Schumacher,M., Schmidhauser,T.J., Selker,E.U. and Macino,G. (1996) Transgene silencing of the al-1 gene in vegetative cells of Neurospora is mediated by a cytoplasmic effector and does not depend on DNA–DNA interactions or DNA methylation. EMBO J., 15, 3153–3163. [PMC free article] [PubMed] [Google Scholar]
- Dalmay T., Hamilton,A., Rudd,S., Angell,S. and Baulcombe,D.C. (2000) An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell, 101, 543–553. [DOI] [PubMed] [Google Scholar]
- De Block M. et al. (1987) Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J., 6, 2513–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho F., Gheysen,G., Kushnir,S., Van Montagu,M., Inze,D. and Castresana,C. (1992) Suppression of β-1,3-glucanase transgene expression in homozygous plants. EMBO J., 11, 2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Niebel F., Frendo,P., Van Montagu,M. and Cornelissen,M. (1995) Post-transcriptional cosuppression of β-1,3-glucanase genes does not affect accumulation of transgene nuclear mRNA. Plant Cell, 7, 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depicker A. and Van Montagu,M. (1997) Post-transcriptional gene silencing in plants. Curr. Opin. Cell Biol., 9, 373–382. [DOI] [PubMed] [Google Scholar]
- Dernburg A.F., Zalevsky,J., Colaiacovo,M.P. and Villeneuve,A.M. (2000) Transgene-mediated cosuppression in the C.elegans germ line. Genes Dev., 14, 1578–1583. [PMC free article] [PubMed] [Google Scholar]
- Dorlhac de Borne F., Vincentz,M., Chupeau,Y. and Vaucheret,H. (1994) Co-suppression of nitrate reductase host genes and transgenes in transgenic tobacco plants. Mol. Gen. Genet., 243, 613–621. [DOI] [PubMed] [Google Scholar]
- Dougherty W.G. and Parks,T.D. (1995) Transgenes and gene suppression: telling us something new? Curr. Opin. Cell Biol., 7, 399–405. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001a) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 428–429. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M., Lendeckel,W. and Tuschl,T. (2001b) RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev., 15, 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmayan T. et al. (1998) Arabidopsis mutants impaired in cosuppression. Plant Cell, 10, 1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English J.J., Mueller,E. and Baulcombe,D.C. (1996) Suppression of virus accumulation in transgenic plants exhibiting silencing of nuclear genes. Plant Cell, 8, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., Xu,S., Montgomery,M.K., Kostas,S.A., Driver,S.E. and Mello,C.C. (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 391, 806–811. [DOI] [PubMed] [Google Scholar]
- Hamilton A.J. and Baulcombe,D.C. (1999) A species of small antisense RNA in posttranscriptional gene silencing in plants. Science, 286, 950–952. [DOI] [PubMed] [Google Scholar]
- Hamilton A.J., Brown,S., Yuahai,H., Ishizuka,M., Lowe,A., Alpuche Solis,A.-G. and Grierson,D. (1998) A transgene with repeated DNA causes high frequency, post-transcriptional suppression of ACC-oxidase gene expression in tomato. Plant J., 15, 737–746. [DOI] [PubMed] [Google Scholar]
- Hammond S.M., Bernstein,E., Beach,D. and Hannon,G.J. (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature, 404, 293–296. [DOI] [PubMed] [Google Scholar]
- Hammond S.M., Caudy,A.A. and Hannon,G.J. (2001) Post-transcriptional gene silencing by double-stranded RNA. Nat. Rev. Genet., 2, 110–119. [DOI] [PubMed] [Google Scholar]
- Han Y.H. and Grierson,D. (2002) Relationship between small antisense RNAs and aberrant RNAs associated with sense transgene mediated gene silencing in tomato. Plant J., 29, 509–519. [DOI] [PubMed] [Google Scholar]
- Jacobs J.J., Sanders,M., Bots,M., Andriessen,M., van Eldik,G.J., Litiere,K., Van Montagu,M. and Cornelissen,M. (1999) Sequences throughout the basic β-1,3-glucanase mRNA coding region are targets for homology dependent post-transcriptional gene silencing. Plant J., 20, 143–152. [DOI] [PubMed] [Google Scholar]
- Jones J.D.G., Dunsmuir,P. and Bedbrook,J. (1985) High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J., 4, 2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L., Hamilton,A.J., Voinnet,O., Thomas,C.L., Maule,A.J. and Baulcombe,D.C. (1999) RNA–DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell, 11, 2291–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell J.R. and Carthew,R.W. (2000) Heritable gene silencing in Drosophila using double-stranded RNA. Nat. Biotechnol., 18, 896–898. [DOI] [PubMed] [Google Scholar]
- Kooter J.M., Matzke,M.A. and Meyer,P. (1999) Listening to the silent genes: transgene silencing, gene regulation and pathogen control. Trends Plant Sci., 4, 340–347. [DOI] [PubMed] [Google Scholar]
- Lipardi C., Wei,Q. and Paterson,B.M. (2001) RNAi as random degradative PCR: siRNA primers convert mRNA into dsRNAs that are degraded to generate new siRNAs. Cell, 107, 297–307. [DOI] [PubMed] [Google Scholar]
- Matzke M., Matzke,A.J. and Kooter,J.M. (2001) RNA: guiding gene silencing. Science, 293, 1080–1083. [DOI] [PubMed] [Google Scholar]
- Meins F. Jr (2000) RNA degradation and models for post-transcriptional gene-silencing. Plant Mol. Biol., 43, 261–273. [DOI] [PubMed] [Google Scholar]
- Meulewaeter F., Cornelissen,M. and van Emmelo,J. (1992) Subgenomic RNAs mediate expression of cistrons located internally on the genomic RNA of tobacco necrosis virus strain A. J. Virol., 66, 6419–6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourrain P. et al. (2000) Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell, 101, 533–542. [DOI] [PubMed] [Google Scholar]
- Napoli C., Lemieux,C. and Jorgensen,R. (1990) Introduction of a chimaeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell, 2, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palauqui J.C. and Vaucheret,H. (1998) Transgenes are dispensable for the RNA degradation step of cosuppression. Proc. Natl Acad. Sci. USA, 95, 9675–9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Bhadra M., Bhadra,U. and Birchler,J.A. (1997) Cosuppression in Drosophila: gene silencing of alcohol dehydrogenase by white-Adh transgenes is Polycomb dependent. Cell, 90, 479–490. [DOI] [PubMed] [Google Scholar]
- Pal-Bhadra M., Bhadra,U. and Birchler,J.A. (1999) Cosuppression of nonhomologous transgenes in Drosophila involves mutually related endogenous sequences. Cell, 99, 35–46. [DOI] [PubMed] [Google Scholar]
- Parrish S., Fleenor,J., Xu,S., Mello,C. and Fire,A. (2000) Functional anatomy of a dsRNA trigger: differential requirement for the two trigger strands in RNA interference. Mol. Cell, 6, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Romano N. and Macino,G. (1992) Quelling: transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol. Microbiol., 6, 3343–3353. [DOI] [PubMed] [Google Scholar]
- Ruiz F., Vayssie,L., Klotz,C., Sperling,L. and Madeddu,L. (1998) Homology-dependent gene silencing in Paramecium. Mol. Biol. Cell, 9, 931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Seymour G.B., Fray,R.G., Hill,P. and Tucker,G.A. (1993) Down-regulation of two non-homologous endogenous tomato genes with a single chimaeric sense gene construct. Plant Mol. Biol., 23, 1–9. [DOI] [PubMed] [Google Scholar]
- Sijen T., Fleenor,J., Simmer,F., Thijssen,K.L., Parrish,S., Timmons,L., Plasterk,R.H. and Fire,A. (2001) On the role of RNA amplification in dsRNA-triggered gene silencing. Cell, 107, 465–476. [DOI] [PubMed] [Google Scholar]
- Smardon A., Spoerke,J.M., Stacey,S.C., Klein,M.E., Mackin,N. and Maine,E.M. (2000) EGO-1 is related to RNA-directed RNA polymerase and functions in germ-line development and RNA interference in C.elegans. Curr. Biol., 10, 169–178. [DOI] [PubMed] [Google Scholar]
- Smith C.J., Watson,C.F., Bird,C.R., Ray,J., Schuch,W. and Grierson,D. (1990) Expression of a truncated tomato polygalacturonase gene inhibits expression of the endogenous gene in transgenic plants. Mol. Gen. Genet., 224, 477–481. [DOI] [PubMed] [Google Scholar]
- Thomas C.L., Jones,L., Baulcombe,D.C. and Maule,A.J. (2001) Size constraints for targeting post-transcriptional gene silencing and for RNA-directed methylation in Nicotiana benthamiana using a potato virus X vector. Plant J., 25, 417–425. [DOI] [PubMed] [Google Scholar]
- Tuschl T., Zamore,P.D., Lehmann,R., Bartel,D.P. and Sharp,P.A. (1999) Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev., 13, 3191–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaistij F.E., Jones,L. and Baulcombe,D.C. (2002) Spreading of RNA targeting and DNA methylation in RNA silencing requires transcription of the target gene and a putative RNA-dependent RNA polymerase. Plant Cell, 14, 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance V. and Vaucheret,H. (2001) RNA silencing in plants—defense and counterdefense. Science, 292, 2277–2280. [DOI] [PubMed] [Google Scholar]
- van der Krol A.R., Mur,L.A., Beld,M., Mol,J.N. and Stuitje,A.R. (1990) Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell, 2, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eldik G.J., Litiere,K., Jacobs,J.J., Van Montagu,M. and Cornelissen,M. (1998) Silencing of β-1,3-glucanase genes in tobacco correlates with an increased abundance of RNA degradation intermediates. Nucleic Acids Res., 26, 5176–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret H., Beclin,C., Elmayan,T., Feuerbach,F., Godon,C., Morel,J.B., Mourrain,P., Palauqui,J.C. and Vernhettes,S. (1998) Transgene-induced gene silencing in plants. Plant J., 16, 651–659. [DOI] [PubMed] [Google Scholar]
- Vaucheret H., Beclin,C. and Fagard,M. (2001) Post-transcriptional gene silencing in plants. J. Cell Sci., 114, 3083–3091. [DOI] [PubMed] [Google Scholar]
- Voinnet O. (2001) RNA silencing as a plant immune system against viruses. Trends Genet., 17, 449–459. [DOI] [PubMed] [Google Scholar]
- Voinnet O., Vain,P., Angell,S. and Baulcombe,D.C. (1998) Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell, 95, 177–187. [DOI] [PubMed] [Google Scholar]
- Voinnet O., Lederer,C. and Baulcombe,D.C. (2000) A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell, 103, 157–167. [DOI] [PubMed] [Google Scholar]
- Wang M.B., Wesley,S.V., Finnegan,E.J., Smith,N.A. and Waterhouse, P.M. (2001) Replicating satellite RNA induces sequence-specific DNA methylation and truncated transcripts in plants. RNA, 7, 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenegger M. and Pelissier,T. (1998) A model for RNA-mediated gene silencing in higher plants. Plant Mol. Biol., 37, 349–362. [DOI] [PubMed] [Google Scholar]
- Waterhouse P.M., Graham,M.W. and Wang,M.B. (1998) Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc. Natl Acad. Sci. USA, 95, 13959–13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P.M., Wang,M.B. and Lough,T. (2001) Gene silencing as an adaptive defence against viruses. Nature, 411, 834–842. [DOI] [PubMed] [Google Scholar]
- Zamore P.D., Tuschl,T., Sharp,P.A. and Bartel,D.P. (2000) RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell, 101, 25–33. [DOI] [PubMed] [Google Scholar]