Abstract
Engagement of the B-cell antigen receptor (BCR) leads to activation of the Raf–MEK–ERK pathway and Raf kinases play an important role in the modulation of ERK activity. B lymphocytes express two Raf isoforms, Raf-1 and B-Raf. Using an inducible deletion system in DT40 cells, the contribution of Raf-1 and B-Raf to BCR signalling was dissected. Loss of Raf-1 has no effect on BCR-mediated ERK activation, whereas B-Raf-deficient DT40 cells display a reduced basal ERK activity as well as a shortened BCR-mediated ERK activation. The Raf-1/B-Raf double deficient DT40 cells show an almost complete block both in ERK activation and in the induction of the immediate early gene products c-Fos and Egr-1. In contrast, BCR-mediated activation of nuclear factor of activated T cells (NFAT) relies predominantly on B-Raf. Furthermore, complementation of Raf-1/B-Raf double deficient cells with various Raf mutants demonstrates a requirement for Ras-GTP binding in BCR-mediated activation of both Raf isoforms and also reveals the important role of the S259 residue for the regulation of Raf-1. Our study shows that BCR-mediated ERK activation involves a cooperation of both B-Raf and Raf-1, which are activated specifically in a temporally distinct manner.
Keywords: B-cell antigen receptor signalling/B-Raf/conditional gene targeting/DT40/Raf-1
Introduction
Engagement of the B-cell antigen receptor (BCR) is a critical event during activation and development of B lymphocytes (Rajewsky, 1996). BCR engagement results in activation of protein tyrosine kinases (PTKs) such as Lyn, Syk and Btk followed by activation of the ERK pathway (Gold, 2000). The ERK pathway transmits survival signals during B lymphopoiesis (Iritani et al., 1997; Fleming and Paige, 2001). BCR engagement results in rapid and maximum ERK activation and is followed by a sustained phase with moderate ERK activity (Shirakata et al., 1999). However, some functional elements between the BCR and the ERK pathway as well as the mechanisms of the fine tuning of BCR-mediated ERK activation are not characterized yet. ERK is activated by many receptors that promote formation of active Ras (Ras-GTP). Ras-GTP recruits the serine/threonine-kinase Raf (MAPKKK) to the plasma membrane, where Raf activity is controlled by various protein kinases and the protein phosphatase PP2A (Kolch, 2000). Activated Raf phosphorylates the dual-specificity kinase MEK (MAPKK), which in turn phosphorylates ERK (MAPK) at a TEY motif in the activation loop. Phosphorylation of the TEY motif correlates with ERK activity, which can be quantified indirectly with a phosphospecific antibody (Yung et al., 1997). Activated ERK regulates cytoplasmic and nuclear effectors by phosphorylation.
Whereas Caenorhabditis and Drosophila possess only one raf gene, mammals express three raf paralogues: A-Raf, B-Raf and Raf-1 (Hagemann and Rapp, 1999). So far, only raf1 and B-raf but no A-raf orthologues have been identified in bony fish, amphibians and birds (Koenen et al., 1988; Calogeraki et al., 1993; T.Brummer, M.Reth and Y.Misawa, unpublished data), suggesting that a gene duplication leading to the raf-1 and B-raf paralogues occurred in early vertebrate evolution. B-raf displays higher sequence homology to invertebrate raf genes than A-raf and raf-1, and is likely to be the primordial vertebrate raf gene. All Raf isoforms share three highly conserved regions (CRs), which are also invariant features of all alternatively spliced B-Raf subtypes (Barnier et al., 1995). The N-terminal CR1 contains two domains, the Ras-binding domain (RBD), which initiates the interaction between Ras-GTP and Raf, and a cysteine-rich domain (CRD), which is involved in Ras binding, phospholipid interaction and full Raf activation (Kolch, 2000). The CR2 is rich in serine and threonine residues, which are potential targets for Raf-regulating kinases, whereas the CR3 contains the catalytic domain and important phosphorylation sites for Raf activation (Chong et al., 2001). Recent studies identified an amino acid stretch (N-region) at the N-terminal end of the CR3 domain that fulfils a critical role in Raf regulation. Mason et al. (1999) demonstrated that negative charges in this region, provided by either phosphorylation or charged amino acid residues, are required for Raf activation. Maximal activation of Raf-1 involves a two-step mechanism leading to the phosphorylation of S338 and Y341. In B-Raf, the equivalent serine (S445) is constitutively phosphorylated and the Y341 position is replaced by an aspartate residue.
The Raf isoforms share similar properties in vitro, but exhibit limited redundancy in vivo (Hagemann and Rapp, 1999). Due to its constitutively negative charged N-region, B-Raf displays a higher basal kinase activity and transforming capacity than Raf-1 (Papin et al., 1998). Also, gain-of-function mutations of the human B-raf gene have been identified recently in 66% of malignant melanomas (Davies et al., 2002).
Raf-1 is ubiquitously expressed, whereas B-Raf is expressed mainly in neuroectoderm-derived tissues and testis but is also detected in mammalian haematopoetic cells (Eychene et al., 1995). However, tissue-specific expression is not sufficient to explain the role of each Raf isoform, since all Raf isoforms are co-expressed within certain cell types. Unique functions of Raf isoforms have been shown by the phenotypes of Raf-deficient mice. A-Raf-deficient mice display postnatal lethality (Pritchard et al., 1996), whereas B-Raf-deficient mice die of vascular and neuronal defects during midgestation (Wojnowski et al., 1997; Wiese et al., 2001). Multiple organ abnormalities are responsible for the lethal phenotype of Raf-1-deficient mice (Hüser et al., 2001; Mikula et al., 2001). The exacerbated phenotype of Raf-1/B-Raf double deficient mouse embryos demonstrates overlapping functions of both isoforms in early development (Wojnowski et al., 2000).
So far, only Raf-1 has been investigated in B cells. BCR engagement results in Raf-1-mediated ERK activation followed by transcription of immediate early genes such as egr-1 or c-fos (for a review see Gold, 2000). However, other MAPKKKs have been postulated to be involved in MEK/ERK activation in B cells (Li and Carter, 1998; Purkerson and Parker, 1998), raising the possibility that B-Raf represents this unknown MAPKKK. We have detected B-Raf in all mammalian B-cell lines tested as well as in murine lymphatic organs (Y.Misawa, T.Brummer, F.Losch and M.Reth, in preparation). Chicken B-Raf is expressed in the bursal lymphoma DT40 and bursa of Fabricius, suggesting a conserved but still unknown function for B-Raf in B cells. Thus, we asked whether Raf-1 or B-Raf have unique or redundant functions in BCR signalling. Due to their high rate of homologous recombination, DT40 cells allow the generation of loss-of-function mutants by conventional gene targeting techniques (Buerstedde and Takeda, 1991). Indeed, the role of various PTKs and adaptor proteins in BCR-mediated activation of MAPK pathways has been analysed with DT40 lines deficient in these proteins (Hashimoto et al., 1998; Jiang et al., 1998). We have generated a DT40 line expressing the tightly controlled 4-hydroxy-tamoxifen (4-HT)-inducible Cre recombinase MerCreMer (MCM; Zhang et al., 1996). This line, DT40MCM, allows rapid and efficient recombination of loxP-flanked (floxed) gene segments and is used for inducible deletion of genes as well as for the inducible expression of transgenes (T.Brummer, H.Naegele, M.Reth and Y.Misawa, in preparation). In the present study, we generated DT40 cells allowing a conditional loss-of-function mutation of either the raf-1 or B-raf gene in order to dissect the contribution of each Raf isoform to BCR signalling. We show that B-Raf is the dominant ERK activator in DT40 B cells whereas Raf-1 serves as an accessory ERK activator that is activated only transiently during BCR signalling. Furthermore, we demonstrate that not only Raf-1 but also B-Raf is involved in the BCR-mediated induction of Egr-1 and c-Fos, whereas BCR-mediated activation of nuclear factor of activated T cells (NFAT) depends mainly on B-Raf. In addition, this study provides the first biochemical analysis of a vertebrate cell lacking both Raf-1 and B-Raf.
Results
The time course of the regulatory phosphorylation of Raf-1 and B-Raf following BCR engagement is different
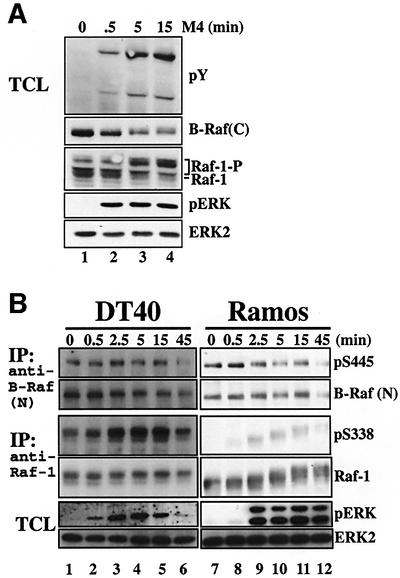
Chickens have two raf orthologues, also known as c-mil and c-Rmil (Koenen et al., 1988; Calogeraki et al., 1993), which we here refer to as raf-1 and B-raf, respectively. Both Raf isoforms become hyperphosphorylated upon BCR engagement by treatment with anti-IgM antibody (Ab) M4 (Figure 1A). This can be seen by the electrophoretic mobility shift of Raf-1 resulting from a MEK/ERK-dependent feedback phosphorylation (Wartmann et al., 1997) (our own observations). Likewise, B-Raf is also subjected to a MEK/ERK-dependent feedback phosphorylation indicated by an apparent decrease in the amount of B-Raf detected by the anti-B-Raf Ab C-19 (Figure 1A, second panel). We confirmed that ERK2 phosphorylates B-Raf within the C-terminal epitope sequence and that this phosphorylation interferes with the recognition of chicken B-Raf by anti-B-Raf C-19 Abs (T.Brummer, H.Naegele, M.Reth and Y.Misawa, in preparation). Both the C-terminal phosphorylation of B-Raf and the mobility shift of Raf-1 can be blocked by the MEK inhibitor U0126 (data not shown). The feedback phosphorylation of B-Raf is already observed after 0.5 min of BCR engagement. In contrast, the mobility shift of Raf-1 becomes visible at ∼3–5 min after stimulation (Figure 1A, third panel), suggesting that these feedback phosphoryl ations reflect temporally distinct waves of B-Raf and Raf-1 activation.
Fig. 1. The course of the phosphorylation of Raf-1 and B-Raf following BCR engagement is different. (A) Western blot analysis of DT40 cells stimulated with anti-IgM Ab (M4). Detection of tyrosine-phosphorylated proteins (pY) indicates successful stimulation. This result is representative of at least five independent experiments. (B) DT40 and Ramos B cells were stimulated with anti-IgM Abs as indicated, and Raf proteins were purified using anti-B-Raf H-145 or anti-Raf-1 Abs, respectively. The immunocomplexes were subjected to western blot analysis. Phosphorylation at S445 (B-Raf) and S338 (Raf-1) was detected by the anti-pS338 Ab. This result is representative of at least four (DT40) or two (Ramos) independent experiments.
Recently, a regulatory residue within the N-region of Raf-1, S338, has been identified, whose phosphorylation is a prerequisite for Raf-1 activation (King et al., 1998; Mason et al., 1999). The phosphorylation of Raf-1 at S338 is Ras-GTP dependent and occurs at the plasma membrane, and the equivalent residue in B-Raf, S445, was reported to be phosphorylated constitutively in B-Raf isolated from COS and PC12 cells (Mason et al., 1999). The phosphorylation of these residues can be monitored by the anti-pS338 monoclonal Ab. Figure 1B demonstrates that B-Raf is constitutively phosphorylated at S445 in DT40 and human Ramos B cells, whereas phosphorylation of Raf-1 at S338 is induced upon BCR engagement. This suggests that B-Raf is already pre-activated to some extent in B cells, while Raf-1 activation is induced upon BCR engagement. Importantly, prominent phosphorylation of Raf-1 at S338 appears at 2.5 min when the ERK pathway is already activated, suggesting that Raf-1 is not involved in the early phase of ERK activation (Figure 1B). We also observed a transient dephosphorylation of Raf-1 at the inhibitory residue S259 upon BCR engagement, which coincided with the onset of S338 phosphorylation (data not shown). This agrees with recent observations in NIH-3T3 cells (Dhillon et al., 2002a), suggesting that BCR-mediated dephosphorylation at S259 precedes phosphorylation at S338 and activation of Raf-1. Together, both Raf-1 and B-Raf display temporally distinct phosphorylation patterns that could reflect isoform-specific functions. Therefore, we adopted a genetic approach to dissect the individual roles of Raf-1 and B-Raf in BCR signalling.
Mutagenesis of the chicken raf-1 and B-raf genes
As the expression level of Raf might affect cellular viability (Rommel et al., 1997; Murakami and Morrison, 2001), we applied the Cre–loxP technology for the inducible deletion of the raf genes in DT40 cells. The following considerations were applied for the targeting strategy. First, we flanked exons, which are invariant features of the transcripts of raf-1 and B-raf, by two loxP sites. The Cre-mediated deletion of these exons removes critical information required for protein function and results in a frameshift, thereby generating premature stop codons (PSCs). Secondly, the targeting cassettes were designed in such a way that the FRT-flanked neomycin resistance gene (neoR) cassette could be excised by FLP-e-mediated recombination (Schaft et al., 2001). This not only allows recycling of targeting vectors, but also minimizes the risk of positional effects.
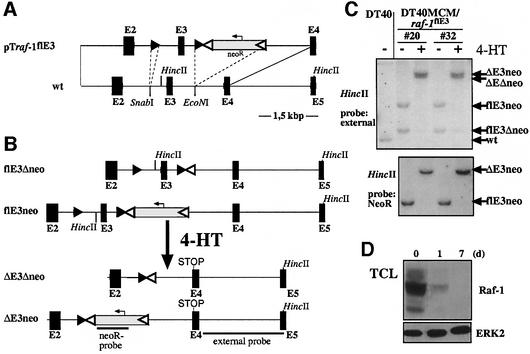
For the raf-1 locus, these criteria are met by exon 3, which encodes almost the entire RBD. Inducible deletion of exon 3 generates a transcript with a PSC at the splice junction between exons 2 and 4. The targeting vector, pTraf-1flE3 was transfected into DT40MCM cells (Figure 2A). Figure 2B and C demonstrates the result of the modification of raf-1 loci by homologous recombination as well as FLP-e- and MCM-mediated deletion of the neoR cassette and exon 3, respectively. Southern blot analysis shows the complete recombination of the modified raf-1 loci upon exposure to 4-HT for 24 h, which is also reflected by the decay of Raf-1 (Figure 2D). However, longer exposures of the western blot revealed the presence of a faint 60 kDa band (∼0.5% of the intensity derived from Raf-1 proper) immunoreactive to the anti-Raf-1 antibody, which was only visible after 4-HT treatment (data not shown). This band is most probably explained by minor production of an aberrant protein generated by alternative translational initiation on the mutated Raf-1 transcript. As the aberrant Raf-1 protein is expressed at very low levels (∼0.5% of Raf-1 proper) and lacks a functional RBD, we do not consider that this protein confers biological activity.
Fig. 2. Conditional targeting of the chicken raf-1 locus. (A) Map of the wild-type raf-1 locus (wt) with exons 2–5 (black boxes). The targeting vector pTraf-1flE3 contains a loxP site and a loxP-FRT-neo-FRT cassette inserted into the SnabI and EcoNI sites, respectively. LoxP and FRT sites are indicated by black and white triangles, respectively. (B) Map of the modified raf-1 alleles. The flE3Δneo allele respresents the first targeted allele after excision of the neoR gene by FLP-e expression. The flE3neo allele is the result of the second round of transfection containing the neoR gene. Following 4-HT treatment, MCM excises E3 from the alleles flE3Δneo and flE3neo, thereby generating the ΔE3Δneo and ΔE3neo alleles, respectively. (C) MCM-mediated recombination in DT40MCM/raf-1flE3 cells was examined by Southern blot analysis. HincII-digested genomic DNA derived from two clones, which were either exposed to 4-HT for 24 h (+) or left untreated (–), was detected by the indicated probes. MCM-mediated recombination results in excision of genomic sequences around exon 3 and concomitant loss of a HincII site as indicated by the increased fragment size. The polymorphism (by the neoR cassette) of the modified raf-1 loci in these clones was used here to demonstrate the efficient and autonomous recombination of both alleles. (D) Western blot analysis of Raf-1 expression in total cellular lysates after induction with 4-HT.
The aforementioned criteria were applied for construction of the targeting vector pTB-rafflE6, which was used to generate B-raf alleles allowing the inducible deletion of exon 6 (Figure 3A). This exon encodes the CRD, which is critical for activation of Raf kinases (Kolch, 2000) and its deletion would lead to a transcript with exon 5 spliced to exon 7 thereby generating a PSC at the beginning of exon 7 (Figure 3B). Southern blot analysis illustrates the modification of the B-raf locus by homologous, FLP-e- and MCM-mediated recombination (Figure 3C). Figure 3D shows the disappearance of B-Raf at day 5 after induction. However, a longer exposure of the western blot revealed the presence of low amounts of an aberrant protein. According to its size, this protein could be encoded by an mRNA generated by an aberrant splice from exon 4 to exon 7, and lacks the C-terminal portion of the RBD as well as the entire CRD. In addition, minor production of an N-terminal peptide corresponding to the amino acids encoded by exons 1–5 is observed. However, as both aberrant B-Raf proteins are expressed at very low levels (<5%) and lack the CRD, which is required for a stable Ras–Raf interaction in vivo (Bondeva et al., 2002), we rule out a potent dominant-negative effect of these proteins on Ras signalling. In addition, using a coupled in vitro kinase assay Alessi et al., 1995) with immunoprecipitates (IPs) from B-Raf-deficient cells, the B-Raf activity that is present in the IP was negligible compared with that of the B-Raf-positive population (data not shown).
Fig. 3. Conditional targeting of the chicken B-raf locus. (A) Map of the wild-type B-raf locus with exons 4–7 (black boxes). The targeting vector pTB- rafflE6 contains a loxP site and a loxP-FRT-neo-FRT cassette inserted into the EcoRV and PacI sites, respectively. LoxP and FRT sites are indicated by black and white triangles, respectively. After removal of the FRT-flanked neoR by transient FLP-e expression, the same construct was used for targeting of the second allele. (B) Map of the modified B-raf allele after homologous and FLP-e-mediated recombination. (C) MCM-mediated recombination was confirmed by Southern blot analysis. VspI-digested genomic DNA fragments derived from either parental DT40MCM or DT40MCM/B-rafflE6 cells [either exposed to 4-HT (+) for 24 h or left untreated (–)] were detected with the E6/E7 probe. Homologous recombination of both alleles (lane 3) is indicated by the increased size of the fragment. MCM-mediated recombination results in excision of exon 6 indicated by the decreased fragment size (lane 4). (D) Western blot analysis using anti-B-Raf H-145 Abs. DT40MCM/ B-rafflE6 cells were harvested at 5 days post-induction. The aberrant B-Raf (B-Raf*) and the N-terminal peptide (N-term.) are only detected after MCM-mediated recombination.
Raf-1 is not required for a prominent BCR-mediated ERK pathway activation
The Ras–Raf-1–MEK–ERK pathway represents the canonical MAPK cascade. Therefore, we tested whether ERK activation was altered in Raf-1-deficient, BCR-stimulated DT40 cells. In this and the following experiments, Raf-positive and -deficient populations of the same clone displayed the same pattern of tyrosine phosphorylation and BCR expression (data not shown), indicating that both populations received the same stimulus. Unexpectedly, the kinetics of BCR-mediated ERK activation as well as basal ERK activity were not affected by loss of Raf-1 (Figure 4). These surprising results imply that Raf-1 contributes only to a minor extent or not at all to BCR-mediated ERK activation and show that Raf-1 is dispensable for prominent BCR-mediated ERK activation. Since Raf-1 has been implicated as the classical ERK activator in many cell types, including B lymphocytes, these data question the established role of Raf-1 as the canonical ERK activator.
Fig. 4. Loss of Raf-1 does not affect the kinetics of BCR-mediated ERK activation. Populations of DT40MCM/raf-1flE3 cells, which were either left untreated (–) or exposed to 4-HT for 24 h (+), were stimulated with M4 at 8 days post-induction. Total cellular lysates were subjected to western blot analysis. This result is representative of at least seven independent, comparable experiments.
B-Raf-deficient DT40 cells display a shortened BCR-mediated ERK activation
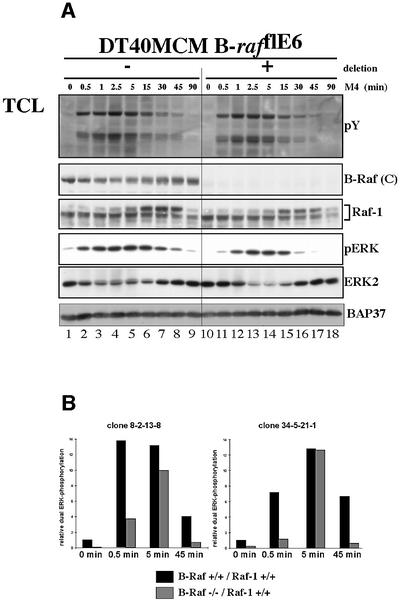
In contrast to Raf-1-deficient DT40 cells, B-Raf-deficient cells display a reduced ERK activation during the immediate early (0.5 min) and late (45 min) phases of BCR-induced ERK activation (Figure 5). This demonstrates an important role for B-Raf in the initiation as well as the maintenance of BCR-mediated ERK activation. Moreover, basal ERK phosphorylation is strongly reduced in B-Raf-deficient DT40 cells, suggesting that B-Raf generates basal ERK activity in DT40 cells. Furthermore, the kinetics of the feedback phosphorylation of Raf-1, as indicated by its electrophoretic mobility shift, are not influenced by B-Raf deficiency.
Fig. 5. The early and late phases of BCR-mediated ERK activation are impaired in B-Raf-deficient DT40 cells. (A) Western blot analysis of B-Raf-positive and -negative populations of DT40MCM/B-rafflE6 cells, which were conducted as described in Figure 4. This result is representative of three independent experiments with an identical procedure and several other experiments with a similar design. The apparent decrease in the level of ERK2 is caused by sequential detection of phospho-ERK followed by detection with anti-ERK2 antibody on the same western blot membrane. (B) The ERK phosphorylation kinetics of the B-Raf-positive and -negative populations from two independent clones were quantified by Lumiimager analysis. The values represent the fold activation over the dual ERK phosphorylation measured in unstimulated B-Raf-positive cells. This figure shows two representative results obtained from the analysis of several clones.
However, a prominent, but transient ERK activation occurs in the absence of B-Raf (Figure 5A, lanes 11–16), showing that a B-Raf-independent ERK pathway is involved during the intermediate phase of BCR-mediated ERK activation. These findings demonstrate that B-Raf is a dominant but not the only ERK activator in DT40 cells.
ERK activation is severely impaired in Raf-1/B-Raf double deficient DT40 cells
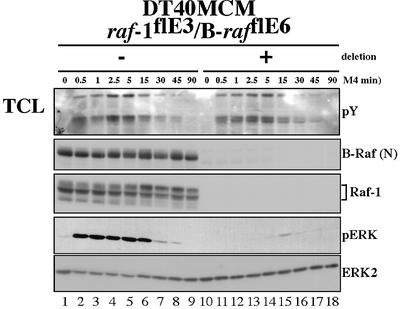
The data above show that, even in the absence of B-Raf, a considerable amount of ERK becomes transiently activated upon BCR engagement. In order to test whether this ERK activation was mediated by Raf-1, conditional Raf-1/B-Raf double deficient cells (DT40MCM/raf-1flE3/ B-rafflE6 cells) were generated. Indeed, Raf-1/B-Raf double deficient DT40 cells exhibit a severe block in BCR-mediated ERK activation, indicating that Raf-1 is involved during the intermediate phase of BCR-mediated ERK activation (5–15 min). Nevertheless, traces of ERK activation are still observed in Raf-1/B-Raf double deficient cells at ∼15 min (Figure 6). To rule out that this residual ERK activation is caused by the low amounts of aberrant B-Raf protein present in these cells, the B-raf gene was also constitutively disrupted in DT40MCM/ raf-1flE3 cells by insertion of resistance gene cassettes into exon 6. Although this targeting approach abolished expression of B-Raf completely, traces of active ERK were still detectable in stimulated cells after inducible exon 3 deletion from the raf-1 locus (data not shown). Thus, this residual ERK activation observed in Raf-1/B-Raf double deficient cells is more likely to be explained by activation of non-Raf-family MAPKKKs such as MEKK1 (Roy et al., 2002).
Fig. 6. Severe block in BCR-mediated ERK activation in Raf-1/B-Raf double deficient DT40 cells. Populations of DT40MCM/raf-1flE3/ B-rafflE6 cells were harvested at 5 days after induction of MCM-mediated deletion (+) and stimulated with M4. Total cellular lysates were subjected to western blot analysis. This result is representative of two experiments with an identical procedure (two independent clones) and at least five other experiments with a similar design. The apparent decrease in the level of ERK2 is caused by sequential detection of phospho-ERK followed by detection with anti-ERK2 antibody on the same western blot membrane.
The importance of Ras–Raf interaction for BCR-mediated ERK activation
Structural and genetic analyses have revealed an evolutionarily conserved arginine residue within the RBD that is critical for Ras–Raf interaction and for proper Raf activation (Nassar et al., 1995). However, Ras-independent activation of the Raf–MEK–ERK pathway has also been described (Sakatsume et al., 1998). Thus, it was of interest to determine whether Ras–Raf interaction is required for BCR-mediated ERK activation. Therefore, Raf-1/B-Raf double deficient DT40 cells were transfected with expression vectors encoding either wild-type human Raf-1 or its mutated counterpart where arginine R89 in the RBD has been replaced by a leucine residue (Raf-1 R89L). Wild-type Raf-1 but not Raf-1 R89L can partially restore BCR-mediated ERK activation (Figure 7A), which demonstrates that, upon BCR engagement, Raf-1 activates the ERK pathway in a strictly Ras-GTP-dependent manner.
Fig. 7. The significance of Ras–Raf interaction for BCR-mediated ERK activation. Western blot analysis of total cellular lysates. (A) DT40MCM/ raf-1flE3/B-rafflE6 cells were treated with 4-HT to delete both raf-1 and B-raf, and transfected with expression vectors for either wild-type Raf-1 (R), Raf-1 R89L (L) or Raf-1 S259D (S). These cell lines were stimulated with M4 together with parental, Raf-deficient 4-HT-treated DT40MCM/raf-1flE3/B-rafflE6 cells (Δ) and their non-4-HT-treated, Raf-positive sister population (W) as indicated. (B) After the deletion of both raf genes, DT40MCM/ raf-1flE3/B-rafflE6 cells were transfected with expression vectors for either wild-type HA-tagged B-Raf (R) or HA-B-Raf R188L (L). These cell lines were stimulated with M4 together with parental, Raf-deficient cells (Δ) and their non-4-HT-treated, Raf-positive counterpart (W) as indicated. Detection of BAP37 serves a loading control. (C) Detection of S445 phosphorylation in HA-B-Raf and HA-B-Raf R188L by anti-pS338 Ab. DT40MCM/raf-1flE3/B-rafflE6 cells were transfected with expression vectors for HA-B-Raf or HA-B-Raf R188L and then treated with 4-HT. Nine days after induction, the cells were stimulated with M4 for the indicated times and HA-B-Raf proteins were purified as in (B). For each panel, representative results of at least three independent, comparable experiments are shown.
Recent data show that a phosphorylated S259 allows a 14-3-3 dimer to keep Raf-1 in an inactive conformation. Upon membrane recruitment, Ras-GTP displaces 14-3-3 from S259, which leads to its dephosphorylation by PP2A followed by S338 phosphorylation and full Raf-1 activation (Zimmermann and Moelling, 1999; Dhillon et al., 2002a; Kubicek et al., 2002; Light et al., 2002). Therefore, a Raf-1 mutant with S259 replaced by an aspartate (S259D) displays enhanced S338 phosphorylation and increased in vitro kinase activity (Dhillon et al., 2002b; Dumaz et al., 2002). Hence, we asked to what extent Raf-1 S259D would rescue BCR-mediated ERK activation in Raf-1/B-Raf double deficient DT40 cells (Figure 7A). Astonishingly, Raf-1 S259D is sufficient to compensate for the loss of both endogenous Raf isoforms. Importantly, in contrast to wild-type Raf-1, Raf-1 S259D restores the immediate early phase of ERK activation (Figure 7A, compare lanes 6–8 and 10), suggesting that 14-3-3 displacement and dephosphorylation of S259 are early and rate-limiting steps in BCR-mediated activation of Raf-1. Thus, the S259D mutation circumvents these critical steps and renders Raf-1 hyper-responsive to BCR engagement.
Replacement of the R89 equivalent in B-Raf, R188, by leucine prevents membrane recruitment by oncogenic Ras but does not affect its high basal in vitro kinase activity (Marais et al., 1997). In light of isoform-specific differences in the activation of Raf-1 and B-Raf, it was of interest to determine whether R188 is required for BCR-mediated ERK activation in vivo. Accordingly, Raf-1/B-Raf double deficient cells were transfected with expression vectors encoding an N-terminal haemagglutinin (HA)-tagged, wild-type B-Raf (HA-B-Raf) or its mutated counterpart, HA-B-Raf R188L. Chemoluminescence analysis revealed that both HA-B-Raf proteins are overexpressed ∼8-fold in comparison with endogenous B-Raf (Figure 7B). Overexpression of wild-type HA-B-Raf rescues BCR-mediated ERK activation (Figure 7B, lanes 7, 11 and 15). In contrast, HA-B-Raf R188L cannot restore BCR-mediated ERK activation to the same extent as is observed in DT40 cells expressing endogenous Raf-1 and B-Raf (Figure 7B, compare lanes 5 and 9 with lanes 8 and 12). Thus, BCR-mediated activation of both Raf-1 and B-Raf is dependent on interaction with Ras family members.
Using the Raf-1 R89L mutant, Mason et al. (1999) have demonstrated that S338 phosphorylation of Raf-1 requires interaction with Ras-GTP. In order to test if the constitutive phosphorylation of B-Raf at S445 is also Ras dependent, HA-B-Raf or HA-B-Raf R188L proteins were purified and analysed for their phosphorylation at S445. Figure 7C shows that the constitutive phosphorylation of S445 is not affected by its ability to interact with Ras-GTP and suggests that, in contrast to S338 in Raf-1, phosphorylation of the N-region of B-Raf occurs prior to its interaction with Ras-GTP. This observation is supported by experiments showing that oncogenic Ras does not enhance S445 phosphorylation of B-Raf in COS cells (Mason et al., 1999). Together, these data suggest that S338 of Raf-1 and S445 of B-Raf are phosphorylated by different kinases or in different subcellular locations.
B-Raf is important for BCR-mediated NFAT activation
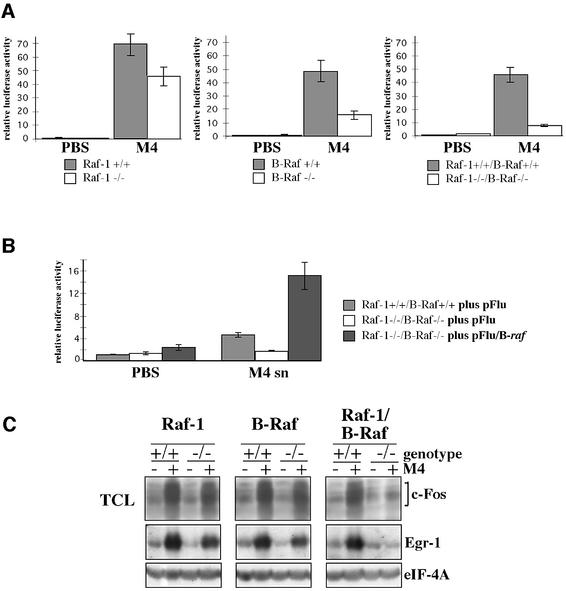
An important BCR-triggered event is the activation of the NFAT transcription factor complex, which consists of the Ca2+-regulated NFAT proteins, as well as the AP-1 components c-Fos and c-Jun (Gold, 2000). Thus, NFAT activation in T cells requires not only a sustained Ca2+ influx but also simultaneous activation of multiple Ras effectors such as the JNK and ERK pathways (Genot et al., 1996). As BCR-mediated Ca2+ influx is not affected in Raf-deficient DT40 cells (data not shown), we asked to what extent Raf deficiency would influence NFAT-driven reporter gene expression. NFAT reporter expression is reduced by 30% in the Raf-1-deficient clone shown in Figure 8A, whereas no reduction was observed in another clone that exhibited a generally weaker NFAT response towards BCR engagement (data not shown). This suggests that Raf-1 plays only a minor role in BCR-mediated NFAT activation. In contrast, B-Raf-deficient DT40 cells consistently displayed >60% reduction in NFAT reporter activity, suggesting that B-Raf is the dominant Ras effector responsible for NFAT activation (Figure 8A). Raf-1/B-Raf double deficient DT40 cells showed the strongest reduction (>80%) in BCR-mediated NFAT activation, suggesting that this event relies heavily on ERK activation. Indeed, pre-treatment of DT40 cells with the MEK inhibitor U0126 blocks both ERK and NFAT reporter activity (data not shown). Conversely, co-transfection of the NFAT reporter plasmid with HA-B-Raf expression vectors overcompensates BCR-mediated NFAT activation in Raf-1/B-Raf double deficient DT40 cells (Figure 8B).
Fig. 8. BCR-mediated induction of the transcription factors NFAT, c-Fos and Egr-1 is regulated by both Raf kinases. (A) DT40 lines allowing inducible deletion of either raf-1, B-raf or both raf genes were exposed to 4-HT for 24 h (white bars) or left untreated (grey bars) and cultivated for an additional 4 days. The cells were then transfected with the NFAT reporter plasmid. Cells were stimulated with 10 µg of M4 for 6 h. (B) Raf-1/B-Raf double-deficient DT40 cells were transfected with the NFAT reporter plasmid and 10 µg of the expression vector pFlu/B-raf or the empty vector pFlu as indicated. As wild-type reference, uninduced DT40MCM/raf-1flE3/B-rafflE6 cells were also included. The DT40 lines were treated as described in (A) and stimulated with M4 hybridoma supernatant (a stimulus equivalent to 5–10 µg M4/ml) for 6 h. The mean of the standardized luciferase activity derived from three independent, simultaneously performed transfections is shown. Standard deviation is indicated by an error bar. For each panel, representative results of at least three independent experiments are shown in both (A) and (B). (C) Western blot analysis of BCR-mediated synthesis of c-Fos and Egr-1. The DT40 lines were treated as described in (A), stimulated with M4 hybridoma supernatant (a stimulus equivalent to 5–10 µg M4/ml) for 1 h and lysed in RIPA buffer. This result is representative of three independent experiments.
B-Raf and Raf-1 cooperate in the induction of c-Fos and Egr-1 expression
The defect in BCR-mediated NFAT activation could be explained by insufficient synthesis of c-Fos, which is required to form stable NFAT–AP-1 complexes on many NFAT elements, including those of the NFAT reporter plasmid (Gold, 2000; Macian et al., 2001). Thus, we analysed how Raf deficiency would affect BCR-mediated c-Fos expression by using pan c-Fos Abs. Interestingly, Raf-1 or B-Raf single deficient DT40 cells still display a prominent induction of c-Fos synthesis, whereas c-Fos induction is strongly reduced in Raf-1/B-Raf double deficient cells (Figure 8C). As Raf-1 and B-Raf single deficient DT40 cells show a similar reduction in c-Fos synthesis, it is conceivable that B-Raf also contributes to BCR-mediated NFAT activation through another effector, e.g. the transcriptional coactivator CBP/p300 (Avots et al., 1999).
Finally, we tested whether the synergy of Raf-1 and B-Raf in c-Fos induction could be extended to Egr-1. Loss of Raf-1 or B-Raf results only in reduced Egr-1 synthesis, whereas the absence of both Raf isoforms is neccessary to block Egr-1 induction completely (Figure 8C). These findings show that Raf-1 and B-Raf act synergistically in the BCR-mediated induction of immediate early genes such as c-Fos and Egr-1.
Discussion
On the basis of the phenotypes of Raf-deficient embryos (Murakami and Morrison, 2001), we expected an impaired viability of the DT40 lines deficient for one or both Raf isoforms. However, neither morphological alterations nor increased cell death were observed following inducible deletion of raf-1, B-raf or of both raf genes. These lines, as well as the DT40 line, in which both B-raf alleles have been constitutively disrupted, can be cultured over several weeks without observing a prominent defect in proliferation. In addition, important BCR signalling events, e.g. phosphorylation of PTK substrates (Figures 4, 5A and 6) and activation of the PI-3K/PKB pathway (data not shown), are not affected by the absence of Raf-1 and B-Raf.
For most cell types, including B lymphocytes, Raf-1 is regarded as the dominant transducer from plasma membrane-associated receptors to the ERK pathway (Gold, 2000). Therefore, the finding that loss of Raf-1 does not influence BCR-mediated ERK activation significantly was unexpected. However, while this work was in progress, Hüser et al. (2000) and Mikula et al. (2001) reported unaffected ERK activation in Raf-1-deficient mouse embryonic fibroblasts (MEFs) in response to various stimuli. In addition, both groups demonstrated comparable basal ERK activity in Raf-1-positive and -deficient MEFs, as is the case for Raf-1-deficient DT40 cells. Furthermore, Raf-1 is not essential for the ERK activation that occurs during the invasion of bone marrow-derived macrophages by Salmonella (Jesenberger et al., 2001). Taken together, these studies and our work suggest that Raf-1 is indeed dispensable for prominent ERK activation mediated in different cell types derived from birds and mammals.
Hüser et al. (2001) and Mikula et al. (2001) suggest that B-Raf, rather than Raf-1, is the primary Raf isoform that activates the ERK pathway in MEFs. Indeed, Wojnowski et al. (2000) observed a block in epidermal growth factor (EGF)-mediated ERK activation in B-Raf-deficient MEFs, although time-course experiments were not performed in their study. Unlike Raf-1-deficient DT40 cells, B-Raf- deficient DT40 cells exhibit a strongly reduced basal ERK phosphorylation. Furthermore, our results demonstrate an indispensable function for B-Raf for the immediate early and late phases of BCR-mediated ERK activation. Thus, B-Raf, but not Raf-1, is the dominant ERK activator in DT40 cells. In view of the prominent expression of B-Raf in other B-cell lines and lymphatic tissues, it is likely that B-Raf also serves here as the major ERK activator. Indeed, MAPKKKs other than Raf-1 have been postulated to contribute to the synergic activation of MEK/ERK by the BCR and CD19 or CD40, respectively, suggesting that B-Raf mediates these processes (Li and Carter, 1998; Purkerson and Parker, 1998). Thus, the role of B-Raf in B cells is reminiscent of neuronal cells in which B-Raf is the principal MEK/ERK activator (Kao et al., 2001).
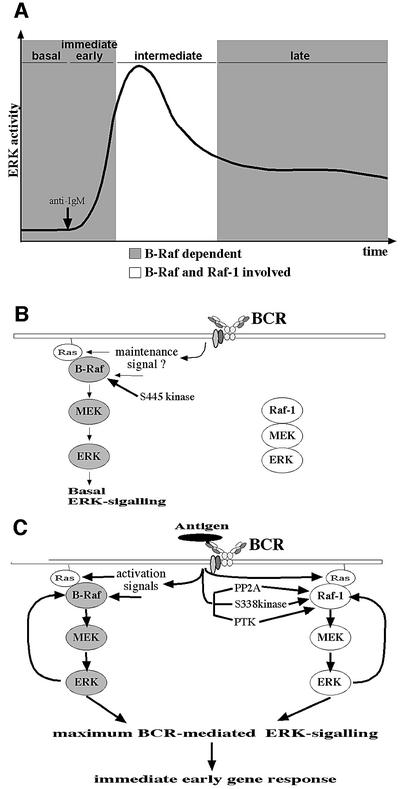
Nevertheless, intermediate BCR-mediated ERK activation is also achieved by a B-Raf-independent pathway. The severe block in BCR-mediated ERK activation of Raf-1/B-Raf double-deficient cells shows that Raf-1 is indeed responsible for the transient ERK activity observed in B-Raf-deficient DT40 cells. As B-Raf contributes to a larger extent to BCR-mediated ERK activation than Raf-1, the minor contribution of Raf-1 is not discerned in Raf-1-deficient but B-Raf-positive DT40 cells. Recent studies have demonstrated that Raf-1 and B-Raf can be assembled as heterodimers, and such heterodimers have been proposed to be required for EGF-mediated ERK activation in MEFs (Wojnowski et al., 2000; Weber et al., 2001). In BCR-mediated ERK activation, a physical interaction of both isoforms within the same signalosome is also conceivable. However, our comparison of the ERK activation in Raf-1- or B-Raf-deficient DT40 cells with Raf-1/B-Raf double-deficient cells shows that both Raf kinases can function independently. This is supported by the observation that the kinetics of ERK-mediated feedback phosphorylation of one Raf isoform are not affected by the absence of the other isoform. In addition, we demonstrate temporally distinct ERK-mediated feedback phosphorylation kinetics for Raf-1 and B-Raf (Figures 1, 4 and 5). The early onset of the ERK-mediated feedback phosphorylation of B-Raf compared with the 5-fold slower onset of the Raf-1 feedback phosphorylation fits well with the observation that B-Raf expression is required for the immediate early ERK activation following BCR engagement. This suggests not only temporally distinct recruitment/activation mechanisms for B-Raf and Raf-1 upon BCR engagement but also the autonomous action of both isoforms during BCR signalling. Together, these findings suggest that Raf-1 and B-Raf act in separate signalling modules but cooperate functionally during the intermediate phase of BCR-mediated ERK activation (Figure 9).
Fig. 9. A new model for BCR-mediated ERK activation. (A) Kinetic model of the different requirements for B-Raf and Raf-1 in BCR-mediated ERK activation as defined by the phenotypes of B-Raf or Raf-1 single deficient DT40 cells. The x-axis is not drawn to scale. The immediate early (<2.5 min) and late (>30 min) phases of ERK activation as well as the basal ERK activity are dependent on B-Raf (grey areas). In contrast, Raf-1 and B-Raf cooperate during the intermediate phase of BCR-mediated ERK activation, which is characterized by maximum ERK activity (white area). Raf-1 can partially compensate B-Raf deficiency in the intermediate phase and this genetically defined time window for the contribution of Raf-1 to BCR-mediated ERK activation correlates with the kinetics of S338 phosphorylation (Figure 1B). (B) Basal ERK activity is maintained in DT40 cells by B-Raf. Phosphorylation of B-Raf at S445 by an unknown kinase primes B-Raf for activation prior to its interaction with Ras-GTP. (C) Upon BCR engagement, more primed B-Raf molecules are recruited and activated at the plasma membrane by increasing levels of Ras-GTP and other activators (Chong et al., 2001). This pool of BCR-activated B-Raf increases ERK activity, leading to its rapid feedback phosphorylation by activated ERK. With persistent BCR engagement, Raf-1 is also recruited to the plasma membrane by Ras-GTP and subsequently is activated by dephosphorylation of S259 followed by phosphorylation of its N-region and other events. This pool of BCR-activated Raf-1 contributes to intermediate ERK activation leading to its own feedback phosphorylation, which is proposed as a negative feedback loop involved in its detachment from the plasma membrane (Wartmann et al., 1997).
What is the molecular basis for the different kinetics in B-Raf and Raf-1 activation? One possible scenario is that activation of B-Raf and Raf-1 occurs in different subcellular locations. Indeed, the BCR enters a detergent-insoluble plasma membrane fraction after antigen binding (Weintraub et al., 2000), and it is conceivable that, during this process, the BCR encounters different Raf/MEK/ERK2 modules that are enriched specifically in certain membrane subdomains. Alternatively, but not contradictory to the first model, the temporally distinct waves of B-Raf and Raf-1 activation could reflect different requirements of both isoforms for upstream activators. The fact that B-Raf is constitutively phosphorylated at S445 whereas phosphorylation of Raf-1 at S338 is induced transiently upon BCR engagement could explain why expression of Raf-1 can only compensate for B-Raf deficiency in the intermediate but not in the early and late phases of BCR-mediated ERK activation. According to this model, BCR engagement not only leads to Ras-dependent recruitment of Raf-1 to the plasma membrane but must also initiate dephosphorylation of S259 followed by phosphorylation of the N-region at S338 (and maybe Y341) before Raf-1 can contribute to ERK activation (Figure 9C). The importance of S259 dephosphorylation in BCR-mediated ERK activation is supported by the fact that Raf-1/B-Raf-deficient DT40 cells complemented with the Raf-1S259D mutant show a faster and stronger ERK activation than those expressing wild-type Raf-1 (Figure 7A). This observation is in line with the work of Dhillon et al. (2002a,b) suggesting that S259 controls Raf-1 activation by regulating S338 phosphorylation. In contrast, B-Raf is constitutively phosphorylated at S445, the equivalent of S338, and contains aspartate at the Y341 equivalent. Thus, the N-region in B-Raf is already highly negatively charged in unstimulated B lymphocytes and this ‘pre-activated’ B-Raf could immediately translate BCR engagement into ERK activation after its encounter with Ras-GTP (Figure 9). Our observation that the Ras binding-defective B-Raf R188L mutant is phosphorylated at S445 implies that this critical phosphorylation occurs prior to the interaction of B-Raf with Ras-GTP. Upon binding to Ras-GTP, full B-Raf activation could then be achieved rapidly by other events, e.g. by phosphorylation of its activation loop (Chong et al., 2001). Thus, the extent of cellular B-Raf activity may be regulated primarily by the availability of Ras-GTP, and the ‘pre-activated state’ of B-Raf would also explain why expression of B-Raf but not of Raf-1 is required for maintaining basal ERK activity in DT40 cells. Indeed, unstimulated DT40 cells contain low levels of Ras-GTP (Ishiai et al., 1999), which could be sufficient to trigger basal ERK phosphorylation through ‘pre-activated’ B-Raf.
Analyses of BCR-deficient mice have suggested a maintenance signal derived from the BCR in the absence of antigen (Lam et al., 1997). Therefore, it is an attractive hypothesis that basal ERK activity, which could be part of this maintenance signal, is transmitted through low levels of Ras-GTP and B-Raf (Figure 9A). Likewise, if the signal perceived by the BCR, e.g. antigen encounter, is strong enough to activate N-region kinases such as the S338 kinase or PTKs, Raf-1 is used as an accessory MAPKKK in order to achieve maximum ERK activation (Figure 9B). This role for Raf-1 as an accessory ERK activator in B cells may be applied to other cell types and would reconcile the conflict between the phenotype of Raf-1-deficient MEFs and a plethora of biochemical studies demonstrating Raf-1 as an ERK activator (Murakami and Morrison, 2001). Nevertheless, the phenotype of Raf-1-deficient mice as well as the biological activity of a mutant Raf-1 protein defective in MEK binding (Pearson et al., 2000) suggest that Raf-1 has also evolved functions that are not related to its role as an accessory ERK activator. The DT40 lines generated in this study might help to identify additional targets of Raf-1 and to redefine its biological function. In this context, we analysed whether BCR-mediated NF-κB activation, which is discussed as a MEK-independent target of Raf-1, is influenced by Raf deficiency. However, loss of Raf-1 does not affect NF-κB reporter gene expression, and loss of B-Raf or both Raf kinases leads only to a slight or modest reduction in NF-κB reporter gene expression, respectively (data not shown).
Taken together, we have shown that BCR-mediated ERK activation is modulated in a complex manner by B-Raf and Raf-1. Indeed, two Raf isoforms could allow a greater plasticity in BCR-mediated ERK activation depending on the nature and concentration of the antigen. In PC12 cells, Raf-1 and B-Raf not only are regulated differently, but their distinct activation kinetics also play an important role in the proliferation versus differentiation decision (Marshall, 1995; York et al., 1998; Kao et al., 2001). Since there is accumulating evidence that the Raf–ERK pathway is involved at various checkpoints during B-cell development (Iritani et al., 1997; Fleming and Paige, 2001), it is conceivable that these cell fate decisions could be influenced by differential activation of B-Raf and Raf-1. In this respect, our work shows that the synthesis or activation of the transcription factors Egr-1, c-Fos and NFAT, which are all implicated in B-cell activation and development (Gold, 2000), have different requirements for Raf-1 and B-Raf. Whilst Raf-1 and B-Raf are somewhat redundant for the induction of c-Fos and Egr-1, BCR-mediated NFAT activation relies mainly on B-Raf. Although the DT40 lines generated in the present work are not suitable for studying B-cell development, these lines may allow further insight into the molecular mechanisms underlying the regulation and plasticity of BCR-mediated ERK activation and may help to design suitable mouse experiments.
Materials and methods
The detailed processes for generating the raf-1 and B-raf targeting vectors shown in Figures 2 and 3, respectively, are provided as Supplementary data, available at The EMBO Journal Online, together with the establishment of conditional Raf-1, B-Raf and Raf-1/B-Raf double deficient DT40 clones and their complementation with various cDNAs.
Cell lines, cell culture and transfections
Ramos cells, DT40 and DT40MCM cells have been described previously (Buerstedde and Takeda, 1991) (T.Brummer, H.Naegele, M.Reth and Y.Misawa, in preparation). DT40 cells were cultivated in RPMI 1640 medium (10% FCS, 1% chicken serum, 100 U/ml penicillin/streptomycin, 2 mM glutamine, 0.05 mM 2-mercaptoethanol, 20 mM HEPES). For transfections, 2 × 107 DT40 cells were electroporated with plasmid DNA using a Bio-Rad Gene Pulser II set at 300 V/975 µF.
Southern blot analysis
Restriction enzyme-digested genomic DNA was separated on 0.9% agarose gels and transferred to nylon membranes (Schleicher and Schuell). Probe labelling was performed with the Megaprime labelling kit (Amersham).
4-HT treatment
DT40 cells were cultured with 200 nM 4-HT (Sigma) for 24 h. Thereafter, cells were kept in normal culture medium as indicated. No 4-HT side effects were observed (data not shown).
Antibodies
The rabbit Abs recognizing the C- (C-19) and N-terminus (H-145) of B-Raf as well as the anti-Raf-1 (C-12), anti-c-Fos (K-25) and anti-EGR-1 (C-19) Abs were purchased from Santa Cruz Biotechnology. The anti-phospho-Raf-1 S338 and anti-phosphotyrosine Ab 4G10 were purchased from Upstate Biotechnology. ERK2 and phosphorylated ERK were detected with anti-ERK2 clone 33 (Transduction Laboratories) and anti-activated MAPK 12D4 (Nanotools), respectively. The anti-BAP37 Ab and the anti-eIF-4A Ab were kindly provided by Dr Mitsuaki Kimura and Dr Peter J.Nielsen, respectively. The anti-Shc Ab was purchased from Transduction Laboratories.
Stimulation of B cells
If not stated otherwise, DT40 cells were stimulated with 5 µg of anti-IgM Ab M4 (Southern Biotechnology or kindly provided by Dr Max Cooper) as described. Ramos cells were stimulated with 5 µg of goat anti-human IgM Abs (Southern Biotechnologies). Cells were lysed in lysis buffer [50 mM Tris–HCl pH 7.4, 1% NP-40, 137.5 mM NaCl, 1% glycerol, 1 mM Na-orthovanadate, 0.5 µg/µl leupeptin, 1 mM aminoethylbenzenesulfonyl fluoride (AEBSF), 0.1 µg/ml aprotinin] and cleared lysates were boiled with 2× sample buffer (20% glycerol, 6% SDS, 6% 2-mercaptoethanol, 0.6% bromophenol blue) for 5 min. Thereafter, 7.5 × 105 cell equivalents were separated by SDS–PAGE.
Western blot analysis
SDS–PAGE and western blot analysis using the enhanced chemiluminescence (ECL) system (Amersham) were performed as described (T.Brummer, H.Naegele, M.Reth and Y.Misawa, in preparation). Signals of immunoreactive proteins on western blot membranes were quantified by a Lumiimager (Boehringer). The signal intensity (e.g. pERK) was standardized on a loading control.
Raf purification
For isolation of Raf-1 and B-Raf, 1.2 × 107 cells were stimulated as indicated and lysed in 600 µl of RIPA buffer (50 mM Tris–HCl pH 7.4, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 137.5 mM NaCl, 1% glycerol, 2 mM sodium orthovanadate, 0.5 mM EDTA pH 8.0 plus the aforementioned protease inhibitors). A 100 µl aliquot of total cellular lysates was used for western blot analysis and the remainder was incubated with 2 µg of anti-B-Raf H-145 or anti-Raf-1 C-12 Abs, respectively, at 4°C for 1 h followed by binding to protein G–Sepharose beads (4°C, overnight). After an intense wash, purified proteins were denatured as described above.
Luciferase assay
For the measurement of NFAT activity, 2 × 107 cells were transiently transfected with 10 µg of β-galactosidase (β-gal) expression vector pCMVβ (Clonetech) and 30 µg of pNFATluc (kindly provided by Dr G.Crabtree). Following electroporation, the cells were kept for an additional 18 h in culture medium and were then starved (1% FCS, 0.25% chicken serum) for 12 h. The cells were then treated with M4 as indicated, lysed and luciferase and β-gal activities were assayed as described previously (Frost et al., 1997). Luciferase activity was standardized against β-gal activity as an internal control for transfection efficiency.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Dr K.Rajewsky, Dr F.Stewart and Dr D.Stehelin for providing pFrt2-neo, pCAGGSFLPe-IRESpuro and genomic subclones of the raf-1 locus, respectively, Dr Peter J.Nielsen, Dr Hassan Jumaa, Dr Michael Hueber and Elias Hobeika for helpful discussions, and Eva Dengler for technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft through SFB 388 and the Leibniz Prize to M.R.
References
- Alessi D.R., Cohen,P., Ashworth,A., Cowley,S., Leevers,S.J. and Marshall,C.J. (1995) Assay and expression of mitogen-activated protein kinase, MAP kinase kinase, and Raf. Methods Enzymol., 255, 279–290. [DOI] [PubMed] [Google Scholar]
- Avots A., Buttmann,M., Chuvpilo,S., Escher,C., Smola,U., Bannister,A.J., Rapp,U.R., Kouzarides,T. and Serfling,E. (1999) CBP/p300 integrates Raf/Rac-signaling pathways in the transcriptional induction of NF-ATc during T cell activation. Immunity, 10, 515–524. [DOI] [PubMed] [Google Scholar]
- Barnier J.V., Papin,C., Eychene,A., Lecoq,O. and Calothy,G. (1995) The mouse B-raf gene encodes multiple protein isoforms with tissue-specific expression. J. Biol. Chem., 270, 23381–23389. [DOI] [PubMed] [Google Scholar]
- Bondeva T., Balla,A., Varnai,P. and Balla,T. (2002) Structural determinants of ras–raf interaction analyzed in live cells. Mol. Biol. Cell, 13, 2323–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerstedde J.M. and Takeda,S. (1991) Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell, 67, 179–188. [DOI] [PubMed] [Google Scholar]
- Calogeraki I., Barnier,J.V., Eychene,A., Felder,M.P., Calothy,G. and Marx,M. (1993) Genomic organization and nucleotide sequence of the coding region of the chicken c-Rmil (B-raf-1) proto-oncogene. Biochem. Biophys. Res. Commun., 193, 1324–1331. [DOI] [PubMed] [Google Scholar]
- Chong H., Lee,J. and Guan,K.L. (2001) Positive and negative regulation of Raf kinase activity and function by phosphorylation. EMBO J., 20, 3716–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. et al. (2002) Mutations of the BRAF gene in human cancer. Nature, 417, 949–954. [DOI] [PubMed] [Google Scholar]
- Dhillon A.S., Meikle,S., Yazici,Z., Eulitz,M. and Kolch,W. (2002a) Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J., 21, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon A.S., Pollock,C., Steen,H., Shaw,P.E., Mischak,H. and Kolch,W. (2002b) Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol. Cell. Biol., 22, 3237–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaz N., Light,Y. and Marais,R. (2002) Cyclic AMP blocks cell growth through Raf-1-dependent and Raf-1-independent mechanisms. Mol. Cell. Biol., 22, 3717–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eychene A., Dusanter-Fourt,I., Barnier,J.V., Papin,C., Charon,M., Gisselbrecht,S. and Calothy,G. (1995) Expression and activation of B-Raf kinase isoforms in human and murine leukemia cell lines. Oncogene, 10, 1159–1165. [PubMed] [Google Scholar]
- Fleming H.E. and Paige,C.J. (2001) Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity, 15, 521–531. [DOI] [PubMed] [Google Scholar]
- Frost J.A., Steen,H., Shapiro,P., Lewis,T., Ahn,N., Shaw,P.E. and Cobb,M.H. (1997) Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J., 16, 6426–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genot E., Cleverley,S., Henning,S. and Cantrell,D. (1996) Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO J., 15, 3923–3933. [PMC free article] [PubMed] [Google Scholar]
- Gold M.R. (2000) Intermediary signaling effectors coupling the B-cell receptor to the nucleus. Curr. Top. Microbiol. Immunol., 245, 77–134. [DOI] [PubMed] [Google Scholar]
- Hagemann C. and Rapp,U.R. (1999) Isotype-specific functions of Raf kinases. Exp. Cell Res., 253, 34–46. [DOI] [PubMed] [Google Scholar]
- Hashimoto A., Okada,H., Jiang,A., Kurosaki,M., Greenberg,S., Clark,E.A. and Kurosaki,T. (1998) Involvement of guanosine triphosphatases and phospholipase C-γ2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J. Exp. Med., 188, 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüser M. et al. (2001) MEK kinase activity is not necessary for Raf-1 function. EMBO J., 20, 1940–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani B.M., Forbush,K.A., Farrar,M.A. and Perlmutter,R.M. (1997) Control of B cell development by Ras-mediated activation of Raf. EMBO J., 16, 7019–7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiai M. et al. (1999) BLNK required for coupling Syk to PLCγ 2 and Rac1-JNK in B cells. Immunity, 10, 117–125. [DOI] [PubMed] [Google Scholar]
- Jesenberger V., Procyk,K.J., Ruth,J., Schreiber,M., Theussl,H.C., Wagner,E.F. and Baccarini,M. (2001) Protective role of Raf-1 in Salmonella-induced macrophage apoptosis. J. Exp. Med., 193, 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A., Craxton,A., Kurosaki,T. and Clark,E.A. (1998) Different protein tyrosine kinases are required for B cell antigen receptor-mediated activation of extracellular signal-regulated kinase, c-Jun NH2-terminal kinase 1 and p38 mitogen-activated protein kinase. J. Exp. Med., 188, 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S., Jaiswal,R.K., Kolch,W. and Landreth,G.E. (2001) Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J. Biol. Chem., 276, 18169–18177. [DOI] [PubMed] [Google Scholar]
- King A.J., Sun,H., Diaz,B., Barnard,D., Miao,W., Bagrodia,S. and Marshall,M.S. (1998) The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature, 396, 180–183. [DOI] [PubMed] [Google Scholar]
- Koenen M., Sippel,A.E., Trachmann,C. and Bister,K. (1988) Primary structure of the chicken c-mil protein: identification of domains shared with or absent from the retroviral v-mil protein. Oncogene, 2, 179–185. [PubMed] [Google Scholar]
- Kolch W. (2000) Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J., 351, 289–305. [PMC free article] [PubMed] [Google Scholar]
- Kubicek M., Pacher,M., Abraham,D., Podar,K., Eulitz,M. and Baccarini,M. (2002) Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J. Biol. Chem., 277, 7913–7919. [DOI] [PubMed] [Google Scholar]
- Lam K.P., Kuhn,R. and Rajewsky,K. (1997) In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell, 90, 1073–1083. [DOI] [PubMed] [Google Scholar]
- Li X. and Carter,R.H. (1998) Convergence of CD19 and B cell antigen receptor signals at MEK1 in the ERK2 activation cascade. J. Immunol., 161, 5901–5908. [PubMed] [Google Scholar]
- Light Y., Paterson,H. and Marais,R. (2002) 14-3-3 antagonizes ras-mediated raf-1 recruitment to the plasma membrane to maintain signaling fidelity. Mol. Cell. Biol., 22, 4984–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F., Lopez-Rodriguez,C. and Rao,A. (2001) Partners in transcription: NFAT and AP-1. Oncogene, 20, 2476–2489. [DOI] [PubMed] [Google Scholar]
- Marais R., Light,Y., Paterson,H.F., Mason,C.S. and Marshall,C.J. (1997) Differential regulation of Raf-1, A-Raf and B-Raf by oncogenic ras and tyrosine kinases. J. Biol. Chem., 272, 4378–4383. [DOI] [PubMed] [Google Scholar]
- Marshall C.J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Mason C.S., Springer,C.J., Cooper,R.G., Superti-Furga,G., Marshall,C.J. and Marais,R. (1999) Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J., 18, 2137–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikula M. et al. (2001) Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J., 20, 1952–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M.S. and Morrison,D.K. (2001) Raf-1 without MEK? Sci. STKE, 2001, PE30. [DOI] [PubMed] [Google Scholar]
- Nassar N., Horn,G., Herrmann,C., Scherer,A., McCormick,F. and Wittinghofer,A. (1995) The 2.2 Å crystal structure of the Ras-binding domain of the serine/threonine kinase c-Raf1 in complex with Rap1A and a GTP analogue. Nature, 375, 554–560. [DOI] [PubMed] [Google Scholar]
- Papin C., Denouel-Galy,A., Laugier,D., Calothy,G. and Eychene,A. (1998) Modulation of kinase activity and oncogenic properties by alternative splicing reveals a novel regulatory mechanism for B-Raf. J. Biol. Chem., 273, 24939–24947. [DOI] [PubMed] [Google Scholar]
- Pearson G., Bumeister,R., Henry,D.O., Cobb,M.H. and White,M.A. (2000) Uncoupling Raf1 from MEK1/2 impairs only a subset of cellular responses to Raf activation. J. Biol. Chem., 275, 37303–37306. [DOI] [PubMed] [Google Scholar]
- Pritchard C.A., Bolin,L., Slattery,R., Murray,R. and McMahon,M. (1996) Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-Raf protein kinase gene. Curr. Biol., 6, 614–617. [DOI] [PubMed] [Google Scholar]
- Purkerson J.M. and Parker,D.C. (1998) Differential coupling of membrane Ig and CD40 to the extracellularly regulated kinase signaling pathway. J. Immunol., 160, 2121–2129. [PubMed] [Google Scholar]
- Rajewsky K. (1996) Clonal selection and learning in the antibody system. Nature, 381, 751–758. [DOI] [PubMed] [Google Scholar]
- Rommel C., Radziwill,G., Moelling,K and Hafen, E. (1997) Negative regulation of Raf activity by binding of 14-3-3 to the amino terminus of Raf in vivo. Mech. Dev., 64, 95–104. [DOI] [PubMed] [Google Scholar]
- Roy S.K. et al. (2002) MEKK1 plays a critical role in activating the transcription factor C/EBP-β-dependent gene expression in response to IFN-γ. Proc. Natl Acad. Sci. USA, 99, 7945–7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakatsume M., Stancato,L.F., David,M., Silvennoinen,O., Saharinen,P., Pierce,J., Larner,A.C. and Finbloom,D.S. (1998) Interferon γ activation of Raf-1 is Jak1-dependent and p21ras-independent. J. Biol. Chem., 273, 3021–3026. [DOI] [PubMed] [Google Scholar]
- Schaft J., Ashery-Padan,R., van der Hoeven,F., Gruss,P. and Stewart,A.F. (2001) Efficient FLP recombination in mouse ES cells and oocytes. Genesis, 31, 6–10. [DOI] [PubMed] [Google Scholar]
- Shirakata Y., Ishii,K., Yagita,H., Okumura,K., Taniguchi,M. and Takemori,T. (1999) Distinct subcellular localization and substrate specificity of extracellular signal-regulated kinase in B cells upon stimulation with IgM and CD40. J. Immunol., 163, 6589–6597. [PubMed] [Google Scholar]
- Wartmann M., Hofer,P., Turowski,P., Saltiel,A.R. and Hynes,N.E. (1997) Negative modulation of membrane localization of the Raf-1 protein kinase by hyperphosphorylation. J. Biol. Chem., 272, 3915–3923. [DOI] [PubMed] [Google Scholar]
- Weber C.K., Slupsky,J.R., Kalmes,H.A. and Rapp,U.R. (2001) Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res., 61, 3595–3598. [PubMed] [Google Scholar]
- Weintraub B.C., Jun,J.E., Bishop,A.C., Shokat,K.M., Thomas,M.L. and Goodnow,C.C. (2000) Entry of B cell receptor into signaling domains is inhibited in tolerant B cells. J. Exp. Med., 191, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese S., Pei,G., Karch,C., Troppmair,J., Holtmann,B., Rapp,U.R. and Sendtner,M. (2001) Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat. Neurosci., 4, 137–142. [DOI] [PubMed] [Google Scholar]
- Wojnowski L., Zimmer,A.M., Beck,T.W., Hahn,H., Bernal,R., Rapp,U.R. and Zimmer,A. (1997) Endothelial apoptosis in Braf-deficient mice. Nat. Genet., 16, 293–297. [DOI] [PubMed] [Google Scholar]
- Wojnowski L., Stancato,L.F., Larner,A.C., Rapp,U.R. and Zimmer,A. (2000) Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech. Dev., 91, 97–104. [DOI] [PubMed] [Google Scholar]
- York R.D., Yao,H., Dillon,T., Ellig,C.L., Eckert,S.P., McCleskey,E.W. and Stork,P.J. (1998) Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature, 392, 622–626. [DOI] [PubMed] [Google Scholar]
- Yung Y. et al. (1997) Detection of ERK activation by a novel monoclonal antibody. FEBS Lett., 408, 292–296. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Riesterer,C,. Ayrall,A.M., Sablitzky,F., Littlewood,T.D. and Reth,M. (1996) Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res., 24, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S. and Moelling,K. (1999) Phosphorylation and regulation of Raf by Akt (protein kinase B). Science, 286, 1741–1744. [DOI] [PubMed] [Google Scholar]