Abstract
The identification of transcriptional targets of the tumor suppressor p53 is crucial in understanding mechanisms by which it affects cellular outcomes. Through expression array analysis, we identified cyclooxygenase 2 (Cox-2), whose expression was inducible by wild-type p53 and DNA damage. We also found that p53-induced Cox-2 expression results from p53-mediated activation of the Ras/Raf/MAPK cascade, as demonstrated by suppression of Cox-2 induction in response to p53 by dominant-negative Ras or Raf1 mutants. Furthermore, heparin-binding epidermal growth factor-like growth factor (HB- EGF), a p53 downstream target gene, induced Cox-2 expression, implying that Cox-2 is an ultimate effector in the p53→HB-EGF→Ras/Raf/MAPK→Cox-2 pathway. p53-induced apoptosis was enhanced greatly in Cox-2 knock-out cells as compared with wild-type cells, suggesting that Cox-2 has an abrogating effect on p53-induced apoptosis. Also, a selective Cox-2 inhibitor, NS-398, significantly enhanced genotoxic stress-induced apoptosis in several types of p53+/+ normal human cells, through a caspase-dependent pathway. Together, these results demonstrate that Cox-2 is induced by p53-mediated activation of the Ras/Raf/ERK cascade, counteracting p53-mediated apoptosis. This anti-apoptosis effect may be a mechanism to abate cellular stresses associated with p53 induction.
Keywords: apoptosis/cell survival/Cox-2/MAPK/p53
Introduction
The p53 gene is the most frequently inactivated tumor suppressor identified in human cancer. Depending on cell type or context, wild-type p53 limits cellular proliferation by inducing cell cycle arrest, apoptosis or senescence (Levine, 1997; Vogelstein et al., 2000). Inhibition of cell proliferation by p53 is due largely to its ability to transcriptionally activate genes that directly control the fate of the cell. p53-induced cell cycle arrest is mediated mainly by the p53 target gene p21, an inhibitor of cyclin-dependent kinases (El-Deiry et al., 1993; Bunz et al., 1998). Bax, IGF-BP3, p53AIP1, DR5, PREP, Pidd and PUMA, which are transcriptional targets of p53, are thought to be mediators of p53-induced apoptosis (Buckbinder et al., 1995; Miyashita and Reed, 1995; Wu et al., 1997; Attardi et al., 2000; Lin et al., 2000; Oda et al., 2000; Nakano and Vousden, 2001). In addition, recent reports have demonstrated the possibility of a novel type of gene targeted by p53, such as heparin-binding epidermal growth factor-like growth factor (HB-EGF), which is induced as a cellular stress response and lessens p53-mediated apoptosis through Ras/Raf/MAPK activation (Lee et al., 2000; Fang et al., 2001).
Prostaglandin endoperoxide H synthases (PGHSs) catalyze the conversion of arachidonic acid and O2 to PGH2, the committed step in prostanoid biosynthesis (Smith et al., 1996). Two isoforms of PGHS, PGHS-1 (Cox-1) and PGHS-2 (Cox-2), have been identified. Cox-1 is responsible for basal and constitutive prostaglandin synthesis, whereas Cox-2 is an inducible enzyme whose expression is increased rapidly in response to growth factors (Chen et al., 1997; Perkins and Kniss, 1997), cytokines (Feng et al., 1995; Misko et al., 1995), tumor promoters (DuBois et al., 1994; Ledwith et al., 1997), oncogene products (Xie and Herschman, 1995; Sheng et al., 1998a), bacterial endotoxins (Feng et al., 1995), hypertonic agents (Yang et al., 2000), microtubule-interfering agents (Subbaramaiah et al., 2000) and anti-cancer agents, including γ-radiation (Steinauer et al., 2000). It is well documented that the Ras/Raf/MAPK signaling pathway is necessary for transcriptional induction of Cox-2 by several kinds of stimuli and that Cox-2 is linked to cell survival (Xie and Herschman, 1995; Adderley and Fitzgerald, 1999; Subbaramaiah et al., 2000; Yang et al., 2000; Van Putten et al., 2001). Cox-2 has been reported to protect (i) cardiomyocytes from H2O2-, doxorubicin- or ischemia-induced apoptosis (Adderley and Fitzgerald, 1999; Shinmura et al., 2000; Dowd et al., 2001); (ii) neuronal cells from nerve growth factor (NGF) withdrawal apoptosis (Chang et al., 2000; McGinty et al., 2000); and (iii) renal cells from hypertonicity-induced cell death (Yang et al., 2000); these effects have been demonstrated by the use of selective Cox-2 inhibitors. Intriguingly, recent reports have suggested that Cox-2 overexpression exerts anti-proliferative effects through the induction of p53 as well as p21 and p27 (Trifan et al., 1999; Zahner et al., 2002), implying functional interactions of Cox-2 with p53. Recently, specific Cox-2 inhibitors have been generated for the treatment of arthritis (Geba et al., 2002). Non-steroidal anti-inflammatory drugs (NSAIDs), which are Cox inhibitors, reduced the formation of polyposis in familial adenomatous polyposis (FAP) patients (Phillips et al., 2002), prevented colorectal tumor growth and induced apoptosis in in vitro and in vivo models (Kawamori et al., 1998). However, observations relating to the pro-apoptosis effect of NSAIDs have led to contradictory conclusions, as well as evidence, that they act via both Cox-dependent and -independent mechanisms (Grosch et al., 2001; Hansen-Petrik et al., 2002).
Our previous studies have shown that p53 can induce sustained activation of the Ras/Raf/ERK cascade. Such activation is mediated by HB-EGF, induced both by p53 and in association with the cellular survival response (Lee et al., 2000; Fang et al., 2001). To identify downstream target genes regulated by p53, in particular those that might be also involved in downstream signaling of ERK, we performed expression array analysis using tetracycline-regulatable p53-expressing EJ tumor cells that have lost p53 function.
In the present study, we show that Cox-2 is an ultimate downstream target of p53, and that Cox-2 activation is mediated by p53 induction of HB-EGF, which activates the Ras/Raf/MAPK pathway. In addition, inhibition of Cox-2 function by NS-398 potentiated DNA damage-/p53-induced apoptosis in several types of human primary cells, including epithelial cells, fibroblasts and endothelial cells. Moreover, p53-induced apoptosis was significantly enhanced in Cox-2-null mouse fibroblasts as compared with Cox-2+/+ cells. These results point to a novel pathway by which, within the cellular stress response program mediated by DNA damage, Cox-2 can act as a survival factor by controlling the major anti-apoptosis Ras/Raf/MAPK pathway, whose activation results from p53 induction of HB-EGF.
Results
Cox-2 induction by p53
We previously showed that a human bladder tumor cell line, EJ, which has lost p53 function, undergoes permanent growth arrest/senescence following expression of exogenous wild-type p53 under the control of a tetracycline-regulated promoter (Sugrue et al., 1997). To identify p53-regulated genes that might be involved in this permanent growth arrest process, we used a DNA chip expression array to compare genes expressed in the presence or absence of p53 using EJ-p53 cells. Affymetrix GeneChips were used for hybridization. Among upregulated genes detected, the transcript for Cox-2 was increased in response to p53 induction. To quantitate the level of Cox-2 induction, we performed northern and western blot analysis using several p53 expression systems.
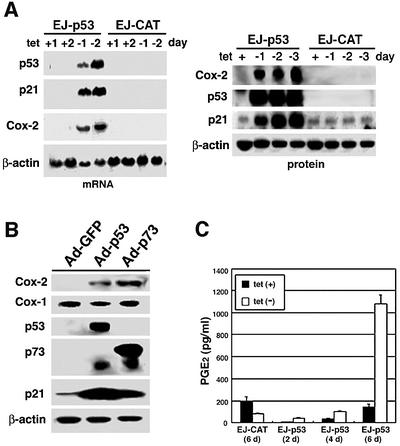
As shown in Figure 1A, as early as 24 h after tetracycline removal in EJ-p53 cells, the expression of both Cox-2 and a known p53 target gene, p21CIP1/WAF1, was induced. The Cox-2 transcript was induced >8-fold with kinetics similar to those of p21. In contrast, Cox-2 was not induced in EJ-CAT (chloramphenicol acetyltransferase) control cells after tetracycline removal. To confirm further the p53-mediated upregulation of Cox-2, we infected p53-null Saos2 cells with recombinant adenoviruses (Ad) expressing p53 or green fluorescent protein (GFP). We also evaluated whether Cox-2 was induced in response to p73, a gene related to p53 (Yang and McKeon, 2000). Western blotting showed that p53, p73α and their transcriptional target, p21, were markedly induced after adenovirus infection. Cox-2 expression was also induced in p53- or p73α-infected Saos2 cells, but not in Ad–GFP-infected cells. Unlike Cox-2, Cox-1 expression levels remained unchanged in the setting of p53 or p73α expression (Figure 1B).
Fig. 1. Induction of Cox-2 and PGE2 production by expression of p53. (A) Cox-2 mRNA (left panel) and protein (right panel) were induced by tetracycline removal in EJ-p53 cells. EJ-CAT was used as a control cell line. Total RNA and protein extracts were prepared from each cell line grown in the presence or absence of tetracycline (1 µg/ml) for 1, 2, 3 or 4 days. Northern blots were performed as described in Materials and methods. 36B4 and β-actin were used as loading controls for northern and western blot analysis, respectively. (B) Cox-2 expression was induced by infections with recombinant adenoviruses, Ad-p53 and Ad-p73α, but it was not induced by Ad–GFP control adenoviruses in Saos2 (p53-null) cells. (C) The PGE2 level was increased in response to p53 induction in EJ-p53 cells. EJ-p53 or EJ-CAT cells were grown in the presence or absence of tetracycline for 2, 4 or 6 days. Culture media were collected and analyzed for PGE2 production using a PGE2-specific radioimmunoassay.
In addition, the level of prostaglandin E2 (PGE2), one of the major enzymatic products of Cox-2, was significantly increased after tetracycline removal in EJ-p53 cells, whereas no major difference in the level of PGE2 was found in the absence or presence of tetracycline in EJ-CAT cells (Figure 1C). These results demonstrated that Cox-2 function was upregulated specifically by the tumor suppressor p53, consistent with the results of the expression array analysis.
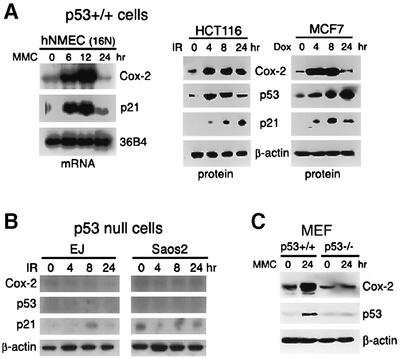
p53 becomes activated following physiological stimuli such as DNA damage, leading to transcriptional activation of its target genes. To investigate whether Cox-2 expression was induced in response to endogenous p53 activation, as well as exogenous p53, we analyzed Cox-2 induction in response to DNA damage. These included human normal mammary epithelial cells (16N hNMECs), HCT116 (a human colon cancer cell line), MCF-7 (a human breast cancer cell line) cells and p53+/+ mouse embryonic fibroblasts (MEFs). Treatment with genotoxic agents included mitomycin C (MMC), doxorubicin and γ-irradiation with a mark 135I Cs source (5 Gy). The treatments resulted in consistent and stepwise increases in Cox-2 expression levels. Similar increases were observed with p21CIP1/WAF1 or p53, whereas the levels of 36B4 mRNA and β-actin protein remained unchanged (Figure 2A and C). In contrast, EJ and Saos2 cells, which contain mutant forms or null of p53, as well as p53–/– MEFs did not show any detectable upregulation of Cox-2 expression in response to γ-irradiation or MMC treatment (Figure 2B and C). These findings indicate that Cox-2 can be induced by endogenous wild-type p53 activated under DNA-damaging stress conditions.
Fig. 2. Induction of Cox-2 in response to DNA damage. (A) Cox-2 expression in wild-type p53-containing cells treated with DNA-damaging agents. 16N hNMECs were exposed to 2.5 µg/ml MMC for 0, 6, 12 or 24 h. Total RNA was then extracted and analyzed by northern blotting. HCT116 and MCF-7 cells were exposed to 5 Gy of γ-irradiation (IR) or 0.3 µg/ml of doxorubicin (Dox), and total proteins were extracted at the indicated times after treatment and subjected to western blot analysis. (B) Cox-2 expression in p53-null cells in response to DNA damage. EJ (bladder cancer cell line) and Saos2 (osteosarcoma cell line) cells were γ-irradiated (5 Gy), and total protein lysates were isolated for western blot analysis. β-actin was used as a loading control. (C) p53+/+ or p53–/–MEFs were treated with 2.5 µg/ml MMC for 24 h. Total cell extracts were isolated for western blot analysis.
Cox-2 induction is mediated by the ERK/MAPK activation induced by p53
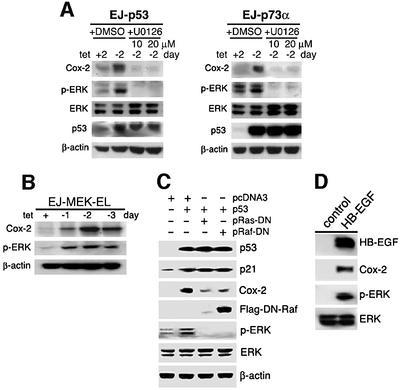
Wild-type p53 functions as a transcription factor capable of binding in a sequence-specific manner to well-defined DNA elements and inducing transcription of genes that contain these elements (Levine, 1997). Although Cox-2 induction was shown to be p53 dependent, the consensus sequence for p53 binding was not found in the Cox-2 promoter or intron regions, suggesting that Cox-2 expression was induced by a different mechanism in response to p53. We have shown previously that the Ras/Raf/MAPK signaling pathway is activated by p53 induction of HB-EGF (Lee et al., 2000; Fang et al., 2001). In addition, recent studies indicate that the activated ERK induces Cox-2 expression (Sheng et al., 1998b; Adderley and Fitzgerald, 1999; Subbaramaiah et al., 2000). Therefore, we first investigated whether Cox-2 induction was mediated by p53-induced activation of MAPK/ERK. As shown in Figure 3A, a substantial increase of Cox-2 protein was observed following p53 or p73α expression in EJ-p53 or EJ-p73α cells. This p53- or p73α-mediated Cox-2 induction was significantly inhibited by pre-incubation with U0126 (a specific MEK1/2 inhibitor) at a concentration that also inhibited ERK activation by p53 (Figure 3A). To assess the effect of MAPK activation on Cox-2 expression more directly, we generated a tetracycline-regulatable inducible system for a constitutively active MEK mutant (F53L) in EJ cells. The expression of constitutively active MEK by tetracycline removal resulted in an increase of phosphorylated ERK (p-ERK) and Cox-2 (Figure 3B). To confirm that activation of the MAPK signaling cascade led to Cox-2 induction, we used dominant-negative (DN) mutants of Ras and Raf, whose products block the functions of upstream activators of MAPK/ERKs. When p53 and either a DN mutant form of Ras (N17Ras) or Raf1 (DN-Raf-Flag), a direct downstream effector of Ras, were transiently transfected into 293T cells, Cox-2 induction in response to p53 was substantially decreased (Figure 3C). To determine the role of HB-EGF in p53→Ras/Raf/MAPK-mediated Cox-2 induction, we tested whether HB-EGF expression had any effect on Cox-2 induction. As shown in Figure 3D, when full-length HB-EGF cDNA under the cytomegalovirus (CMV) promoter was transfected into 293T cells known to have intact EGF receptor signaling (Thomas and Bradshaw, 1997), increasing levels of HB-EGF expression resulted in increased levels of Cox-2 and the phospho-specific MAPK, whereas the total MAPK level remained constant. All these data support the conclusion that p53 induction of Cox-2 is mediated by p53-mediated activation of the Ras/Raf/MAPK cascade via HB-EGF.
Fig. 3. Induction of Cox-2 expression by HB-EGF-mediated ERK/MAPK activation induced by p53. (A) ERK was activated and Cox-2 expression was increased by induction of p53 or p73 after tetracycline removal (within 2 days); these effects were abolished by the MEK inhibitor U0126 (20 µM). As a control, cells were treated with the same amounts of dimethylsulfoxide (DMSO), the solvent of U0126. Western blots were carried out with the specific antibodies indicated. (B) Cox-2 was induced in response to activated MEK1 expression. A tetracycline-regulated constitutively active MEK1 mutant (F53L)-inducible system was generated in EJ cells. EJ-MEK1-EL cells were grown with or without tetracycline for the indicated times. Cell lysates were then isolated for western blot analysis. Note that constitutively active MEK expression induced by removal of tetracycline resulted in increased phosphorylated ERK (p-ERK) and Cox-2. (C) p53-induced Cox-2 expression was suppressed by DN-Ras and DN-Raf. Wild-type p53 was transfected into 293T cells with the vector (pcDNA3), DN-Raf or DN-Ras as shown on the top of each lane. Western blot analysis was performed with antibodies against p53, p21, Cox-2, flag tag (DN-Raf), p-ERK, total ERK or β-actin. (D) HB-EGF induces Cox2 expression. 293T cells were transfected with vector (pcDNA3) or HB-EGF expression plasmid (pcDNA3-HB-EGF). Cell lysates were harvested at 48 h post-transfection. Immunoblots were performed using antibodies against HB-EGF, Cox-2, phospho-ERK and total ERK, respectively.
p53-induced apoptosis is enhanced in Cox-2-null cells
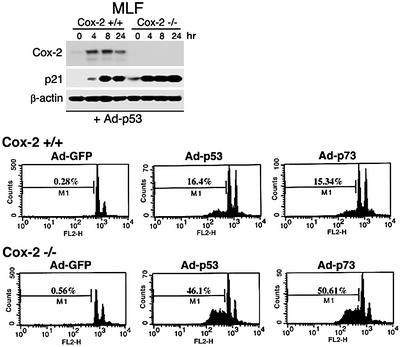
To understand the effects of Cox-2, whose induction is p53 mediated, we examined p53-induced responses such as apoptosis in cells in which Cox-2 was deleted. We compared the apoptosis response to p53 or p73 overexpression in Cox-2+/+ and Cox-2–/– mouse lung fibroblasts (MLFs) derived from adult mouse lung tissues from wild-type and Cox-2 knock-out mice, respectively. Infection with adenovirus-expressing p53 resulted in dramatic induction of both Cox-2 and p21, whereas no induction of Cox-2 was observed in Cox-2 null MLFs (Figure 4). Expression of p53 or p73α resulting from infection with recombinant adenoviruses expressing either gene in wild-type MLFs resulted in an increased apop tosis response (p53, ∼16%; p73, ∼15%) as compared with control cells (0.28%) (Figure 4). However, expression of p53 or p73α in Cox-2–/– MLFs enhanced the rate of apoptosis from 16 to 46% for p53, and from 15 to 50% for p73α, respectively, as compared with effects in wild-type MLFs. These results indicate that p53-induced Cox-2 counteracts p53-mediated apoptosis in p53+/+ cells and that the absence of Cox-2 can enhance p53-mediated cell death.
Fig. 4. p53-mediated apoptosis was enhanced in Cox-2-null cells. Cox-2+/+ or Cox-2–/– MLFs were infected with Ad–GFP, Ad-p53 or Ad-p73α for 24 h. Upper panel: a western blot analysis using lysates prepared from cells infected with Ad-p53 adenoviruses. Lower panels: the patterns of apoptosis analysis by FACScan. The M1 cell population represents apoptotic cells from each sample.
Inhibition of Cox-2 function enhances genotoxic stress-mediated apoptosis
To characterize further the Cox-2-mediated protective effect on DNA damage-/p53-induced apoptosis, we determined the effects of Cox-2 inhibition by a selective Cox-2 inhibitor, NS-398, on DNA damage-induced apoptosis. For this purpose, we utilized three types of normal human cells with wild-type p53, including 16N hNMECs, human normal lung fibroblasts (IMR90) and human umbilical vein endothelial cells (HUVECs).
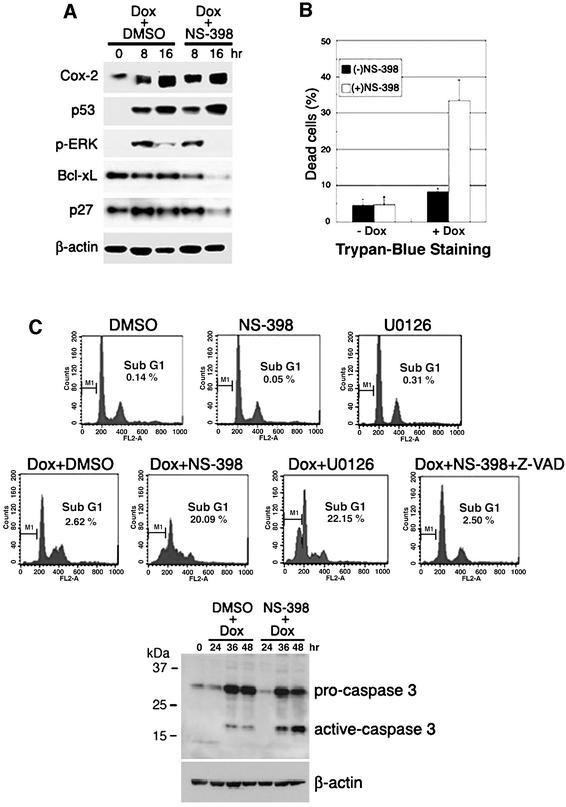
16N hNMECs were exposed to a genotoxic drug, doxorubicin (0.5 µg/ml), with or without NS-398. Doxorubicin treatment increased Cox-2 expression as early as 8 h, similar to results for p53 and p21 expression as determined by western blot analysis (Figure 5A). After 48 h, cells were collected for Trypan Blue staining to measure the population of dead cells and for FACScan to determine sub-G1 DNA content. Exposure of NMECs to both doxorubicin and NS-398 resulted in a marked increase in cell death, whereas cells exposed to doxorubicin alone showed a low apoptosis frequency. As shown in Figure 5B, co-treatment with doxorubicin and NS-398 increased the percentage of dead cells from ∼8.0 to ∼33%, as compared with treatment with doxorubicin alone. Fluorescence-activated cell sorting (FACS) analysis also showed that co-treatment with doxorubicin and NS-398 increased the cell death/apoptosis rate from ∼2.6 to 20%, but that NS-398 itself had no significant effect on the apoptosis rate (Figure 5C, upper panel). These results demonstrate that Cox-2 protects cells from doxorubicin-induced apoptosis and that impairment of Cox-2 function leads to a pronounced increase in apoptosis.
Fig. 5. Effects of Cox-2 inactivation on apoptosis after DNA damage in 16N hNMECs. 16N cells were exposed to either doxorubicin (0.5 µg/ml) and DMSO (a solvent for NS-398) or doxorubicin and NS-398 (20 µM), the Cox-2 selective inhibitor, for 48 h. Cells were (A) harvested at the indicated times for western blot analysis using antibodies against Cox-2, p53, phospho-ERK, Bcl-xL and p27, or (B) harvested after 48 h for Trypan Blue staining. The percentages of dead cells were compared. Error bars indicate means ± SD of three independent experiments with duplicate plates. (C) FACS analysis of drug-treated cells. Z-VAD-fmk (50 µM), the general caspase inhibitor, abolished the effect of NS-398 (upper panels). The activation of caspase 3 induced by doxorubicin was increased further by NS-398 (lower panel). Cells were pre-treated with DMSO, NS-398 or Z-VAD-fmk for 1 h and then co-treated with doxorubicin for 48 h.
To address whether the ERK signaling pathway that can be activated by p53 plays an important role in protecting cells from doxorubicin-induced apoptosis, we monitored the apoptosis rate by FACS analysis after co-treatment with U0126, the MEK1/2 inhibitor, and doxorubicin. The level of p-ERK protein was significantly increased as early as 8 h by doxorubicin treatment, and NS-398 reduced the potency and duration of ERK phosphorylation (Figure 5A). It is of note that U0126 increased the apoptosis rate up to 22% when it was combined with doxorubicin, whereas U0126 treatment itself had no toxic effect on cells (Figure 5C, upper panel). These results support the concept that the ERK signaling pathway activated by p53 induces Cox-2 expression, which protects cells from apoptosis caused by genotoxic stresses.
We also investigated whether the death-enhancing effects of Cox-2 inhibition were mediated through a caspase-dependent pathway. Experiments were carried out in the presence of the general caspase inhibitor, Z-VAD-fmk. As shown in Figure 5C, addition of Z-VAD blocked enhanced cell death induced by inhibition of Cox-2. Moreover, the death-enhancing effects of Cox-2 inhibition in 16N cells following genotoxic stress resulted in a further increase in cleavage of procaspase 3 to the active caspase 3 as compared with control cells (Figure 5C, lower panel). These data confirm that doxorubicin-induced cell death follows a caspase-dependent apoptosis pathway and that NS-398 increases the extent of apoptosis in a caspase 3-dependent manner.
To elucidate further the mechanism by which Cox-2 protects cells from genotoxic stress-induced apoptosis, we next examined expression of several apoptosis- and cell cycle-regulated genes. When cells were co-treated NS-398 and doxorubicin, we observed that expression levels of anti-apoptotic proteins, Bcl-xL and the cell cycle inhibitor p27, were both decreased, as compared with cells treated with doxorubicin alone (Figure 5A), suggesting that Cox-2 may enhance cell survival by activating anti-apoptosis gene(s).
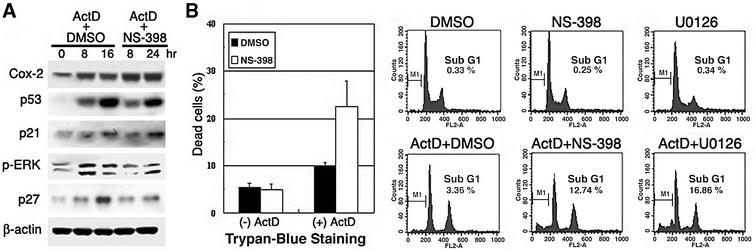
When IMR90 normal diploid lung fibroblasts were exposed to a genotoxic drug, actinomycin D (10 ng/ml), increased Cox-2 expression was observed as early as 8 h, accompanied by increased expression of p53, p21 and phosphorylated ERK in western blot analysis (Figure 6A). When actinomycin D was combined with NS-398 in Trypan Blue staining, the resulting population of dead cells increased from ∼10 to ∼23% (Figure 6B, left panel). FACS analysis also showed that the apoptosis rate increased from ∼3.4 to 12.7% with co-treatment with actinomycin D and NS-398, but NS-398 alone had no significant effect on the apoptosis rate (Figure 6B, right panel). Moreover, actinomycin D treatment increased ERK phosphorylation as early as 8 h (Figure 6A), and addition of 20 µM U0126 increased the cell death rate up to 16.9% when it was given as co-treatment with actinomycin D, even though U0126 treatment alone showed no toxic effect on cells (Figure 6B, right panel). Unlike the situation in epithelial cells, a reduced level of the anti-apoptosis protein Bcl-xL was not observed in normal diploid fibroblast cells (IMR90) when they were co-treated with NS-398 and actinomycin D (data not shown). However, the level of p27 expression was also decreased in response to co-treatment with doxorubicin and NS-398 in IMR90 cells as observed in epithelial cells (Figure 6A).
Fig. 6. Enhanced apoptosis by Cox-2 inhibition in response to DNA-damaging agents in human normal lung fibroblasts (IMR90). (A) IMR90 cells were exposed to either actinomycin D (10 ng/ml) and DMSO (solvent for NS-398) or actinomycin D and NS-398 (20 µM) for 48 h. Cells were harvested at the indicated times for western blot analysis using antibodies against Cox-2, p53, p21, phospho-ERK, p27 or β-actin. (B) Drug-treated cells were harvested after 48 h for the Trypan Blue exclusion experiment. The percentages of dead cells were compared. Error bars indicate means ± SD of three independent experiments with duplicate plates (left panel). Effects of Cox-2 inactivation on apoptosis after actinomycin D (ActD). Cells treated with each indicated drug were collected for FACS analysis, and sub-G1 DNA content was analyzed by FACScan. DNA content, as measured by propidium iodide fluorescence, is depicted on the x-axis. The sub-G1 population (M1) is shown as apoptotic cells (right panel). Cells were pre-treated with DMSO, NS-398 or U0126 for 1 h and then co-treated with actinomycin D for 48 h.
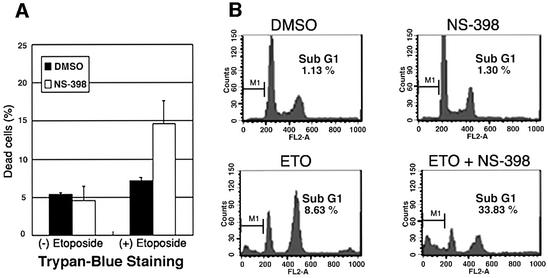
We further investigated whether Cox-2 protected cells from DNA damage-induced apoptosis in human vascular endothelial cells (HUVECs). We treated HUVECs with a DNA-damaging agent, etoposide (2 µM), in the presence or absence of NS-398. Co-treatment with etoposide and NS-398 increased the cell death rate from ∼7.2 to ∼14.7% as determined by Trypan Blue exclusion staining, as compared with a treatment with etoposide alone (Figure 7A). Co-treatment with doxorubicin and NS-398 increased the apoptosis rate from ∼8.6 to ∼33% as determined by FACS analysis (Figure 7B), also demonstrating that Cox-2 protects HUVECs from etoposide-induced apoptosis.
Fig. 7. Etoposide-induced apoptosis by Cox-2 inactivation in HUVECs. (A) HUVECs were exposed to either etoposide (2 µM) and DMSO, or etoposide and NS-398 (20 µM) for 48 h. Cells were then harvested for Trypan Blue staining. The percentages of dead cells were compared. Error bars indicate means ± SD of three independent experiments with duplicate plates. (B) FACS analysis. Cells were pre-treated with DMSO or NS-398 for 1 h and then co-treated with etoposide for 48 h.
These results demonstrate that Cox-2 and the ERK signaling pathway are critically important for the prevention of DNA damage-induced apoptosis in normal human cells, which might be mediated by potentiation of HB-EGF-mediated MAPK/ERK activation and increased expression of anti-apoptosis or cell cycle-inhibitory genes.
Discussion
Accumulating evidence indicates that Cox-2 activity and prostaglandin synthesis play an important role in the promotion of various proliferative diseases including cancer. It is well documented that Cox-2 can be induced by growth factors and cytokines, inflammatory stimuli and tumor promoters (DuBois et al., 1994; Misko et al., 1995; Smith et al., 1996; Chen et al., 1997; Perkins and Kniss, 1997). Recent studies have provided evidence that oxidative stresses as well as DNA-damaging agents, including ionizing radiation, can induce Cox-2 expression (Adderley and Fitzgerald, 1999; Shinmura et al., 2000; Steinauer et al., 2000). However, the mechanism of induction of Cox-2 in response to oxidative stress or DNA damage remains unknown. We report here that the tumor suppressor p53 or DNA-damaging agents that induce p53 can induce Cox-2 expression. Further, we demonstrate that Cox-2 was induced by activation of the Ras/MEK/MAPK cascade through p53 induction of HB-EGF.
p53 has been thought to inhibit cellular proliferation through two mechanisms, apoptosis or cell cycle arrest/senescence, in response to cellular stresses, including DNA damage. However, the molecular processes that determine the fate of cells—cell death versus arrest—in which p53 mediation plays a role are not known. A number of target genes induced by p53 have been described. They include p21, mdm2, Bax, GADD45, cyclin G, DR5, IGF-BP-3, Fas, PERP, Noxa, Pidd, PTEN and PUMA. The roles of these diverse genes in mediating p53 functions are beginning to be understood. For example, p21 is a cyclin-dependent kinase inhibitor known to be essential for mediating the G1 arrest checkpoint function of p53. Mdm2, which binds p53 and consequently causes it to be degraded by the proteasome, is involved in negative feedback regulation of p53 (Levine, 1997; Vogelstein et al., 2000; Vousden, 2000). Other p53 target genes, including Bax, DR5, Noxa, Fas, Pigs, PERP, Pidd and PUMA, have been proposed to play roles in apoptosis (Miyashita and Reed, 1995; Polyak et al., 1997; Wu et al., 1997; Lin et al., 2000; Oda et al., 2000; Nakano and Vousden, 2001; Yu et al., 2001). In the present study, we showed that Cox-2 was induced by p53 or by DNA damage in a p53-dependent manner. We further demonstrated that activation of the Ras/MEK/MAP kinase pathway in response to p53 is required for Cox-2 induction. Finally, we showed that inhibition of Cox-2 function by use of a Cox-2 inhibitor or Cox-2-null cells markedly increased DNA damage-induced apoptosis. These results imply that p53-mediated Cox-2 induction promotes cell survival in response to p53 or DNA damage and suggest that induction of Cox-2 favors the cellular outcome of survival over apoptosis in response to p53. Recent reports indicate that enhanced Cox-2 expression may play a significant role in resistance to chemotherapy-induced apoptosis (Morham et al., 1995; Hida et al., 2000; Hsu et al., 2000; Lin et al., 2001). Moreover, all three members of the MAPK family (ERKs, JNKs and p38 MAPK) and Akt belong to signaling pathways leading to Cox-2 induction by various stimuli (Sheng et al., 1998a; Subbaramaiah et al., 2000; Van Putten et al., 2001).
Previous studies have established a biochemical link between p53 signaling and activation of the Ras/Raf/MAPK cascade. MAPK activation required the p53 transcription function and occurred in a p53-dependent manner (Lee et al., 2000). HB-EGF, which activates the EGF receptor and other members of the erbB family, has recently been shown to be induced in a p53-dependent manner in response to DNA damage and to account, at least in part, for activation of MAPK as well as Akt in response to p53 (Fang et al., 2001). Furthermore, cell lines and strains used in this study, which respond to the p53-mediated Cox-2 induction, are known to have intact EGF receptor signaling (see Materials and methods), and MAPK activation in response to HB-EGF could be inhibited by EGF receptor antagonists, implying that HB-EGF activates the Ras/Raf/MAPK cascade through the EGF receptor. Our finding that inhibition of MAPK and Cox-2 enhanced p53-/DNA damage-induced apoptosis is consistent with findings of previous studies indicating that inhibition of MAPK or HB-EGF function in wild-type p53 cells leads to increased cell death caused by oxidative or genotoxic stresses (Fang et al., 2001). These findings imply that not only HB-EGF but also Cox-2 is a downstream mediator of this prosurvival component of the induced p53-dependent program, a program that favors cell survival rather than apoptosis in response to genotoxic stresses.
Several groups have reported that Cox-2 and its major final product, PGE2, protect cells against apoptosis. Rat intestinal epithelial (RIE) cells overexpressing Cox-2 were resistant to butyrate-induced apoptosis and had elevated Bcl2 protein expression, which were reversed by a Cox inhibitor, sulindac sulfide (Tsujii and DuBois, 1995). PGE2 inhibited apoptosis caused by a Cox-2 inhibitor, SC-58125, and induced Bcl2 expression in human colon cancer cells (HCA-7) (Sheng et al., 1998b). Human lung adenocarcinoma CL1.0 cells with high levels of Cox-2 showed a remarkable resistance to apoptosis induced by UVB irradiation and vinblastine by inducing a Mcl-1-dependent survival mechanism (Lin et al., 2001). These studies are consistent with our data that Cox-2 inhibits apoptosis, which might be through regulation of apoptosis-related proteins such as Bcl-xL or cell cycle inhibitors such as p27 (Figures 5A and 6A), even though the mechanism for the anti-apoptosis effect of Cox-2 requires further study.
There is one report that Cox-2 expression was repres sed by ectopic p53 expression in an MEF cell line (Subbaramaiah et al., 1999). However, our finding of p53-dependent induction of Cox-2 in response to oxidative or genotoxic stress strongly argues against an inhibitory effect of p53 on Cox-2 transcription. Our results demonstrated a physiological response of the Cox-2 gene to genotoxic stress in a p53-dependent manner associated with other downstream effectors.
In summary, our findings indicate that Cox-2 is induced by p53, mediated by activation of the Ras/Raf/ERK cascade, which counteracts p53-mediated apoptosis. This effect may abate cellular stresses, which both cause and result from p53 induction. As such, these findings establish a novel role for Cox-2 as an important effector of cell survival in response to genotoxic stresses that induce p53. Furthermore, our results suggest that anti-tumorigenic effects of NSAIDs may be due in part to potentiation of p53-induced apoptosis by antagonizing cell survival signaling caused by Cox-2. In fact, a possible mechanism for enhanced apoptosis by Cox-2 inhibitors was discussed nicely in a study demonstrating that apoptosis due to NSAIDS is not likely to be related to a reduction in prostaglandins but rather to elevation of the prostaglandin precursor arachidonic acid (AA), which stimulates ceramide production, a strong mediator of apoptosis (Chan et al., 1998).
The cooperative effects of Cox-2 inhibitors with DNA-damaging agents in inducing cell death in wild-type p53 cells demonstrated in our present studies provides a mechanistic basis for use of Cox-2 inhibitors in combination with agents such as radiation in the localized treat ment of wild-type p53-containing tumors. Moreover, the increased toxicity of Cox-2 inhibitors in combination with DNA-damaging agents to wild-type p53-containing vascular endothelial cells shown here suggests that Cox-2 inhibitors might be advantageous in combination with chemotherapeutic agents in efforts to target tumor angiogenesis.
Materials and methods
Cell culture and transient transfection
EJ-p53, EJ-CAT, EJ-p73α, EJ-p73β and EJ-MEK cells were cultured in the presence or absence of tetracycline (1 µg/ml) in Dulbecco’s modified Eagle’s medium (DMEM) containing fetal bovine serum (FBS; 10%), penicillin (100 U/ml), streptomycin (100 U/ml), hygromycin (100 µg/ml) and geneticin (400 µg/ml). MEFs, HCT116, EJ, Saos-2, 293T and IMR90 cells were maintained in DMEM plus 10% FBS. hNMECs were established from reduction mammoplasties obtained through the Cooperative Human Tissue Network (CHTN) and designated 16N, as described previously (Band et al., 1990). These cells were grown in DFCI-1 medium (D complete) as described previously (Band et al., 1990) and were used at early to mid passage, i.e. 5–10 population doublings. HUVECs were obtained from Clonetics Co. and grown in EGM-2 medium as recommended by the vendor. 293T cells (90% confluent) were transfected with the indicated plasmids using Lipofectamine 2000 (Gibco-BRL). In transient co-transfection experiments, the total amounts of plasmid DNAs were kept constant with empty vector. The Cox-2 inhibitor NS-398 was obtained from Cayman Chemical Co. and the MEK inhibitor U0126 was purchased from Cell Signaling Technologies. Cell lines and strains used in this study including MCF7, 16N, IMR90, EJ, HCT116, 293T, MEF and HUVEC are known to express EGF receptor and respond to EGF-like growth factors (Gunn et al., 1983; Band et al., 1990; Bates et al., 1990; Thomas and Bradshaw, 1997; Howell et al., 1998; Ji et al., 1998; Modjtahedi et al., 1998; Russell et al., 1999; Fang et al., 2001; McClelland et al., 2001; Sawhney et al., 2002).
Expression array screening
Affymetrix GeneChips were used for hybridization. Two sets of human expression arrays (human genome U95A, Affymetrix Inc.) were hybridized with fluorescently labeled cRNA probes derived from total RNAs extracted from EJ-p53 cells grown in the presence or absence of tetracycline for 2 days.
Western blot analysis
Cells were lysed in RIPA buffer (150 mM NaCl, 100 mM Tris–HCl, 1% Tween-20, 1% sodium deoxycholate and 0.1% SDS) with 0.5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 µg/ml leupeptin, 10 µg/ml aprotinin and 1 µg/ml pepstatin. Proteins were resolved in SDS–PAGE and transferred to nitrocellulose membranes, which were probed with the appropriate antibodies. The immunoreactive protein complexes were detected by enhanced chemiluminescence (Amersham Pharmacia). Anti-Cox-1 and anti-Cox-2 antibodies were obtained from Santa Cruz Biotechnology and Transduction Laboratories, respectively. p53 (AB6) and p21 (AB6) antibodies were obtained from Calbiochem-Novabiochem. Antibodies against phospho-p44/42 MAPK (Thr202/Tyr204), phospho-Akt (Ser473), p44/42 MAPK, Akt and activated caspase 3 were purchased from Cell Signaling Technologies.
PGE2 production
Cox enzyme activity was determined by radioimmunoassay for PGE2 produced in the indicated cells (EJ-p53) with or without tetracycline as described previously (Kirtikara et al., 1998).
Northern blot analysis
Total RNA was extracted using an RNA isolation kit (Quiagen). Total RNA, 20 µg per lane, was electrophoresed in a 1% agarose–formaldehyde gel and transferred to nylon membranes (Bio-Rad). Hybridization was performed using 32P-labeled probes, and washed membranes were sub jected to autoradiography.
FACS analysis
Cells were pelleted at 1000 r.p.m. and washed once with 10 ml of ice-cold phosphate-buffered saline (PBS). The resultant pellets were resuspended in 1 ml of cold PBS. Ethanol (80%), pre-chilled at –20°C, was added dropwise with periodical vortexing to mix the cells. The resultant mixture was kept on ice for 60 min. Cells were permeabilized in a reagent consisting of 0.5% Triton X-100, 230 µg/ml RNase A and propidium iodide to 50 µg/ml in PBS. Samples were kept at 37°C for 30 min followed by flow cytometry analysis (Becton Dickinson FACScan), and apoptotic populations were analyzed using the CellQuest program.
γ-irradiation
HCT116, EJ and Saos2 cells were seeded at a cell density of 2 × 105 in p100 culture dishes with DMEM and 10% FBS. Cells were irradiated with various doses of γ-irradiation and lysed at the indicated times.
Acknowledgments
Acknowledgements
We thank J.Kwak and M.Meyer for proofreading the manuscript, B.Vogelstein for the adenovirus expression system and Ad-p53 virus, and Toru Ouchi for Ad–GFP. This work was supported in part by National Institutes of Health grants, CA85214, CA80058 and CA82211.
References
- Adderley S.R. and Fitzgerald,D.J. (1999) Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J. Biol. Chem., 274, 5038–5046. [DOI] [PubMed] [Google Scholar]
- Attardi L.D., Reczek,E.E., Cosmas,C., Demicco,E.G., McCurrach,M.E., Lowe,S.W. and Jacks,T. (2000) PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev., 14, 704–718. [PMC free article] [PubMed] [Google Scholar]
- Band V., Zajchowski,D., Swisshelm,K., Trask,D., Kulesa,V., Cohen,C., Connolly,J. and Sager,R. (1990) Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res., 50, 7351–7357. [PubMed] [Google Scholar]
- Bates S.E., Valverius,E.M., Ennis,B.W., Bronzert,D.A., Sheridan,J.P., Stampfer,M.R., Mendelsohn,J., Lippman,M.E. and Dickson,R.B. (1990) Expression of the transforming growth factor-α/epidermal growth factor receptor pathway in normal human breast epithelial cells. Endocrinology, 126, 596–607. [DOI] [PubMed] [Google Scholar]
- Buckbinder L., Talbott,R., Velasco-Miguel,S., Takenaka,I., Faha,B., Seizinger,B.R. and Kley,N. (1995) Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature, 377, 646–649. [DOI] [PubMed] [Google Scholar]
- Bunz F., Dutriaux,A., Lengauer,C., Waldman,T., Zhou,S., Brown,J.P., Sedivy,J.M., Kinzler,K.W. and Vogelstein,B. (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science, 282, 1497–1501. [DOI] [PubMed] [Google Scholar]
- Chan T.A., Morin,P.J., Vogelstein,B. and Kinzler K.W. (1998) Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc. Natl Acad. Sci. USA, 95, 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.W., Jakobi,R., McGinty,A., Foschi,M., Dunn,M.J. and Sorokin,A. (2000) Cyclooxygenase 2 promotes cell survival by stimulation of dynein light chain expression and inhibition of neuronal nitric oxide synthase activity. Mol. Cell. Biol., 20, 8571–8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.R., Miyaura,C., Higashi,S., Murakami,M., Kudo,I., Saito,S., Hiraide,T., Shibasaki,Y. and Suda,T. (1997) Activation of cytosolic phospholipase A2 by platelet-derived growth factor is essential for cyclooxygenase-2-dependent prostaglandin E2 synthesis in mouse osteoblasts cultured with interleukin-1. J. Biol. Chem., 272, 5952–5958. [DOI] [PubMed] [Google Scholar]
- Dowd N.P., Scully,M., Adderley,S.R., Cunningham,A.J. and Fitzgerald,D.J. (2001) Inhibition of cyclooxygenase-2 aggravates doxorubicin-mediated cardiac injury in vivo. J. Clin. Invest., 108, 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois R.N., Awad,J., Morrow,J., Roberts,L.J.,2nd and Bishop,P.R. (1994) Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-α and phorbol ester. J. Clin. Invest., 93, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deiry W.S. et al. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell, 75, 817–825. [DOI] [PubMed] [Google Scholar]
- Fang L., Li,G., Liu,G., Lee,S.W. and Aaronson,S.A. (2001) p53 induction of heparin-binding EGF-like growth factor counteracts p53 growth suppression through activation of MAPK and PI3K/Akt signaling cascades. EMBO J., 20, 1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Xia,Y., Garcia,G.E., Hwang,D. and Wilson,C.B. (1995) Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-α and lipopolysaccharide. J. Clin. Invest., 95, 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geba G.P., Weaver,A.L., Polis,A.B., Dixon,M.E. and Schnitzer,T.J. (2002) Efficacy of rofecoxib, celecoxib and acetaminophen in osteoarthritis of the knee: a randomized trial. JAMA, 287, 64–71. [DOI] [PubMed] [Google Scholar]
- Grosch S., Tegeder,I., Niederberger,E., Brautigam,L. and Geisslinger,G. (2001) COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J., 15, 2742–2744. [DOI] [PubMed] [Google Scholar]
- Gunn J.M., Bodner,J.B., Knowles,S.E. and Ballard,F.J. (1983) Inhibition of protein breakdown by epidermal growth factor in IMR90 human fibroblasts and other mammalian cell lines. Biochem. J., 210, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Petrik M.B., McEntee,M.F., Jull,B., Shi,H., Zemel,M.B. and Whelan,J. (2002) Prostaglandin E(2) protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in Apc(Min/+) mice. Cancer Res., 62, 403–408. [PubMed] [Google Scholar]
- Hida T., Kozaki,K., Muramatsu,H., Masuda,A., Shimizu,S., Mitsudomi,T., Sugiura,T., Ogawa,M. and Takahashi,T. (2000) Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin. Cancer Res., 6, 2006–2011. [PubMed] [Google Scholar]
- Howell G. et al. (1998) Regulation of transforming growth factor α expression in a growth factor-independent cell line. Mol. Cell. Biol., 18, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A.L., Ching,T.T., Wang,D.S., Song,X., Rangnekar,V.M. and Chen,C.S. (2000) The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol. Chem., 275, 11397–11403. [DOI] [PubMed] [Google Scholar]
- Ji Q.-s., Ermini,S., Baulida,J., Sun,F.-l. and Carpenter,G. (1998) Epidermal growth factor signaling and mitogenesis in Plcg1 null mouse embryonic fibroblasts. Mol. Biol. Cell, 9, 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori T., Rao,C.V., Seibert,K. and Reddy,B.S. (1998) Chemo preventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res., 58, 409–412. [PubMed] [Google Scholar]
- Kirtikara K., Morham,S.G., Raghow,R., Laulederkind,S.J., Kanekura,T., Goorha,S. and Ballou,L.R. (1998) Compensatory prostaglandin E2 biosynthesis in cyclooxygenase 1 or 2 null cells. J. Exp. Med., 187, 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledwith B.J., Pauley,C.J., Wagner,L.K., Rokos,C.L., Alberts,D.W. and Manam,S. (1997) Induction of cyclooxygenase-2 expression by peroxisome proliferators and non-tetradecanoylphorbol 12,13-myristate-type tumor promoters in immortalized mouse liver cells. J. Biol. Chem., 272, 3707–3714. [DOI] [PubMed] [Google Scholar]
- Lee S.W., Fang,L., Igarashi,M., Ouchi,T., Lu,K.P. and Aaronson,S.A. (2000) Sustained activation of Ras/Raf/mitogen-activated protein kinase cascade by the tumor suppressor p53. Proc. Natl Acad. Sci. USA, 97, 8302–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A.J. (1997) p53, the cellular gatekeeper for growth and division. Cell, 88, 323–331. [DOI] [PubMed] [Google Scholar]
- Lin M.T., Lee,R.C., Yang,P.C., Ho,F.M. and Kuo,M.L. (2001) Cyclooxygenase-2 inducing a Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1.0 cells: involvement of PI 3-K/Akt pathway. J. Biol. Chem., 276, 48997–49002. [DOI] [PubMed] [Google Scholar]
- Lin Y., Ma,W. and Benchimol,S. (2000) Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nat. Genet., 26, 122–127. [DOI] [PubMed] [Google Scholar]
- McClelland R.A., Barrow,D., Madden,T.A., Dutkowski,C.M., Pamment,J., Knowlden,J.M., Gee,J.M. and Nicholson,R.I. (2001) Enhanced epidermal growth factor receptor signaling in MCF7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ICI 182,780 (Faslodex). Endocrinology, 142, 2776–2788. [DOI] [PubMed] [Google Scholar]
- McGinty A., Chang,Y.W., Sorokin,A., Bokemeyer,D. and Dunn,M.J. (2000) Cyclooxygenase-2 expression inhibits trophic withdrawal apoptosis in nerve growth factor-differentiated PC12 cells. J. Biol. Chem., 275, 12095–12101. [DOI] [PubMed] [Google Scholar]
- Misko T.P., Trotter,J.L. and Cross,A.H. (1995) Mediation of inflammation by encephalitogenic cells: interferon γ induction of nitric oxide synthase and cyclooxygenase 2. J. Neuroimmunol., 61, 195–204. [DOI] [PubMed] [Google Scholar]
- Miyashita T. and Reed,J.C. (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell, 80, 293–299. [DOI] [PubMed] [Google Scholar]
- Modjtahedi H., Komurasaki,T., Toyoda,H. and Dean,C. (1998) Anti-EGFR monoclonal antibodies which act as EGF, TGFα, HB-EGF and BTC antagonists block the binding of epiregulin to EGFR-expressing tumours. Int. J. Cancer, 75, 310–316. [DOI] [PubMed] [Google Scholar]
- Morham S.G. et al. (1995) Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell, 83, 473–482. [DOI] [PubMed] [Google Scholar]
- Nakano K. and Vousden,K.H. (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell, 7, 683–694. [DOI] [PubMed] [Google Scholar]
- Oda E., Ohki,R., Murasawa,H., Nemoto,J., Shibue,T., Yamashita,T., Tokino,T., Taniguchi,T. and Tanaka,N. (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science, 288, 1053–1058. [DOI] [PubMed] [Google Scholar]
- Perkins D.J. and Kniss,D.A. (1997) Rapid and transient induction of cyclo-oxygenase 2 by epidermal growth factor in human amnion-derived WISH cells. Biochem. J., 321, 677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R.K. et al. (2002) A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut, 50, 857–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Xia,Y., Zweier,J.L., Kinzler,K.W. and Vogelstein,B. (1997) A model for p53-induced apoptosis. Nature, 389, 300–305. [DOI] [PubMed] [Google Scholar]
- Russell K.S., Stern,D.F., Polverini,P.J. and Bender,J.R. (1999) Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am. J. Physiol., 277, H2205–H2211. [DOI] [PubMed] [Google Scholar]
- Sawhney R.S., Zhou,G.K., Humphrey,L.E., Ghosh,P., Kreisberg,J.I. and Brattain,M.G. (2002) Differences in sensitivity of biological functions mediated by epidermal growth factor receptor activation with respect to endogenous and exogenous ligands. J. Biol. Chem., 277, 75–86. [DOI] [PubMed] [Google Scholar]
- Sheng H., Williams,C.S., Shao,J., Liang,P., DuBois,R.N. and Beauchamp,R.D. (1998a) Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J. Biol. Chem., 273, 22120–22127. [DOI] [PubMed] [Google Scholar]
- Sheng H., Shao,J., Morrow,J.D., Beauchamp,R.D. and DuBois,R.N. (1998b) Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res., 58, 362–366. [PubMed] [Google Scholar]
- Shinmura K., Tang,X.L., Wang,Y., Xuan,Y.T., Liu,S.Q., Takano,H., Bhatnagar,A. and Bolli,R. (2000) Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc. Natl Acad. Sci. USA, 97, 10197–10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W.L., Garavito,M. and DeWitt,D.L. (1996) Prostaglandin endoperoxide synthase (cyclooxygenase)-1 and -2. J. Biol. Chem., 271, 33157–33160. [DOI] [PubMed] [Google Scholar]
- Steinauer K.K., Gibbs,I., Ning,S., French,J.N., Armstrong,J. and Knox,S.J. (2000) Radiation induces upregulation of cyclooxygenase-2 (COX-2) protein in PC-3 cells. Int. J. Radiat. Oncol. Biol. Phys., 48, 325–328. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K., Altorki,N., Chung,W.J., Mestre,J.R., Sampat,A. and Dannenberg,A.J. (1999) Inhibition of cyclooxygenase-2 gene expression by p53. J. Biol. Chem., 274, 10911–10915. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K., Hart,J.C., Norton,L. and Dannenberg,A.J. (2000) Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 and p38 mitogen-activated protein kinase pathways. J. Biol. Chem., 275, 14838–14845. [DOI] [PubMed] [Google Scholar]
- Sugrue M.M., Shin,D.Y., Lee,S.W. and Aaronson,S.A. (1997) Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc. Natl Acad. Sci. USA, 94, 9648–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. and Bradshaw,R.A. (1997) Differential utilization of ShcA tyrosine residues and functional domains in the transduction of epidermal growth factor-induced mitogen-activated protein kinase activation in 293T cells and nerve growth factor-induced neurite outgrowth in PC12 cells. J. Biol. Chem., 272, 22293–22299. [DOI] [PubMed] [Google Scholar]
- Trifan O.C., Smith,R.M., Thompson,B.D. and Hla,T. (1999) Over expression of cyclooxygenase-2 induces cell cycle arrest. Evidence for a prostaglandin-independent mechanism. J. Biol. Chem., 274, 34141–34147. [DOI] [PubMed] [Google Scholar]
- Tsujii M. and DuBois,R.N. (1995) Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell, 83, 493–501. [DOI] [PubMed] [Google Scholar]
- Van Putten V., Refaat,Z., Dessev,C., Blaine,S., Wick,M., Butterfield,L., Han,S.Y., Heasley,L.E. and Nemenoff,R.A. (2001) Induction of cytosolic phospholipase A2 by oncogenic Ras is mediated through the JNK and ERK pathways in rat epithelial cells. J. Biol. Chem., 276, 1226–1232. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Lane,D. and Levine,A.J. (2000) Surfing the p53 network. Nature, 408, 307–310. [DOI] [PubMed] [Google Scholar]
- Vousden K.H. (2000) p53: death star. Cell, 103, 691–694. [DOI] [PubMed] [Google Scholar]
- Wu G.S. et al. (1997) KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nature Genet., 17, 141–143. [DOI] [PubMed] [Google Scholar]
- Xie W. and Herschman,H.R. (1995) v-src induces prostaglandin synthase 2 gene expression by activation of the c-Jun N-terminal kinase and the c-Jun transcription factor. J. Biol. Chem., 270, 27622–27628. [DOI] [PubMed] [Google Scholar]
- Yang A. and McKeon,F. (2000) P63 and P73: P53 mimics, menaces and more. Nat. Rev. Mol. Cell. Biol., 1, 199–207. [DOI] [PubMed] [Google Scholar]
- Yang T., Huang,Y., Heasley,L.E., Berl,T., Schnermann,J.B. and Briggs,J.P. (2000) MAPK mediation of hypertonicity-stimulated cyclooxygenase-2 expression in renal medullary collecting duct cells. J. Biol. Chem., 275, 23281–23286. [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang,L., Hwang,P.M., Kinzler,K.W. and Vogelstein,B. (2001) PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell, 7, 673–682. [DOI] [PubMed] [Google Scholar]
- Zahner G., Wolf,G., Ayoub,M., Reinking,R., Panzer,U., Shankland,S.J. and Stahl,R.A. (2002) Cyclooxygenase-2 overexpression inhibits platelet-derived growth factor induced mesangial cell proliferation through induction of the tumor suppressor p53 and the cyclin dependent kinase inhibitors p21cip1 and p27kip1. J. Biol. Chem., 277, 9763–9771. [DOI] [PubMed] [Google Scholar]