Abstract
The exosome is a complex of 3′→5′ exoribonucleases which is involved in many RNA metabolic processes. To regulate these functions distinct proteins are believed to recruit the exosome to specific substrate RNAs. Here, we demonstrate that M-phase phosphoprotein 6 (MPP6), a protein reported previously to co-purify with the TAP-tagged human exosome, accumulates in the nucleoli of HEp-2 cells and associates with a subset of nuclear exosomes as evidenced by co-immunoprecipitation and biochemical fractionation experiments. In agreement with its nucleolar accumulation, siRNA-mediated knock-down experiments revealed that MPP6 is involved in the generation of the 3′ end of the 5.8S rRNA. The accumulation of the same processing intermediates after reducing the levels of either MPP6 or exosome components strongly suggests that MPP6 is required for the recruitment of the exosome to the pre-rRNA. Interestingly, MPP6 appeared to display RNA-binding activity in vitro with a preference for pyrimidine-rich sequences, and to bind to the ITS2 element of pre-rRNAs. Our data indicate that MPP6 is a nucleolus-specific exosome co-factor required for its role in the maturation of 5.8S rRNA.
INTRODUCTION
The exosome complex consists of 3′→5′ exoribonucleases and putative RNA-binding proteins and was originally identified in yeast during a study investigating the 3′ end maturation of 5.8S rRNA (1,2). Since, the yeast exosome has also been demonstrated to be required for the processing of small nuclear RNA (snRNA), small nucleolar RNA (snoRNA) and the degradation of aberrant pre-mRNAs in the nucleus (3–6), as well as the turnover of mRNAs in the cytoplasm (7–11).
In 1999, the yeast exosome components Rrp6p and Rrp45p were found to be homologous to the human PM/Scl-100 and PM/Scl-75 autoantigens. Moreover, human homologues of the yeast exosome components Rrp4p, Rrp40p, Rrp41p and Rrp46p were shown to physically interact with the polymyositis/scleroderma (PM/Scl) complex, an autoantigenic multiprotein complex containing PM/Scl-100 and PM/Scl-75, demonstrating that the yeast exosome and the human PM/Scl complex are very similar (12,13). Nine components of the human exosome were shown to be shared by the nuclear and cytoplasmic forms of the complex and are collectively referred to as the core exosome (9). Six of these nine proteins (hRrp41p, hRrp42p, hRrp46p, hMtr3p, OIP2 and PM/Scl-75) show homology to the Escherichia coli exonuclease RNase PH, the three other core exosome components (hRrp4p, hRrp40p and hCsl4p) contain a putative S1 RNA-binding domain. The exosome core components assemble into a doughnut-like structure, characterized by a six-membered ring formed by the RNase PH subunits (14–16).
In the nucleolus, three out of the four rRNA molecules are transcribed as a single precursor by RNA polymerase I. This precursor is processed by a series of endo- and exonucleolytic cleavages to form the mature 18S, 5.8S and 25S/28S rRNAs [reviewed in ref. (17)]. In yeast, deletion of core exosome components as well as the nuclear exosome-associated co-factor Mtr4p/Dob1p leads to accumulation of both precursor 5.8S rRNAs extended at their 3′ ends and 5′ETS fragments (1,3,6,12,18). Moreover, deletion of one of the yeast exosome components prevents cleavage at the early pre-rRNA cleavage sites A0, A1, A2 and A3, leading to depletion of mature 18S and 25S rRNAs (3,19). These processing steps do not require 3′→5′ exoribonuclease activity implying an indirect requirement for the exosome. Also upon depletion of the nuclear exosome-associated exoribonuclease Rrp6p and co-factor Rrp47p, defects in rRNA processing are observed (5,6,20,21). However, the effects are distinct from depletion of core exosome components, indicating that the functions of Rrp6p and the core exosome are not identical.
Four yeast exosome components and two human exosome components have proven 3′→5′ exonuclease activity, while the other exosome components with a RNase PH domain are predicted to possess such an activity (1,22,23). Besides the core exosome components, several additional exosome-associated proteins have been identified and these are most probably involved in the recruitment of the exosome to specific classes of substrate RNAs, its association with other processing complexes, or the modulation of its activity. An early identified exosome-associated protein, PM/Scl-100 (Rrp6p in yeast), is homologous to E.coli RNase D. KIAA0052/hMtr4p is a putative helicase and its yeast homologue Dob1p/Mtr4p acts in concert with the exosome in the processing of several types of nuclear RNA substrates. The M-phase phosphoprotein 6 (MPP6) was found to co-purify with the human exosome, when the latter was isolated via a TAP-tag purification approach (9). MPP6, which resides in the nucleus of interphase cells, was originally identified by virtue of its phosphorylation during mitosis (24). More recently, MPP6 was shown to interact with KIAA0052/hMtr4p and PM/Scl-100 using a yeast-two-hybrid approach (25). MPP6 is the only exosome-associated protein currently identified for which no yeast counterpart is known.
Here we show that MPP6 is associated with a subset of nuclear exosome complexes and that knock down of MPP6 leads to an accumulation of 3′ end extended 5.8S rRNAs, which are also accumulating upon knock down of PM/Scl-100 and hRrp41p. Moreover, we show that MPP6 is a RNA-binding protein in vitro, which is able to bind to (pre)-rRNAs and preferentially binds to poly(C) and poly(U).
MATERIALS AND METHODS
cDNA cloning and recombinant protein expression
The human MPP6 cDNA (GenBank accession number: NM_005792) was cloned by a PCR-based approach using a placenta cDNA library and oligonucleotides MPP6-forward, 5′-GCGGATCCGGAATTCAATGGCGGCCGAGAGAAAGAC-3′, and MPP6-reverse, 5′-GCCCCGGGTTATCTAGAATCCTGGGGCTTTAAGAACA-3′. The PCR products were cloned into the pCR4-TOPO vector according the manufacturer's procedure (Invitrogen) and the integrity of the resulting construct was verified by DNA sequencing. In order to obtain the N-terminally GST- or His6-tagged MPP6 protein, the MPP6 open reading frame was cloned into pGEX-4T3 (Amersham Pharmacia Biotech) and pQE32 (Qiagen), respectively. The tagged proteins were expressed in E.coli BL21(DE3)pLysS and purified by affinity chromatography using either glutathione–Sepharose or an immobilized nickel resin. The purity and quantity of the recombinant proteins were determined by SDS–PAGE.
GST pull-down assay
For the analysis of protein–RNA interactions, 20 ml of glutathione–Sepharose (Amersham Pharmacia Biotech) beads (50% slurry) were washed three times with 200 µl of pull-down buffer 100 [PB-100: 20 mM HEPES–KOH, pH 7.6, 100 mM KCl, 0.5 mM EDTA, 0.05% NP-40, 1 mM DTT, 0.02% BSA, 0.5 mM phenylmethlysulfonyl fluoride (PMSF)]. GST or GST-MPP6 (∼1 µg) was coupled to the beads in 200 µl of PB-100 by incubating at room temperature for 1 h under continuous agitation. After centrifugation, the supernatant was discarded and the beads were resuspended in 100 µl pull-down buffer. 32P-labelled RNAs and 20 U RNasin were added and the mixture was incubated for 1 h at 4°C under continuous agitation. The beads were washed three times with PB-200 (composition like PB-100, but containing 200 mM KCl) and the co-precipitating RNAs were extracted and analysed by denaturing PAGE and autoradiography.
Generation and affinity purification of rabbit anti-MPP6 antibodies
GST-MPP6 fusion protein was used to immunize rabbit M87. The serum from rabbit M87 recognized recombinant His6-MPP6 (data not shown). Anti-MPP6 antibodies were affinity-purified using His6-MPP6 recombinant protein coupled to CNBr-activated Sepharose as described by the manufacturer (Amersham Biosciences).
Preparation of HEp-2 cell extracts
Cytoplasmic and nuclear extracts were prepared from HEp-2 cells by resuspension of the harvested cells in lysis buffer (25 mM Tris–HCl, pH 7.5, 150 mM KCl, 1 mM EDTA, 0.5 mM PMSF and 1 mM DTT), permeabilization by the addition of digitonin to a final concentration of 0.025%, incubation for 10 min at room temperature, and centrifugation at 1000 g. The supernatant was transferred to a new tube and is referred to as the cytoplasmic fraction. After resuspending the pellet in lysis buffer the nuclear extract was prepared by sonication using a Branson microtip. Both extracts were clarified by centrifugation at 12 000 g for 10 min.
Immunoprecipitation
Per immunoprecipitation 10 µl of rabbit or patient serum was coupled to 10 µl of protein A-agarose beads (Kem-En-Tec) in IPP500 (0.5 M NaCl, 10 mM Tris–HCl, pH 8.0 and 0.05% NP-40) at room temperature for 1 h. Beads were washed once with IPP500 and twice with IPP150 (same as IPP500, but containing 150 mM NaCl). Subsequently, cell extract was added to the beads in IPP150 and incubated for 2 h at 4°C. After washing the beads three times with IPP150 the precipitated proteins were eluted by the addition of SDS sample buffer, separated by SDS–PAGE and blotted on nitrocellulose membranes.
Immunoblotting
For the detection of proteins on western blots, monoclonal anti-hRrp4p antibody (culture supernatant) (ModiQuest, Nijmegen, The Netherlands) and rabbit antisera were diluted 25- and 500-fold, respectively, in blocking buffer (5% skimmed milk, phosphate-buffered saline (PBS) and 0.05% NP-40). As secondary antibody, horseradish peroxidase-conjugated rabbit anti-mouse IgG or swine anti-rabbit IgG (Dako Immunoglobulins) were used after 2500-fold dilution in blocking buffer. Bound antibodies were visualized by chemiluminescence.
Northern blot analysis
Total RNA from HEp-2 cells was extracted 48 h after transfection with or without siRNA using the TRIzol reagent (Invitrogen). Per sample 5 µg of total RNA was separated on a denaturing 6% polyacrylamide gel, and after transfer to Hybond N+ membranes (Amersham Biosciences) the blots were hybridized with several 32P-labelled antisense RNA probes in hybridization solution (6× SSC, 10× Denhardt's, 100 µg/ml herring sperm DNA and 0.1% SDS). After overnight incubation at 65°C, the blots were washed three times with 2× SSC/0.1% SDS and analysed by autoradiography. Oligonucleotides used in this study are 5′ETS-1, 5′-CGCGCGAGAGAACAGCAGGC-3′, and 5′ETS-2, 5′-TCGTGATTCTCGTCCATCCTCCGAC-3′. The antisense 5.8S rRNA probe was generated by linearizing a pGEM-3Zf(+) plasmid containing a full-length 5.8S rRNA EcoRI–BamHI PCR product with EcoRI and in vitro transcription by SP6 RNA polymerase. A pCR4-TOPO vector containing a XhoI–SalI PCR product that covers the most 5′ 300 nt of the ITS2 was linearized with XhoI and transcribed by T7 RNA polymerase to yield a 337 nt antisense probe. The antisense ITS1 probe was generated as described by Cohen et al. (26)
Transient transfection of HEp-2 cells and fluorescence microscopy
For transfection, the cDNAs encoding hMtr3p and MPP6 were cloned into EGFP vectors (Clontech) allowing expression of the protein fused to the C-terminus of the EGFP protein. HEp-2 cells were grown to 70% confluency in DMEM containing 10% fetal calf serum (FCS) (DMEM+). For fluorescence microscopy ∼3 × 106 cells were transfected with 10 µg of plasmid DNA in 800 µl DMEM+ by electroporation at 260 V and 950 µF. The cells were seeded on coverslips and cultured overnight. Subsequently, the cells were washed twice with PBS and fixed with 4% paraformaldehyde in PBS for 20 min. After fixation, cells were washed three times with PBS and mounted with 50% glycerol in PBS. The EGFP-fusion proteins were visualized by fluorescence microscopy.
For siRNA transfection ∼3 × 105 cells were grown in DMEM+. Per transfection 100 pmol of siRNA was transfected into HEp-2 cells using Oligofectamine transfection reagent (Invitrogen), as described by the manufacturer with the exception that during transfection 10% FCS was present in the medium. As a control, cells were treated in the same way but without adding siRNA to the transfection reagent.
siRNAs
Three MPP6 siRNA duplexes were designed based upon the coding region of the human MPP6 sequence (GenBank accession number: NM_005792). The 21 nt siRNA duplexes containing dTdT overhangs were purchased from IDT (Coralville, IA): siMPP6-1, 5′-GAGCACUGGUACUUGGAUUTT-3′; siMPP6-2, 5′-CAGUAGAGCUUGAUGUGUCTT-3′; and siMPP6-3, 5′-GAUAUGAGACCUUGGUGGGTT-3′. siRNAs against human hRrp41p and PM/Scl-100 were described previously by LeJeune et al. (27). As a control an siRNA specific for a melanoma cell marker, which is not expressed in the cell lines used in this study, was used.
Glycerol gradient analysis
HEp-2 cell extracts were prepared as described above and loaded on 5–40% (v/v) glycerol gradients prepared in gradient buffer (25 mM Tris–HCl, pH 7.5, 150 mM KCl, 1 mM DTT and 0.02% Triton X-100). Gradients were centrifuged in a TH641 rotor (Sorvall) for 16 h at 25 000 r.p.m. at 4°C and subsequently 23 fractions per gradient were collected. The proteins in these fractions were separated by SDS–PAGE and transferred to nitrocellulose membranes for further analysis.
RNase protection assay
Total RNA was extracted from HEp-2 cells transfected with siRNAs as described above and resuspended in 100 µl of hybridization buffer (40 mM PIPES, pH6.4, 400 mM NaCl, 1 mM EDTA and 80% formamide). A radiolabelled antisense ITS2 probe was synthesized by in vitro transcription, treated with RNase-free DNase I (Invitrogen) for 15 min at 37°C and after precipitation dissolved in hybridization buffer. Subsequently, an excess of probe RNA was hybridized with 5 µg of total RNA overnight at 52°C. Free probe and non-protected RNA was digested in ribonuclease digestion buffer (10 mM Tris–HCl, pH7.4, 300 mM NaCl and 5 mM EDTA) containing 40 µg/ml ribonuclease A and 2 µg/ml ribonuclease T1 for 1 h at 37°C. The protected RNA was purified, dissolved in RNA loading buffer and analysed on a 10% sequencing gel and visualized by autoradiography.
RESULTS
The MPP6 protein localizes to the nucleoli and interacts with the nuclear exosome
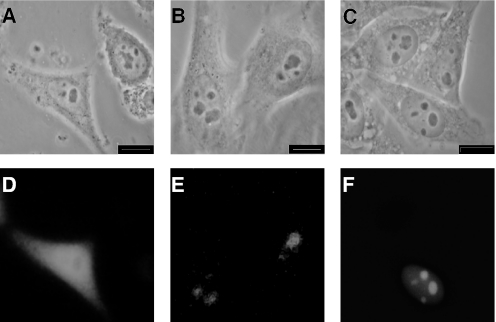
During TAP-tag purifications of the human exosome, MPP6 was found to co-fractionate with the human exosome (9). To investigate whether MPP6 is truly associated with the human exosome, its subcellular localization and interaction with the human exosome complex were studied in more detail. HEp-2 cells were transiently transfected with constructs coding for the EGFP-fusion proteins of MPP6 and hMtr3p as well as EGFP alone as a control. The subcellular localization of these proteins was examined by fluorescence microscopy 24 h later. EGFP was found throughout the cell, whereas MPP6 and hMtr3p both accumulated in the nucleoli (Figure 1). As has been observed before for other exosome core proteins, a weak but significant staining of the nucleoplasm was observed for EGFP-hMtr3p in addition to the nucleolar staining (Figure 1F).
Figure 1.
Subcellular localization of MPP6. HEp-2 cells were transfected with constructs encoding EGFP alone (A and D), EGFP-MPP6 (B and E) and EGFP-hMtr3p (C and F) and after 24 h the cells were fixed in 4% paraformaldehyde/PBS and the expressed fusion proteins were analysed by fluorescence microscopy. Phase-contrast and fluorescence images are displayed in (A–C and D–F), respectively. Each bar corresponds to 10 µm.
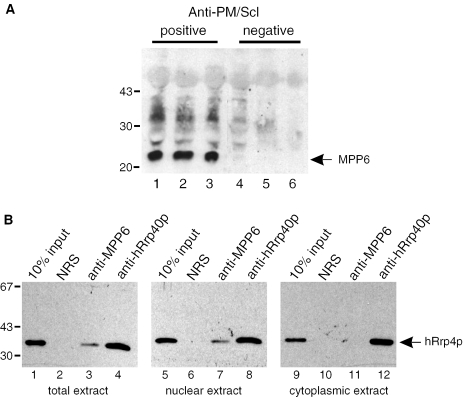
To confirm the association of MPP6 with the human exosome, co-immunoprecipitation experiments were performed using a total HEp-2 cell extract and three anti-PM/Scl-positive patient sera. These sera are strongly reactive with the exosome components PM/Scl-75 and PM/Scl-100 and to a lesser extent with other exosome components, but not with the MPP6 protein. Three anti-PM/Scl-negative sera served as controls (28,29). After immunoprecipitation, the precipitated material was analysed by western blotting using the rabbit anti-MPP6 antiserum. The results show that MPP6 is precipitated with all anti-PM/Scl-positive sera (Figure 2A, lanes 1–3) but not with the anti-PM/Scl-negative sera (Figure 2A, lanes 4–6). In the reciprocal experiment, the anti-MPP6 antiserum was used for immunoprecipitation of human exosome components from a total, nuclear and cytoplasmic HEp-2 cell extract. Co-precipitation of the exosome was monitored with a monoclonal antibody to the human exosome component hRrp4p. As expected, an antibody to the core exosome component hRrp40p co-precipitated hRrp4 from all three extracts (Figure 2B, lanes 4, 8 and 12). Anti-MPP6, however, co-precipitated hRrp4p from the total as well as the nuclear extract (Figure 2B, lanes 3 and 7), but not from the cytoplasmic extract (Figure 2B, lane 11). The normal rabbit serum failed to precipitate hRrp4p from all three extracts (Figure 2B, lanes 2, 6 and 10). Taken together, these results indicate that MPP6 is a nuclear protein, which associates with the exosome in the nucleoli.
Figure 2.
MPP6 is associated with nuclear exosome complexes. (A) HEp-2 cell extracts were subjected to immunoprecipitation with three anti-PM/Scl-positive (lanes 1–3) and three anti-PM/Scl-negative patient sera (lanes 4–6). Precipitated proteins were analysed by western blotting using a rabbit anti-MPP6 serum. The positions of molecular mass markers (kDa) are indicated on the left. (B) Reciprocal experiment in which a normal rabbit serum (lanes 2, 6 and 10), a polyclonal anti-MPP6 serum (lanes 3, 7 and 11) and a polyclonal anti-hRrp40p serum (lanes 4, 8 and 12) were used for immunoprecipitations. A monoclonal antibody to hRrp4p was used for the detection of a co-precipitated exosome component. For the immunoprecipitations total (lanes 1–4), nuclear (lanes 5–8) and cytoplasmic (lanes 9–12) HEp-2 cell extracts were used. Input material (10%) of the extracts was loaded in lanes 1, 5 and 9, respectively. The positions of molecular mass markers (kDa) are indicated on the left.
MPP6 co-sediments with nuclear exosome complexes at 60S–80S in glycerol gradients
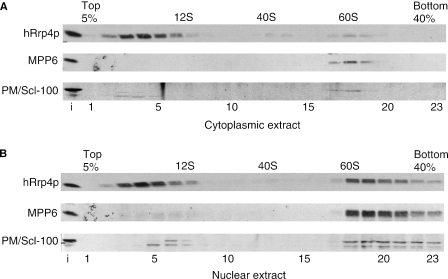
Previous reports have shown that human exosome core components are in part associated with high molecular weight complexes (1,12,13). To determine the size of MPP6-associated complexes, nuclear and cytoplasmic extracts from HEp-2 cells were fractionated by glycerol gradient sedimentation. Fractions were analysed by western blotting with rabbit anti-MPP6, anti-hRrp4p and anti-PM/Scl-100 antisera and by agarose gel electrophoresis to monitor the distribution of RNA. The U1 snRNA was used as marker for 12S complexes, whereas the large rRNAs were used to identify 40S and 60S ribosomal subunits. The results in Figure 3 show that for the cytoplasmic extract the majority of hRrp4p was present in fractions 2–7 corresponding to ∼10S, whereas PM/Scl-100 and MPP6 were almost completely absent from these fractions (Figure 3A). Interestingly, all three proteins, including MPP6 were detected in cytoplasmic 60S complexes, in spite of the fact that the immunofluorescence and immunoprecipitation data did not show any MPP6 in the cytoplasm. For the nuclear extract hRrp4p also sedimented in 10S complexes, but approximately half of the nuclear hRrp4p molecules was found in complexes corresponding to 60S–80S as well (Figure 3B). The majority of both PM/Scl-100 and MPP6 co-sedimented with hRrp4p in 60S–80S fractions, whereas these proteins were hardly or not detected at 10S. These data indicate that MPP6, together with PM/Scl-100, is mainly associated with higher molecular weight complexes in the nucleus.
Figure 3.
MPP6 predominantly co-sediments with nuclear exosome complexes at 60S–80S in glycerol gradients. Cytoplasmic (A) and nuclear (B) extracts from HEp-2 cells were fractionated in a 5–40% (v/v) glycerol gradient. The sedimentation of hRrp4, MPP6 and PM/Scl-100 was determined by immunoblotting. The sedimentation of the large rRNAs was determined by agarose gel electrophoresis and ethidium bromide staining and used as markers (40S and 60S) in the gradient. U1 snRNA was used as marker for 12S complexes.
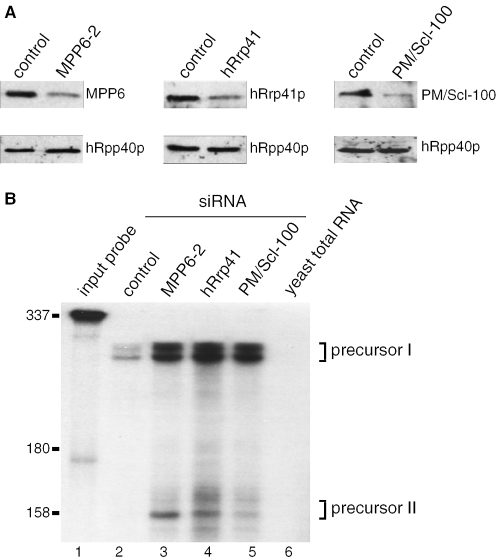
Knock-down of MPP6 leads to accumulation of 5.8S rRNA precursors extended at the 3′ end
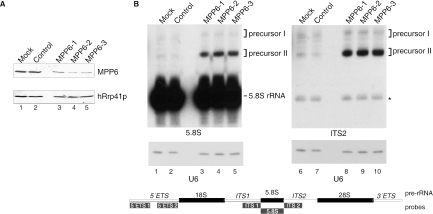
The nucleolar accumulation of MPP6, as well as its association with pre-rRNA sized complexes in the nucleus suggested that MPP6 might play a role in the biogenesis of ribosomes. Based upon the situation in yeast, the precursor of rRNA has been proposed to be one of the major substrates for the nuclear exosome in human cells. In yeast it has been demonstrated that the maturation of the 3′ end of the 5.8S rRNA requires the exosome, the exosome-associated TRAMP complex and several other co-factors (3,5,6,18–20,30–33). To investigate the role of MPP6 and the human exosome in the processing of pre-rRNA, RNAi experiments were performed. siRNAs were designed and synthesized for MPP6 and as a control, an siRNA specific for a melanoma marker protein was included. The effect of each MPP6–siRNA was assayed by transfection of HEp-2 cells with each of these siRNAs and 2 days after transfection protein as well as RNA was isolated. The efficiency of MPP6 knock-down was assessed by western blotting. A polyclonal antibody to hRrp41p was used as a control. The results in Figure 4A show that all three MPP6-siRNAs led to significantly reduced levels of the MPP6 protein at 48 h after transfection, while the levels of hRrp41p remained unaffected (Figure 4A). Subsequently, RNA isolated from MPP6 siRNA-treated cells was analysed by gel electrophoresis and northern blot hybridization using radiolabelled probes specific for different regions of the pre-rRNA (Figure 4B). The results demonstrate that knock-down of MPP6 leads to the accumulation of 5.8S rRNA precursors, which hybridize with the ITS2 probe in addition to the 5.8S coding sequence probe (Figure 4B). These processing intermediates were not detected with a probe specific for ITS1 (data not shown), which indicates that the precursors accumulating upon MPP6 knock-down are extended at their 3′ end. Also, no accumulation of processing intermediates was detected using the probes specific for 5′ETS (data not shown). Hybridization with a U6 snRNA probe, which was used as a control, showed that there were no significant loading differences between the different samples (Figure 4B, lanes 1–10). Note that under these conditions the total amount of mature 5.8S rRNA was not significantly reduced.
Figure 4.
Knock down of MPP6 by RNAi leads to the accumulation of 5.8S rRNA precursors. (A) HEp-2 cells were transiently transfected with three different siRNAs to MPP6 (100 pmol), control siRNA or buffer (mock). Cells were harvested 2 days after transfection and 75 µg of total protein was analysed by western blotting using a polyclonal anti-MPP6 serum or a polyclonal anti-hRrp41p serum (control). (B) Northern analysis of 5.8S rRNA processing upon MPP6 knock down. Total RNA (5 µg) from (mock) transfected cells was analysed by northern blot hybridization using radiolabelled probes specific for 5.8S rRNA (left) or ITS2 (right). The relative positions of these probes, and also the other probes used, with respect to the primary rRNA transcript are depicted in the scheme below the autoradiographs. Note that the size of the probes is not proportional to that of the pre-rRNA. As a control, a U6 snRNA probe was used. The positions of 5.8S rRNA and its precursors (I and II) are indicated. The asterisk in the right panel points to a weak cross-hybridization of the ITS2 probe with the mature 5.8S rRNA.
Based upon the role of the exosome in the maturation of the 3′ end of 5.8S rRNA in yeast and the association of MPP6 with the exosome in the nucleus, we hypothesized that the observed accumulation of processing intermediates resulting from MPP6 knock-down might be due to the abrogation of the function of the exosome. However, the effects of abrogation of the function of the human exosome on 5.8S rRNA maturation have not been documented so far. To shed more light on this issue the effect of MPP6 RNAi on the 3′ end of the 5.8S rRNA was compared with those of knock down of the exosome components hRrp41p and PM/Scl-100 in an RNase protection assay. The reduction in protein levels was monitored by western blotting, which indeed confirmed the efficiency of the siRNAs used (Figure 5A). The RNAs isolated from the cells treated with the MPP6, hRrp41p and PM/Scl-100 siRNAs were hybridized with a probe comprising the sequence complementary to the most 5′ 300 nt of ITS2. After RNase digestion, the reaction products were analysed by denaturing PAGE (Figure 5B). Precursor RNAs detected in this experiment were of similar size as those observed in the northern blot hybridization. Note that low levels of precursor I were observed also in the cells treated with the control siRNA, whereas precursor II products were only detected for RNA from MPP6 and exosome knock-down cells. The size of the products of the RNase protection experiments indicated that the 3′ ends of the processing intermediates are located ∼156 and 250 nt downstream of the 3′ end of the mature 5.8S rRNA. Hybridization of the probe with yeast total RNA indicated that the signals observed in lanes 2–5 are specific and not owing to background signals resulting from self-complementary sequences within the probe (lane 6).
Figure 5.
MPP6, hRrp41 and PM/Scl-100 knock down lead to the accumulation of the same 5.8S rRNA precursors. (A) HEp-2 cells were transiently transfected with control siRNA (100 pmol), MPP6-2 siRNA (100 pmol), hRrp41 siRNA (200 pmol) and PM/Scl-100 siRNA (100 pmol). After 36 h transfection cells were retransfected with the same amount of siRNA and after an additional 36 h the cells were harvested. The proteins were analysed by western blotting using 75 µg of total protein extracts from transfected cells and a polyclonal anti-MPP6 serum, a polyclonal anti-hRrp41 serum, and a polyclonal anti-PM/Scl-100 serum. A polyclonal anti-hRpp40 serum was used as a control. (B) A radiolabelled antisense ITS2 probe, complementary to the first 300 nt of ITS2, was hybridized to 5 µg of total RNA from siRNA-treated cells. After digestion with RNase A and RNase T1 the RNA was analysed on a 10% denaturing polyacrylamide gel. The radiolabelled ITS2 probe was loaded in lane 1. As a control, the same probe was incubated with 5 µg of yeast total RNA and further treated by the same procedure (lane 6). Lanes 4–6 show protected RNAs from cells treated with control, MPP6-2, Rrp41 and PM/Scl-100 siRNA, respectively. The positions of the 5.8S precursor rRNAs (I and II) are indicated. Positions of marker RNAs are indicated on the left.
MPP6 interacts with RNA in vitro and binds preferentially to polypyrimidines
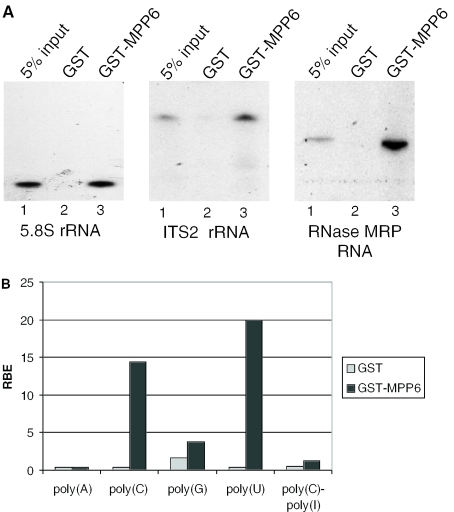
The results of the experiments described above suggest that the exosome and MPP6 are involved in the same processing steps in ITS2 to generate the mature 3′ end of the human 5.8S rRNA. This raises the question how they cooperate in this process. The activities that are predicted to be involved are RNA-binding for the recognition of processing sites in ITS2, endoribonuclease activities for cleavage(s) in ITS2, 3′→5′ exoribonuclease for trimming of the cleaved precursor RNA, and RNA helicase activities for resolving secondary structures that interfere with nucleolytic processing. Several exosome components have been shown to contain 3′→5′ exoribonuclease activity, but the recombinant MPP6 did not appear to have such an activity (data not shown). The amino acid sequence of MPP6 is also devoid of endoribonuclease and helicase motifs. Therefore, we investigated whether MPP6 can bind to RNA. GST pull-down experiments were performed with GST-tagged MPP6 protein and three distinct 32P-labelled, in vitro transcribed RNAs, 5.8S rRNA, a transcript containing part of the ITS2 and the RNA component of RNase MRP. As shown in Figure 6A, GST-MPP6 bound to all three RNAs efficiently (compare lanes 3 with the corresponding lanes 1), in contrast to GST, which did not precipitate any of the RNAs (lanes 2). In addition, a GST pull-down experiment using a HEp-2 cell extract showed that GST-MPP6 was able to associate with RNAs in this extract including 5.8S rRNA and 5.8S rRNA precursors (data not shown). These data demonstrate that MPP6 does contain RNA-binding activity and suggest that the specificity of binding may be low. To study this in more detail, a similar experiment was performed with different radiolabelled homopolynucleotides. The results showed that GST-MPP6 preferentially binds to poly(C) and poly(U). No binding to poly(A) could be detected and the relatively weak binding to poly(G) should be interpreted with care, because also GST displayed some binding to poly(G) and poly(G) is known to adopt higher order structures which may affect background levels. GST-MPP6 also did not appear to bind to poly(C)–poly(I), which was included as a double-stranded RNA in these analyses (Figure 6B).
Figure 6.
GST-tagged MPP6 binds to in vitro transcribed RNAs and prefers polypyrimidines. (A) GST and GST-MPP6 recombinant proteins were incubated with radiolabeled, in vitro transcribed full-length 5.8S rRNA, ITS2 rRNA (a fragment corresponding to the most 5′ 300 nt) and RNase MRP RNA. Binding to these RNAs was assayed by GST pull-down followed by denaturing gel electrophoresis and autoradiography. Five percent of the input RNA was loaded in lanes 1. Lanes 2 and 3 contain the material precipitated by GST alone and GST-MPP6, respectively. (B) Binding of GST and GST–MPP6 fusion proteins to radiolabelled homopolynucleotides was analysed as described above and the bound RNAs were quantified in a scintillation counter. The binding efficiency of GST or GST-MPP6 with poly(A), poly(C), poly(G), poly(U) and poly(C)–ply(I) is depicted as a percentage of input RNA (RBE: relative binding efficiency). These results are the averages of two independent experiments.
DISCUSSION
In this study we have characterized the previously identified association of MPP6 with exosome complexes in human cells. The association with the exosome was confirmed by co-immunoprecipitation experiments and shown to be restricted to the nuclear compartment, more specifically the nucleoli. In logarithmically growing HEp-2 cells the majority of MPP6 appeared to be associated with 60S–80S complexes which most probably represent pre-ribosomes and knock-down experiments indeed demonstrated that MPP6 participates, in conjunction with the exosome, in the processing of human pre-rRNA.
The association of MPP6 with the nuclear exosome
Although the highest concentrations of human core exosome components can be detected in the nucleolus by immunofluorescence microscopy, subcellular fractionation experiments have shown that core exosome components are present in both nuclear and cytoplasmic fractions (12,13,34–37). Similar to other exosome components hMtr3p, the subcellular distribution of which has not been described before, showed a nuclear fluorescence pattern, with an accumulation in the nucleoli. MPP6 also displayed a subcellular localization which is mainly restricted to the nucle(ol)us both by fluorescence microscopy and biochemical fractionation. The relatively low level of co-precipitation of hRrp4p with anti-MPP6 in comparison with anti-hRrp40p from nuclear extracts indicated that only a relatively small fraction of nuclear exosome complexes is stably associated with MPP6. This is substantiated by the results of co-immunoprecipitation experiments performed with anti-EGFP antibodies using extracts from transiently transfected HEp-2 cells expressing EGFP-tagged MPP6 or, as a reference, EGFP-tagged hCsl4p (data not shown). Fractionation of the nuclear extract by glycerol gradient sedimentation revealed that MPP6 is not associated with the low molecular weight exosome complexes at ∼10S, but only associates with the exosome complexes at 60S–80S. Also the majority of PM/Scl-100 appeared to be associated with 60S–80S complexes in the nuclear extract.
In yeast, the exosome has been shown to function in pre-rRNA, pre-sn(o)RNA and pre-mRNA processing and/or degradation in the nucleus, whereas in the cytoplasm it is involved in mRNA degradation (38). Currently, it is unclear whether the small, though significant fraction of MPP6, PM/Scl-100 and hRrp4p molecules in the cytoplasmic extract that sediment at 60S are associated with a cytoplasmic complex, or whether this is due to some leakage of nuclear material to the cytoplasm during preparation of the extracts. Interestingly, the high molecular weight peak in the cytoplasmic material is observed at 60S, whereas the peak with the nuclear material extends from 60S to 80S, suggesting that they might represent different complexes.
Because neither co-immunoprecipitation nor density gradient sedimentation data demonstrate a direct interaction of MPP6 with the exosome, the possibility exists that other proteins mediate their interaction, especially because it only seems to occur in the large preribosomal complexes. The results of an analysis of possible interactions between MPP6 and exosome core components in a mammalian two-hybrid system were all negative (G. Schilders and G.J.M. Pruijn, unpublished data). However, yeast two-hybrid data have suggested direct interactions of MPP6 with both PM/Scl-100 and hMtr4p (25). Moreover, all three proteins have also been identified as components of the nucleolar proteome (http://www.lamondlab.com/NOPdb/). Therefore, it seems most probable that the interaction of MPP6 with the exosome depends on its binding to PM/Scl-100, which is believed to interact directly with the core of the exosome.
Still, the almost exclusive association of the MPP6 protein with nuclear 60S–80S complexes strongly suggested that MPP6 is specifically involved in the function of the exosome in pre-rRNA processing. Indeed, we found that upon knock down of MPP6 the maturation of 5.8S rRNA is affected. The accumulation of the same processing intermediates in cells with reduced levels of either MPP6 or the exosome components hRrp41p and PM/Scl-100 provides further evidence that they act in concert in these processing steps.
The role of MPP6 in the maturation of the 3′ end of the 5.8S rRNA
Although the exosome complex has been shown to function in a wide variety of RNA processing pathways, it is still largely unknown how the exosome recognizes its different substrates and how these substrates are subsequently subjected to either precise 3′ end processing or complete degradation. Most probably, other proteins, which transiently associate with the exosome, mediate the recruitment of the exosome to specific substrate RNAs. In mammalian cells so far no evidence has been presented for the involvement of the exosome in rRNA processing pathways. Our data show for the first time that the human exosome, like the yeast exosome, is required for the maturation of the 3′ end of the 5.8S rRNA. Both the hRrp41p and PM/Scl-100 proteins appeared to be involved in this process. In yeast depletion of Rrp6p and core exosome components (including Rrp41p) have distinct effects on pre-rRNA processing, because their depletion leads to the accumulation of different processing intermediates. Based upon these observations a hand-over mechanism was proposed, in which the core exosome components remove ITS2 nucleotides up to a position 30 nt beyond the 3′ end of the 5.8S rRNA and Rrp6p then takes over and processes most of the remaining ITS2 nucleotides (3,20). In contrast to the situation in yeast, our data did not reveal differences in the accumulation of 5.8S rRNA precursors after knocking down PM/Scl-100 or hRrp41p. Although the functional activities of these proteins may have changed over time, we believe that these differences are more probably explained by the depletion of the whole exosome from the site of processing. The accumulation of the same processing intermediates after MPP6 knock down supports this idea. Furthermore, the observed accumulation of distinct 5.8S intermediates indicates that there are endonucleolytic cleavage sites within the human ITS2, which are not affected by MPP6 or exosome knock down. These putative cleavage sites are ∼156 and 250 nt downstream of the 3′ end of the 5.8S rRNA coding sequence and might give rise to the 8S and 12S pre-rRNAs observed previously in other mammalian cells (39–41).
A database search did not reveal an MPP6 homologue in the yeast Saccharomyces cerevisiae, but the amino acid sequence of the hypothetical protein SPACUNK4.11c of Schizosaccharomyces pombe is a putative homologue of MPP6 (27% identity; 39% similarity). This indicates that there might be differences in pre-rRNA architecture between both yeast strains which require at least in part distinct processing machineries.
There are no known protein motifs that can be discerned in the primary structure of MPP6 which could give a clue about its function. The only sequence motif that can be discerned is a bipartite nuclear localization signal (amino acid 116–132). The bacterially expressed recombinant GST–MPP6 fusion protein did not display 3′→5′ exoribonuclease activity in vitro (G. Schilders and G.J.M. Pruijn, unpublished data). However, our in vitro RNA-binding experiments show that MPP6 is able to interact with the 5.8S-proximal ITS2 region of pre-rRNA, and displays a binding preference for polypyrimidines. Interestingly, the 3′ end of the 5.8S precursor II, which accumulates upon MPP6 knock down, contains an oligopyrimidine tract (∼20 nt). The effects on pre-rRNA processing suggest that MPP6 is an essential co-factor for the exosome in the nucleolus. Nevertheless, our RNA-binding data show that the binding specificity of MPP6 is low, implying that this protein may bind to various other RNAs and may also be involved in the 3′ end processing of other RNAs.
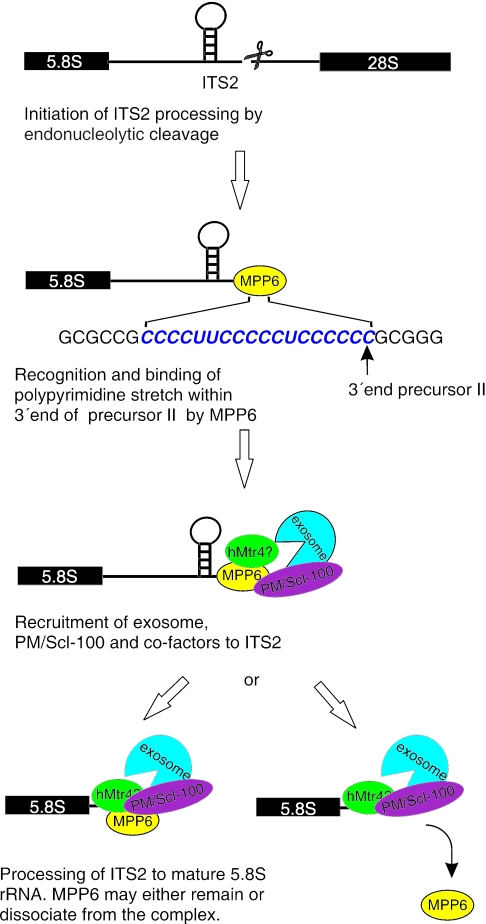
The reduction of MPP6 levels did not significantly affect the expression levels or subcellular localization of exosome components (data not shown), indicating that MPP6 is probably not required for exosome assembly or for its subcellular targeting. Taken together, our data support a model in which MPP6 mediates the recruitment of the exosome to the ITS2 for further processing (Figure 7). The RNA-binding properties of MPP6 suggest that MPP6 first interacts with the pre-rRNA, thereby creating a binding site for the PM/Scl-100-containing exosome. The exosome subsequently processes ITS2, and at this stage MPP6 may either remain associated or may be released from the exosome at the start of the exonucleolytic digestion. Recently, the Mtr4p-containing TRAMP complex was shown to be involved in exosome-mediated pre-rRNA processing in yeast (31–33). It would therefore be interesting to investigate whether MPP6 also plays a role in the association of the human counterpart of the TRAMP complex with the pre-rRNA-exosome complex.
Figure 7.
Model for the role of MPP6 in pre-rRNA processing. Before, or directly after cleavage of the pre-rRNA in ITS2 by a yet unknown endoribonuclease, MPP6 binds to oligopyrimidine stretches in the ITS2 RNA and subsequently recruits the PM/Scl-100-containing exosome and probably several additional factors, like hMtr4p or an hMtr4p containing complex. This is in agreement with the reported two-hybrid interactions between MPP6 and PM/Scl-100 and between MPP6 and hMtr4p (25). The exosome will then process the ITS2 RNA to generate the mature 5.8S rRNA. MPP6 may either remain associated with the processing complex or dissociate once the exosome starts the digestion.
Acknowledgments
We would like to thank Sonja Schoenmakers for the subcellular localization experiments and David Tollervey (Wellcome Trust Centre, Edinburgh, Scotland, UK) for providing the rabbit anti-hRrp4p antibodies. This work was supported in part by the Council for Chemical Sciences of the Netherlands Organization for Scientific Research (NWO-CW). Funding to pay the Open Access publication charges for this article was provided by the Radboud University Nijmegen.
Conflict of interest statement. None declared.
REFERENCES
- 1.Mitchell P., Petfalski E., Shevchenko A., Mann M., Tollervey D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′→5′ exoribonucleases. Cell. 1997;91:457–466. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P., Petfalski E., Tollervey D. The 3′ end of yeast 5.8S rRNA is generated by an exonuclease processing mechanism. Genes Dev. 1996;10:502–513. doi: 10.1101/gad.10.4.502. [DOI] [PubMed] [Google Scholar]
- 3.Allmang C., Kufel J., Chanfreau G., Mitchell P., Petfalski E., Tollervey D. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 1999;18:5399–5410. doi: 10.1093/emboj/18.19.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kufel J., Allmang C., Chanfreau G., Petfalski E., Lafontaine D., Tollervey D. Precursors to the U3 small nucleolar RNA lack small nucleolar RNP proteins but are stabilized by La binding. Mol. Cell. Biol. 2000;20:5415–5424. doi: 10.1128/mcb.20.15.5415-5424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell P., Petfalski E., Houalla R., Podtelejnikov A., Mann M., Tollervey D. Rrp47p is an exosome-associated protein required for the 3′ processing of stable RNAs. Mol. Cell. Biol. 2003;23:6982–6992. doi: 10.1128/MCB.23.19.6982-6992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van-Hoof A., Lennertz P., Parker R. Yeast exosome mutants accumulate 3′-extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs. Mol. Cell. Biol. 2000;20:441–452. doi: 10.1128/mcb.20.2.441-452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira C.C., Gonzales F.A., Zanchin N.I. Temperature-sensitive mutants of the exosome subunit Rrp43p show a deficiency in mRNA degradation and no longer interact with the exosome. Nucleic Acids Res. 2002;30:4186–4198. doi: 10.1093/nar/gkf545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van-Hoof A., Staples R.R., Baker R.E., Parker R. Function of the ski4p (Csl4p) and Ski7p proteins in 3′-to-5′ degradation of mRNA. Mol. Cell. Biol. 2000;20:8230–8243. doi: 10.1128/mcb.20.21.8230-8243.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C.Y., Gherzi R., Ong S.E., Chan E.L., Raijmakers R., Pruijn G.J., Stoecklin G., Moroni C., Mann M., Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107:451–464. doi: 10.1016/s0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee D., Gao M., O'Connor J.P., Raijmakers R., Pruijn G., Lutz C.S., Wilusz J. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 2002;21:165–174. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z., Kiledjian M. Functional link between the mammalian exosome and mRNA decapping. Cell. 2001;107:751–762. doi: 10.1016/s0092-8674(01)00592-x. [DOI] [PubMed] [Google Scholar]
- 12.Allmang C., Petfalski E., Podtelejnikov A., Mann M., Tollervey D., Mitchell P. The yeast exosome and human PM-Scl are related complexes of 3′→5′ exonucleases. Genes Dev. 1999;13:2148–2158. doi: 10.1101/gad.13.16.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwer R., Allmang C., Raijmakers R., van-Aarssen Y., Egberts W.V., Petfalski E., van-Venrooij W.J., Tollervey D., Pruijn G.J. Three novel components of the human exosome. J. Biol. Chem. 2001;276:6177–6184. doi: 10.1074/jbc.M007603200. [DOI] [PubMed] [Google Scholar]
- 14.Raijmakers R., Egberts W.V., van Venrooij W.J., Pruijn G.J. Protein–protein interactions between human exosome components support the assembly of RNase PH-type subunits into a six-membered PNPase-like ring. J. Mol. Biol. 2002;323:653–663. doi: 10.1016/s0022-2836(02)00947-6. [DOI] [PubMed] [Google Scholar]
- 15.Pruijn G.J. Doughnuts dealing with RNA. Nature Struct. Mol. Biol. 2005;12:562–564. doi: 10.1038/nsmb0705-562. [DOI] [PubMed] [Google Scholar]
- 16.Lorentzen E., Walter P., Fribourg S., Evguenieva-Hackenberg E., Klug G., Conti E. The archaeal exosome core is a hexameric ring structure with three catalytic subunits. Nature Struct. Mol. Biol. 2005;12:575–581. doi: 10.1038/nsmb952. [DOI] [PubMed] [Google Scholar]
- 17.Venema J., Tollervey D. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 1999;33:261–311. doi: 10.1146/annurev.genet.33.1.261. [DOI] [PubMed] [Google Scholar]
- 18.de-la-Cruz J., Kressler D., Tollervey D., Linder P. Dob1p (Mtr4p) is a putative ATP-dependent RNA helicase required for the 3′ end formation of 5.8S rRNA in Saccharomyces cerevisiae. EMBO J. 1998;17:1128–1140. doi: 10.1093/emboj/17.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanchin N.I., Goldfarb D.S. The exosome subunit Rrp43p is required for the efficient maturation of 5.8S, 18S and 25S rRNA. Nucleic Acids Res. 1999;27:1283–1288. doi: 10.1093/nar/27.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briggs M.W., Burkard K.T., Butler J.S. Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8 S rRNA 3′ end formation. J. Biol. Chem. 1998;273:13255–13263. doi: 10.1074/jbc.273.21.13255. [DOI] [PubMed] [Google Scholar]
- 21.Peng W.T., Robinson M.D., Mnaimneh S., Krogan N.J., Cagney G., Morris Q., Davierwala A.P., Grigull J., Yang X., Zhang W., et al. A panoramic view of yeast noncoding RNA processing. Cell. 2003;113:919–933. doi: 10.1016/s0092-8674(03)00466-5. [DOI] [PubMed] [Google Scholar]
- 22.Burkard K.T., Butler J.S. A nuclear 3′–5′ exonuclease involved in mRNA degradation interacts with Poly(A) polymerase and the hnRNA protein Npl3p. Mol. Cell. Biol. 2000;20:604–616. doi: 10.1128/mcb.20.2.604-616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang T., Altman S. A protein subunit of human RNase P, Rpp14, and its interacting partner, OIP2, have 3′→5′ exoribonuclease activity. Proc. Natl Acad. Sci. USA. 2002;99:5295–5300. doi: 10.1073/pnas.072083699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto-Taniura N., Pirollet F., Monroe R., Gerace L., Westendorf J.M. Identification of novel M phase phosphoproteins by expression cloning. Mol. Biol. Cell. 1996;7:1455–1469. doi: 10.1091/mbc.7.9.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehner B., Sanderson C.M. A protein interaction framework for human mRNA degradation. Genome Res. 2004;14:1315–1323. doi: 10.1101/gr.2122004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen A., Reiner R., Jarrous N. Alterations in the intracellular level of a protein subunit of human RNase P affect processing of tRNA precursors. Nucleic Acids Res. 2003;31:4836–4846. doi: 10.1093/nar/gkg691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lejeune F., Li X., Maquat L.E. Nonsense-mediated mRNA decay in mammalian cells involves decapping, deadenylating, and exonucleolytic activities. Mol. Cell. 2003;12:675–687. doi: 10.1016/s1097-2765(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer R., Egberts W.V., Hengstman G.J.D., Raijmakers R., van Engelen B.G.M., Seelig H.P., Renz M., Mierau R., Gendt E., Pruijn G.J.M., et al. Autoantibodies directed to novel components of the PM/Scl complex, the human exosome. Arthritis Res. 2002;4:134–138. doi: 10.1186/ar389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raijmakers R., Renz M., Wiemann C., Egberts W.V., Seelig H.P., van Venrooij W.J., Pruijn G.J. PM-Scl-75 is the main autoantigen in patients with the polymyositis/scleroderma overlap syndrome. Arthritis Rheum. 2004;50:565–569. doi: 10.1002/art.20056. [DOI] [PubMed] [Google Scholar]
- 30.Allmang C., Mitchell P., Petfalski E., Tollervey D. Degradation of ribosomal RNA precursors by the exosome. Nucleic Acids Res. 2000;28:1684–1691. doi: 10.1093/nar/28.8.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyers F., Rougemaille M., Badis G., Rousselle J.C., Dufour M.E., Boulay J., Regnault B., Devaux F., Namane A., Seraphin B., et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121:725–737. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 32.LaCava J., Houseley J., Saveanu C., Petfalski E., Thompson E., Jacquier A., Tollervey D. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121:713–724. doi: 10.1016/j.cell.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Vanacova S., Wolf J., Martin G., Blank D., Dettwiler S., Friedlein A., Langen H., Keith G., Keller W. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS. Biol. 2005;3:e189. doi: 10.1371/journal.pbio.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alderuccio F., Chan E.K., Tan E.M. Molecular characterization of an autoantigen of PM-Scl in the polymyositis/scleroderma overlap syndrome: a unique and complete human cDNA encoding an apparent 75-kD acidic protein of the nucleolar complex. J. Exp. Med. 1991;173:941–952. doi: 10.1084/jem.173.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bluthner M., Bautz F.A. Cloning and characterization of the cDNA coding for a polymyositis-scleroderma overlap syndrome-related nucleolar 100-kD protein. J. Exp. Med. 1992;176:973–980. doi: 10.1084/jem.176.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raijmakers R., Noordman Y.E., van Venrooij W.J., Pruijn G.J. Protein–protein interactions of hCsl4p with other human exosome subunits. J. Mol. Biol. 2002;315:809–818. doi: 10.1006/jmbi.2001.5265. [DOI] [PubMed] [Google Scholar]
- 37.Targoff I.N., Reichlin M. Nucleolar localization of the PM-Scl antigen. Arthritis Rheum. 1985;28:226–230. doi: 10.1002/art.1780280221. [DOI] [PubMed] [Google Scholar]
- 38.Raijmakers R., Schilders G., Pruijn G.J. The exosome, a molecular machine for controlled RNA degradation in both nucleus and cytoplasm. Eur. J. Cell Biol. 2004;83:175–183. doi: 10.1078/0171-9335-00385. [DOI] [PubMed] [Google Scholar]
- 39.Reddy R., Rothblum L.I., Subrahmanyam C.S., Liu M.H., Henning D., Cassidy B., Busch H. The nucleotide sequence of 8 S RNA bound to preribosomal RNA of Novikoff hepatoma. The 5′-end of 8 S RNA is 5.8 S RNA. J. Biol. Chem. 1983;258:584–589. [PubMed] [Google Scholar]
- 40.Bowman L.H., Goldman W.E., Goldberg G.I., Hebert M.B., Schlessinger D. Location of the initial cleavage sites in mouse pre-rRNA. Mol. Cell. Biol. 1983;3:1501–1510. doi: 10.1128/mcb.3.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowman L.H., Rabin B., Schlessinger D. Multiple ribosomal RNA cleavage pathways in mammalian cells. Nucleic Acids Res. 1981;9:4951–4966. doi: 10.1093/nar/9.19.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]