Abstract
Mitochondrial dysfunction, with an estimated incidence of 1 in 5,000 births, is associated with a wide variety of multisystem degenerative diseases. Among the most prevalent forms of dysfunction are defects in the NADH:ubiquinone oxidoreductase (complex I). Caenorhabditis elegans strains with complex I mutations exhibit characteristic features of human mitochondrial disease including decreased rates of respiration and lactic acidosis. We hypothesized that introducing an additional pathway for the direct oxidation of lactate would be beneficial for energy metabolism. The yeast CYB2 gene encodes an l-lactate:cytochrome c oxidoreductase that oxidizes lactate, donates electrons directly into the mitochondrial respiratory chain, and supports lactate-dependent respiration. Cyb2p expression markedly increases lifespan, fertility, respiration rates, and ATP content in complex I-deficient animals. Our results indicate that metabolic imbalance leading to lactic acidosis and energy depletion are central mechanisms of pathogenesis in mitochondrial dysfunction and that introduction of an additional pathway for lactate oxidation should be considered as a treatment.
Keywords: complex I, energy metabolism, mitochondria, NADH:ubiquinone oxidoreductase, nematode
Mammalian complex I, embedded in the mitochondrial inner membrane, is the largest respiratory chain complex. It is composed of at least 46 subunits, 7 of which are encoded in the mitochondrial DNA (mtDNA) (1, 2). The complex contains a flavin mononucleotide (FMN) cofactor that serves as the first electron acceptor upon NADH oxidation. Electron transfer from NADH to ubiquinone is coupled to proton pumping across the mitochondrial inner membrane and the formation of a proton gradient (3–5). Complex I dysfunction is associated with myopathies, encephalomyopathies, and neurodegenerative disorders such as Parkinson's disease and Leigh syndrome (6–9). Currently, there are no cures for diseases resulting from mitochondrial respiratory chain (MRC) dysfunction (10, 11).
Complex I is the primary route for the oxidation of NADH generated by glycolysis and the citric acid cycle in aerobic cells. NADH accumulates when complex I activity is impaired (12). Excess NADH inhibits glycolysis, the citric acid cycle, and the pyruvate dehydrogenase complex, resulting in a build-up of pyruvate in the cell. Cytosolic lactate dehydrogenases use NADH to reduce pyruvate to lactate, resulting in hyperlactatemia and lactic acidosis, which can directly cause malaise, muscle weakness, exercise intolerance, and vomiting (13–16). Chronic redox imbalance likely modulates gene expression and may account for the often progressive nature of mitochondrial diseases (16–19).
To better understand the molecular mechanisms of pathogenesis of mitochondrial dysfunction, we developed a Caenorhabditis elegans model of complex I deficiency (20). The nuo-1 gene is the nematode ortholog of the human NDUFV1 gene; these genes encode the 51-kDa active site subunit of complex I (21). Modified nuo-1 genes containing the disease-causing, missense mutations A352V, T434M, or A443F were introduced into a nematode strain with a nuo-1 deletion background (20). The resulting transgenic strains demonstrate hallmark features of complex I dysfunction such as vitamin-responsive lactic acidosis and decreased NADH-dependent mitochondrial respiration. Addressing the lactic acidosis produces substantial improvements in mutant fitness, suggesting that redox imbalance and acidosis are key pathogenic mechanisms in complex I deficiency (20).
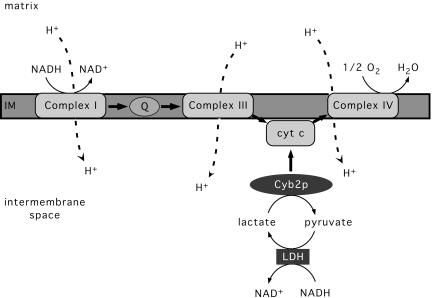
We have developed an alternative gene therapy strategy using the Saccharomyces cerevisiae CYB2 gene. CYB2 encodes a homotetrameric heme and FMN-containing l-lactate:cytochrome c oxidoreductase, known as cytochrome b2 (Cyb2p) (22). Cyb2p is normally located in the yeast mitochondrial intermembrane space, where it oxidizes lactate to pyruvate and directly reduces cytochrome c (Fig. 1) (23). Cyb2p-like enzymes are not found in C. elegans or in mammals. We hypothesized that Cyb2p would both oxidize lactate and contribute to ATP generation. When coupled with an endogenous NADH-linked cytosolic lactate dehydrogenase, Cyb2p activity will provide an additional pathway for NADH oxidation coupled to ATP synthesis by the MRC.
Fig. 1.
Cyb2p-mediated oxidation of lactate and NADH. Complexes I, III, and IV are found in the mitochondrial inner membrane (IM), and they translocate protons to the intermembrane space. LDH, an endogenous cytosolic lactate dehydrogenase; Q, ubiquinone; cyt c, cytochrome c.
CYB2 expression resulted in significantly increased reproductive capabilities, respiration rates, ATP levels, and lifespans. Cyb2p also decreases lactate concentrations. CYB2 expression is an example of a stable gene therapy strategy with considerable benefits in a complex I-deficient animal model system. Our data emphasize the importance of addressing redox imbalance and lactic acidosis as a means of improving energy generation in cases of mitochondrial dysfunction.
Materials and Methods
Strains. Worms were cultured as described in ref. 24. We used the following C. elegans strains: N2 (Bristol) wild type; LB25, nuo-1(ua-1) II, unc-119(ed3) III, uaEx25[p016bA352V]; and LB27, nuo-1(ua-1) II, unc-119(ed3) III, uaEx27[p016bA443F]. uaEx25 and uaEx27 are extrachromosomal arrays that encode the nuo-1(A352V) or the nuo-1(A443F) point mutations, respectively (20).
Plasmid Constructs. For construction of the Plet-858::CYB2 expression plasmid, a 1.8-kb fragment of DNA encoding the CYB2 ORF was amplified by PCR from S. cerevisiae genomic DNA. The oligonucleotides encoded restriction enzyme sites (underlined; forward, SstI; reverse, SalI) to facilitate cloning into the C. elegans expression vector pEXPlet-858 (25): forward, 5′-CTGATGTCGACGTCGCTAATACAGTTCCC-3′ and reverse, 5′-TAGCTGAGCTCGGCTATAATCATGCATCCTC-3′. pEXPlet-858 is a Gateway Cloning Technology-compatible (Invitrogen) vector that contains a 3.5-kb fragment encoding the let-858 promoter. The Pnuo-1::CYB2 expression vector places the CYB2 gene downstream of a 0.6-kb fragment encoding the nuo-1 promoter region. All sequences amplified by PCR were confirmed by DNA sequencing.
Generation of Transgenic C. elegans. CYB2 expression constructs (17 ng/μl) were coinjected into the syncytial gonads of young adult hermaphrodites with pDP #SU006 (170 ng/μl), a reporter plasmid that carries the green fluorescent protein gene under the control of the F25B3.3 promoter (26). F25B3.3 encodes the C. elegans ortholog of the Ca2+-regulated ras nucleotide exchange factor CalDAG-GEFII/RasGRP, which is ubiquitously expressed in the nervous system (27). A minimum of three independent transgenic lines were generated for each strain and CYB2 construct.
Phenotypic Analyses. Lifespan measurement and brood size determination were performed with GFP-expressing (transgene-containing) animals as described in ref. 20. For the analysis of animal morphology, adult animals were mounted on 2% agarose pads and observed under a Zeiss Axioskop-2 research microscope equipped with Nomarski and fluorescence optics and a SPOT-2 digital camera (Zeiss).
Electrophoresis. To detect Cyb2p expression, trichloroacetic acid-precipitated nematode protein was resolved by SDS/polyacrylamide gel electrophoresis and transferred electrophoretically to nitrocellulose membrane. A rabbit polyclonal antiserum raised against S. cerevisiae Cyb2p (28) followed by peroxidase-labeled goat anti-rabbit secondary antibody allowed detection by enhanced chemiluminescence (Amersham Pharmacia Biosciences). Blots were stripped and reprobed with a rabbit polyclonal antiserum raised against the S. cerevisiae ATP synthase β-subunit (21, 28).
Polarographic Analyses. Oxygen consumption rates were measured by using a Strathkelvin 1302 oxygen electrode with an MT200 Mitocell respiration chamber (Strathkelvin Instruments, Glasgow, U.K.). For whole-animal respiration rates, synchronized late L4 to early adult worms were washed extensively and resuspended to ≈10,000 animals per ml. Sixty-microliter aliquots of worms were introduced into the Mitocell chamber, which was maintained at 22°C, and oxygen consumption was measured for at least 10 min. Samples were carefully recovered from the chamber, and the worms were lysed with 1.85 M NaOH/7.4% 2-mercaptoethanol. Total protein was recovered by trichloroacetic acid precipitation and solubilized in 50 μl of 5% SDS/62.5 mM Tris·HCl, pH 6.8 for quantitation by the Bio-Rad DC Protein Assay. A minimum of four replicates were performed for each strain.
Mitochondria were isolated from worms grown in liquid culture as described in ref. 20. l-lactate-dependent respiration was measured in isolated mitochondria that had been diluted 10-fold in 0.1 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hepes), pH 7.4, and disrupted by sonication in an ice-water bath (20). To 20 μg of mitochondrial protein were added rotenone, antimycin A, and l-lactate to 100 nM, 75 nM, and 10 mM, respectively, and the volume was adjusted to 60 μl with 0.1 M Hepes, pH 7.4. Respiration rates obtained in the absence of l-lactate were measured and subtracted. Five replicates for each strain and construct were performed to calculate average rates.
Enzyme Assays. Rotenone-sensitive NADH:decylubiquinone oxidoreductase activity (complex I) and KCN-sensitive cytochrome c oxidase activities were determined as described in ref. 20. l-lactate:cytochrome c oxidoreductase assays were performed at 30°C by using 50 μg of disrupted mitochondria as for the succinate:cytochrome c oxidoreductase assays except that 10 mM lactate was used as substrate, the inhibitor malonate was omitted, and activity in the absence of lactate was subtracted (20). Reported activities represent the mean of at least five determinations.
l-Lactate Dehydrogenase Histochemistry. Permeabilization and fixation of worms were performed as described in ref. 29. An l-lactate dehydrogenase staining protocol was adapted for C. elegans (30). Synchronized, fixed, and permeabilized young adults were incubated in 1 ml of 5 mM potassium phosphate, pH 7.4/0.4 mM phenazine methosulfate/0.5 mM tetranitro blue tetrazolium/0.05 M sodium l-lactate for 2 h at 37°C in the dark with constant rotation. Control samples were incubated in the absence of lactate. Stained worms were centrifuged for 5 min at 360 × g and washed three times for 5 min in phosphate buffer before photography.
Measurement of Metabolite Concentrations. Worms were cultured in liquid medium, harvested, extensively washed, and resuspended in 27 ml of water at a concentration of ≈20 mg of protein per ml. Protein-free extracts were obtained by trichloroacetic acid precipitation (20). To determine total ATP content, synchronized late L4 to early adult animals were washed repeatedly in S Basal buffer (0.1 M NaCl/0.05 M potassium phosphate buffer, pH 6.0), resuspended in a final volume of 100 μl, and frozen at –80°C. The frozen worms were immediately immersed in a boiling water bath for 15 min, cooled, and centrifuged to pellet lysed nematodes. The supernatant was removed to a fresh tube and serially diluted. ATP content was determined by using the ATP bioluminescence assay kit CLS II (Roche Molecular Biochemicals) with a Lumat LB 9501 luminometer (Berthold Technologies, Oak Ridge, TN). The values reported are means of at least three independent determinations.
Oxidative Stress Assays. A minimum of 50 L1 larvae were placed onto seeded nematode growth medium (NGM) plates and incubated at 22°C in an atmosphere of 100% oxygen or on plates containing 0.2 mM paraquat (20). Survival, measured as the fraction of L1 larvae that developed into adults, was scored after 5 days. A minimum of 10 trials were performed for each strain and construct.
Results
Generation of Transgenic C. elegans Strains Expressing CYB2. The S. cerevisiae gene CYB2 was cloned into C. elegans expression vectors under the control of the promoter for the let-858 gene or the nuo-1 gene. The let-858 gene is a member of the lethal gene class and encodes nucampholin, a ubiquitously expressed protein required for early embryogenesis and tissue differentiation (31). Both the let-858 and the nuo-1 promoters result in the ubiquitous expression of CYB2. LB25 carries on the uaEx25 extrachromosomal array a mutated version of nuo-1 encoding an A352V substitution. Similarly, LB27 expresses a version of nuo-1 with an A443F substitution. LB25 and LB27 are both homozygous for the nuo-1(ua1) allele, a lethal deletion (21). LB25 and LB27 were chosen because their overall fitness is markedly impaired and their phenotypes include features typical of mitochondrial diseases such as riboflavin- and dichloroacetate-responsive lactic acidosis. In the strains we chose to investigate in detail, the Plet-858::CYB2 and Pnuo-1::CYB2 transgenes in N2 strains were transmitted to progeny at rates of 70 ± 8% and 64 ± 9%, respectively, 51 ± 7% and 62 ± 8% for the LB25 strains, respectively, and 62 ± 12% and 51 ± 9% for the LB27 strains, respectively.
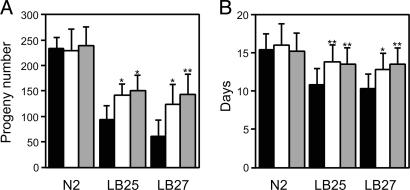
Characterization of CYB2 Transgenic Strains. CYB2 expression is strongly beneficial to overall animal health and fertility. We noted significant increases in the average brood size of 44–54% in LB25 and 92–123% in LB27 strains expressing CYB2 compared with their parental mutant strains (Fig. 2A). Similarly, the average lifespan of LB25 and LB27 is increased 25–28% and 24–31%, respectively, with CYB2 expression (Fig. 2B). The CYB2 constructs do not affect the N2 strain.
Fig. 2.
Effects of CYB2 expression on brood sizes and lifespans. (A) Wild-type (N2) and mutant (LB25 and LB27) animals were allowed to lay eggs at 20°C, and all progeny were counted. The values reported are averages of at least 18 counted broods. (B) Transgenic animals cultured at 20°C were monitored daily and scored as dead when they no longer responded to gentle prodding. The values reported are averages of a minimum of 24 animals. Black bars, no CYB2 transgene; white bars, Pnuo-1::CYB2-containing transgenes; gray bars, Plet-858::CYB2-containing transgenes. All P values (*, P < 0.05; **, P < 0.01) are derived from a two-sample t test when comparing with the corresponding strain without CYB2.
LB25 and LB27 nematodes display gross abnormalities in gonad morphology and muscle tissue integrity (20). Mutant adults often have shortened or malformed gonad arms with disorganized syncytia that appear shriveled, leaving space between somatic gonad tissue and the body wall. LB25 and LB27 strains carrying CYB2 transgenes displayed significantly lower frequencies of abnormal gonad morphology and fewer signs of tissue degeneration in 1-day-old adult hermaphrodites. Abnormal gonad morphology is present in ≈69% and 78% of 1-day-old adult LB25 and LB27 hermaphrodites, respectively, and decreases to 32–34% and 36–44%, respectively, with CYB2 expression. The CYB2 constructs do not affect the gonad morphology of N2 strains. Many young adult mutants have large vacuole-like structures throughout the body, beneath the cuticle, suggesting premature degeneration of body wall muscle tissue (20). About 60% and 67% of 1-day-old adult LB25 and LB27 hermaphrodites, respectively, display prominent signs of tissue degeneration in the head region. Only 38–40% and 39–41% of similarly aged animals do so when carrying the CYB2 transgene.
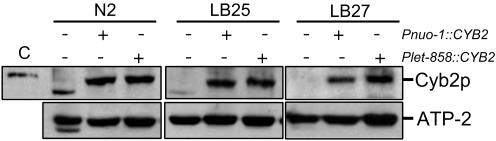
Cyb2p Is Expressed and Functionally Active. Cyb2p expression in the transgenic C. elegans strains was confirmed by Western blot analysis (28). Cyb2p is synthesized as a protein precursor of 591 aa (65.5 kDa) that includes an 80-residue bipartite N-terminal mitochondrial presequence (32). The Cyb2p presequence is removed by proteolysis upon successful import into the mitochondrial matrix and subsequent sorting to the intermembrane space, resulting in the production of mature Cyb2p (511 residues, 56.6 kDa). A Cyb2p-specific signal was detected only in C. elegans samples from Cyb2p-expressing strains (Fig. 3). The signal corresponded to a protein with an apparent molecular mass of just under 58 kDa, similar in size to native Cyb2p in yeast mitochondria. We believe this species to be the mature form of Cyb2p, indicating its successful import, sorting, and processing into nematode mitochondria. We did not detect higher molecular mass forms that might correspond to the precursor. As a loading control, we monitored the steady-state protein levels of ATP-2, the β-subunit of the ATP synthase, which do not vary in the complex I-deficient mutants (20).
Fig. 3.
Detection of Cyb2p in whole-nematode lysates by Western blot analysis. One hundred micrograms of protein were loaded per lane. C, yeast mitochondrial protein control. The blot was stripped and reprobed with antiserum against ATP-2.
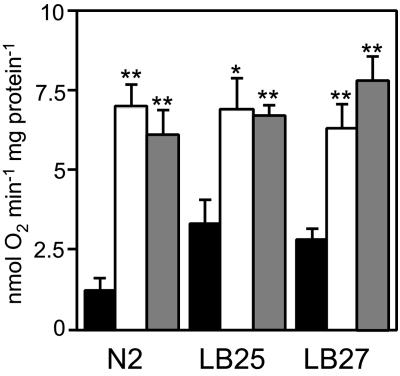
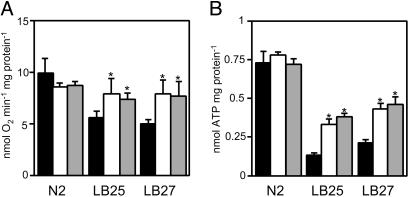
Our data strongly suggest that Cyb2p is functional in nematode mitochondria and contributes bioenergetically. We measured the l-lactate-dependent cytochrome c reductase activity in isolated worm mitochondria. Significant activity was detectable only in mitochondria from Cyb2p-expressing strains (Table 1). Cyb2p expression did not affect the activities of complexes I or IV (data not shown), which are compromised in LB25 and LB27 (20). Polarographic analysis of oxygen consumption rates revealed dramatically higher l-lactate-dependent respiration in isolated mitochondria of Cyb2p-expressing strains (Fig. 4). l-lactate dehydrogenase activity, detected as the black precipitate formed upon the phenazine methosulfate-mediated reduction of tetranitro blue tetrazolium, was also assessed histochemically in transgenic strains. l-lactate dehydrogenase activity appears most abundant in the anterior body wall muscles surrounding the pharyngeal bulbs of young adult hermaphrodites (data not shown). The nuo-1 promoter appeared to result in lower activity in the anterior regions, but more activity in muscle tissue throughout the body (data not shown). Finally, we measured whole-animal respiration by monitoring oxygen consumption in live nematodes. LB25 and LB27 are characterized by significantly decreased respiration rates to approximately half of wild type (20). Oxygen consumption rates increased 32–41% and 54–58% in LB25 and LB27, respectively, when Cyb2p was expressed (Fig. 5A). Respiration in Cyb2p-expressing N2 animals was not affected.
Table 1. Cyb2p activity affects lactate and pyruvate concentrations of transgenic animals.
| Strain | Cyb2p† | Lactate, mM | Pyruvate, mM | Lactate/pyruvate ratio |
|---|---|---|---|---|
| N2 | ND | 2.43 ± 0.51 | 0.148 ± 0.026 | 16.4 ± 4.5 |
| N2 + Pnuo-1::CYB2 | 3.9 ± 1.4 | 1.95 ± 0.18 | 0.117 ± 0.010 | 16.7 ± 2.1 |
| N2 + Plet-858::CYB2 | 3.8 ± 1.1 | 1.92 ± 0.12 | 0.114 ± 0.011 | 16.8 ± 1.9 |
| LB25 | ND | 3.06 ± 0.32 | 0.063 ± 0.007 | 48.6 ± 7.4 |
| LB25 + Pnuo-1::CYB2 | 4.2 ± 1.6 | 2.36 ± 0.12* | 0.068 ± 0.003 | 34.7 ± 2.5* |
| LB25 + Plet-858::CYB2 | 6.8 ± 2.7 | 2.00 ± 0.17* | 0.062 ± 0.004 | 32.3 ± 3.4* |
| LB27 | ND | 4.23 ± 0.75 | 0.052 ± 0.004 | 81.3 ± 15.6 |
| LB27 + Pnuo-1::CYB2 | 7.3 ± 2.2 | 3.03 ± 0.13 | 0.076 ± 0.007* | 39.9 ± 4.0* |
| LB27 + Plet-858::CYB2 | 8.4 ± 3.0 | 2.37 ± 0.53* | 0.063 ± 0.007 | 37.6 ± 9.3* |
The values reported are the means ± SD of a minimum of four determinations. *, P < 0.05, compared with corresponding parental strain by using a two-sample t test. ND, not detected.
Cyb2p activity was measured as l-lactate-dependent cytochrome c reductase activity. The values reported are nanomoles of cytochrome c per minute per milligram of protein.
Fig. 4.
l-lactate-dependent respiration in isolated mitochondria. The values reported are the means of at least five measurements. Black bars, no CYB2 transgene; white bars, Pnuo-1::CYB2-containing transgenes; gray bars, Plet-858::CYB2-containing transgenes. All P values (*, P < 0.05; **, P < 0.01) are derived from a two-sample t test when comparing with the corresponding strain without CYB2.
Fig. 5.
Cyb2p improves the overall metabolism of transgenic strains. (A) Oxygen consumption rates of live nematodes. The values reported are the means of five measurements. (B) Total ATP contents of transgenic animals. The values reported are the averages of three independent determinations. Black bars, no CYB2 transgene; white bars, Pnuo-1::CYB2-containing transgenes; gray bars, Plet-858::CYB2-containing transgenes. All P values (*, P < 0.05; **, P < 0.01) are derived from a two-sample t test when comparing with the corresponding strain without CYB2.
Cyb2p Contributes to Energy Generation. CYB2 expression improves animal health and increases respiration rates and thus likely contributes to energy production in transgenic strains. We determined ATP levels from extracts of synchronized, late L4-young adult hermaphrodites. LB25 and LB27 have severely depressed total ATP concentrations that are 20–30% of wild type (Fig. 5B). All mutant strains expressing CYB2 demonstrated considerable elevations in ATP concentrations of ≈2- to 3-fold. These levels, however, remain below wild-type levels, suggesting that Cyb2p-mediated respiration cannot fully compensate for the diminished complex I function. Taken together, our data demonstrate that CYB2 expression leads to increased respiration rates that contribute to the increased production of ATP through oxidative phosphorylation.
CYB2 Expression Moderates Lactic Acidosis. A key indicator of mitochondrial dysfunction is metabolic acidosis resulting from a diminished ability to oxidize NADH (10, 13, 33, 34). LB25 and LB27 have notably increased molar ratios of lactate to pyruvate, reflecting the metabolic imbalance in the metabolism of NADH (20). Protein-free extracts of CYB2-expressing strains contain significantly lower concentrations of lactate than control strains without Cyb2p (23–35% in LB25 and 28–44% in LB27) and correspondingly lower lactate-to-pyruvate ratios (Table 1). The lactate concentrations in the CYB2-expressing strains approach those seen in wild-type animals, although the molar ratios of lactate to pyruvate do not fully return to wild type. Pyruvate concentrations are relatively unchanged in all LB25 strains but are increased in LB27 strains, suggesting that the A352V and A443F mutations differ in the metabolic details of pathogenesis. No effects on lactate or pyruvate levels were observed in transgenic N2 animals. Our data strongly suggest that CYB2 expression can provide an alternate pathway for oxidizing lactate and NADH and thus partially correct the redox imbalance seen in the complex I-deficient mutants.
CYB2-Expressing C. elegans Have Increased Hypersensitivity to Oxidative Stress. Mitochondrial dysfunction can result in accelerated rates of reactive oxygen species generation and hypersensitivity to oxidative stress (35). LB25 and LB27 are hypersensitive to oxidative stress caused by hyperoxia or exposure to paraquat, a known generator of superoxide radicals (20). Surprisingly, transgenic strains expressing CYB2 showed increased sensitivity to oxidative stress (Fig. 6, which is published as supporting information on the PNAS web site). Transgenic N2 strains developed a hypersensitivity to paraquat. These data suggest that CYB2 expression may exacerbate reactive oxygen species generation in C. elegans mitochondria under conditions of oxidative stress.
Discussion
Elucidating the relationships between genotype and phenotype is a major challenge in the field of mitochondrial medicine. MRC dysfunction can result in at least four mechanisms of pathology: (i) abnormal cellular redox states, (ii) the impairment of numerous metabolic pathways that require MRC function, (iii) the generation of reactive oxygen species, and (iv) decreased ATP formation (12). The complex I-deficient strains of C. elegans we have generated provide evidence that all four of these mechanisms contribute to mutant phenotypes.
The pathogenic effects of MRC deficiency cannot always be explained by a lack of cellular energy. In nematodes with the gas-1(fc21) mutation in the 49-kDa subunit of complex I, lifespan correlates with the extent of oxidative damage to mitochondrial proteins rather than to the rates of oxidative phosphorylation (36). In 12 cell lines from complex I-deficient patients, there was little correlation between the degree of decrease in complex I activity and the severity of the symptoms; rather, there was a correlation with the lactate-to-pyruvate ratios (37). In another study, the rates of ATP production with complex I-linked substrates were found to be similar in mitochondria from patient or from control fibroblasts (38). Our results suggest that an abnormal redox state is a major determinant of the phenotype of complex I-deficient nematodes. The nuo-1 mutations we studied impair NADH-dependent mitochondrial respiration, leading to elevated NADH/NAD+ ratios and the NADH-dependent reduction of pyruvate to lactate. Mutant fitness is responsive to pharmacological agents that stimulate complex I activity and reduce the levels of NADH, or stimulate pyruvate dehydrogenase activity, which should reduce the levels of pyruvate (20). The severity of the mutant phenotypes is correlated to the level of lactic acidosis, although defects in the ability to generate ATP likely also contribute to pathology. These observations underlie the rationale for developing a gene therapy strategy that provides an alternative pathway for the metabolism of lactate.
Gene therapy strategies for complex I deficiency have focused on replacing the functionality of complex I rather than on correcting the enzyme itself. The S. cerevisiae NDI1 gene encodes a single-subunit, matrix-oriented NADH dehydrogenase that oxidizes NADH and reduces ubiquinone (23). Ndi1p does not however couple NADH oxidation to proton pumping and thus cannot fully replace complex I (39). When expressed in respiration-deficient Chinese hamster or human cell lines, Ndi1p could fully restore NADH-dependent respiration and the ability to grow on galactose (40–42). NDI1 has been efficiently and functionally expressed in nondividing human cells (43) and in dopaminergic neuronal cells by using a recombinant adeno-associated virus vector (44). Recently, NDI1 was successfully expressed in vivo in the skeletal muscle and brains of rodents, with sustained expression observed for at least 7 months (45).
In this study, we demonstrate that expression of the yeast Cyb2p, a lactate:cytochrome c oxidoreductase localized to the mitochondrial intermembrane space, introduces an additional pathway for lactate and NADH oxidation. This metabolic ability results in considerable improvement in animal fitness as measured by fertility and lifespan. CYB2 expression better supports the highly organized developmental processes needed to produce the adult germ line and somatic gonad. It also results in markedly fewer signs of premature body wall muscle tissue degeneration, suggesting that addressing metabolic imbalances in disease can result in a broad range of beneficial effects.
Mechanisms of import, processing, and targeting of proteins to mitochondria are highly conserved among widely divergent eukaryotes (46–49). Several lines of evidence suggest that the heterologous expression of the yeast CYB2 produced functional Cyb2p in nematode mitochondria. Immunologically, we detected Cyb2p that comigrated with mature Cyb2p from yeast mitochondria, indicating it was properly processed. l-lactate dependent cytochrome c reductase activity was present only in mitochondria of CYB2-expressing strains. Cyb2p activity was also detected in situ by means of histochemical staining. Finally, we demonstrated that CYB2 expression resulted in lactate-dependent respiration in isolated mitochondria and in increased total respiration in live animals.
Cyb2p expression may result in beneficial effects in at least two ways. First, lactate oxidation is coupled to the reduction of cytochrome c and complex IV activity. Complex IV pumps protons and contributes to the electrochemical proton gradient that drives ATP synthesis. We showed that Cyb2p expression results in the elevation of ATP levels in transgenic strains. This elevation is not surprising, given that mitochondria are the major site of ATP synthesis. Second, Cyb2p functions as an l-lactate dehydrogenase. When coupled with an endogenous NADH-linked cytosolic lactate dehydrogenase, Cyb2p reduces the cellular levels of lactate and NADH, thus correcting the redox imbalance (Fig. 1). We demonstrated significantly decreased lactate concentrations and decreased molar ratios of lactate to pyruvate in CYB2-expressing mutants. Correcting the redox imbalance may also influence the activities of other pathways such as glycolysis, the pentose phosphate pathway, the citric acid cycle, and fatty acid oxidation, all pathways that require NAD+ or NADP+ for their operation. The increased live animal respiration rates we observed in CYB2-expressing strains support our contention that overall metabolism is stimulated by the correction in redox imbalance.
Chronic redox imbalance may lead to deleterious effects on gene expression, contributing to the progressive nature of mitochondrial diseases. For example, the Sir2p family of proteins consists of NAD+-dependent histone deacetylases that affect gene silencing (17). The mammalian homolog of Sir2p, SIRT1, deacetylates not only histones but also key transcription factors (50). Furthermore, SIRT3, another homolog of Sir2p, can activate mitochondrial function and is localized to the mitochondrial inner membrane (51). Addressing redox imbalance may provide both short-term metabolic benefits and longer-term benefits in the treatment of mitochondrial disease by avoiding maladaptive regulation of gene expression.
Complex I is a known site of reactive oxygen species production in the MRC (52, 53). The pharmacological agents that addressed lactic acidosis also provided protection from oxidative stress, suggesting a mechanistic connection between oxidative stress and redox imbalance (20). Although Cyb2p clearly ameliorates the redox imbalance and leads to an elevation of ATP levels, it also increases sensitivity to oxidative stress. This phenomenon is independent of complex I dysfunction because wild-type nematodes expressing CYB2 were also rendered hypersensitive to paraquat. The Cyb2p-dependent production of reactive oxygen species must be mechanistically different during exposure to paraquat or to hyperoxia because only the former affects the survival of wild-type worms. One possible explanation is that Cyb2p is itself a site of reactive oxygen species production, possibly through its flavin or heme cofactors. Alternatively, the increased flow of electrons in the Cyb2p-containing mitochondria or the increased membrane potential as a result of the increased electron flow may stimulate the production of reactive oxygen species at other sites in the MRC. Further study will be needed to elucidate the exact mechanisms by which Cyb2p leads to sensitivity to oxidative stress.
In conclusion, we have demonstrated the successful use of a gene therapy strategy that introduces an alternative metabolic pathway to mitigate the effects of mitochondrial dysfunction. The yeast CYB2 gene targets the redox imbalance and lactic acidosis caused by complex I dysfunction, resulting in significant improvements in animal fitness. Our data strongly suggest that the disruption of cellular energy metabolism associated with redox imbalance is central to the pathology of mitochondrial diseases.
Supplementary Material
Acknowledgments
We thank Sam Szeto for his generous help in constructing plasmids and for many fruitful discussions. This work was supported by the Canadian Institutes of Health Research and the United Mitochondrial Disease Foundation. We acknowledge the Caenorhabditis Genetics Center, which is funded in part by the National Institutes of Health National Center for Research Resources, for providing strains.
Author contributions: B.D.L. designed research; L.I.G. and L.C.S. performed research; L.I.G. and B.D.L. analyzed data; and L.I.G. and B.D.L. wrote the paper.
Conflict of interest statement: No conflict declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MRC, mitochondrial respiratory chain; Cyb2p, cytochrome b2.
References
- 1.Carroll, J., Fearnley, I. M., Shannon, R. J., Hirst, J. & Walker, J. E. (2003) Mol. Cell Proteomics 2, 117–126. [DOI] [PubMed] [Google Scholar]
- 2.Gabaldón, T., Rainey, D. & Huynen, M. A. (2005) J. Mol. Biol. 348, 857–870. [DOI] [PubMed] [Google Scholar]
- 3.Yano, T. (2002) Mol. Aspects Med. 23, 345–368. [DOI] [PubMed] [Google Scholar]
- 4.Walker, J. E. (1992) Q. Rev. Biophys. 25, 253–324. [DOI] [PubMed] [Google Scholar]
- 5.Yagi, T. & Matsuno-Yagi, A. (2003) Biochemistry 42, 2266–2274. [DOI] [PubMed] [Google Scholar]
- 6.Robinson, B. H. (1998) Biochim. Biophys. Acta 1364, 271–286. [DOI] [PubMed] [Google Scholar]
- 7.Triepels, R. H., van den Heuvel, L. P., Trijbels, J. M. & Smeitink, J. A. (2001) Am. J. Med. Genet. 106, 37–45. [DOI] [PubMed] [Google Scholar]
- 8.Smeitink, J. A., Loeffen, J. L., Triepels, R. H., Smeets, R. J., Trijbels, J. M. & van den Heuvel, L. P. (1998) Hum. Mol. Genet. 7, 1573–1579. [DOI] [PubMed] [Google Scholar]
- 9.Shoubridge, E. A. (2001) Hum. Mol. Genet. 10, 2277–2284. [DOI] [PubMed] [Google Scholar]
- 10.Dimauro, S., Mancuso, M. & Naini, A. (2004) Ann. N.Y. Acad. Sci. 1011, 232–245. [DOI] [PubMed] [Google Scholar]
- 11.Smith, P. M., Ross, G. F., Taylor, R. W., Turnbull, D. M. & Lightowlers, R. N. (2004) Biochim. Biophys. Acta 1659, 232–239. [DOI] [PubMed] [Google Scholar]
- 12.Munnich, A. & Rustin, P. (2001) Am. J. Med. Genet. 106, 4–17. [DOI] [PubMed] [Google Scholar]
- 13.Chinnery, P. F. & Turnbull, D. M. (2001) Am. J. Med. Genet. 106, 94–101. [DOI] [PubMed] [Google Scholar]
- 14.Schuelke, M., Smeitink, J., Mariman, E., Loeffen, J., Plecko, B., Trijbels, F., Stockler-Ipsiroglu, S. & van den Heuvel, L. (1999) Nat. Genet. 21, 260–261. [DOI] [PubMed] [Google Scholar]
- 15.Bénit, P., Chretien, D., Kadhom, N., de Lonlay-Debeney, P., Cormier-Daire, V., Cabral, A., Peudenier, S., Rustin, P., Munnich, A. & Rötig, A. (2001) Am. J. Hum. Genet. 68, 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace, D. C. (1994) J. Bioenerg. Biomembr. 26, 241–250. [DOI] [PubMed] [Google Scholar]
- 17.Guarente, L. (2000) Genes Dev. 14, 1021–1026. [PubMed] [Google Scholar]
- 18.Starai, V. J., Celic, I., Cole, R. N., Boeke, J. D. & Escalante-Semerena, J. C. (2002) Science 298, 2390–2392. [DOI] [PubMed] [Google Scholar]
- 19.Lane, N. (2003) J. Theor. Biol. 225, 531–540. [DOI] [PubMed] [Google Scholar]
- 20.Grad, L. I. & Lemire, B. D. (2004) Hum. Mol. Genet. 13, 303–314. [DOI] [PubMed] [Google Scholar]
- 21.Tsang, W. Y., Sayles, L. C., Grad, L. I., Pilgrim, D. B. & Lemire, B. D. (2001) J. Biol. Chem. 276, 32240–32246. [DOI] [PubMed] [Google Scholar]
- 22.Mowat, C. G. & Chapman, S. K. (2000) in Enzyme-Catalyzed Electron and Radical Transfer, Subcellular Biochemistry, eds. Holzenburg, A. & Scrutton, N. S. (Kluwer, New York), Vol. 35, pp. 279–295. [Google Scholar]
- 23.de Vries, S. & Marres, C. A. (1987) Biochim. Biophys. Acta 895, 205–239. [DOI] [PubMed] [Google Scholar]
- 24.Lewis, J. A. & Fleming, J. T. (1995) Methods Cell Biol. 48, 3–29. [PubMed] [Google Scholar]
- 25.Ndegwa, S. & Lemire, B. D. (2004) Biochem. Biophys. Res. Commun. 319, 1307–1313. [DOI] [PubMed] [Google Scholar]
- 26.Mello, C. & Fire, A. (1995) Methods Cell Biol. 48, 451–482. [PubMed] [Google Scholar]
- 27.Altun-Gultekin, Z., Andachi, Y., Tsalik, E. L., Pilgrim, D., Kohara, Y. & Hobert, O. (2001) Development (Cambridge, U.K.) 128, 1951–1969. [DOI] [PubMed] [Google Scholar]
- 28.Dibrov, E., Fu, S. & Lemire, B. D. (1998) J. Biol. Chem. 273, 32042–32048. [DOI] [PubMed] [Google Scholar]
- 29.Xie, G., Jia, Y. & Aamodt, E. (1995) Genet. Anal. 12, 95–100. [DOI] [PubMed] [Google Scholar]
- 30.Owen, P., Graeme-Cook, K. A., Crowe, B. A. & Condon, C. (1982) Bacterial Membranes: Preparative Techniques and Criteria of Purity, Techniques in Lipid and Membrane Biochemistry, eds. Hasketh, T. R., Kornberg, H. L., Metcalfe, J. C., Northcote, D. H., Pogson, C. I. & Tipton, K. F. (Elsevier, Amsterdam), Part 1, pp. 1–69.
- 31.Kelly, W. G., Xu, S., Montgomery, M. K. & Fire, A. (1997) Genetics 146, 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neupert, W. (1997) Annu. Rev. Biochem. 66, 863–917. [DOI] [PubMed] [Google Scholar]
- 33.Scaglia, F., Towbin, J. A., Craigen, W. J., Belmont, J. W., Smith, E. O., Neish, S. R., Ware, S. M., Hunter, J. V., Fernbach, S. D., Vladutiu, G. D., et al. (2004) Pediatrics 114, 925–931. [DOI] [PubMed] [Google Scholar]
- 34.Munnich, A., Rötig, A., Chretien, D., Saudubray, J. M., Cormier, V. & Rustin, P. (1996) Eur. J. Pediatr. 155, 262–274. [DOI] [PubMed] [Google Scholar]
- 35.Senoo-Matsuda, N., Yasuda, K., Tsuda, M., Ohkubo, T., Yoshimura, S., Nakazawa, H., Hartman, P. S. & Ishii, N. (2001) J. Biol. Chem. 276, 41553–41558. [DOI] [PubMed] [Google Scholar]
- 36.Kayser, E. B., Sedensky, M. M. & Morgan, P. G. (2004) Mech. Ageing Dev. 125, 455–464. [DOI] [PubMed] [Google Scholar]
- 37.Pitkänen, S., Feigenbaum, A., Laframboise, R. & Robinson, B. H. (1996) J. Inherited Metab. Dis. 19, 675–686. [DOI] [PubMed] [Google Scholar]
- 38.Pitkänen, S., Merante, F., McLeod, D. R., Applegarth, D., Tong, T. & Robinson, B. H. (1996) Pediatr. Res. 39, 513–521. [DOI] [PubMed] [Google Scholar]
- 39.de Vries, S. & Grivell, L. A. (1988) Eur. J. Biochem. 176, 377–384. [DOI] [PubMed] [Google Scholar]
- 40.Bai, Y., Hajek, P., Chomyn, A., Chan, E. K. L., Seo, B. B., Matsuno-Yagi, A., Yagi, T. & Attardi, G. (2001) J. Biol. Chem. 276, 38808–38813. [DOI] [PubMed] [Google Scholar]
- 41.Seo, B. B., Kitajima-Ihara, T., Chan, E. K. L., Scheffler, I. E., Matsuno-Yagi, A. & Yagi, T. (1998) Proc. Natl. Acad. Sci. USA 95, 9167–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seo, B. B., Matsuno-Yagi, A. & Yagi, T. (1999) Biochim. Biophys. Acta 1412, 56–65. [DOI] [PubMed] [Google Scholar]
- 43.Seo, B. B., Wang, J., Flotte, T. R., Yagi, T. & Matsuno-Yagi, A. (2000) J. Biol. Chem. 275, 37774–37778. [DOI] [PubMed] [Google Scholar]
- 44.Seo, B. B., Nakamaru-Ogiso, E., Flotte, T. R., Yagi, T. & Matsuno-Yagi, A. (2002) Mol. Ther. 6, 336–341. [DOI] [PubMed] [Google Scholar]
- 45.Seo, B. B., Nakamaru-Ogiso, E., Cruz, P., Flotte, T. R., Yagi, T. & Matsuno-Yagi, A. (2004) Hum. Gene Ther. 15, 887–895. [DOI] [PubMed] [Google Scholar]
- 46.Glerum, D. M. & Tzagoloff, A. (1994) Proc. Natl. Acad. Sci. USA 91, 8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, Z. G., White, P. S. & Ackerman, S. H. (2001) J. Biol. Chem. 276, 30773–30778. [DOI] [PubMed] [Google Scholar]
- 48.Rodríguez-Aguilera, J. C., Asencio, C., Ruiz-Ferrer, M., Vela, J. & Navas, P. (2003) Biofactors 18, 237–244. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto, M., Shinohara, Y., Majima, E., Hatanaka, T., Yamazaki, N. & Terada, H. (1999) Biochim. Biophys. Acta 1409, 113–124. [DOI] [PubMed] [Google Scholar]
- 50.Bordone, L. & Guarente, L. (2005) Nat. Rev. Mol. Cell Biol. 6, 298–305. [DOI] [PubMed] [Google Scholar]
- 51.Shi, T., Wang, F., Stieren, E. & Tong, Q. (2005) J. Biol. Chem. 280, 13560–13567. [DOI] [PubMed] [Google Scholar]
- 52.Raha, S. & Robinson, B. H. (2001) Am. J. Med. Genet. 106, 62–70. [DOI] [PubMed] [Google Scholar]
- 53.Raha, S. & Robinson, B. H. (2000) Trends Biochem. Sci. 25, 502–508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.