Abstract
The responses to sound of auditory-nerve fibers are well known in many animals but are topics of conjecture for humans. Some investigators have claimed that the auditory-nerve fibers of humans are more sharply tuned than are those of various experimental animals. Here we invalidate such claims. First, we show that forward-masking psychophysical tuning curves, which were used as the principal support for those claims, greatly overestimate the sharpness of cochlear tuning in experimental animals and, hence, also probably in humans. Second, we calibrate compound action potential tuning curves against the tuning of auditory-nerve fibers in experimental animals and use compound action potential tuning curves recorded in humans to show that the sharpness of tuning in human cochleae is not exceptional and that it is actually similar to tuning in all mammals and birds for which comparisons are possible. Third, we note that the similarity of frequency of tuning across species with widely diverse cochlear lengths and auditory bandwidths implies that for any given stimulus frequency the “cochlear amplifier” is confined to a highly localized region of the cochlea.
Keywords: auditory nerve, basilar membrane, masking, compound action potential, psychophysical tuning curve
The perception of acoustic signals begins in the cochlea, where sounds are encoded in the activity of frequency-tuned auditory-nerve fibers (ANFs). The filtering characteristics of ANFs are well known for experimental animals (1) but are essentially unknown for humans, from whom recordings of individual ANFs are unavailable. Recently, renewed interest in the filtering characteristics of human ANFs was sparked by a study that derived measures of the sharpness of tuning of human ANFs from group delays of stimulus frequency otoacoustic emissions and forward-masking (FM) psychophysical tuning curves (PsychTCs) (2). That study has been enormously influential, and one of its central conclusions, namely that the sharpness of ANF frequency-threshold curves (ANFTCs) is much greater in humans than in experimental animals (2), has been cited in many papers (e.g., refs. 3-10). That conclusion coincides with an earlier study, based on the measurement of compound action potential tuning curves (APTCs) (11), but is at odds with an investigation that measured frequency selectivity in humans and chinchillas using the same psychophysical task (12).
In this article we review the literature of psychophysics and physiology addressing the relative sharpness of cochlear filtering in humans and other species. First, we explore the claims that FM PsychTCs correctly estimate ANFTCs in humans and that ANFTCs are sharper in humans than in experimental animal species (2). We disprove such claims by showing that FM PsychTCs greatly overestimate the sharpness of ANFTCs in experimental animals.
Second, we review the literature on APTCs in humans and experimental animals. We derive equations relating sharpness of tuning in ANFTCs and APTCs in experimental animals and use those equations to predict the sharpness of tuning of ANFTCs in humans on the basis of APTCs in humans. The predicted ANFTCs turn out to be unexceptional, with sharpness of tuning comparable to that of ANFs in all mammals and birds for which comparisons are possible.
Third, we note that the similarity of frequency of tuning across species with widely varying cochlear lengths and auditory bandwidths implies that for any given stimulus frequency the “cochlear amplifier” (13) is confined to a highly localized region of the cochlea.
Methods
Estimates of bandwidth and/or sharpness of tuning of ANFTCs, FM PsychTCs, simultaneous-masking (SM) PsychTCs, and APTCs were drawn from the literature. Estimates not explicitly stated in texts or tables were extracted from figures by digitization (digimatic, FEB Software, Chesterfield, VA). To facilitate comparisons across studies, sharpness of tuning is usually expressed in this article as Q10, i.e., center frequency divided by the 10-dB bandwidth. Q20 values were translated to Q10 values by using Eq. 1 [derived from a large data set of chinchilla ANFTCs (A.N.T., N. C. Rich, and M.A.R., unpublished observations)]. This adjustment is valid because Q20/Q10 is nearly constant with respect to characteristic frequency.
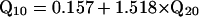 |
[1] |
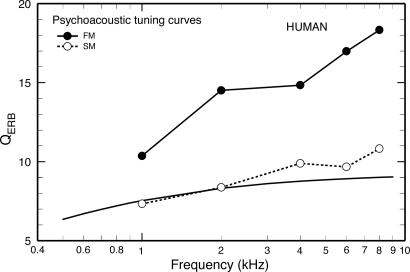
In Humans, FM PsychTCs Are More Sharply Tuned than SM PsychTCs. Fig. 1 compares the sharpness of tuning of SM and FM PsychTCs for humans, expressed as QERB [probe frequency divided by the equivalent rectangular bandwidth (ERB)]. Each of the QERB values for FM PsychTCs (filled symbols) is an average for eight subjects (14). The open symbols indicate average SM PsychTCs for four of the same subjects (open symbols) measured in the same study (14). In addition, the solid line indicates the trend (15) for SM PsychTCs measured in other studies (16-19). Fig. 1 illustrates the general finding in humans that FM PsychTCs are more sharply tuned than are SM PsychTCs (e.g., refs. 14 and 20-22). The sharper tuning of FM PsychTCs has been attributed to the absence of nonlinear effects that spuriously increase the apparent bandwidth of SM PsychTCs (reviewed in ref. 23, but see ref. 24). Therefore, it has been suggested that the FM PsychTCs of Fig. 1 (rather than the SM PsychTCs) should be taken as the true indicators of frequency tuning of ANFs in humans (14).
Fig. 1.
Sharpness of PsychTCs in humans. Filled symbols, QERB values of FM PsychTCs (from figure 7 of ref. 14); open symbols, QERB values of SM PsychTCs (from figure 7 of ref. 14); solid line with no symbols, trend for the QERB values of SM PsychTCs reported by several studies (16-19) (from figure 7 of ref. 15).
In Experimental Animals, FM PsychTCs Overestimate the Sharpness of Tuning of Cochlear Afferent Neurons. To test the validity of FM PsychTCs as measures of cochlear filtering in humans, we compared the Q10 values of FM PsychTCs and ANFTCs measured in the same experimental animal species (Fig. 2). Fig. 2 surveys the relationship among the sharpness of frequency tuning of ANFTCs, SM PsychTCs, and FM PsychTCs for the few mammals and birds for which adequate data exist in the literature.
Fig. 2.
Sharpness of PsychTCs and of ANFTCs ANFTCs in experimental animals. The Q10 values of ANFTCs (lines), SM PsychTCs (open symbols), and FM PsychTCs (filled symbols) are compared for monkeys (A), chinchillas (B), and birds (C). In addition, the Q10 values of ANFTCs and SM PsychTCs are compared for cats (D), gerbils (E), and guinea pigs (F). (A)Q10 values of ANFTCs for squirrel monkey (Saimiri sciureus) computed from data extracted from figure 3 of ref. 48; Q10 values of SM (○) and FM (•) PsychTCs for macaque monkeys (Macaca nemestrina) from figures 3 and 5 of ref. 49, and Q10 values of FM PsychTCs for patas monkeys (Erythrocebus patas)(▪) from table 1 of ref. 50. (B)Q10 values of ANFTCs for chinchilla (Chinchilla lanigera) from unpublished data of A.N.T., N. C. Rich, and M.A.R.; Q10 values of FM PsychTCs (•) (means from 10-dB data of table 4 of ref. 25); Q10 values of SM PsychTCs (○) (means from 10-dB data of table 4 of ref. 25); derived from ERBs from rippled (⋄) and notched noise (□) data in table 2 of ref. 26; ▿ from ref. 28 and▵ from ref. 27. (C)Q10 values of ANFTCs for pigeon (Columba livia) from ref. 32 and starling (Sturnus vulgaris) from ref. 33; •, Q10 values of FM PsychTCs of parakeets (Melopsittacus undulatus) from ref. 29; ○, Q10 values of SM PsychTCs of parakeets (Melopsittacus undulatus) from ref. 29; □, from ref. 30; ⋄, from ref. 31. (D)Q10 values of ANFTCs of cat (Felis catus) from ref. 44 as presented in figure 11 of ref. 51; ○, SM PsychTCs from ref. 52. [Pickles states that the mean Q10 at 1 kHz was 4.9, but measurements taken from his figure 7 yields a value of 4.3, as illustrated here.] (E) Gerbil (Meriones unguiculatus) ANF Q10 values from ref. 53 as presented in figure 11 of ref. 51; ○, SM PsychTC Q10 from ref. 54. (F)Q10 values of ANFTCs of guinea pig (Cavia porcellus) from ref. 43 as presented in figure 11 of ref. 51. Shown are SM PsychTC Q10 values derived from the ERBs of ref. 55; ○, comb-filtered noise (CFN); □, band-stop noise (BSN).
The SM and FM PsychTCs of macaque and patas monkeys are more sharply tuned than the ANFTCs of squirrel monkeys (Saimiri). In macaque monkey (Fig. 2 A) FM PsychTCs are more sharply tuned than are SM PsychTCs.
PsychTCs for chinchilla (Fig. 2B) have been measured in several laboratories (25-28). The only study in chinchilla that measured both FM and SM PsychTCs reported that they had similar bandwidths (25). However, Q10 values recomputed from those data, although confirming the reported similarity for 1-kHz probe tones, indicate sharper tuning of FM (compared with SM) PsychTCs for higher probe frequencies (Fig. 3B). The FM PsychTCs of ref. 25 are also much more sharply tuned than the ANFTCs and the SM PsychTCs reported by other studies.
Fig. 3.
Sharpness of SM APTCs in humans. The symbols represent mean Q10 values reported in refs. 41 (○), 11 (□), and 42 (⋄). The line is a least-squares fit to the Q10 values of refs. 11 and 41.
Parakeet FM PsychTCs are much more sharply tuned than SM PsychTCs (29-31) in the same species (Fig. 2C) and than ANFTCs of pigeons and starlings (32, 33). FM PsychTCs have not been measured in cats, gerbils, and guinea pigs (Fig. 2 D-F). In guinea pigs (Fig. 2F), SM PsychTCs and ANFTCs have similar Q10 values.
Fig. 2 may be summarized as follows:
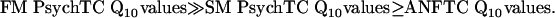 |
One claim (2) that ANFTCs are sharper in humans than in experimental animals was based on two key assumptions: (i) the group delay of the cochlear traveling wave at its peak equals half of the group delay of stimulus-frequency otoacoustic emissions; and (ii) FM PsychTCs are accurate indicators of cochlear tuning (2, 14, 23). The first assumption was recently shown to be incorrect (34). The second assumption is contradicted by Fig. 2, which shows that FM PsychTCs greatly overestimate the sharpness of ANFTCs in experimental animals. The implication is that cochlear tuning in humans is not exceptionally sharp. The only other alternative is that the relative magnitudes of FM PsychTCs and ANFTCs differ fundamentally between humans and other species. This alternative seems unlikely because the auditory systems of humans and other mammals are so similar. For example, analyses of the cochleae of mammals have been used to advantage to account for the mechanical properties of the human cochlea (for example, see chapter 6 of ref. 35).
The results of Fig. 2 are consistent with a recent psychophysical study that estimated spectral resolvability in humans and chinchillas using identical phase-discrimination tasks and concluded that the cochlear filters of humans and chinchillas are similar (12).
Can PsychTCs Validly Estimate the Tuning of Cochlear Afferent Neurons? Because of cochlear compressive nonlinearities, which produce level-dependent filtering (13), PsychTCs can validly estimate ANFTCs (or other iso-response functions) only if the central auditory processes that mediate PsychTCs themselves function as linear systems. There is some evidence favoring that possibility. In humans, the stapedial reflex grows with stimulus level at rates reminiscent of those of input-output functions in the cochleae of experimental animals (see figure 1 of ref. 36). Also, psychophysical FM functions in humans grow at rates similar to those of cochlear input-output functions in experimental animals (37). These findings have been interpreted as evidence that “for substantial parts of the auditory system, the compressive nonlinearity is limited to the periphery” (36) and “forward masking itself is a linear process” (37).
Even if central auditory masking is linear (36), the estimation of ANFTCs by using PsychTCs is at least potentially vitiated by “off-frequency listening,” i.e., combining auditory information from multiple cochlear sites with different characteristic frequencies. It seems likely that off-frequency listening is responsible for the large differences between FM PsychTCs and ANFTCs in any given species (Fig. 2 A-C). Insight into this issue comes from a physiological study of ANFs that used an FM paradigm identical to the one used to measure FM PsychTCs (38). In ANFs, “although the (forward) masker does reduce the detectability of the probe tone... the threshold shifts are much less than those observed behaviorally, particularly for intense maskers” (38). The disproportionately greater increase of behavioral FM (vis-à-vis FM in ANFs) at higher masker levels implies that FM PsychTCs must be more sharply tuned than ANFTCs and that “behavioral forward masked thresholds must be the result of processes that occur central to the auditory nerve” (38). Relkin and Smith (39), noting that the growth of FM of the compound action potential “more closely corresponds to that observed psychophysically than does FM observed in the response of a single neuron,” hypothesized that detection of a forward masked probe is “due to a summation of responses across a population of fibers with a wide range of characteristic frequencies” (p. 139 of ref. 39). Such summation amounts to a physiological basis for off-frequency listening.
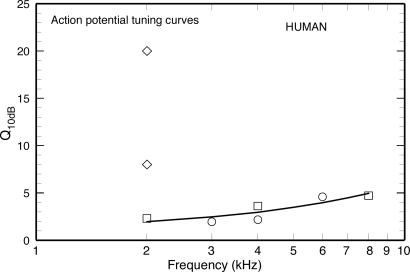
APTCs in Humans. By their very nature, APTCs (40) should estimate ANFTCs more accurately than PsychTCs: APTCs reflect the activity of limited populations of ANFs whereas PsychTCs presumably derive from the activity of neuronal networks distributed throughout the brain. Fortunately, measurements of APTCs are available for humans, as well as experimental animals. Therefore, to the extent that systematic quantitative relationships exist between the Q10 values of ANFTCs and APTCs in experimental animals, it should be possible to predict the Q10 values of human ANFTCs. Three studies (11, 41, 42) have used SM procedures to measure APTCs in humans. Their results are summarized in Fig. 3.
Two of the studies of SM APTCs in humans (circles and squares in Fig. 3) reported similar Q10 values for probe tones between 2 and 8 kHz (11, 41). A third study, by Rutten (42), reported Q10 values of 8 and 20 for a 2-kHz probe frequency (diamonds), values that are far out of line with the other results (11, 41). Rutten's estimates may reflect cochlear pathology (as suggested by Rutten himself for the Q10 value of 20; see p. 201 of ref. 42). Therefore, Rutten's estimates were not included in the computation of Eq. 2, which expresses SM APTC Q10 values in humans as a function of frequency (in kHz) and is illustrated as the solid line in Fig. 3.
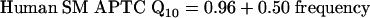 |
[2] |
APTCs in Experimental Animals. To use Eq. 2 to predict Q10 values for human ANFTCs it is necessary to first establish the relationship(s) between the Q10 values of ANFTCs and SM APTCs in experimental animals. Before the present review, only one study [by Harrison et al. (11)] derived ANFTCs from APTCs in humans by using correlated measurements of APTCs and ANFTCs in an experimental species, guinea pig (11). That study reached the conclusion that ANFs are more sharply tuned in humans than in guinea pigs or chinchillas.
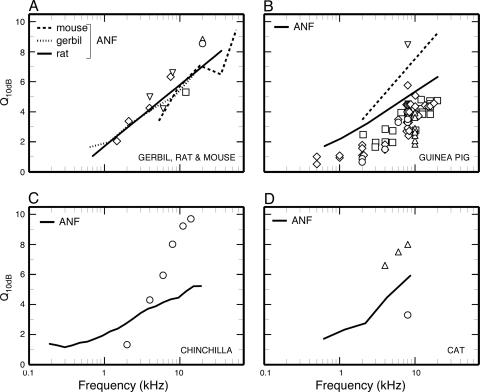
The Q10 values of ANFTCs and SM APTCs in several experimental animals are plotted in Fig. 4 (lines and symbols, respectively) against frequency. As may be seen in Fig. 4B, the Q10 values of ANFTCs recorded by Harrison et al. (11) in guinea pig (dashed line) by using manual control of stimulus frequency and intensity and audiovisual threshold detection are larger and increasingly depart with increasing frequency from those of a more recent study (43) (solid line), which used an automated procedure (e.g., ref. 44). We consider the latter data (43) to be more accurate, and we use them to compare with the SM APTCs. The many Q10 values of SM APTCs reported for guinea pigs (open symbols) are almost universally smaller than the Q10 values of ANFTCs by a roughly constant amount in the entire frequency range for which comparisons are possible.
Fig. 4.
Sharpness of ANFTCs and SM APTCs in experimental animals. (A) Gerbil: ANFTC Q10 values from ref. 53 as presented in figure 11 of ref. 51; ⋄, SM APTCs from figure 3 of ref. 56. Mouse: ANFTC Q10 values from figure 11 of ref. 51; □, SM APTC Q10 value computed from figure 3 of ref. 57;▵,Q20 values for probe levels ≤25-dB SPL (sound pressure level compared with 20 μPa) from figure 4 of ref. 58; ○, wild-type Q20 values from figure 4 of ref. 59. Rat: ANFTC Q10 values computed from Q20 values of ref. 60; ▿, SM APTCs from ref. 61. (B) Guinea pig. Solid line, ANFTC Q10 values (43) as presented in figure 11 of ref. 51; dashed line, least-squares fit to the ANFTC Q10 values of figure 3 of ref. 11. SM APTCs: Q10 values from figure 4 of ref. 40 (⋄), table 1 of ref. 62 (○), ref. 63 (▵), ref. 64 (▿), and ref. 11 (□). (C) Chinchilla. ANFTCs: unpublished data of A.N.T., N. C. Rich, and M.A.R. SM APTCs: ○,Q10 values computed from the APTCs of figure 2 of ref. 65. (D) Cat: Shown are ANFTC Q10 values from ref. 44 as presented in figure 11 of ref. 51. SM APTCs are from ref. 61 (▵) and ref. 66 (○).
The Q10 values of ANFTCs (lines) and SM APTCs (symbols) of gerbil, rat, and mouse (Fig. 4A) are about the same at any given frequency, so that a single trend line (data not shown) accounts for the variations of both ANFTC and SM-APTC Q10 values as a function of frequency in all three species.
The relation between ANFTC and SM-APTC Q10 values is more complex in chinchilla (Fig. 4C). In this species, the SM-APTC Q10 values grow larger and smaller than the ANFTC Q10 values, respectively, at frequencies higher and lower than 3 kHz by amounts proportional to the separation from 3 kHz. The results for cat (Fig. 4D) are inconclusive: three values of SM-APTC Q10 values are larger, but another is smaller, than the Q10 values of ANFTCs.
The relationship between the Q10 values of ANFTCs and SM APTCs in gerbil, rat, mouse, guinea pig, and chinchilla are summarized by the following equations.
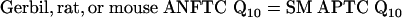 |
[3] |
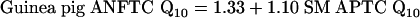 |
[4] |
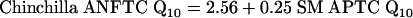 |
[5] |
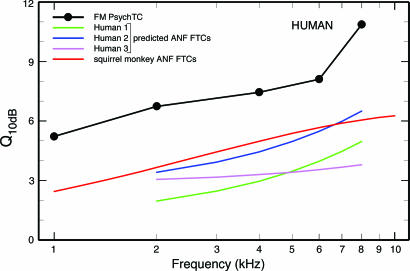
Sharpness of Tuning of Cochlear Afferent Neurons in Humans Predicted on the Basis of the Sharpness of Tuning of APTCs. On the assumption that the quantitative relations between the Q10 values of ANFTCs and SM APTCs in experimental animals also hold for humans, Eqs. 3, 4, 5 can be used to predict Q10 values for the ANFTCs of humans. Those Q10 values are shown in Fig. 5 (blue, magenta, and green curves). Fig. 5 also shows Q10 values for FM PsychTCs of humans (solid black symbols), which grossly exceed the Q10 values of ANFTCs predicted on the basis of APTC measurements. This result confirms the corresponding finding for experimental animals (Fig. 2).
Fig. 5.
Sharpness of PsychTCs in humans and ANFTCs in humans and monkey. Shown are Q10-vs.-frequency curves for human ANFTCs derived from equations 3 (green), 4 (blue), and 5 (magenta and the fit line for APTCs in humans (Fig. 3). For comparison, also shown are the Q10 values of ANFTCs measured in squirrel monkey (red), reproduced from Fig. 2, and the Q10 values of FM PsychTCs in humans, derived from figure 6 of ref. 14.
Fig. 5 also shows that the Q10 values predicted for human ANFTCs are similar to, or smaller than, the measured Q10 values for ANFTCs of squirrel monkeys (red curve). This comparison has special significance because monkeys are evolutionarily more closely related to humans than any other experimental animal for which information exists on the responses of ANFs. It has been suggested, on the basis of the similarity between the group delays of stimulus frequency otoacoustic emissions of humans (2) and monkeys (45), that the cochlear filters of primates are similar and much more sharply tuned than the cochlear filters of other species (see footnote on p. 3322 of ref. 2). Such a suggestion is proven incorrect by Fig. 6A.
Fig. 6.
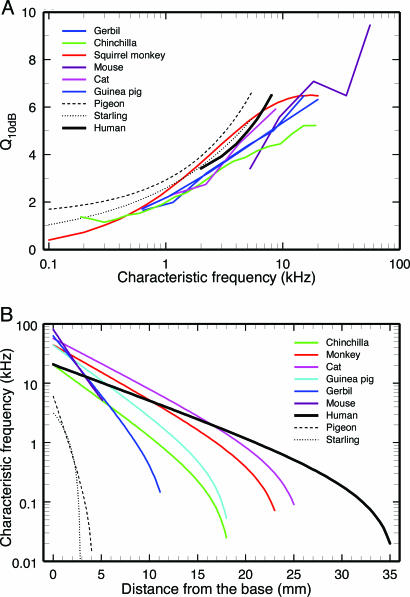
Similarity of frequency tuning of cochlear neurons of several species despite large differences in the cochlear maps. (A) Q10 values of cochlear neurons of experimental animals are compared with the largest predicted Q10 values for the ANFTCs of humans (reproduced from the blue curve of Fig. 5). (B) Cochlear maps for several mammalian and avian species, including humans. Data for most animals are from ref. 67; data for starling are from ref. 68, and data for pigeon are from ref. 69.
Similarity of Frequency Tuning in the Auditory Organs of Mammals and Birds Despite Large Differences in Length and Bandwidth. Fig. 6A gathers the highest Q10 values predicted for the ANFTCs of humans (blue curve of Fig. 5) and of the experimental species represented in Figs. 2 and 4. Fig. 6A shows that, in all represented species, including humans (black solid curve), the Q10 values vary similarly as functions of characteristic frequency. At most frequencies, the Q10 values of ANFTCs are confined to a relatively narrow band. Pigeons have the largest Q10 values, and chinchillas have the smallest. At most frequencies, even the highest Q10 estimates for humans occupy an intermediate position among estimates for other species.
The similarity of Q10 values across species is remarkable because their auditory organs are so different (Fig. 6B): (i) lengths range from 2.9 mm (in starling) to 35 mm (in humans); (ii) the distances allocated to any given octave vary between 0.6 mm (in pigeon or starling) and 5 mm (in humans); (iii) bandwidths range from only 2.9 kHz (in starling) to ≈80 kHz (in mouse).
It has been conjectured that the sharpness of cochlear frequency tuning is negatively correlated “with the frequency range of the... cochlea compared with its length” (p. 1382 of ref. 11). In other words, the greater the length of the basilar membrane dedicated to a given frequency range, the sharper the expected frequency tuning. This conjecture applies to certain highly specialized animals, such as echolocating bats, whose cochleae have “acoustic foveas” that dedicate as much as 60 mm to a single octave (46). However, Fig. 6 shows that such conjecture does not apply to less specialized species. For example, humans and chinchillas have almost identical hearing ranges, but, because the cochlea is almost twice as long in humans as in chinchillas, a given octave occupies nearly twice as much space in the former. Yet sharpness of frequency tuning (Fig. 6A) is similar in these two species.
The relative uniformity of sharpness of frequency tuning across species with enormously different basilar membrane lengths indicates that for any given frequency the cochlear amplifier that determines tuning in each ANF resides in a very restricted cochlear span (see pp. 1340-1341 of ref. 13), apparently occupying 0.5 mm (or less) in mammals (47) and even shorter distances in birds (see p. 271 of ref. 46).
Conclusions
(i) FM PsychTCs greatly overestimate the sharpness of tuning of ANFTCs in experimental animals and, hence, probably also in humans. (ii) The tuning of ANFTCs that we have derived from APTCs in humans (on the basis of the relationship between Q10 values of ANFTCs and APTCs in experimental animals) is similar to the tuning of directly measured ANFTCs in other vertebrates species. (iii) In the face of great differences of basilar membrane lengths and auditory ranges, the similarity of tuning across species indicates that sharpness of tuning does not depend on basilar membrane length per octave and suggests that for any given frequency the cochlear amplifier resides in a very short region of the cochlea.
Acknowledgments
We thank Hongxue Cai, Mary Ann Cheatham, Sumitrajit Dhar, Yun-Hui Fan, Jonathan Siegel, Beverly Wright, and Jozef Zwislocki for reading and criticizing previous drafts of this article. The writing of this article was supported by National Institutes of Health Grant DC-00419 from the National Institute on Deafness and Other Communication Disorders.
Author contributions: M.A.R. designed research; M.A.R. and A.N.T. performed research; M.A.R. and A.N.T. analyzed data; and M.A.R. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: APTC, compound action potential tuning curve; ANF, auditory-nerve fiber; ANFTC, ANF frequency-threshold curve; SM, simultaneous-masking; FM, forward-masking; PsychTC, psychophysical tuning curve; ERB, equivalent rectangular bandwidth.
References
- 1.Ruggero, M. A. (1992) in The Mammalian Auditory Pathway: Neurophysiology, eds. Popper, A. N. & Fay, R. R. (Springer, New York), pp. 34-93.
- 2.Shera, C. A., Guinan, J. J. & Oxenham, A. J. (2002) Proc. Natl. Acad. Sci. USA 99, 3318-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin, G. K., Villasuso, E. I., Stagner, B. B. & Lonsbury-Martin, B. L. (2003) Hear. Res. 177, 111-122. [DOI] [PubMed] [Google Scholar]
- 4.Withnell, R. H., Shaffer, L. A. & Talmadge, C. L. (2003) Hear. Res. 178, 106-117. [DOI] [PubMed] [Google Scholar]
- 5.LePage, E. L. (2003) J. Acoust. Soc. Am. 114, 896-906. [DOI] [PubMed] [Google Scholar]
- 6.Nelson, P. C. & Carney, L. H. (2004) J. Acoust. Soc. Am. 116, 2173-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes, S. D., Sumner, C. J., O'Mard, L. P. & Meddis, R. (2004) J. Acoust. Soc. Am. 116, 3534-3545. [DOI] [PubMed] [Google Scholar]
- 8.Withnell, R. H. & McKinley, S. (2005) J. Acoust. Soc. Am. 117, 281-291. [DOI] [PubMed] [Google Scholar]
- 9.Cedolin, L. & Delgutte, B. (2005) J. Neurophysiol. 94, 347-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonsbury-Martin, B. L. & Martin, G. K. (2003) Curr. Opin. Otolaryngol. Head Neck Surg. 11, 361-366. [DOI] [PubMed] [Google Scholar]
- 11.Harrison, R. V., Aran, J. M. & Erre, J. P. (1981) J. Acoust. Soc. Am. 69, 1374-1385. [DOI] [PubMed] [Google Scholar]
- 12.Shofner, W. P., Sparks, K., Wu, Y. E. & Pham, E. (2005) Acoust. Res. Lett. Online 6, 35-40. [Google Scholar]
- 13.Robles, L. & Ruggero, M. A. (2001) Physiol. Rev. 81, 1305-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oxenham, A. J. & Shera, C. A. (2003) J. Assoc. Res. Otolaryngol. 4, 541-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasberg, B. R. & Moore, B. C. (1990) Hear. Res. 47, 103-138. [DOI] [PubMed] [Google Scholar]
- 16.Moore, B. C. & Glasberg, B. R. (1983) J. Acoust. Soc. Am. 74, 750-753. [DOI] [PubMed] [Google Scholar]
- 17.Dubno, J. R. & Dirks, D. D. (1989) J. Acoust. Soc. Am. 85, 1666-1675. [DOI] [PubMed] [Google Scholar]
- 18.Shailer, M. J., Moore, B. C., Glasberg, B. R., Watson, N. & Harris, S. (1990) J. Acoust. Soc. Am. 88, 141-148. [DOI] [PubMed] [Google Scholar]
- 19.Moore, B. C., Peters, R. W. & Glasberg, B. R. (1990) J. Acoust. Soc. Am. 88, 132-140. [DOI] [PubMed] [Google Scholar]
- 20.Moore, B. C., Glasberg, B. R. & Roberts, B. (1984) J. Acoust. Soc. Am. 76, 1057-1066. [DOI] [PubMed] [Google Scholar]
- 21.Moore, B. C. (1978) J. Acoust. Soc. Am. 63, 524-532. [DOI] [PubMed] [Google Scholar]
- 22.Moore, B. C. & Glasberg, B. R. (1981) J. Acoust. Soc. Am. 70, 1003-1014. [DOI] [PubMed] [Google Scholar]
- 23.Pickles, J. O. (1988) An Introduction to the Physiology of Hearing (Academic, London).
- 24.Weber, D. L. & Patterson, R. D. (1984) J. Acoust. Soc. Am. 75, 925-931. [DOI] [PubMed] [Google Scholar]
- 25.McGee, T., Ryan, A. & Dallos, P. (1976) J. Acoust. Soc. Am. 60, 1146-1150. [DOI] [PubMed] [Google Scholar]
- 26.Niemiec, A. J., Yost, W. A. & Shofner, W. P. (1992) J. Acoust. Soc. Am. 92, 2636-2649. [DOI] [PubMed] [Google Scholar]
- 27.Salvi, R. J., Ahroon, W. A., Perry, J. W., Gunnarson, A. D. & Henderson, D. (1982) Am. J. Otolaryngol. 3, 408-416. [DOI] [PubMed] [Google Scholar]
- 28.Clark, W. W. & Bohne, B. A. (1986) in Sensorineural Hearing Loss: Mechanisms, Diagnosis, and Treatment, eds. Collins, M. J., Glattke, T. J. & Harker, L. A. (Univ. of Iowa Press, Iowa City).
- 29.Kuhn, A. & Saunders, J. C. (1980) J. Acoust. Soc. Am. 68, 1892-1894. [Google Scholar]
- 30.Saunders, J. C., Rintelmann, W. F. & Bock, G. R. (1979) Hear. Res. 1, 303-323. [DOI] [PubMed] [Google Scholar]
- 31.Saunders, J. C. & Else, P. V. (1976) Trans. Sect. Otolaryngol. Am. Acad. Ophthalmol. Otolaryngol. 82, 356-362. [PubMed] [Google Scholar]
- 32.Sachs, M. B., Young, E. D. & Lewis, R. H. (1974) Brain Res. 70, 431-447. [DOI] [PubMed] [Google Scholar]
- 33.Manley, G. A., Gleich, O., Leppelsack, H. J. & Oeckinghaus, H. (1985) J. Comp. Physiol. A 157, 161-181. [DOI] [PubMed] [Google Scholar]
- 34.Siegel, J. H., Cerka, A. J., Recio-Spinoso, A., Temchin, A. N., van Dijk, P. & Ruggero, M. A. (2005) J. Acoust. Soc. Am. 118, 2434-2443. [DOI] [PubMed] [Google Scholar]
- 35.Zwislocki, J. J. (2002) Auditory Sound Transmission: An Autobiographical Perspective (Lawrence Erlbaum, Mahwah, NJ).
- 36.Zwislocki, J. J. (2002) Proc. Natl. Acad. Sci. USA 99, 14601-14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oxenham, A. J. & Bacon, S. P. (2004) in Compression: From Cochlea to Cochlear Implants, eds. Bacon, S. P., Popper, A. N. & Fay, R. R. (Springer, New York), pp. 62-106.
- 38.Relkin, E. M. & Turner, C. W. (1988) J. Acoust. Soc. Am. 84, 584-591. [DOI] [PubMed] [Google Scholar]
- 39.Relkin, E. M. & Smith, R. L. (1991) Hear. Res. 53, 131-140. [DOI] [PubMed] [Google Scholar]
- 40.Dallos, P. & Cheatham, M. A. (1976) J. Acoust. Soc. Am. 59, 591-597. [DOI] [PubMed] [Google Scholar]
- 41.Eggermont, J. J. (1977) J. Acoust. Soc. Am. 62, 1247-1251. [DOI] [PubMed] [Google Scholar]
- 42.Rutten, W. L. (1986) Hear. Res. 21, 195-204. [DOI] [PubMed] [Google Scholar]
- 43.Tsuji, J. & Liberman, M. C. (1997) J. Comp. Neurol. 381, 188-202. [PubMed] [Google Scholar]
- 44.Liberman, M. C. (1978) J. Acoust. Soc. Am. 63, 442-455. [DOI] [PubMed] [Google Scholar]
- 45.Martin, G. K., Lonsbury-Martin, B. L., Probst, R. & Coats, A. C. (1988) Hear. Res. 33, 49-68. [DOI] [PubMed] [Google Scholar]
- 46.Manley, G. A. (1990) Peripheral Hearing Mechanisms in Reptiles and Birds (Springer, Berlin).
- 47.Cody, A. R. (1992) Hear. Res. 62, 166-172. [DOI] [PubMed] [Google Scholar]
- 48.Rose, J. E., Hind, J. E., Anderson, D. J. & Brugge, J. F. (1971) J. Neurophysiol. 34, 685-699. [DOI] [PubMed] [Google Scholar]
- 49.Serafin, J. V., Moody, D. B. & Stebbins, W. C. (1982) J. Acoust. Soc. Am. 71, 1513-1518. [DOI] [PubMed] [Google Scholar]
- 50.Smith, D. W., Moody, D. B. & Stebbins, W. C. (1987) J. Acoust. Soc. Am. 82, 63-68. [DOI] [PubMed] [Google Scholar]
- 51.Taberner, A. M. & Liberman, M. C. (2005) J. Neurophysiol. 93, 557-569. [DOI] [PubMed] [Google Scholar]
- 52.Pickles, J. O. (1979) J. Acoust. Soc. Am. 66, 1725-1732. [DOI] [PubMed] [Google Scholar]
- 53.Ohlemiller, K. K. & Echteler, S. M. (1990) J. Comp. Physiol. A 167, 329-338. [DOI] [PubMed] [Google Scholar]
- 54.Burkey, J. & Gans, D. (1991) J. Acoust. Soc. Am. 89, 1822-1823. [PubMed] [Google Scholar]
- 55.Evans, E. F., Pratt, S. R., Spenner, H. & Cooper, N. P. (1992) in Auditory Physiology and Perception, eds. Cazals, Y., Demany, L. & Horner, K. (Pergamon, Oxford), pp. 159-164.
- 56.Dolan, T. G., Mills, J. H. & Schmiedt, R. A. (1985) Hear. Res. 17, 259-266. [DOI] [PubMed] [Google Scholar]
- 57.Cheatham, M. A., Huynh, K. H., Gao, J., Zuo, J. & Dallos, P. (2004) J. Physiol. 560, 821-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohlemiller, K. K. (2002) J. Assoc. Res. Otolaryngol. 3, 444-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohlemiller, K. K., Hennig, A. K., Lett, J. M., Heidbreder, A. F. & Sands, M. S. (2002) Hear. Res. 169, 69-84. [DOI] [PubMed] [Google Scholar]
- 60.el Barbary, A. (1991) Hear. Res. 54, 91-104. [DOI] [PubMed] [Google Scholar]
- 61.Carlier, E. (1979) Hear. Res. 1, 197-201. [Google Scholar]
- 62.Mitchell, C. & Fowler, C. (1980) J. Acoust. Soc. Am. 68, 896-900. [DOI] [PubMed] [Google Scholar]
- 63.Rajan, R., Robertson, D. & Johnstone, B. M. (1990) Hear. Res. 44, 195-207. [DOI] [PubMed] [Google Scholar]
- 64.Bonfils, P., Remond, M. C. & Pujol, R. (1986) Hear. Res. 24, 277-283. [DOI] [PubMed] [Google Scholar]
- 65.Spagnoli, S. D. & Saunders, J. C. (1987) Otolaryngol. Head Neck Surg. 96, 99-105. [DOI] [PubMed] [Google Scholar]
- 66.van Heusden, E. & Smoorenburg, G. F. (1981) Hear. Res. 5, 25-48. [DOI] [PubMed] [Google Scholar]
- 67.Greenwood, D. D. (1990) J. Acoust. Soc. Am. 87, 2592-2605. [DOI] [PubMed] [Google Scholar]
- 68.Gleich, O. (1989) Hear. Res. 37, 255-267. [DOI] [PubMed] [Google Scholar]
- 69.Smolders, J. W., Ding-Pfennigdorff, D. & Klinke, R. (1995) Hear. Res. 92, 151-169. [DOI] [PubMed] [Google Scholar]