Abstract
If an individual can learn to directly control activation of localized regions within the brain, this approach might provide control over the neurophysiological mechanisms that mediate behavior and cognition and could potentially provide a different route for treating disease. Control over the endogenous pain modulatory system is a particularly important target because it could enable a unique mechanism for clinical control over pain. Here, we found that by using real-time functional MRI (rtfMRI) to guide training, subjects were able to learn to control activation in the rostral anterior cingulate cortex (rACC), a region putatively involved in pain perception and regulation. When subjects deliberately induced increases or decreases in rACC fMRI activation, there was a corresponding change in the perception of pain caused by an applied noxious thermal stimulus. Control experiments demonstrated that this effect was not observed after similar training conducted without rtfMRI information, or using rtfMRI information derived from a different brain region, or sham rtfMRI information derived previously from a different subject. Chronic pain patients were also trained to control activation in rACC and reported decreases in the ongoing level of chronic pain after training. These findings show that individuals can gain voluntary control over activation in a specific brain region given appropriate training, that voluntary control over activation in rACC leads to control over pain perception, and that these effects were powerful enough to impact severe, chronic clinical pain.
Keywords: anterior cingulate cortex, plasticity
Individuals exhibit voluntary but unwitting control over brain activation all of the time: Every voluntary action engages the activation of specific brain mechanisms. However, the extent to which an individual can learn to directly, consciously control the activation of specific brain regions or brain mechanisms is not yet known. Although initially surprising, it has now been known for some time that subjects can learn to control a variety of autonomic measures such as heart rate, skin conductance, and muscle tone (1). Subjects have also learned to control brain electroencephalogram (EEG) rhythms (2-4). The mechanisms of these changes in autonomic tone or EEG rhythm are likely widespread, spanning multiple brain systems and reflecting general processes such as overall relaxation. Whether subjects can learn precise control over a discrete, anatomically localized brain region with a highly specialized function is another matter.
Real-time functional MRI (rtfMRI) allows measurement of localized processes within the brain as they take place (5), providing information that could serve as the basis for learning to control specific neurophysiological mechanisms. Although fMRI is an indirect measure of neural activation with spatial and temporal resolution limited by hemodynamics and signal variability, recent results have demonstrated that subjects can successfully learn to control fMRI activation in a localized brain region by using rtfMRI as the basis of training (5-8). It is not known, however, whether learned control over fMRI activation corresponds with changes in underlying neurophysiological processes that in turn lead to predicted changes in behavior, cognition, or potentially to impact on clinical disorders. Here, we investigated whether learned, deliberate manipulation of rostral anterior cingulate cortex (rACC) activation by subjects leads to predicted effects on pain perception.
The brain systems that mediate pain may be particularly relevant clinical candidates for rtfMRI-based training. Chronic pain is one of the most ubiquitous and important clinical problems facing society and is the primary complaint resulting in physician visits and healthcare resource use (9). Chronic pain patients often fail to find relief even after pursuing multiple treatment options and incurring tens of thousands of dollars per year in healthcare costs. Further, pain perception can be substantially affected by cognitive processes including placebo effects (10), anticipation (11, 12), and attention (9, 13, 14). There is evidence that subregions within rACC are involved in mediating the conscious perception of pain in consort with a matrix of other brain structures (9, 15-17). For example, rACC activation is altered by manipulations that reduce the perception of pain, such as hypnosis (18-20) or placebo conditions (21). In the present study, we examined whether subjects can learn to control the brain's pain system and whether learned control over rACC activation would alter pain perception in healthy subjects and also in patients with chronic pain. If so, this could ultimately lead to the development of a form of neuroimaging therapy.
Methods
Subjects, Stimuli, and Ratings. Healthy volunteer subjects (20 male, 16 female, mean age of 23.5 yr, range of 18-37 yr) were recruited from the community, and chronic pain patients were selected from the Stanford University Pain Management Service (eight male, four female, mean age of 36.7 yr, range of 31-38 yr, mean duration of pain of 42 mo). Healthy volunteers, but not pain patients, were presented with nociceptive stimuli for 30 s by using a30 × 30-mm Peltier thermode on the subject's left palm (thenar eminence). Temperature levels were individually selected for each subject before scanning (group mean of 47.9°C, range of 46.8-48.6°C) by using a psychometric thresholding procedure that is designed to yield the maximally painful stimulus that each subject can tolerate without moving, rated as “7 out of 10” (see Supporting Text, which is published as supporting information on the PNAS web site). Subjects were thoroughly screened and monitored throughout for any potential adverse events, with immediate referral to Stanford University physicians should an adverse event or side effects have been noted (none were noted). Subjects rated the intensity and unpleasantness of stimuli on a 1-10 continuous visual analog scale (VAS) by using a computer mouse and a graphical analog slider reverse-projected inside the scanner. This study was approved by the Stanford University human subjects panel, and all subjects completed written, informed consent.
Subject Pretraining Information. It was explained to subjects that the goal of training was for them to learn to enhance their control over activation in a localized brain region associated with pain. Pilot experiments indicated that to learn enhanced control over activation of a specific neural mechanism in a limited training period, subjects required cognitive strategy guidelines. Given the large number of cognitive processes associated with different brain areas, trial and error alone, even with rtfMRI feedback, was found to be ineffective. Subjects were told that during scanning, they would be attempting to alternately increase and then decrease activation in the target brain region and that they would view real-time feedback of their success. The potential effects on subject expectations created through the training procedure were evaluated through comparison with results from a variety of control subject groups who did not receive valid rtfMRI information. All subjects received identical written instructions regarding strategies for use in increasing/decreasing brain activation or pain. The strategy instructions provided to all subject groups included instructions to change the following.
Attention. Attend toward the painful stimulus vs. away from it (to the other side of the body).
Stimulus quality. Attempt to perceive the stimulus as a neutral sensory experience vs. a tissue-damaging, frightening, or overwhelming experience.
Stimulus severity. Attempt to perceive the stimulus as either low or high intensity.
Control. Attempt to control the painful experience, or allow the stimulus to control the percept.
It was explained to subjects that rtfMRI information includes random noise and that it is inherently delayed relative to cognitive and brain events due to biologically inherent hemodynamics (≈3-5 s) and computer processing time (1-2 s).
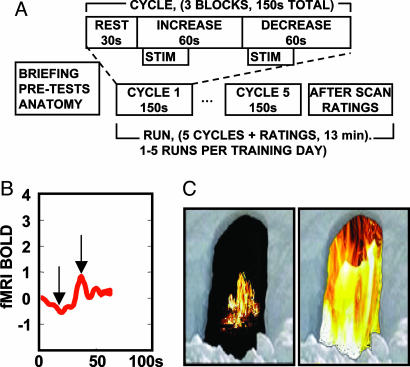
rtfMRI and Subject Training Protocol. After anatomical and fMRI localizer scans, eight healthy subjects underwent a series of training runs inside the scanner while receiving rtfMRI information from the target region of interest (ROI) in the rACC as a scrolling line graph of signal from the entire ROI (Fig. 1B) and a continuous video display depicting the same information as a larger or smaller virtual fire image (Fig. 1C). 2D or 3D images of brain activation can be confusing to subjects and were not presented. Each 13-min scanning run consisted of five increase/decrease cycles. Each cycle consisted of a 30-s rest block, followed by a 60-s increase block during which subjects were trained to increase ROI activation, followed by a 60-s decrease block during which subjects were trained to decrease activation in the target ROI. An identical noxious thermal stimulus was applied for 30 s during each increase and decrease block, beginning 10 s after the beginning of the block. Text cues were presented for each block (“Rest,” “Increase,” and “Decrease”). Healthy subjects received a localizer scan, three training runs, and a posttest run. The posttest run was identical to the training runs except that during the posttest run, subjects rated each stimulus immediately after the stimulus was presented. During training runs, ratings were made only after the scan was completed to avoid activations caused by the rating process itself interfering with training. Chronic pain patients underwent similar training, except that the thermal stimulus was not used (STIM in Fig. 2A). In addition, for ethical considerations, the pain patients themselves chose when to end scanning. Four patients completed three total training runs, two patients completed two training runs, and two patients completed one training run.
Fig. 1.
Pain control task. (A) Task diagram (STIM not present for pain patients). (B) Scrolling line chart of rtfMRI activation viewed by subjects during training. Chart units are percent signal change for BOLD signal (fMRI BOLD) vs. time in seconds. (C) Two sample images taken from a continuum of video images presented to subjects depicting low (Left) to high (Right) levels of activation in the target ROI, corresponding to the arrows in B.
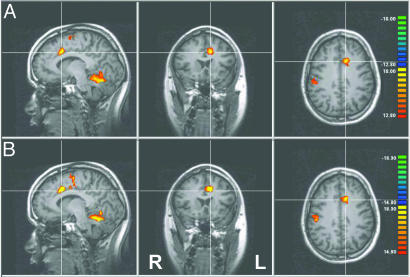
Fig. 2.
Volumetric analysis of the spatial pattern of learned control over activation. (A) Change in activation comparing the last training session to the first training session showing activation in rACC, the targeted brain region. Seven total clusters were observed at this threshold level (t > 12.80, top of scale t = 18.00; for coordinates, see Table 1, which is published as supporting information on the PNAS web site). (B) Repeat of the same analysis comparing the posttest session (performed after the last training session) to the initial training session, showing similar results. Data are presented as thresholded, Bonferroni-corrected t-maps superimposed on high-resolution T1 data. The crosshairs indicate the three planes of section displayed and the group mean of the target ROI y and z coordinates used for rACC rtfMRI-based training (x coordinate for training ROI was midline). Color designates the t value, using a general linear model comparing different time periods convolved with a canonical hemodynamic response function. All data are experimental group averages after normalization to Talairach-Tournoux coordinates.
Control Groups. Four separate healthy subject control groups were trained and tested using similar or identical procedures but in the absence of valid rACC rtfMRI information.
Group I (n = 8) received identical instructions to the experimental group and the same period of training, except without rtfMRI information and with attempted cognitive control over pain alone to test the effects of repeated practice.
Group II (n = 8) received purely behavioral training for twice as long as the experimental group but with no rtfMRI information. These subjects were additionally instructed to overtly focus attention on the thermal stimuli during “increase” blocks and away during the “decrease” blocks, rather than using any other strategy.
Group III (n = 8) received identical training to the experimental group, but using rtfMRI information derived from a different brain region in posterior cingulate cortex that is not believed to be involved in pain processing to examine spatial and physiological specificity.
Group IV (n = 4) received identical training to the experimental group, but, unknown to the subjects, the rtfMRI displays that they viewed corresponded to activation from a previously tested experimental subject's rACC, rather than their own rACC brain activation. Therefore, the displays were visually identical to what had been shown previously to the experimental subject.
A patient control group (n = 4) received autonomic biofeedback information rather than rtfMRI, and they were trained to control their autonomic tone, viewing continuous scrolling graphs of skin conductance, heart rate, and respiration and following methods in use to decrease arousal and induce relaxation (3).
fMRI Imaging and Analysis. Spiral fMRI volume data were collected on a 3.0 Tesla General Electric Signa scanner at Stanford University and processed in real time by using in-house software. Analysis included spiral reconstruction, motion correction, and continuous measurement of the level of ROI activation [measured as percent signal change from running average of the ROI, the fMRI blood oxygen level-dependent (BOLD) signal, bandpass-filtered 1/5-1/120 s, no spatial smoothing]. ROIs were individually selected during an initial physiological localizer scan based on activation observed within the rACC by using an increase vs. decrease pain contrast (see Supporting Text). The level of activation in the target ROI, a large background ROI, and the difference between the two were presented to subjects inside the scanner as three separate scrolling line charts (Fig. 1B depicts a single example). For offline analysis, data were 3D motion-corrected, smoothed by using a 4-mm Gaussian kernel, bandpass-filtered (1/5-1/120 s), and transformed into standard Talairach-Tournoux coordinates (22) by using brain voyager 2000 and qx (Brain Innovation, Maastricht, The Netherlands). Group data were analyzed by using Bonferroni-corrected fixed effects analysis, and all reported volumetric activations showed P values <0.001.
Results
Through the course of training, experimental group subjects (n = 8) learned to control the fMRI signal in the target ROI. The primary index of learning was the enhancement in the difference in fMRI signal between the increase (inc) and decrease (dec) periods comparing the final run of training with the initial run of training [i.e., (fMRIinc vs. fMRIdec)final - (fMRIinc vs. fMRIdec)initial]. Periods when the stimuli were not present and when ratings were performed were excluded. Group analysis demonstrated increased activation after training in a spatially localized region corresponding to the trained rACC target ROI, which showed the most significant activation of any forebrain region [ACC, Brodmann's area (BA) 32/24, t = 18.35; P < 0.001 corrected; Fig. 2A). This finding was replicated for a posttest run (Fig. 2B). Additional brain areas showing increased activation included secondary somatosensory cortex (BA 2), insula (BA 21/22/13), supplementary motor cortex (BA 6), superior cerebellum, and superior temporal gyrus (BA 22) (all coordinates are provided in Supporting Text).
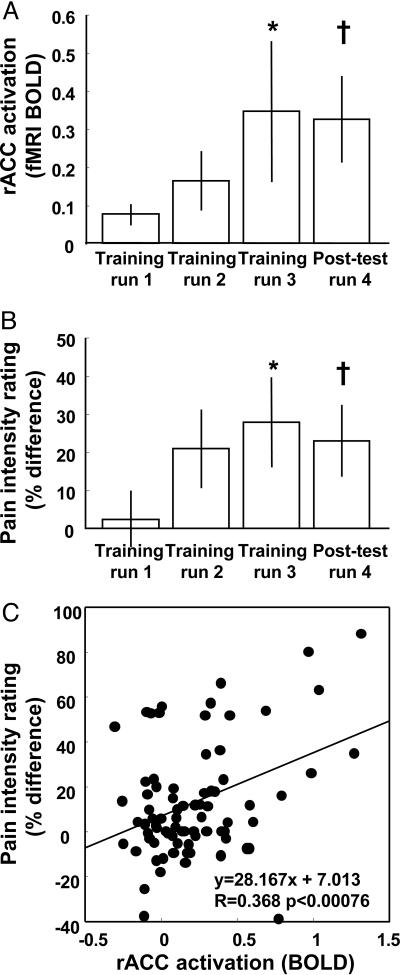
The subjects' control over fMRI activation in the target ROI increased monotonically over three training runs of 13 min each (Fig. 3A, bars 1-3; P < 0.05, linear regression) and was replicated in the posttest run (Fig. 3A). As subjects learned to produce increases and decreases of activation in rACC, there was a corresponding change in their perception of pain measured through pain intensity ratings (Fig. 3B). During the first rtfMRI training run, there was little difference in rated pain intensity for stimuli presented during increase vs. decrease periods (Fig. 3B), corresponding to the small amount of control over rACC activation (Fig. 3A). By the last training run, when subjects were successfully controlling rACC activation, stimuli presented when subjects were increasing rACC activation were rated as significantly more painful than when subjects were decreasing rACC activation, leading to a greater percentage difference between these two ratings (Fig. 3B). The enhancement in pain intensity difference was also reproduced during the posttest run (Fig. 3B). Changes in fMRI activation and perception were both measured as the difference between paired increase and decrease blocks, rather than using absolute magnitude measures, so that the results would be directly comparable and baseline fluctuations common in both types of measures would be eliminated. After training, experimental subjects showed both an increase in absolute pain ratings during the increase periods and a decrease in absolute pain ratings during the decrease periods (see supporting information for details).
Fig. 3.
Learned enhancement of control over fMRI BOLD activation and pain. (A) Control over fMRI BOLD activation in rACC ROI activation increased significantly through training (*, P < 0.05, linear regression; †, P < 0.05, t test run 3/4 vs. run 1). (B) In parallel, control over pain increased significantly through training (*, P < 0.05, linear regression; †, P < 0.05, t test run 3/4 vs. run 1). (C) The difference in BOLD activation induced by the subject correlated with the difference in reported pain intensity (P < 0.00076, linear regression) for each individual cycle during which subjects increased and then decreased brain activation and rated the intensity of individual stimuli (all experimental subjects). fMRI BOLD plotted in A is percent signal change, measured as the group mean and standard errors of the difference in T2*-weighted MRI intensity during stimuli presented during increase periods vs. during decrease periods, shifted by5sto allow for hemodynamic delay and averaged over all voxels within the ROI and averaged over five repeated blocks per training run. Bars in B represent the group mean and standard errors of a pain intensity percentage difference index, defined as 100% × (Rinc - Rdec)/((Rinc + Rdec)/2), where Rinc and Rdec correspond to the pain rating for increase and decrease periods, respectively.
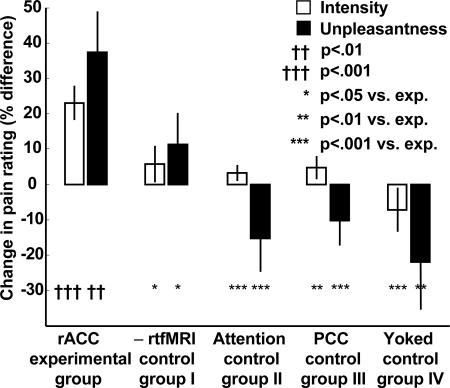
A linear regression analysis examined the relation between the activation level produced by subjects on individual trials (percent fMRI signal change) and the resultant pain ratings. There was a significant correlation between the induced changes in rACC activation and the corresponding difference in pain intensity ratings (Fig. 3C; P < 0.0007, linear regression). After training, subjects showed a 23% enhancement in control over pain intensity, a sensory measure of pain (Fig. 4, rACC experimental group open bar; P < 0.001, t test of all data from Fig. 3B, bars 3 and 4 vs. bar 1), and a 38% enhancement in control over pain unpleasantness, an affective measure of pain (Fig. 4, rACC experimental group filled bar, P < 0.01, t test).
Fig. 4.
Percentage change in control over perceived pain intensity and unpleasantness for experimental group and four comparison control groups. Training included 36 total subjects among all groups and 140 total pain training, posttesting, and scanning runs. Each bar plots the group mean and standard errors of percentage change in pain intensity difference ratings (open bars) or pain unpleasantness difference ratings (filled bars). These values correspond to the change in the pain intensity percentage difference index, as defined in Fig. 2, between run 1 and the average of runs 3/4. Results were similar when runs 3 and 4 were analyzed individually. †, t test for experimental group; *, paired t test compared with experimental group for control groups.
To determine whether this effect is specifically due to rtfMRI-induced learning, rather than other learning or nonspecific effects, the experimental subjects were compared with four control groups of subjects who received extended practice without rtfMRI information (group I), twice the duration of training at focusing attention away from pain (group II), training using rtfMRI data taken from a different brain area (group III), or training using sham rtfMRI data taken from a different subject's brain, rather than from the experimental subject's brain (group IV). The improvement in control over pain intensity and unpleasantness shown by the experimental (rtfMRI) group was significantly larger than for any of the four independent control groups (Fig. 4).
Eight chronic pain patients following a similar rtfMRI-based training protocol but without externally applied painful stimuli reported substantial decreases in their average baseline pain level assessed with the short-form McGill pain questionnaire (MPQ) (23) as well as pain ratings on a simple 1-10 VAS. Pain patients reported a 64% decrease in MPQ pain rating sums after training (P < 0.00015, paired t test) and a 44% decrease in VAS pain ratings (P < 0.0016, paired t test). All of the eight patients who were trained reported a decrease in pain intensity after the procedure, which was carried out on a single day, and five of eight patients reported a reduction of pain by 50% or greater on the MPQ.
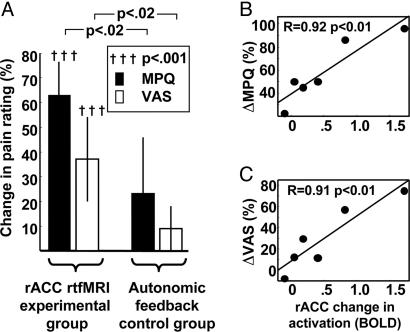
A control group of patients was trained under similar circumstances and for a similar duration inside the scanner but was provided with autonomic biofeedback information rather than rtfMRI. The changes in pain ratings for the experimental group were three times larger than for the autonomic biofeedback control group (Fig. 5A; ΔMPQexperimental = 2.6 × ΔMPQcontrol; P < 0.02, t test; ΔVASexperimental = 3.4 × ΔVAScontrol; P < 0.02, t test). The difference between experimental and control subjects was statistically significant for both MPQ and VAS pain ratings (P < 0.02 and P < 0.009, respectively, t test).
Fig. 5.
Changes in pain ratings and rACC activation in chronic pain patients after rtfMRI-based training. (A) Change in experimental and control subject pain ratings after vs. before training. Error bars correspond to standard errors of group means. (B and C) Significant correlation (P < 0.01, linear regression) between individual subject percentage change in MPQ pain rating and VAS pain ratings, respectively, and changes in rACC ROI fMRI BOLD activation (change in signal intensity from increase vs. decrease periods taken from the last vs. the first training run).
Finally, to explore whether patients who learned greater control over brain activation showed greater changes in pain, changes in activation and changes in pain ratings between the first and last training runs were correlated for the patients who completed two or more training runs. This comparison was only possible for the six of eight total patients who completed at least two runs. There was a significant correlation between the extent to which patients learned to control rACC activation and their decrease in pain rated with MPQ (Fig. 4B; P < 0.01, linear regression) and also VAS (Fig. 5C; P < 0.01, linear regression). In interviews after the procedure, patients described an increased sense of control over their pain as well as an overall decrease in pain level when not overtly attempting to exercise control, but they were not able to provide clear details regarding the strategies that they used.
Discussion
To our knowledge, this work provides the first full-group, controlled demonstration that individuals can learn to exert deliberate, voluntary control over localized brain activation by using training based on rtfMRI, leading to a resultant impact on behavior or disease symptoms. Subjects successfully learned to control a brain region that is understood to be involved in pain processing, the rACC, and this process led to significant changes in their pain perception. Four independent control groups trained in similar procedures but without valid rACC fMRI information did not learn enhanced control over pain. Similarly, chronic pain patients who were trained with rtfMRI information from rACC showed significant decreases in pain perception, whereas patients who were trained with autonomic biofeedback did not.
rtfMRI Methods and Training Results. This research builds on a growing body of work that demonstrates that subjects can be trained to control localized brain activation by using real-time neuroimaging (6-8). An early system for real-time analysis of fMRI was developed by Cox et al. (5), and, subsequently, a number of groups have worked to advance this technology (24, 25). Here, the term rtfMRI is used to indicate that fMRI data analysis and display keep pace with fMRI data acquisition (in these data, after a 1- to 2-s processing delay).
The developing field of rtfMRI-based training has shown that individuals can learn to control activation in a region of the brain that is targeted for observation. Yoo and Jolesz (26) conducted an initial pilot study to investigate this approach, using a “near real-time” fMRI paradigm in which subjects were shown the results of fMRI data analysis ≈20 s after they completed a period of finger tapping, and the subjects successfully learned to control their finger tapping behavior to control brain activation. Posse et al. (7) provided fMRI information about the level of amygdala activation to subjects verbally (on a 1-5 scale) after each 60-s block of a mood induction paradigm. Weiskopf et al. (8) were the first to show the feasibility of a training paradigm using rtfMRI and showed increased control over activation in the ACC in a single pilot subject. Our group demonstrated in a group of subjects that it is possible for individuals to learn control over a target brain region by using rtfMRI training (6). In that study, experimental subjects engaged in motor imagery and received training with continuous rtfMRI feedback of activation in the somatomotor cortex. Subjects showed a monotonic increase in control over activation in this brain area, but did not show covert movement as monitored by electromyogram. Members of a control group who received sham rtfMRI information did not improve their control over brain activation, demonstrating that the learned control is specifically due to training with rtfMRI, as opposed to other learning effects. Also, once trained, subjects could control brain activation even in the absence of rtfMRI information.
More recently, several studies have investigated subjects' control over brain activation as a potential brain-computer interface. Weiskopf et al. (8) investigated whether subjects were able to differentially control brain activation in the supplementary motor area and parahippocampal place areas (8) using strategies such as visual vs. motor imagery. Two of four subjects showed an increase in the differential signal. Yoo et al. (27) reported that subjects can learn to navigate a cursor through a 2D maze by using four cognitive tasks associated with four different brain volumes of interest. Subjects closed their eyes and executed an entire sequence of mental strategies to try to produce a sequence of movement commands of a cursor through a maze that were derived from fMRI, and the subjects were provided feedback regarding their success after completion of each trial.
Control Over Cognitive, Behavioral, and Neurophysiological Processes Using rtfMRI Training. The current investigation suggests that when subjects learn to control the fMRI signal in a brain region, this process engages the neurophysiological mechanisms within that brain region, leading to predicted cognitive results. The observed form of pain control required that subjects be trained specifically to regulate the pain modulatory system and receive activation information from this system. Subjects who were trained to use cognitive strategies without brain activation information in a conventional learning paradigm did not learn similar control over pain. Healthy control subjects who received rtfMRI information from the posterior cingulate cortex also did not learn to control pain perception, suggesting that nonspecific strategies affecting global arousal or global brain activation are not effective and that subjects must be trained to control specific neurophysiological systems. Finally, the members of an additional control group were shown the exact same information that had been displayed previously to the experimental subjects, derived from the experimental subjects' brains rather than from the control subjects' brains. Any effects of expectation or suggestion created by the displays themselves or by the subjects' perception of their control over brain activation were identically matched in these control subjects, who nonetheless did not show an improvement in their control over pain.
It is particularly interesting that pain patients need to be able to observe the functioning of the brain's pain system to learn this form of control over these systems because pain patients already have continuously available sensory feedback of their own pain level, they already have a strong motivation to learn to control their pain, and they typically have tried and practiced many strategies to alleviate their pain over many years. These individuals may have remained pain patients because cognitive strategies, practice, and sensory feedback alone were not adequate for them to learn to control their pain. Conventional biofeedback using autonomic measures was not sufficient for training patients to control their pain compared with anatomically localized information derived from the pain control system, again supporting that subjects require information about this specific system and that more general approaches are not equivalently effective. Long-term pain outcomes after similar injuries can vary widely. One possible source of these differences is that, if a patient's pain control system is inherently more efficacious or more strongly engaged, this greater efficacy might lead to a greater likelihood of successful recovery, rather than ongoing chronic pain. The approach described here may provide a different avenue for up-regulation of the pain control system based on targeted neuroplasticity through training.
Although the rtfMRI information presented to subjects here was derived only from rACC, it is possible that subjects learned to engage or control a number of closely associated brain regions that may have acted in consort to produce the behavioral effects observed. Given that placebo effects are mediated in part by the brain's pain control system (28), it is possible that these subjects produced a trained, controllable form of the typically unconscious placebo effect.
The brain mechanisms that translate rtfMRI feedback into learned regulation of focal brain activation are as yet unknown. Learning cognitive control over a particular brain system is a highly complex task likely engaging a constellation of brain regions that depends critically on the target system being trained. Once it has been established that a brain system is subject to learned control, then it will be important to determine what strategies subjects use, what brain regions are engaged in this learning process, what constitutes the underlying physiology, and what potential behavioral impacts can be achieved.
Processing of Pain in the Brain. The rACC was selected as a target for this investigation because its role is likely to be particularly important for both pain perception and pain regulation (15-17), but rACC activation is also associated with a broad variety of cognitive processes, including attention (29), emotion (30), executive function, task difficulty, and motor control (31). The fact that learned control over rACC activation was associated with pain modulation supports a role for rACC and its allied neural system in pain control (32, 33), not just cognitive sequelae to pain such as attention, arousal, or orienting. Multiple brain regions comprising a pain matrix are involved in pain processing, including ACC, primary (SI) and secondary (SII) somatosensory cortices, insula, and thalamus (9, 32-34). Cognitive modulation of pain engages this same group of structures (32, 35). Attention and distraction modulate activation in ACC, as well as other pain-related regions (35). Pain anticipation also affects pain modulatory systems, including ACC, medial orbitofrontal cortex, amygdala, and periacqueductal gray (11, 12, 36). Hypnotic suggestion (18) studies have shown a specific role for ACC in pain unpleasantness, whereas the manipulation of pain intensity produced changes primarily in SI cortex (20). ACC has also been implicated in opioidergic pain modulation (37, 38) and placebo analgesia (16, 28). In addition, patients who have received neurosurgical deafferentation of the ACC for chronic intractable pain report that the experience of pain is maintained but that the affective impact is diminished (39), supporting the interpretation that areas BA 24/ACC are associated with pain unpleasantness.
Clinical Perspective and Potential Applications. Immediately after training with rtfMRI, pain patients exhibited a decrease in the magnitude of experienced chronic pain. These patients had been largely refractory to multiple previously administered pharmacologic, psychological, and behavioral interventions, as is typical in chronic pain patients after years of attempted treatment. However, the potential of this approach for long-term pain treatment is still being investigated. It will also be important to characterize what pain conditions and types of patients show the greatest response to this method.
The approach of monitoring the impact of an intervention on brain activation in real time as the intervention is being applied has a number of potential applications both for research and for clinical use. As a research tool, this approach allows for neuroimaging experiments to investigate the behavioral consequences of activation of a selected brain region or target pattern, in addition to the prospect of better quality control over experiments using real-time monitoring. In the clinical setting, it is possible that a number of types of intervention, including cognitive therapy or psychotherapy, surgery, pharmacologic intervention and screening, or electrical or magnetic stimulation of the nervous system could be investigated or improved through real-time monitoring. Approaches using rtfMRI will have to be fully tested in thorough clinical trials before clinical use.
Supplementary Material
Acknowledgments
We thank James Gross, Rebecca Ray, John Keltner, and Susan Gabrieli for comments on the manuscript or figures and Herschel Toomim for very helpful background discussions. This work was supported by National Institutes of Health Grants R43MH067290, R44NS050642, N43DA-4-7748, and RR09784; the Oxnard Foundation; the Rosekrans Research Fund; and the Stanford University Department of Anesthesia.
Author contributions: R.C.d.C., F.M., G.H.G., J.M.P., J.D.E.G., and S.C.M. designed research; R.C.d.C., F.M., G.H.G., D.L., S.C.M., and D.S. performed research; J.M.P. contributed new reagents/analytic tools; R.C.d.C., F.M., G.H.G., D.S., and S.C.M. analyzed data; and R.C.d.C., J.D.E.G., and S.C.M. wrote the paper.
Conflict of interest statement: This work was funded by the National Institutes of Health (NIH), with NIH funding provided both directly to Stanford University and through grants to Omneuron Inc., a venture developing rtfMRI. C.d.C. has an ownership interest in this venture with pending patents on rtfMRI-based training methods and has initiated further ongoing clinical trials of this approach. Other authors do not have competing interests to disclose. Work was performed both at Omneuron and at Stanford University through a NIH-funded subcontract.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: rtfMRI, real-time functional MRI; rACC, rostral anterior cingulate cortex; VAS, visual analog scale; ROI, region of interest; BOLD, blood oxygen level-dependent; BA, Brodmann's area; MPQ, McGill pain questionnaire.
References
- 1.Manuck, S. B. (1976) Biofeedback Self Regul. 1, 273-284. [DOI] [PubMed] [Google Scholar]
- 2.Lubar, J. F. & Deering, W. M. (1981) Behavioral Approaches to Neurology (Academic, New York).
- 3.Zeier, H. (1984) Biofeedback Self Regul. 9, 497-508. [DOI] [PubMed] [Google Scholar]
- 4.Nowlis, D. P. & Kamiya, J. (1970) Psychophysiology 6, 476-484. [DOI] [PubMed] [Google Scholar]
- 5.Cox, R. W., Jesmanowicz, A. & Hyde, J. S. (1995) Magn. Reson. Med. 33, 230-236. [DOI] [PubMed] [Google Scholar]
- 6.deCharms, R. C., Christoff, K., Glover, G. H., Pauly, J. M., Whitfield, S. & Gabrieli, J. D. (2004) NeuroImage 21, 436-443. [DOI] [PubMed] [Google Scholar]
- 7.Posse, S., Fitzgerald, D., Gao, K., Habel, U., Rosenberg, D., Moore, G. J. & Schneider, F. (2003) NeuroImage 18, 760-768. [DOI] [PubMed] [Google Scholar]
- 8.Weiskopf, N., Veit, R., Erb, M., Mathiak, K., Grodd, W., Goebel, R. & Birbaumer, N. (2003) NeuroImage 19, 577-586. [DOI] [PubMed] [Google Scholar]
- 9.Mackey, S. C. & Maeda, F. (2004) Neurosurg. Clin. N. Am. 15, 269-288. [DOI] [PubMed] [Google Scholar]
- 10.Dubner, R. & Ren, K. (1999) Pain Suppl. 6, S45-S53. [DOI] [PubMed] [Google Scholar]
- 11.Ploghaus, A., Tracey, I., Gati, J. S., Clare, S., Menon, R. S., Matthews, P. M. & Rawlins, J. N. (1999) Science 284, 1979-1981. [DOI] [PubMed] [Google Scholar]
- 12.Fields, H. L. (2000) Prog. Brain Res. 122, 245-253. [DOI] [PubMed] [Google Scholar]
- 13.Brooks, J. C., Nurmikko, T. J., Bimson, W. E., Singh, K. D. & Roberts, N. (2002) NeuroImage 15, 293-301. [DOI] [PubMed] [Google Scholar]
- 14.Tracey, I., Ploghaus, A., Gati, J. S., Clare, S., Smith, S., Menon, R. S. & Matthews, P. M. (2002) J. Neurosci. 22, 2748-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peyron, R., Laurent, B. & Garcia-Larrea, L. (2000) Neurophysiol. Clin. 30, 263-288. [DOI] [PubMed] [Google Scholar]
- 16.Petrovic, P. & Ingvar, M. (2002) Pain 95, 1-5. [DOI] [PubMed] [Google Scholar]
- 17.Rainville, P. (2002) Curr. Opin. Neurobiol. 12, 195-204. [DOI] [PubMed] [Google Scholar]
- 18.Rainville, P., Duncan, G. H., Price, D. D., Carrier, B. & Bushnell, M. C. (1997) Science 277, 968-971. [DOI] [PubMed] [Google Scholar]
- 19.Rainville, P., Carrier, B., Hofbauer, R. K., Bushnell, M. C. & Duncan, G. H. (1999) Pain 82, 159-171. [DOI] [PubMed] [Google Scholar]
- 20.Hofbauer, R. K., Rainville, P., Duncan, G. H. & Bushnell, M. C. (2001) J. Neurophysiol. 86, 402-411. [DOI] [PubMed] [Google Scholar]
- 21.Wager, T. D., Rilling, J. K., Smith, E. E., Sokolik, A., Casey, K. L., Davidson, R. J., Kosslyn, S. M., Rose, R. M. & Cohen, J. D. (2004) Science 303, 1162-1167. [DOI] [PubMed] [Google Scholar]
- 22.Talairach, J. & Tournoux, P. (1988) Co-Planar Stereotaxic Atlas of the Human Brain (Thieme, New York).
- 23.Melzack, R. (1987) Pain 30, 191-197. [DOI] [PubMed] [Google Scholar]
- 24.Voyvodic, J. T. (1999) NeuroImage 10, 91-106. [DOI] [PubMed] [Google Scholar]
- 25.Posse, S., Binkofski, F., Schneider, F., Gembris, D., Frings, W., Habel, U., Salloum, J. B., Mathiak, K., Wiese, S., Kiselev, V., et al. (2001) Hum. Brain Mapp. 12, 25-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo, S. S. & Jolesz, F. A. (2002) NeuroReport 13, 1377-1381. [DOI] [PubMed] [Google Scholar]
- 27.Yoo, S. S., Fairneny, T., Chen, N. K., Choo, S. E., Panych, L. P., Park, H., Lee, S. Y. & Jolesz, F. A. (2004) NeuroReport 15, 1591-1595. [DOI] [PubMed] [Google Scholar]
- 28.Amanzio, M. & Benedetti, F. (1999) J. Neurosci. 19, 484-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis, K. D., Taub, E., Duffner, F., Lozano, A. M., Tasker, R. R., Houle, S. & Dostrovsky, J. O. (2000) J. Neurosurg. 92, 64-69. [DOI] [PubMed] [Google Scholar]
- 30.Allman, J. M., Hakeem, A., Erwin, J. M., Nimchinsky, E. & Hof, P. (2001) Ann. N.Y. Acad. Sci. 935, 107-117. [PubMed] [Google Scholar]
- 31.Carter, C. S., Botvinick, M. M. & Cohen, J. D. (1999) Rev. Neurosci. 10, 49-57. [DOI] [PubMed] [Google Scholar]
- 32.Melzack, R. (1999) Pain Suppl. 6, S121-S126. [DOI] [PubMed] [Google Scholar]
- 33.Melzack, R. (1999) Acta Anaesthesiol. Scand. 43, 880-884. [DOI] [PubMed] [Google Scholar]
- 34.Willis, W. D. & Westlund, K. N. (1997) J. Clin. Neurophysiol. 14, 2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villemure, C. & Bushnell, M. C. (2002) Pain 95, 195-199. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh, J. C., Stone-Elander, S. & Ingvar, M. (1999) Neurosci. Lett. 262, 61-64. [DOI] [PubMed] [Google Scholar]
- 37.Zubieta, J. K., Smith, Y. R., Bueller, J. A., Xu, Y., Kilbourn, M. R., Jewett, D. M., Meyer, C. R., Koeppe, R. A. & Stohler, C. S. (2001) Science 293, 311-315. [DOI] [PubMed] [Google Scholar]
- 38.Casey, K. L., Svensson, P., Morrow, T. J., Raz, J., Jone, C. & Minoshima, S. (2000) J. Neurophysiol. 84, 525-533. [DOI] [PubMed] [Google Scholar]
- 39.Foltz, E. L. & White, L. E., Jr. (1962) J. Neurosurg. 19, 89-100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.