Abstract
Synaptotagmin 1 likely acts as a Ca2+ sensor in neurotransmitter release by Ca2+-binding to its two C2 domains. This notion was strongly supported by the observation that a mutation in the C2A domain causes parallel decreases in the apparent Ca2+ affinity of synaptotagmin 1 and in the Ca2+ sensitivity of release. However, this study was based on a single loss-of-function mutation. We now show that tryptophan substitutions in the synaptotagmin 1 C2 domains act as gain-of-function mutations to increase the apparent Ca2+ affinity of synaptotagmin 1. The same substitutions, when introduced into synaptotagmin 1 expressed in neurons, enhance the Ca2+ sensitivity of release. Mutations in the two C2 domains lead to comparable and additive effects in release. Our results thus show that the apparent Ca2+ sensitivity of release is dictated by the apparent Ca2+ affinity of synaptotagmin 1 in both directions, and that Ca2+ binding to both C2 domains contributes to Ca2+ triggering of release.
Keywords: Ca2+ sensor, exocytosis, gain-of-function, hippocampus, synaptic transmission
Ca2+-evoked neurotransmitter release is a key process in interneuronal communication. The synaptic vesicle protein synaptotagmin 1 is believed to act as a Ca2+ sensor in the major, synchronous component of release (reviewed in refs 1 and 2). Most of the cytoplasmic region of synaptotagmin 1 is formed by two C2 domains (C2A and C2B) that share a similar β-sandwich structure (3, 4). The C2A and C2B domains bind three and two Ca2+ ions, respectively, through loops emerging at the top of the β-sandwich (4-6). Both synaptotagmin 1 C2 domains interact in a Ca2+-dependent manner with negatively charged phospholipids (4, 7, 8), which is the most general property of C2 domains (9). In addition, the synaptotagmin 1 C2 domains exhibit a number of protein-protein interactions in vitro. Synaptotagmin 1 C2 domains interact with, among others, SNARE proteins (10-18), which are critical components of the membrane fusion machinery, and with synaptotagmin 1 itself (19-22).
Perhaps the most compelling evidence supporting the proposed role of synaptotagmin 1 as the major Ca2+ sensor that triggers synchronous neurotransmitter release was provided by the observation that a mutation in a Ca2+-binding loop of the C2A domain (R233Q) causes a parallel decrease in the apparent Ca2+ affinity of synaptotagmin 1 in the presence of phospholipids in vitro and in the Ca2+ sensitivity of release in vivo (23). However, rescue experiments in Drosophila have led to the proposal that synaptotagmin 1 promotes release independently of the C2A domain (24). In addition, loss-of-function studies have inherent limitations because it is often unclear whether an impairment induced by a mutation is specific or results from indirect effects. To address this issue, we studied gain-of-function mutations in synaptotagmin 1 that increase its apparent Ca2+ affinity. We investigated a set of synaptotagmin 1 mutants in which hydrophobic residues in the Ca2+-binding loops of the C2A domain, the C2B domain, or both domains were replaced with tryptophan residues. We found that tryptophan mutations in both C2 domains indeed increased the apparent Ca2+ affinity of synaptotagmin 1 in the presence of phospholipids in vitro and led to approximately parallel increases in the Ca2+ sensitivity of release in vivo. Hence, these constitute true gain-of-function mutations for synaptotagmin 1. Together with the previous data obtained with the R233Q mutant, our results demonstrate that synaptotagmin 1 acts as a major Ca2+ sensor in synchronous neurotransmitter release, and that both of its C2 domains participate in Ca2+ sensing. In addition, our data provide further support for the notion that Ca2+-dependent phospholipid binding is a primary determinant of this function.
Methods
Centrifugation Phospholipid-Binding Assay. Phospholipid-binding assays were done essentially as described (18, 25) by using soluble GST-fusion proteins and heavy liposome reconstituted by synaptic vesicle lipid [composition by weight 41% phosphatidylcholine, 32% phosphatidylethanolamine, 12% phosphatidylserine, 5% phosphatidylinositol, and 10% cholesterol (26)]. All binding assays were done in a Hepes buffer (50 mM Hepes, pH 6.8/100 mM NaCl/4 mM EGTA/2 mM MgCl2), and free Ca2+ concentrations were calculated with eqcal software (Biosoft, Cambridge, U.K.).
Site-Directed Mutagenesis and Viral Preparation. Mutations (C2A3W, M173W, F231W, F234W; C2B3W, V304W, Y364W, I367W) were introduced into rat synaptotagmin 1 by using standard procedures. Semliki forest virus (SFV) production was carried out as described in ref. 27. Briefly, linearized SFV plasmids were transcribed in vitro, and the resulting RNAs were transfected into BHK21 cells by electroporation. One day after transfection, cell culture media containing inactive virus were collected and frozen in aliquots.
Cell Culture and Transfection. Autaptic hippocampal cultures of neonatal synaptotagmin 1-deficient mice were prepared as described in refs. 28 and 29. Experiments were performed on neurons 10-16 days in vitro and 15-22 h after transfection. Only islands containing single neurons were examined. Successfully transfected neurons were detected by development of GFP-based fluorescence, resulting from internal ribosome entry site (IRES)-driven expression of the GFP. Although the viral overexpression could, in principle, impede other cellular functions, we relied on the gain-of-function to determine whether a mutant was present on synaptic vesicles. Immunoblotting showed that Semliki forest virus expression of all synaptotagmin 1 rescue constructs led to strong overexpression, yet the C2A and C2B mutants were not expressed at significantly higher levels than in the rescue experiments with the wild-type form of synaptotagmin 1 (data not shown). In addition, SytWT-IRES-GFP-overexpressing wild-type neurons did not show any noticeable shift in the apparent Ca2+ sensitivity of release compared with GFP-transfected neurons (data not shown), indicating that relative degrees of overexpression of synaptotagmin 1 cannot account for the observed phenotypes.
Electrophysiology and Statistics. Patch-pipette solutions contained 120 mM KCl, 20 mM Hepes, 1 mM EGTA, 4.6 mM MgCl2, 4 mM K2ATP, 15 mM creatine phosphate, and 50 units/ml creatine kinase (0.300 osM, pH 7.3). The extracellular saline solution contained 140 mM NaCl, 2.4 mM KCl, 10 mM Hepes, 10 mM glucose, 4 mM CaCl2, and 4 mM MgCl2, except when otherwise noted (0.305 osM, pH 7.3). All chemicals, except for 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline and tetrodotoxin (Tocris Cookson, Bristol, U.K.), were purchased from Sigma. All solutions were applied by using a fast flow system at room temperature (29). Cells were whole-cell voltage clamped at -70 mV with an Axopatch 200B amplifier under control of the clampex 8.0 program (both Axon Instruments, Union City, CA). Currents were low-pass-filtered at 1 or 5 kHz and stored at either 10 or 20 kHz. The series resistance was compensated to 70-90%. Only cells with series resistances <15 MΩ were analyzed. Data were analyzed by using axograph 4.6 and kaleidagraph 3.0. Deconvolution of evoked neurotransmitter release was performed by a deconvolution algorithm implemented in axograph 4.6 and was provided by John Clements (University of Canberra, Australia). Statistical significance was tested by using unpaired, nonparametric Wilcoxon tests. All values are presented as the mean ± SE.
Results
Synaptotagmin 1 Mutants with Increased Apparent Ca2+ Affinity. The gain-of-function mutants of synaptotagmin 1 were designed based on our knowledge of the mechanism of Ca2+-dependent phospholipid binding to the C2A domain, which involves multiple types of interactions (30) such as: (i) electrostatic forces arising both from the drastic switch in electrostatic potential caused by Ca2+ binding (6, 31) and from direct interactions between basic residues around the Ca2+-binding region and the negatively charged phospholipids (23, 30); (ii) partial coordination of the Ca2+ ions by the phospholipid head groups, because phospholipids dramatically increase the Ca2+ affinity (32); and (iii) hydrophobic interactions due to insertion of exposed hydrophobic residues from the Ca2+-binding loops into the lipid bilayer (33-35). Although less extensively studied, Ca2+-dependent phospholipid binding to the synaptotagmin 1 C2B domain likely involves the same types of interactions because its Ca2+-binding loops share similar structural features with those of the C2A domain (4). Based on these considerations, increasing the exposed hydrophobic surface area of the Ca2+-binding loops of the synaptotagmin 1 C2 domains may enhance phospholipid binding and increase the apparent Ca2+ affinity, much as the R233Q mutation indirectly decreased the apparent Ca2+ affinity by removing favorable electrostatic interactions with the lipids (23). Indeed, we previously showed that replacing three exposed hydrophobic residues from the Ca2+-binding loops of the isolated synaptotagmin 1 C2A domain leads to a substantial increase in its apparent Ca2+ affinity (25).
With the goal of creating a panel of synaptotagmin 1 gain-of-function mutants, we prepared synaptotagmin 1 fragments containing both C2 domains (C2AB) where the same three hydrophobic residues of the C2A domain, the three homologous residues of the C2B domain, or both domains were replaced with tryptophan residues (named SytC2A3W, SytC2B3W, and SytC2A3WB3W mutants, respectively) (see Fig. 1). We then tested Ca2+-dependent phospholipid binding to the wild-type and mutant C2AB domain fragments by using a centrifugation assay (4). A lipid composition that is thought to approximate that of synaptic membranes (26, 36) was used for these experiments. As expected, the triple tryptophan substitutions in either the C2A or C2B domain substantially increased the apparent Ca2+ affinity of the C2AB fragment (Fig. 2). The mutations in the C2B domain had a slightly stronger effect than the mutations in the C2A domain, revealing some degree of asymmetry in the contributions of both C2 domains to the interaction. A further increase in apparent Ca2+ affinity was observed when both C2 domains were mutated (Fig. 2), showing that the effects of both triple mutations are at least partially additive. Because phosphoinositides have been shown to enhance lipid binding to synaptotagmin 1 (30, 37, 38), we also tested the effect of phosphatidylinositol 4-phosphate (PIP) and phosphatidylinositol 4,5-bisphosphate (PIP2) on these experiments and observed an increase in the apparent Ca2+ affinities of all proteins (Fig. 2), but the relative effects of the mutations were similar in the presence and absence of phosphoinositides.
Fig. 1.
Models of the C2A domain (Upper) and C2B domain (Lower) of synaptotagmin 1 before (Left) and after (Right) mutating exposed hydrophobic residues of the Ca2+-binding loops to tryptophans. The mutated residues and the tryptophans are shown as orange space-filling models, and the Ca2+ ions are represented by cyan spheres. The models illustrate the increase in exposed hydrophobic surface area and the drastic change in shape that results from the mutations.
Fig. 2.
Ca2+-dependent phospholipid binding properties of SytWT, SytC2A3W, SytC2B3W, and SytC2A3WC2B3W. Wild-type or mutant forms of GST-synaptotagmin 1 C2AB domains were incubated with liposomes (41% phosphatidylcholine, 32% phosphatidylethanolamine, 10% phosphatidylserine, 5% phosphatidylinositol, 10% cholesterol) containing no PIP and PIP2 (Upper), or 0.25% PIP + 0.05% PIP2 (Lower) in the presence of free Ca2+ at the concentrations shown, clamped by Ca2+/Mg2+/EGTA buffers. Liposomes were centrifuged and washed, and bound proteins were analyzed by SDS/PAGE and Coomassie blue staining. Data shown are representative of experiments performed multiple times.
Increased Apparent Ca2+ Affinity Leads to Gain-of-Function. To test the effects of the tryptophan mutations in the synaptotagmin 1 C2 domains on neurotransmitter release, we performed rescue experiments in excitatory hippocampal neurons from synaptotagmin 1 knockout (Syt-/-) mice. Synaptotagmin 1 wild-type (SytWT) expression in Syt-/- neurons as well as rescue with the three mutant constructs led to almost full restoration of excitatory postsynaptic current (EPSC) amplitudes (ref. 28; and see Fig. 3A), confirming that all three mutants efficiently rescue fast synaptic transmission.
Fig. 3.
Enhanced apparent Ca2+ sensitivity of glutamate release in hippocampal synapses expressing SytC23W mutants. (A) Exemplary evoked synaptic currents from hippocampal Syt-/- neurons rescued by overexpression of SytWT (black), SytC2A3WB3W mutant (red), and negative control mutant SytC2A3DAC2B3DA (gray), which contains 6 C2A/B top loop aspartate-to-alanine substitutions that eliminate Ca2+-dependent phospholipid binding and rescue activity (28). (Inset) Bar plot shows mean EPSC amplitudes of SytWT (black), SytC2A3W (gray), SytC2B3W (blue), and SytC2A3WB3W (red) rescues. Number of cells is indicated within each bar. (B) Exemplary EPSCs from SytWT rescues (Left) and SytC2A3WB3W rescues (Right) at 0.5 mM (gray trace), 4 mM (black), and 8 mM (red) external Ca2+. (C) Mean apparent Ca2+ sensitivity of EPSC amplitudes in SytWT and the three SytC23W rescues. EPSC amplitudes measured at 0.2-12 mM external Ca2+ (with 1 mM Mg2+ held constant) were normalized to the response evoked at the highest Ca2+ concentration, and the data were fitted with a Hill function. SytC2A3WB3W, n = 12; SytWT, n = 18; SytC2A3W, n = 10; and SytC2B3W, n = 12.
We examined the apparent Ca2+ sensitivity of triggered release upon rescue with SytWT and the tryptophan mutants by measuring EPSC amplitudes as a function of external Ca2+ concentration (0.2-12 mM/1 mM Mg2+; Fig. 3 B and C). We found that the synaptic responses of neurons expressing any of the mutants displayed a substantial increase in apparent Ca2+ sensitivity compared with SytWT rescued neurons. When we lowered external Ca2+ to 0.5 mM, the EPSC response of the SytC2A3WB3W was still 51 ± 4% of the control conditions, whereas SytWT responses were barely detectable (5 ± 2% of control) (Fig. 3B). Standard Hill equation fit of the dose-response function showed, in addition to a 3-fold increase in the apparent affinity for external Ca2+ (Kd, SytWT 1.8 ± 0.1 mM vs. SytC2A3WB3W 0.6 ± 0.04 mM), also a substantial decrease in cooperativity (Hill coefficient for SytWT = 2.5 ± 0.3 vs. Hill coefficient for SytC2A3WB3W = 1.6 ± 0.3). Interestingly, among the two individual C23W mutants, the SytC2A3W was closer to the double mutant, whereas the SytC2B3W mutant was between the double mutant and the SytWT (SytC2A3W Hill coefficient = 1.7 ± 0.3, Kd = 0.77 ± 0.07 mM; SytC2B3W Hill coefficient = 2.0 ± 0.2, Kd = 1.11 ± 0.07 mM). Nevertheless, our results clearly demonstrate that strengthening the apparent Ca2+ affinity of either the C2A or the C2B domain of synaptotagmin 1 by increasing the hydrophobic surface area of the Ca2+-binding loops of the C2 domains enhances the apparent Ca2+ sensitivity of the synaptic response. Furthermore, the decreased cooperativity in the tryptophan mutants suggests that the interaction with the membrane became less Ca2+-dependent.
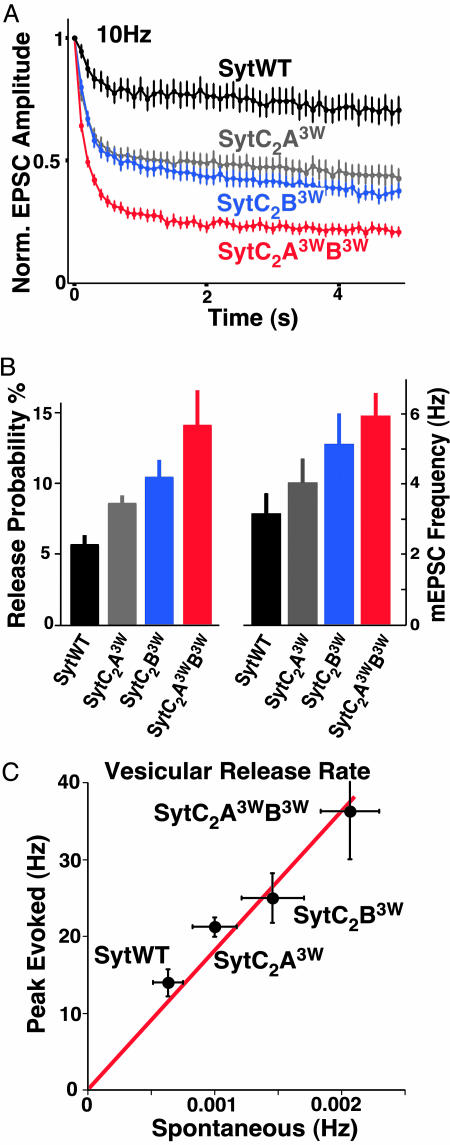
To show that the increased Ca2+ sensitivity of release in the synaptotagmin 1 tryptophan mutants is linked to an increase in the efficiency of triggered release, we studied short-term plasticity in neurons rescued with the synaptotagmin 1 mutants. In general, low release probability synapses display facilitation, whereas more efficient synapses show depression (39). We monitored EPSC amplitudes during 10-Hz action potential (AP) trains. Whereas neurons rescued with SytWT showed moderate depression of EPSC amplitudes, neurons rescued by Syt 3W/6W mutants displayed graded increases in synaptic depression (SytC2A3WB3W > SytC2B3W > SytC2A3W > SytWT; Fig. 4A) indicative of a strongly enhanced release probability in these mutants.
Fig. 4.
Increased vesicular release probability in the gain-of-function synaptotagmin 1 mutants. (A) Plot of mean normalized EPSC amplitudes of SytWT and all three SytC23W mutants rescues during a high stimulation 10-Hz action potential train (5-s duration). (B) Bar plot of the vesicular release probability (Left) and miniature EPSC (mEPSC) frequency (Right). (C) Correlation of vesicular release rates during spontaneous release and at the peak of the evoked response in SytWT and SytC2A3W, SytC2B3W, and SytC2A3WB3W mutant rescues. The linear fit corresponds to a constant increase in release rate by the Ca2+-triggering event by ≈18,200-fold.
Next, we measured vesicular release probability by computing the ratio of the EPSC charge to the readily releasable vesicle pool (RRP) charge. The RRP itself can be readily determined by short, pulsed application of hypertonic solution to the entire synapse population (40). In addition, this method of evoking vesicle release has the important advantage of not depending on elevation of intracellular Ca2+. We found that, compared with the wild-type synaptotagmin 1 rescues, the neurons rescued with SytC23W mutants had moderate but significant reductions of the RRP (SytWT, 0.50 ± 0.07 nC, n = 44; SytC2A3W, 0.40 ± 0.06 nC, n = 37; SytC2B3W, 0.35 ± 0.06, n = 42; SytC2A3WB3W: 0.29 ± 0.04 nC, n = 36, P < 0.01 for SytWT compared with SytC2A3WB3W). The vesicular release probability increased in the order SytWT < SytC2A3W < SytC2B3W < SytC2A3WB3W (Fig. 4B). Thus, the increase in the Ca2+ sensitivity of release results in an increase in the efficiency of release.
Tryptophan Mutations Increase Spontaneous Release but Do Not Affect the Time Course of Release. We also examined the effect of the synaptotagmin 1 tryptophan mutations on spontaneous release. mEPSC amplitudes were comparable in all four groups (SytWT 23.9 ± 2.3 pA, n = 25; SytC2A3W 22.3 ± 1.1, n = 27; SytC2B3W 24.8 ± 1.1, n = 23; SytC2A3WB3W 23.4 ± 1.3 pA, n = 21). Interestingly, we found that the spontaneous release rates in the mutants increased despite their decreased RRP size (Fig. 4B), and that the spontaneous release rate per vesicle (as computed by dividing the mEPSC frequency by the number of vesicles in the RRP) increased to a similar extent as the evoked release rates (Fig. 4C).
The similar impact of the tryptophan mutations on both spontaneous and Ca2+-triggered release suggests that some of their effects may be mediated by a Ca2+-independent mechanism. Alternatively, the increase of spontaneous release may arise from a gain in sensitivity to residual intraterminal Ca2+ concentrations. We tested the latter possibility by analyzing whether the increased mEPSC rate in the SytC2A3WC2B3W mutant persisted after a treatment that should lead at least transiently to lower background intracellular Ca2+ concentrations. Preincubation of neurons with EGTA/acetoxymethyl ester (50 μM for 15 min at 37°C) immediately preceding the experiment did not reduce spontaneous mEPSC release rates in either SytWT (93.7 ± 12.5%, n = 17) or SytC2A3WB3W (81.1 ± 16.2%, n = 19) rescued neurons. In contrast, this treatment led in both groups to a 40-60% reduced probability of Ca2+-triggered release (data not shown), confirming the effectiveness of EGTA/acetoxymethyl ester treatment. Taken together, our data suggest that the tryptophan mutations result in corresponding increases in the probabilities of Ca2+-evoked and spontaneous release.
The available Ca2+-triggered and spontaneous vesicular release rates in this series of synaptotagmin rescues also allow us to estimate the rate increase caused by the Ca2+-triggering event (Fig. 4C). Whereas rescues with even the strongest mutant (SytC2A3WB3W) increased release rates over the SytWT control ≈3-fold, the maximal increase of release rate by the Ca2+ trigger in neurons rescued with SytWT and all three SytC23W mutants was consistently ≈18,200-fold. This rate increase corresponds to a maximal energy contribution of 5.9 kcal/mol for the Ca2+-triggering event.
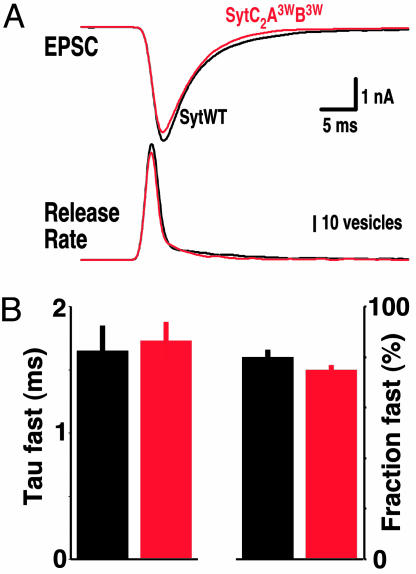
Last, we wanted to test whether the affinity of the Ca2+ sensor synaptotagmin 1 affects the time course of neurotransmitter release. The vesicle release time course will, in principle, be determined by the (i) action potential induced Ca2+ transient, (ii) the affinity/kinetics of Ca2+-Ca2+-receptor interaction, and (iii) the fusion rate once the Ca2+ trigger exerts its function. We thus tested whether a 3-fold increase in apparent Ca2+ sensitivity (by using the SytC2A3WB3W mutant) causes a change in the release time course. To analyze release time course, we deconvolved the EPSC time course in SytWT and SytC2A3WB3W rescues with their mEPSC time courses. Surprisingly, the release time course was not significantly changed (Fig. 5). This result suggests that the time course of release is at most poorly correlated with the intrinsic Ca2+ sensitivity of synaptotagmin 1 and with the Ca2+ sensitivity of release.
Fig. 5.
Enhanced Ca2+ sensitivity of triggered neurotransmitter release does not affect the time course of neurotransmitter release. (A Upper) Exemplary EPSCs from SytWT (black trace) and SytC2A3WB3W rescues (red trace). (A Lower) Corresponding vesicle release rate after deconvolution of EPSC with mean mEPSC time course. (B) Bar plot of mean time course (Left) and relative amplitude of the fast component of release (Right). The time courses of the decay phase were individually fitted with two exponentials to reveal the fast and slow components of vesicular release. Color code as in A. The time course of the slow component of release was not significantly changed. (SytWT, n = 22; Syt6W, n = 26.)
Discussion
Previous studies of the role of synaptotagmin 1 in neurotransmitter release relied on loss-of-function mutations (e.g., see refs. 23, 24, and 41-45). Although these studies established a central role for synaptotagmin 1 in the Ca2+-triggering of release, the loss-of-function approach has intrinsic limitations because it is difficult to rule out that such mutations cause indirect effects that are unrelated to the particular biochemical property that was intended to be perturbed. To address this concern, we have taken the opposite approach and used gain-of-function mutants of synaptotagmin 1, exploiting the wealth of biochemical and structural data available on its C2 domains. Together with our previous study of the R233Q mutant (23), the data obtained with the tryptophan mutants reveal a remarkable correlation between the effects of mutations in synaptotagmin 1 C2 domains on their apparent Ca2+ affinity in vitro and the effects of the same mutations on the Ca2+ sensitivity of neurotransmitter release in vivo. We established this correlation by using point mutations that involve exposed residues and that have defined effects on a well characterized biochemical activity of the synaptotagmin 1 C2 domains and Ca2+/phospholipid binding (9).
The biochemical/functional correlation revealed by our results adds to mounting evidence supporting the notion that Ca2+-dependent phospholipid binding underlies the Ca2+ sensing function of synaptotagmin 1 in release (18, 23, 25, 46). The tryptophan mutations are expected to drastically change the shape of the Ca2+-binding loops of the C2 domains (see Fig. 1). Thus, it is possible, even likely, that if the Ca2+-binding loops participate in specific protein-protein interactions, such interactions may be destabilized by the tryptophan mutations. In contrast, the same mutations enhance phospholipid binding because they increase the hydrophobic surface area of the Ca2+-binding loops (25, 34). We cannot exclude that the tryptophan mutations also enhance SNARE binding to synaptotagmin 1, but testing this possibility is hindered by the multitude of Ca2+-dependent and -independent interactions between synaptotagmin 1 and individual SNAREs and their complexes that have been described.
We observed no apparent change in the time course of release in the strongest gain-of-function synaptotagmin 1 mutant, despite the fact that the mutant displayed an apparent 3-fold increase in Ca2+ affinity of release. This lack of an effect in the time course correlates with recent findings that the Ca2+ concentrations rise and decay very rapidly at the release site, and that the Ca2+ sensor equilibrates with the intraterminal Ca2+ on a submillisecond time scale (47). Ca2+ dissociates from the C2 domains of synaptotagmin 1 with a time constant of 2,000-6,000 s-1 (48, 49), and the Ca2+ sensor at the calyx of held synapse is suggested to unbind Ca2+ with a time constant of ≈3,000 s-1 (47). An increase of affinity by slowing unbinding or increasing binding 3-fold in the SytC2A3WC2B3W mutant would be predicted to lead to a significant increase in EPSC amplitude, consistent with our observation. However, an additionally predicted decrease of 0.5-1 ms in the rise time of the EPSC (47) may be undetectable in our preparation because, in autaptic cultures, measurements of rise times are likely contaminated by the delay in release because of the variable length of the axon. Because we did not observe any changes in the EPSC decay kinetics in the neurons that were rescued with the SytC2A3WC2B3W mutant, we consider it unlikely that EPSC decay kinetics are under major influence by the Ca2+ affinity of synaptotagmin. It is possible that release decay kinetics are determined more by the kinetics of fusion than by the kinetics of Ca2+ binding to the Ca2+ sensor.
Another interesting observation is that spontaneous and Ca2+-triggered release were equally affected by the tryptophan mutants. Although the two different modes of release vary by almost four orders of magnitude, their rates were increased to similar degrees by each individual mutant. This observation provides a strong link between the spontaneous and Ca2+-evoked modes of release. It seems reasonable that the increased membrane affinity resulting from the tryptophan mutations may increase the likelihood of membrane binding at low Ca2+ concentrations or even in the absence of Ca2+. Such increase in intrinsic membrane affinity could underlie the decrease in the Ca2+ cooperativity of release as well as the increase in mEPSC frequency induced by these mutations.
Finally, our data provide insight into the relative contributions of the two C2 domains of synaptotagmin 1 to release. It has been proposed that neurotransmitter release is independent of Ca2+ binding to the synaptotagmin 1 C2A domain (24). However, the comparable effects on release described here for the SytC2A3W and SytC2B3W mutations show that both synaptotagmin 1 C2 domains contribute to the Ca2+ sensitivity of release. The SytC2B3W mutation appears to increase the apparent Ca2+ affinity of synaptotagmin 1 to phospholipids (Fig. 2) and the probability of release (Fig. 4 A and B) more than the SytC2A3W mutation. In contrast, the SytC2A3W mutation appears to increase the Ca2+ sensitivity of release to a larger extent than the SytC2B3W (Fig. 3C). Although the differences between the effects of the tryptophan mutations in the C2A and C2B domains are relatively small, they probably reflect an asymmetry in the contribution of the two C2 domains to synaptotagmin 1 function. This asymmetry is also manifested in the stronger functional effects caused by mutations in the Ca2+-binding residues of the C2B domain than those caused by analogous mutations in the C2A domain (24, 45, 50, 51). The source of this asymmetry is currently not understood. In addition to being useful for deciphering the molecular mechanism of release, these gain-of-function mutants may also serve as tools in studying the role of synaptic efficacy in neural circuits in general, because they produce a unique enhancement of the efficiency of evoked synaptic release.
Acknowledgments
We thank Hui Deng, Dirk Reuter, and Dr. Ralf Nehring for help in the virus preparation. This work was supported by the Brown Foundation, German Research Council Grant Ro1296/5-3 (to C.R.), and National Institutes of Health Grants NS50655 (to C.R.) and NS40944 (to J.R.).
Author contributions: J.-S.R., L.Y.L., O.-H.S., and J.-C.R. performed research and analyzed data; and J.R., T.C.S., and C.R. designed research and wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: EPSC, excitatory postsynaptic current; mEPSC, miniature EPSC; PIP, phosphatidylinositol 4-phosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; RRP, readily releasable vesicle pool.
References
- 1.Chapman, E. R. (2002) Nat. Rev. Mol. Cell Biol. 3, 498-508. [DOI] [PubMed] [Google Scholar]
- 2.Südhof, T. C. (2004) Annu. Rev. Neurosci. 27, 509-547. [DOI] [PubMed] [Google Scholar]
- 3.Sutton, R. B., Davletov, B. A., Berghuis, A. M., Südhof, T. C. & Sprang, S. R. (1995) Cell 80, 929-938. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez, I., Arac, D., Ubach, J., Gerber, S. H., Shin, O. H., Gao, Y., Anderson, R. G., Südhof, T. C. & Rizo, J. (2001) Neuron 32, 1057-1069. [DOI] [PubMed] [Google Scholar]
- 5.Shao, X., Davletov, B. A., Sutton, R. B., Südhof, T. C. & Rizo, J. (1996) Science 273, 248-251. [DOI] [PubMed] [Google Scholar]
- 6.Ubach, J., Zhang, X., Shao, X., Südhof, T. C. & Rizo, J. (1998) EMBO J. 17, 3921-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davletov, B. A. & Südhof, T. C. (1993) J. Biol. Chem. 268, 26386-26390. [PubMed] [Google Scholar]
- 8.Chapman, E. R. & Jahn, R. (1994) J. Biol. Chem. 269, 5735-5741. [PubMed] [Google Scholar]
- 9.Rizo, J. & Südhof, T. C. (1998) J. Biol. Chem. 273, 15879-15882. [DOI] [PubMed] [Google Scholar]
- 10.Bennett, M. K., Calakos, N. & Scheller, R. H. (1992) Science 257, 255-259. [DOI] [PubMed] [Google Scholar]
- 11.Li, C., Ullrich, B., Zhang, J. Z., Anderson, R. G., Brose, N. & Südhof, T. C. (1995) Nature 375, 594-599. [DOI] [PubMed] [Google Scholar]
- 12.Chapman, E. R., Hanson, P. I., An, S. & Jahn, R. (1995) J. Biol. Chem. 270, 23667-23671. [DOI] [PubMed] [Google Scholar]
- 13.Kee, Y. & Scheller, R. H. (1996) J. Neurosci. 16, 1975-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng, Z. H., Yokoyama, C. T. & Catterall, W. A. (1997) Proc. Natl. Acad. Sci. USA 94, 5405-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerona, R. R., Larsen, E. C., Kowalchyk, J. A. & Martin, T. F. (2000) J. Biol. Chem. 275, 6328-6336. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, X., Kim-Miller, M. J., Fukuda, M., Kowalchyk, J. A. & Martin, T. F. (2002) Neuron 34, 599-611. [DOI] [PubMed] [Google Scholar]
- 17.Earles, C. A., Bai, J., Wang, P. & Chapman, E. R. (2001) J. Cell Biol. 154, 1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin, O. H., Rhee, J. S., Tang, J., Sugita, S., Rosenmund, C. & Südhof, T. C. (2003) Neuron 37, 99-108. [DOI] [PubMed] [Google Scholar]
- 19.Sugita, S., Hata, Y. & Südhof, T. C. (1996) J. Biol. Chem. 271, 1262-1265. [DOI] [PubMed] [Google Scholar]
- 20.Chapman, E. R., An, S., Edwardson, J. M. & Jahn, R. (1996) J. Biol. Chem. 271, 5844-5849. [DOI] [PubMed] [Google Scholar]
- 21.Desai, R. C., Vyas, B., Earles, C. A., Littleton, J. T., Kowalchyck, J. A., Martin, T. F. & Chapman, E. R. (2000) J. Cell Biol. 150, 1125-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu, Y., He, Y., Bai, J., Ji, S. R., Tucker, W. C., Chapman, E. R. & Sui, S. F. (2003) Proc. Natl. Acad. Sci. USA 100, 2082-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Chacon, R., Konigstorfer, A., Gerber, S. H., Garcia, J., Matos, M. F., Stevens, C. F., Brose, N., Rizo, J., Rosenmund, C. & Südhof, T. C. (2001) Nature 410, 41-49. [DOI] [PubMed] [Google Scholar]
- 24.Robinson, I. M., Ranjan, R. & Schwarz, T. L. (2002) Nature 418, 336-340. [DOI] [PubMed] [Google Scholar]
- 25.Shin, O. H., Rizo, J. & Südhof, T. C. (2002) Nat. Neurosci. 5, 649-656. [DOI] [PubMed] [Google Scholar]
- 26.Benfenati, F., Greengard, P., Brunner, J. & Bahler, M. (1989) J. Cell Biol. 108, 1851-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashery, U., Betz, A., Xu, T., Brose, N. & Rettig, J. (1999) Eur. J. Cell Biol. 78, 525-532. [DOI] [PubMed] [Google Scholar]
- 28.Han, W., Rhee, J. S., Maximov, A., Lao, Y., Mashimo, T., Rosenmund, C. & Südhof, T. C. (2004) Neuron 41, 85-99. [DOI] [PubMed] [Google Scholar]
- 29.Pyott, S. J. & Rosenmund, C. (2002) J. Physiol. (London) 539, 523-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, X., Rizo, J. & Südhof, T. C. (1998) Biochemistry 37, 12395-12403. [DOI] [PubMed] [Google Scholar]
- 31.Shao, X., Li, C., Fernandez, I., Zhang, X., Südhof, T. C. & Rizo, J. (1997) Neuron 18, 133-142. [DOI] [PubMed] [Google Scholar]
- 32.Südhof, T. C. & Rizo, J. (1996) Neuron 17, 379-388. [DOI] [PubMed] [Google Scholar]
- 33.Chapman, E. R. & Davis, A. F. (1998) J. Biol. Chem. 273, 13995-14001. [DOI] [PubMed] [Google Scholar]
- 34.Gerber, S. H., Rizo, J. & Südhof, T. C. (2002) Diabetes 51, S12-S18. [DOI] [PubMed] [Google Scholar]
- 35.Bai, J., Wang, P. & Chapman, E. R. (2002) Proc. Natl. Acad. Sci. USA 99, 1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deutsch, J. W. & Kelly, R. B. (1981) Biochemistry 20, 378-385. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda, M., Aruga, J., Niinobe, M., Aimoto, S. & Mikoshiba, K. (1994) J. Biol. Chem. 269, 29206-29211. [PubMed] [Google Scholar]
- 38.Bai, J., Tucker, W. C. & Chapman, E. R. (2004) Nat. Struct. Mol. Biol. 11, 36-44. [DOI] [PubMed] [Google Scholar]
- 39.Zucker, R. S. & Regehr, W. G. (2002) Annu. Rev. Physiol. 64, 355-405. [DOI] [PubMed] [Google Scholar]
- 40.Rosenmund, C. & Stevens, C. F. (1996) Neuron 16, 1197-1207. [DOI] [PubMed] [Google Scholar]
- 41.Nonet, M. L., Grundahl, K., Meyer, B. J. & Rand, J. B. (1993) Cell 73, 1291-1305. [DOI] [PubMed] [Google Scholar]
- 42.Littleton, J. T., Stern, M., Schulze, K., Perin, M. & Bellen, H. J. (1993) Cell 74, 1125-1134. [DOI] [PubMed] [Google Scholar]
- 43.DiAntonio, A., Parfitt, K. D. & Schwarz, T. L. (1993) Cell 73, 1281-1290. [DOI] [PubMed] [Google Scholar]
- 44.Geppert, M., Goda, Y., Hammer, R. E., Li, C., Rosahl, T. W., Stevens, C. F. & Südhof, T. C. (1994) Cell 79, 717-727. [DOI] [PubMed] [Google Scholar]
- 45.Mackler, J. M., Drummond, J. A., Loewen, C. A., Robinson, I. M. & Reist, N. E. (2002) Nature 418, 340-344. [DOI] [PubMed] [Google Scholar]
- 46.Arac, D., Murphy, T. & Rizo, J. (2003) Biochemistry 42, 2774-2780. [DOI] [PubMed] [Google Scholar]
- 47.Bollmann, J. H. & Sakmann, B. (2005) Nat. Neurosci. 8, 426-434. [DOI] [PubMed] [Google Scholar]
- 48.Davis, A. F., Bai, J., Fasshauer, D., Wolowick, M. J., Lewis, J. L. & Chapman, E. R. (1999) Neuron 24, 363-376. [DOI] [PubMed] [Google Scholar]
- 49.Millet, O., Bernado, P., Garcia, J., Rizo, J. & Pons, M. (2002) FEBS Lett. 516, 93-96. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Chacon, R., Shin, O. H., Konigstorfer, A., Matos, M. F., Meyer, A. C., Garcia, J., Gerber, S. H., Rizo, J., Südhof, T. C. & Rosenmund, C. (2002) J. Neurosci. 22, 8438-8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishiki, T. & Augustine, G. J. (2004) J. Neurosci. 24, 8542-8550. [DOI] [PMC free article] [PubMed] [Google Scholar]