Abstract
DC-based tumor vaccine research has largely focused on enhancing DC maturation/costimulation and antigen presentation in order to break tolerance against self tumor-associated antigens. DC immunization can activate autoreactive T cells but rarely causes autoimmune pathologies, indicating that self tolerance at the host level is still maintained in the vaccinated hosts. This study in mice reveals a novel regulatory mechanism for the control of self tolerance at the host level by DCs through the restriction of positive cytokine feedback loops by cytokine signaling inhibitor SOCS1. The study further finds the requirement of persistent antigen presentation by DCs for inducing pathological autoimmune responses against normal tissues and tumor, which can be achieved by silencing SOCS1 to unleash the unbridled signaling of IL-12 and the downstream cytokine cascade. However, the use of higher-affinity self peptides, enhancement of DC maturation, and persistent stimulation with cytokines or TLR agonists fail to break tolerance and induce pathological antitumor immunity. Thus, this study indicates the necessity of inhibiting SOCS1, an antigen presentation attenuator, to break self tolerance and induce effective antitumor responses.
Introduction
The mechanisms utilized by professional APCs to sense microbes and initiate immune responses has been well studied (1–3), largely because of the critical role of APCs such as DCs in initiating and regulating immune responses and their therapeutic potential (4–6). When DCs encounter proinflammatory stimuli, such as microbial products, the maturation process is initiated and results in proinflammatory cytokine secretion and upregulation of MHC molecules and costimulatory molecules (4, 5). Following maturation and homing to LNs, DCs establish contact with T cells by forming an immunological synapse, where the TCR/MHC interaction and costimulatory molecules congregate in a central area surrounded by adhesion molecules (7). Once activated, CD8+ CTLs can proliferate for several generations and acquire lytic function (8, 9). It has therefore been proposed that the level and duration of peptide-MHC complexes (signal 1) and costimulatory molecules (signal 2) provided by DCs determines the magnitude and fate of an antigen-specific T cell response (10, 11). In addition, cytokines have been implied as a third signal for the activation of T cells by DCs (12, 13).
Little is known about the negative regulatory mechanisms in DCs used to control the magnitude and overactivation of T cell responses, in contrast to the extensive studies on DC activation. Several studies demonstrate that SOCS1 is a negative regulator of LPS-induced macrophage activation and plays an essential role in suppressing systemic autoimmunity mediated by DCs (14–16). SOCS1 is an inducible negative feedback inhibitor of the JAK/STAT signal pathway (17). Homozygous genetic KO of SOCS1 in mice results in neonatal lethality due to uncontrolled IFN-γ signaling (18, 19). SOCS1-KO DCs are hyperactivated and induce aberrant expansion of B and T cells. Recently we found that SOCS1-silenced DCs were able to induce an enhanced antigen-specific CTL response and antitumor activity (20). We have identified SOCS1 as an important negative regulator of antigen presentation by DCs and demonstrated that silencing of SOCS1 enhances antigen presentation by DCs and antigen-specific antitumor immunity (20). In agreement, Hanada et al. reported that immunization with SOCS1-KO DCs induced a hyper–Th1-type immune response and antitumor activities (21).
Immunologic peripheral tolerance to self antigen reflects the inability of autoreactive T cells that escape censoring in the thymus to cause autoimmune pathologies. The essential requirement for an effective tumor vaccine is its ability to break self tolerance and induce pathological autoimmune responses against tumors and normal nonessential tissues that express self tumor-associated antigens (self TAAs) (22). DC vaccines have been viewed as 1 of the most promising strategies for tumor vaccination (4, 6, 23, 24). A puzzling paradox is that mature DC immunization can effectively break self tolerance at the cellular level, i.e., activate self antigen–specific CTLs, but rarely causes autoimmune pathologies against normal tissues and tumors (25), suggesting that self tolerance at the host level is still maintained in a host’s natural immunosuppressive environment, sustained by various mechanisms such as regulatory T cells and exaggerated by the tumor-derived factors (26, 27), impeding the efficacy and usefulness of DC-based tumor vaccination.
The aims of this study were to investigate the underlying regulatory mechanisms in DCs used to control the magnitude and overactivation of autoreactive CTL responses and to define the requirements for DCs to induce TAA-specific, pathological autoimmune antitumor responses. We investigate autoimmune CTL responses against a natural self antigen, tyrosinase-related protein 2 (TRP2), in syngeneic, WT mice (28). This self TRP2 mouse model may limit the problems associated with the use of transgenic mouse models and/or the use adoptive transfer of high-avidity TCR-transgenic T cells (29). The results of this study demonstrate that SOCS1 functions as an antigen presentation attenuator for the control of self tolerance at the host level through the restriction of IL-12 and the downstream cytokine signaling cascade by SOCS1. This study further establishes a new principle for breaking self tolerance at the host level and inducing self TAA-specific, pathologic antitumor immunity by DC vaccination.
Results
DC immunization and persistent in vivo stimulation with TLR agonists fail to break self tolerance at the host level.
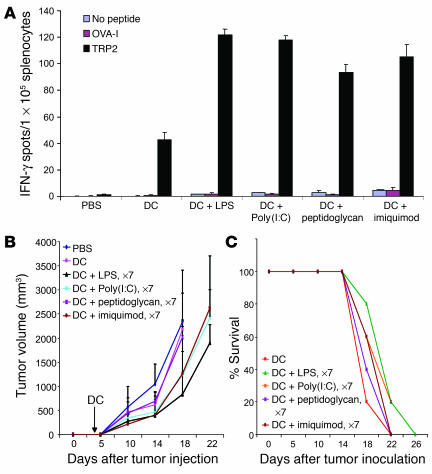
We first tested whether DC immunization, plus persistent in vivo TLR signaling, was able to break self tolerance and induce self antigen–specific autoimmune pathologies. We selected this combined immunization protocol because DCs are the most potent professional APCs, and TLR signaling is the most potent means to activate DCs and immune responses (1, 2, 4). We used the mouse melanocyte differentiation antigen TRP2 as a model self antigen because it is naturally expressed in normal melanocytes as well as weakly immunogenic B16 melanoma, and multiple MHC class I epitopes of varying affinity have been identified within TRP2 (28). WT C57BL/6 mice were administered TRP2-pulsed, LPS-matured DCs derived from mouse BM cells. The mice were then stimulated in vivo (i.p.) with TLR agonists to TLR2, -3, -4, and -7 (LPS [TLR4], poly[I:C] [TLR3], peptidoglycan [TLR2], or imiquimod [TLR7]), which are expressed on mouse myeloid DCs (30), for 7 consecutive days. TRP2-specific CTL responses were efficiently induced in mice immunized with WT DCs and TLR agonists as shown by IFN-γ enzyme-linked immunospot (ELISPOT) analysis (Figure 1A). However, despite persistent in vivo TLR stimulation after DC transfer, the development of autoimmune vitiligo, a self-reactive immune response targeted against TRP2 in host melanocytes that is characterized by the development of coat lightening, depigmentation, and/or hair loss, was absent from all immunized mice. Immunization with TRP2-pulsed, matured DCs, followed by in vivo stimulation with various TLR agonists, failed to effectively control tumor progression (Figure 1, B and C). These results indicate that this immunization protocol of mature DC transfer plus persistent in vivo TLR signaling can effectively break self tolerance at the cellular level, i.e. activate self antigen–specific CTLs, but not at the host level, as manifested by the lack of autoimmune pathologies against self antigen–expressing normal tissues and tumor.
Figure 1.
Persistent TLR stimulation of DC immunization failed to induce pathological autoimmune response and antitumor immunity. (A) TRP2-specific CTL responses induced by persistent in vivo TLR stimulation and DC immunization. C57BL/6 mice were immunized with TRP2-pulsed (100 μg/ml) and LPS-matured (100 ng/ml) DCs and then stimulated with various TLR agonists daily for 7 consecutive days. Splenocytes pooled from immunized mice (2–3 mice) were subjected to IFN-γ ELISPOT assays. An irrelevant peptide (OVA-I peptide) was used as a negative control. (B and C) Inability to control preestablished B16 tumors by persistent in vivo TLR stimulation and DC immunization. Groups of mice were inoculated s.c. with B16 tumor cells (2.5 × 105) and 3 days later were immunized via the rear foot pad with 1.5 × 106 TRP2 peptide–pulsed (100 μg/ml) DCs with ex vivo LPS maturation. After DC transfer, in vivo TLR agonists were administered i.p. daily for 7 days. Tumor growth and percent survival (n = 5–7 mice/group) curves represent 1 of 3 independent experiments. When their tumor volume reached approximately 2,000 mm3 in size, mice were euthanized and recorded as dead.
Silencing of a signaling inhibitor allows DCs to break self tolerance and induce autoimmune pathologies.
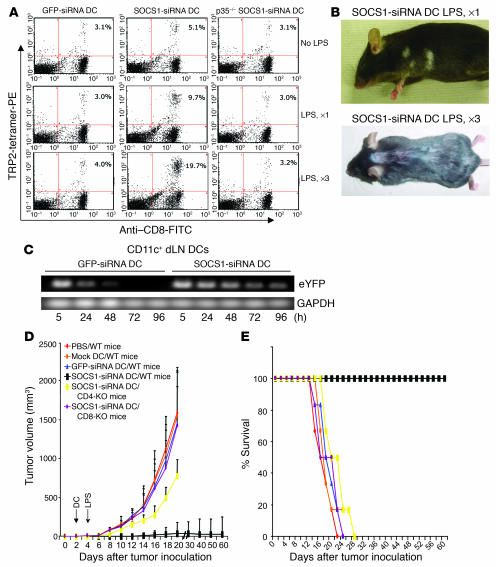
To investigate possible molecular restrictions in DCs to prevent autoimmune pathologies, we tested the effect of silencing SOCS1 in DCs on autoimmune responses based upon our previous findings (20). A lentiviral vector, LV-SOCS1-siRNA (eYFP), with the ability to specifically downregulate approximately 90% of SOCS1 mRNA in BM-derived DCs and a control vector, LV-GFP-siRNA (eYFP), were generated as described previously (20). In agreement, the SOCS1 protein expression in the transduced LV-SOCS1-siRNA DCs was significantly reduced, as demonstrated by Western blotting (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI26169DS1 http://dx.doi.org/10.1172/JCI26169DS1). TRP2-pulsed, transduced DCs were transferred into WT C57BL/6 mice. The mice were then stimulated in vivo with or without a low dose of LPS, and CTL responses were measured. As the number of in vivo LPS stimulations was increased, the amount of TRP2-specific CD8+ T cells also increased in SOCS1-siRNA DC mice (Figure 2A). In contrast, the amount of TRP2-specific CD8+ T cells was only marginally increased in GFP-siRNA DC mice, regardless of the number of LPS stimulations, and was consistently lower than that in SOCS1-siRNA DC mice (Figure 2A, left panels). IFN-γ ELISPOT and CTL assays showed similar results (data not shown). The development of autoimmune vitiligo was apparent at 2–3 months after immunization in many of the SOCS1-siRNA DC mice injected with LPS (Figure 2B). In contrast, autoimmune vitiligo was not observed in any of GFP-siRNA DC mice, even with repeated in vivo LPS administrations. Although the mechanisms for the inability of GFP-siRNA DCs to induce stronger antitumor immunity in response to repeated LPS stimulation are still not clear, SOCS1-restricted JAK/STAT signaling, endotoxin tolerance, and/or other mechanisms may contribute to this outcome. Interestingly, we observed that the duration of the presence of SOCS1-siRNA DCs in draining LNs of immunized mice was increased, compared with that of GFP-siRNA DCs (Figure 2C), suggesting that the enhanced response of SOCS1-siRNA DCs to repeated LPS stimulation is, in part, due to the prolonged duration of these DCs in draining LNs. Taken together, these results suggest a critical role of SOCS1 in DCs for maintaining tolerance to self antigens at the host level and a necessity of silencing the signaling inhibitor to induce pathological autoimmune responses by DC immunization.
Figure 2.
SOCS1-silenced DCs induced pathological autoimmune responses. (A) TRP2-specific CTL responses induced by SOCS1-silenced DCs. Mice were immunized with TRP2-pulsed (50 μg/ml), LV-transduced WT, or IL-12p35–KO DCs with LPS-induced maturation (100 ng/ml) ex vivo. The mice were then stimulated 0, 1 (day 1), or 3 times (days 1, 4, and 7) i.p. with LPS (30 μg/mouse/injection). Percentages of TRP2-tetramer-PE–positive T cells in the gated CD8+ T cells of splenocytes in mice 2 weeks after immunization are shown from 1 of 3 independent experiments. P < 0.01, GFP-siRNA DCs versus SOCS1-siRNA DCs. (B) Representative autoimmune vitiligo of mice 3 months after immunization with TRP2-pulsed LV-SOCS1-siRNA DCs, followed by LPS stimulation once or 3 times. (C) Duration of DCs in draining LNs. Groups of mice were immunized with LV-transduced DCs, and CD11c+ DCs isolated from the draining LNs (dLN) of the mice at different times were used for RT-PCR analysis of the transgene eYFP marker mRNA. GAPDH was used as internal control. Experiments were repeated twice with similar results. (D and E) Inhibition of preestablished B16 tumors by SOCS1-siRNA DCs. WT, CD4-KO, or CD8-KO C57BL/6 mice were inoculated s.c. with B16 tumor cells (2.5 × 105) and 3 days later were immunized via the rear foot pad with 1.5 × 106 TRP2 peptide–pulsed (50 μg/ml), LV-transduced DCs with ex vivo LPS maturation (100 ng/ml). One day after DC transfer, in vivo LPS was administered i.p. (30 μg/mouse) 1 time. Tumor growth (n = 6 mice/group) and percent survival curves represent 1 of 3 independent experiments. P < 0.01, GFP-siRNA DCs compared with SOCS1-siRNA DCs.
SOCS1-silenced DCs induce effective antitumor immunity capable of controlling preestablished B16 tumors when costimulated with a TLR agonist.
We (20) and Hanada et al. (21) have independently shown that SOCS1-siRNA DC or SOCS1-KO DC vaccination of weakly immunogenic (TRP2+) B16 tumor–bearing mice results in a reduction in tumor growth and an increase in survival; however, tumor growth is not fully inhibited, and mice eventually succumb to tumor burden. Therefore, we tested whether SOCS1-silenced DCs more potently induce autoreactive CTL responses capable of controlling the growth of preestablished B16 tumors when boosted in vivo with a TLR agonist such as LPS, a potent inducer of proinflammatory cytokines (1–3). The addition of in vivo stimulation with LPS significantly enhanced the ability to inhibit B16 tumors and promote mouse survival by immunization with SOCS1-siRNA but not GFP-siRNA DCs (Figures 2, D and E). The enhanced antitumor activity was correlated with potent TRP2-specific CTL activities observed in SOCS1-siRNA DC mice (data not shown). By transferring SOCS1-siRNA DCs into CD4- and CD8-KO mice, it was further demonstrated that the antitumor response required both CD8+ and CD4+ cells, although a weak antitumor response was observed in CD4-KO mice (Figures 2, D and E). No apparent toxicity, other than autoimmune vitiligo, was observed in the TRP2-pulsed SOCS1-siRNA DC mice coinjected with LPS more than 6 months after immunization. Histological analysis of major organs and tissues of the immunized mice revealed no pathologic inflammation (data not shown). Collectively, these results suggest that SOCS1-restricted signaling of TLR agonist–induced cytokines in DCs may control their ability to break tolerance at the host level and induce effective antitumor immunity.
Role of SOCS1-restricted cytokine (IL-12) production by DCs in breaking self tolerance at the host level.
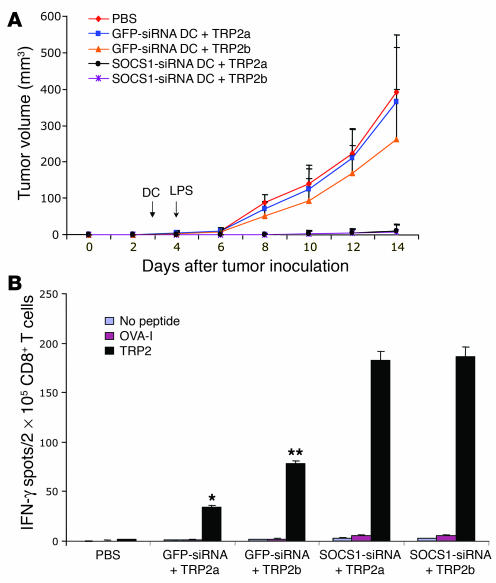
We next investigated possible mechanisms used by SOCS1 in DCs to regulate antigen presentation by examining its influence on 3 major signals provided to T cells: antigenic peptide/MHC presentation (signal 1), costimulatory molecule expression (signal 2), and/or cytokine production (signal 3). By flow cytometric assays, we consistently found that there were only slightly altered surface levels of costimulatory/inhibitory molecules (B7.1, B7.2, OX40L, CD40, or PDL1) on SOCS1-siRNA DCs compared with those on GFP-siRNA DCs both before and after LPS-induced maturation (data not shown), in agreement with our previous observation (20). Comparable levels of MHC class I and II molecules (signal 1) were also detected on SOCS1-siRNA DCs and GFP-siRNA DCs (data not shown). We further investigated whether peptide immunogenicity (TCR affinity) can influence SOCS1-restricted CTL responses in vivo. Since a high-affinity form (TRP2b) and a low-affinity form (TRP2a) of the TRP2 CTL peptide were identified previously (28), we used these 2 TRP2 peptides to test whether the strength of signal 1 can influence the ability of transduced DCs to control B16 tumor growth. Figure 3A shows that GFP-siRNA DCs loaded with either the low- or high-affinity peptide were unable to induce B16 tumor regression with in vivo LPS stimulation, although GFP-siRNA DCs loaded with the TRP2b peptide showed a marginal effect on tumor growth. In contrast, both SOCS1-siRNA DC groups loaded with the low- or high-affinity TRP2 peptide effectively blocked tumor growth. We investigated TRP2-specific CTL activities in vaccinated mice with IFN-γ ELISPOT. Figure 3B shows that GFP-siRNA DCs loaded with the high-affinity peptide induced stronger IFN-γ responses than did GFP-siRNA DCs loaded with the low-affinity peptide. However, both SOCS1-siRNA DCs loaded with low- or high-affinity peptide induced much stronger IFN-γ responses than GFP-siRNA DCs (P < 0.01). These results show that SOCS1 in DCs does not have a significant impact on the expression of costimulatory/inhibitory molecules or MHC class I and II molecules on DCs; and SOCS1-restricted signaling in DCs plays a more dominant role than self peptide affinity in inducing CTL responses and antitumor immunity.
Figure 3.
Effects of SOCS1 silencing on antigen presentation by DCs. (A) Inhibition of preestablished B16 tumor by SOCS1-siRNA DC immunization with TRP2 peptides of different affinities. WT C57BL/6 mice were inoculated s.c. with B16 tumor cells (2.5 × 105) and 3 days later were immunized with 1.5 × 106 TRP2 peptide–pulsed (50 μg/ml; TRP2a or TRP2b), transduced DCs with ex vivo LPS maturation (100 ng/ml). One day after DC transfer, in vivo LPS was administered i.p. (30 μg/mouse) 1 time. Tumor growth curves (n = 6 mice/group) represent 1 of 3 independent experiments. P < 0.01, GFP-siRNA DC compared with SOCS1-siRNA DCs. (B). CD8+ T cell responses induced by SOCS1-siRNA DCs with different TRP2 peptides. CD8+ T cells isolated from the pooled splenocytes of immunized mice (2–3 mice) were subjected to IFN-γ ELISPOT assays stimulated with TRP2a or TRP2b peptide (10 μg/ml). An irrelevant peptide derived from ovalbumin was used as a negative control. *P < 0.01 versus SOCS1-siRNA DC plus TRP2a; **P < 0.01 versus SOCS1-siRNA DC plus TRP2a.
The above observations and known SOCS1 functions as an inhibitor of JAK/STAT signaling (17) imply a critical role of SOCS1-restricted cytokine signaling and production in controlling T cell responses. We initially tested the importance of several candidate cytokines known to influence CTL activation by using DCs derived from the BM of different KO mice. LV-transduced DCs derived from IL-12p35–KO mice (p35–/– SOCS1-siRNA DCs) were no longer able to inhibit the growth of preestablished B16 tumors (Figures 4, A and B). IFN-γ ELISPOT and CTL assays showed that p35–/– SOCS1-siRNA DCs had a substantially reduced ability to induce TRP2-specific CTL responses compared with WT SOCS1-siRNA DCs (Figure 4C and Supplemental Figure 2). In addition, in vivo stimulation with LPS failed to boost CTL responses induced by p35–/– SOCS1-siRNA DCs as measured by TRP2-tetramer analysis (Figure 2A), and autoimmune vitiligo did not develop in these mice (data not shown). In contrast to the essential role of IL-12 produced by antigen-presenting DCs, antitumor CTL responses were still effectively induced in IL-12p35–KO mice immunized with WT SOCS1-siRNA DCs, suggesting that IL-12 produced by host cells is not required for the induction of CTL responses (Figure 4, A–C, and Supplemental Figure 2). These results suggest that IL-12 production by SOCS1-silenced, antigen-presenting DCs is required for inducing a potent TRP2-specific CTL response and breaking self tolerance at the host level.
Figure 4.
The role of IL-12 produced by SOCS1-silenced DCs in breaking self tolerance and inducing antitumor immunity. (A and B) Inability to inhibit preestablished B16 tumor by IL-12–deficient SOCS1-siRNA DCs. WT or IL-12p35–KO mice were inoculated s.c. with B16 tumor cells (2.5 × 105) and 3 days later were immunized with 1.5 × 106 TRP2-pulsed, transduced WT, or IL-12p35–KO DCs with ex vivo LPS maturation. Tumor growth (A) and percent survival (B) (n = 6 mice/group) curves represent 1 of 2 independent experiments. P < 0.01, GFP-siRNA DCs compared with SOCS1-siRNA DCs. (C) CD8+ T cell responses induced by IL-12–deficient SOCS1-siRNA DCs. CD8+ T cells isolated from the pooled splenocytes of immunized WT or IL-12p35–KO mice were subjected to IFN-γ ELISPOT assays. An irrelevant peptide (OVA-I peptide) was used as a negative control. *P < 0.01 versus SOCS1-siRNA DCs.
Prolonged and enhanced production of IL-12 by SOCS1-silenced DCs versus transient and low production by WT DCs.
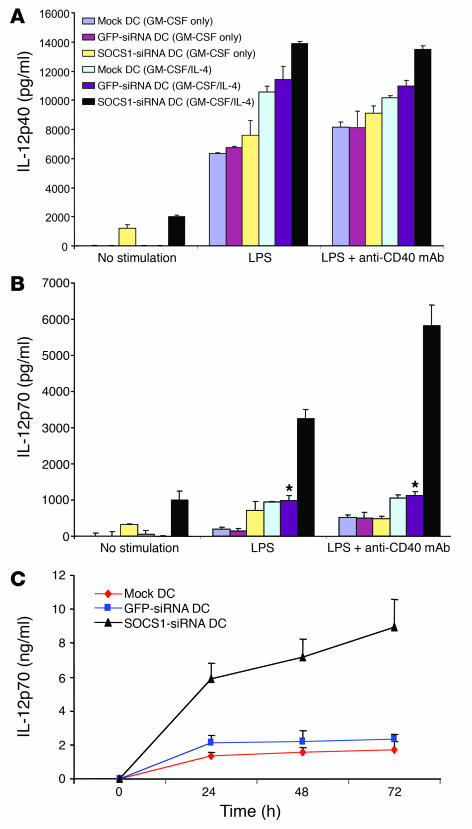
DCs produce significant amounts of heterodimeric IL-12 in response to microbial products and/or CD40 ligand. However, the duration of IL-12 production by stimulated DCs is tightly restricted to a short time period (8–16 hours) (31). We determined the effect of SOCS1 on the concentration and duration of IL-12 produced by DCs following in vitro stimulation. To test this we stimulated SOCS1-silenced DCs or control DCs with LPS alone or in combination with stimulatory anti-CD40 antibody and then measured the production of IL-12. We observed a small but significant (P < 0.05) increase in the production of IL-12p40 by SOCS1-silenced DCs derived from BM culture with GM-CSF and IL-4, compared with control DCs (Figure 5A), which is consistent with a previous observation (32). Strikingly, the expression of IL-12p70 by SOCS1-siRNA DCs was substantially increased after stimulation compared with that by control DCs (Figure 5B), which is consistent with 2 recent reports that SOCS1-KO macrophages produced excessive amounts of IL-12 and other cytokines in response to stimuli and that enhanced levels of IL-12 and other cytokines were found in sera of conditional SOCS1-KO mice (33, 34). Interestingly, BM DCs after culture with only GM-CSF produced less IL-12 (Figure 5A), likely due to the previously described stimulatory effects of IL-4 on IL-12 production by DCs (35). The marked difference in IL-12p70 compared with IL-12p40 secretion is likely due to the upregulation of IL-12p35 expression, which has previously been shown to act as the rate-limiting step for IL-12 heterodimer secretion (36). Indeed, higher levels of IL-12p35 mRNA were also detected in SOCS1-siRNA DCs, compared with control GFP-siRNA DCs (Supplemental Figure 3). We also observed that the levels of the IFN-inducible factors ICSBP and IRF-1 mRNA (37) appeared enhanced in SOCS1-siRNA DCs, while the transcription factors C/EBP-β and c-Rel remained largely unchanged compared with control DCs (Supplemental Figure 3). Consistent with the in vitro data, the expression levels of IL-12p35 as well as other proinflammatory cytokines, IFN-γ and IL-2, were elevated in DCs isolated from the draining LNs of mice receiving SOCS1-siRNA DCs and in vivo LPS stimulation (Supplemental Figure 4). Finally, we found that SOCS1-silenced DCs were able to continuously produce high levels of IL-12p70 for at least 72 hours when stimulated with LPS/anti-CD40 mAb, while control GFP-siRNA DCs produced only low levels of IL-12p70 over the same culture period (Figure 5C). These results indicate that SOCS1 silencing allows DCs to continuously produce increased levels of IL-12p70 in response to inflammatory stimulation.
Figure 5.
SOCS1 restricts the level and duration of IL-12 produced by DCs. (A and B) Levels of IL-12p40 (A) and IL-12p70 (B) secreted by DCs (5 × 105 cells/ml) in response to continuous stimulation with LPS (100 ng/ml) with or without plate-coated anti-CD40 mAb (1:1,000 dilution) for 24 hours from 1 of 3 independent experiments. All BM DCs were cultured with mGM-CSF (20 ng/ml) in the presence or absence of mIL-4 (20 ng/ml). *P < 0.01 versus SOCS1-siRNA DCs. (C). Kinetics of IL-12p70 production by DCs (5 × 105 cells/ml) in response to continuous stimulation with LPS (100 ng/ml) and anti-CD40 mAb for 0–72 hours from 1 of 3 independent experiments. All cells were cultured in the presence of both GM-CSF and IL-4. *P < 0.01 GFP-siRNA DCs versus SOCS1-siRNA DCs.
Increased and continuous production of IL-12 by WT DCs is not sufficient to break self tolerance at the host level.
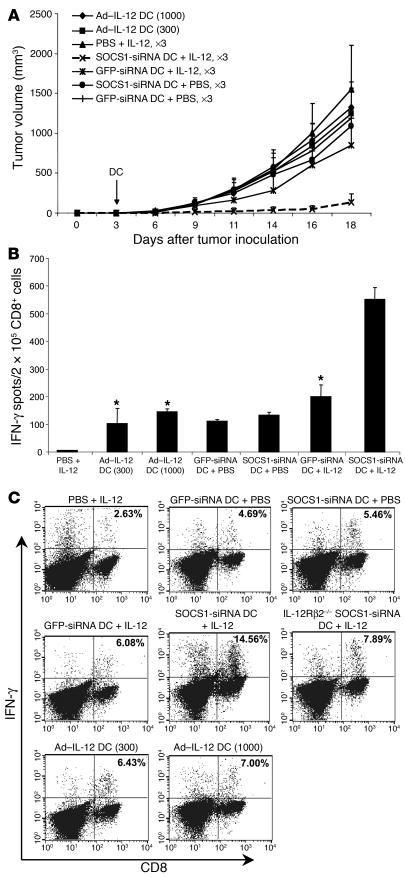
To investigate whether overproduction of IL-12 alone may account for the ability of SOCS1-silenced DCs to break self tolerance at the host level, we compared the ability of WT DCs transfected with recombinant adenovirus constitutively expressing biologically active mouse IL-12 cytokine (Ad–IL-12) and SOCS1-silenced DCs to induce antitumor CTL responses. DCs transfected with Ad–IL-12 at an MOI of 300 produced a high level of IL-12p70, comparable to the IL-12p70 level produced by SOCS1-siRNA DCs after stimulation with LPS. Unexpectedly, immunization with mature TRP2-pulsed DCs transfected with different MOIs of Ad–IL-12 failed to control the growth of preestablished B16 tumors (Figure 6A). In agreement, in mice immunized with Ad–IL-12 DCs (300 or 1,000 MOI), only modest increases in CTL responses were observed (Figure 6, B and C). Furthermore, we found that in vivo stimulation with a low dose of IL-12 did not significantly enhance CTL responses and antitumor activities in GFP-siRNA DC mice but significantly enhanced the antitumor CTL responses in SOCS1-siRNA DC mice (Figure 6, A–C), suggesting the enhanced sensitivity of SOCS1-silenced DCs to IL-12 stimulation. Importantly, vitiligo was not induced in mice immunized with Ad–IL-12 DCs or GFP-siRNA DCs with IL-12 coinjection, while it was observed in about 40% of TRP2-pulsed, SOCS1-siRNA DC mice coinjected with a low dose of IL-12 (Supplemental Figure 5). These results indicate that continuous overexpression of IL-12 by WT DCs is not sufficient to break self tolerance at the host level and that SOCS1-restricted signaling of IL-12 in antigen-presenting DCs and the concentration of IL-12 produced by DCs play a critical role in controlling CTL responses and tolerance.
Figure 6.
WT DCs constitutively expressing IL-12 were insufficient to overcome self tolerance at the host level. (A) Inability to inhibit preestablished B16 tumors by Ad–IL-12 DCs. BM DCs were transduced with Ad–IL-12 (MOIs of 300 or 1,000) or LV-SOCS-siRNA or LV-GFP-siRNA (MOI of 5). WT mice were inoculated s.c. with B16 tumor cells (2.5 × 105) and 3 days later were immunized with 1.5 × 106 TRP2-pulsed DCs with ex vivo TNF-α maturation (50 ng/ml). After transfer of DCs, IL-12 (1 μg/mouse) was administered i.p. 3 times on days 1, 3, and 5. Tumor growth curves (n = 6 mice/group) represent 1 of 3 independent experiments. (B and C). Antigen-specific CD8+ T cell responses. Splenocytes or CD8+ T cells isolated from the pooled splenocytes of immunized mice were subjected to IFN-γ ELISPOT assays (B) or intracellular IFN-γ staining (C). *P < 0.01 versus SOCS1-siRNA DCs plus IL-12.
Unbridled IL-12 signaling in DCs is required to break self tolerance at the host level.
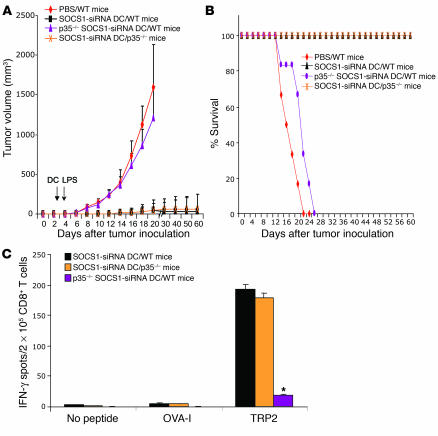
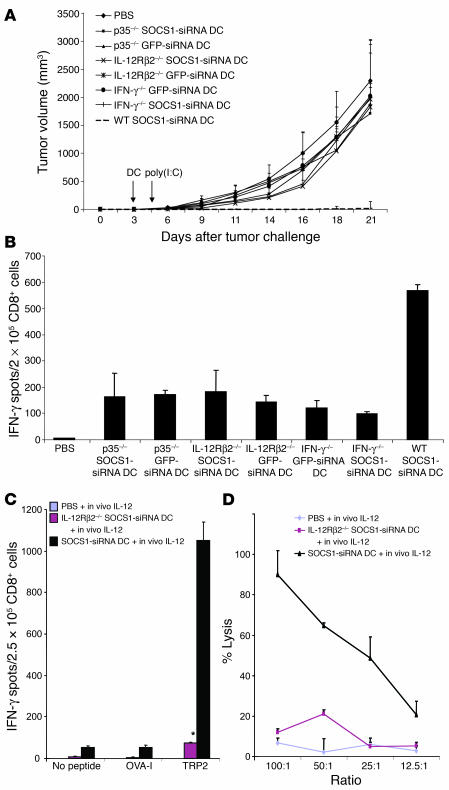
To test the role of IL-12 signaling in DCs for breaking self tolerance at the host level, we compared the CTL responses induced by WT DCs and DCs derived from IL-12 receptor–KO mice (IL-12Rβ2–/–). SOCS1-silenced DCs derived from IL-12Rβ2–/– SOCS-siRNA DCs exhibited a substantially reduced ability to induce TRP2-specific CTLs compared with WT SOCS1-siRNA DCs (Figures 6C and 7B). Furthermore, p35–/– SOCS1-siRNA DCs and IL-12Rβ2–/– SOCS1-siRNA DCs were unable to induce either tumor regression (Figure 7A) or autoimmune vitiligo (data not shown). We found that p35–/– SOCS1-siRNA DCs and IL-12Rβ2–/– SOCS1-siRNA DCs had a substantially reduced ability to induce TRP2-specific CTLs compared with WT SOCS1-siRNA DCs (Figure 7B). To further investigate the role of IL-12 signaling in DCs, we examined the ability of in vivo IL-12 stimulation to enhance CTLs in mice immunized with IL-12Rβ2–/– SOCS1-siRNA DCs. Figures 7, C and D, show that in vivo IL-12 stimulation of IL-12Rβ2–/– SOCS1-siRNA DC mice failed to enhance TRP-specific CTL responses. Collectively, these results indicate that SOCS1-restricted IL-12 signaling in antigen-presenting DCs plays a critical role in controlling antigen presentation and the unbridled signal transduction of IL-12 in antigen-presenting DCs is required to break self tolerance and induce pathological autoimmune responses.
Figure 7.
Persistent cytokine signaling in DCs is required to induce pathological autoimmune pathologies. (A) Inability to inhibit preestablished B16 tumors by IL-12p35–/–, IL-12Rβ2–/–, or IFN-γ–/– DCs. BM DCs derived from either WT, IL-12p35–/–, IFN-γ–/–, or IL-12Rβ2–/– mice were transduced with LV-SOCS1-siRNA or LV-GFP-siRNA. WT mice were inoculated s.c. with B16 tumor cells (2.5 × 105) and 3 days later were immunized with 1.5 × 106 TRP2-pulsed, matured DCs, followed by poly(I:C) stimulation i.p. on days 1, 3, and 5. Tumor growth curves (n = 6 mice/group) represent 1 of 3 independent experiments. (B) Reduced potency to induce CTL responses by SOCS1-silenced, IL-12Rβ2–/– DCs or IFN-γ–/– DCs. CD8+ T cells isolated from the pooled splenocytes of mice immunized with DCs derived from WT, IFN-γ–/–, IL-12p35–/–, or IL-12Rβ2–/– mice, followed by poly(I:C) stimulation 3 times, were subjected to IFN-γ ELISPOT assays. *P < 0.01 versus SOCS1-siRNA DCs. (C and D) In vivo IL-12 stimulation failed to enhance the potency of SOCS1-silenced, IL-12Rβ2–/– DCs. Mice were immunized once with WT or IL-12Rβ2–/– DCs (1.5 × 106/mouse) pulsed with TRP2 and matured with TNF-α, followed by IL-12 (i.p.) 3 times. Two weeks later, splenocytes or CD8+ T cells isolated from the pooled splenocytes were subjected to IFN-γ ELISPOT (C) and CTL assays against B16 tumor cells (D). *P < 0.01 versus SOCS1-siRNA DCs.
SOCS1 restricts IL-12 and cytokine signaling cascade in DCs.
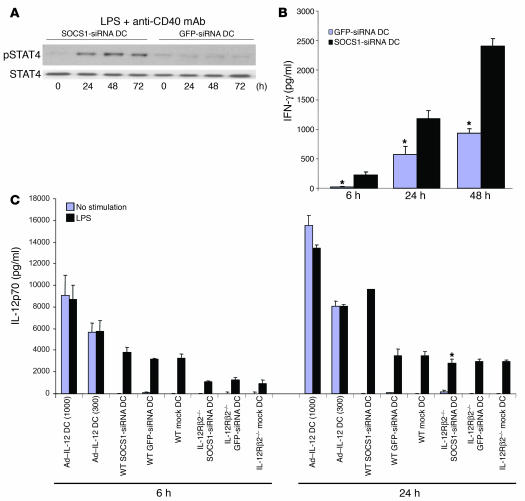
Our data suggest that silencing SOCS1 may allow the establishment of an unbridled signaling cascade of IL-12 and IL-12–induced downstream cytokines in DCs. To further determine the ability of IL-12 to continuously signal in SOCS1-siRNA DCs, we examined the duration of IL-12 signaling in WT and SOCS1-silenced DCs by analyzing the phosphorylation kinetics of Stat4 (phosphorylated Stat4 [pStat4]), which is primarily used by members of the IL-12 cytokine family (36). Figure 8A shows that Stat4 was persistently phosphorylated for at least 72 hours in SOCS1-silenced DCs after LPS/anti-CD40 stimulation, whereas pStat4 was already at basal levels at 24 hours in GFP-siRNA DCs, in agreement with the continuous secretion of IL-12p70 by SOCS1-silenced DCs in response to LPS/anti-CD40 mAb stimulation shown in Figure 5C. Furthermore, Ad–IL-12–transfected DCs were found to only have low levels of pStat4 at the indicated time points, suggesting that SOCS1 inhibits Stat4 activation in Ad–IL-12 DCs that continuously produced and exposed to IL-12 (Supplemental Figure 6). We then examined whether IL-12 induces the enhanced production of downstream cytokines by SOCS1-silenced DCs. Figure 8B shows that IL-12 stimulation induced substantially higher levels of inflammatory cytokines such as IFN-γ by mature SOCS1-siRNA DCs than by mature GFP-siRNA DCs, suggesting that SOCS1-silenced DCs have an increased sensitivity to IL-12 stimulation. Figure 8C shows that GFP-siRNA DCs and mock-transduced DCs only transiently produced IL-12p70. In contrast, SOCS1-siRNA DCs persistently produced IL-12p70 after stimulation, at levels comparable to Ad–IL-12 DCs (MOI, 300). Importantly, this increased level of IL-12p70 secretion by SOCS1-siRNA DCs was dependent on the expression of the IL-12 receptor (Figure 8C), indicating that the marked increase in IL-12p70 secretion by SOCS1-silenced DCs is likely due to autocrine signaling by IL-12p70. IL-12 stimulation induces IFN-γ production by DCs (38), and IFN-γ was recently found to be hyper-produced by SOCS1-KO DCs in response to LPS (21). Therefore, we examined whether an unbridled cytokine network, not just IL-12, in SOCS1-silenced DCs is collectively responsible for inducing effective anti-tumor immunity. Using IFN-γ–KO DCs, we found that IFN-γ–KO SOCS1-siRNA DCs only induced weak CTL responses and did not control the growth of preestablished B16 tumors (Figure 7, A and B), suggesting an important role of IFN-γ in inducing antitumor CTL responses by SOCS1-siRNA DCs as well. Collectively, these data suggested that SOCS1 restricts cytokine signaling and only allows DCs to activate CTLs in a pulsing or transient fashion, thus limiting autoimmune pathologies. These data further suggest that SOCS1 silencing leads to the unbridled signaling of IL-12 and the downstream cytokine network in antigen-presenting DCs, resulting in the breaking of self tolerance at the host level and pathological autoimmune responses.
Figure 8.
Enhanced signaling and sensitivity of SOCS1-silenced DCs to autocrine/paracrine IL-12 stimulation. (A) Western blotting of Stat4 and phosphorylated Stat4 (pStat4) in LV-transduced BM-DCs (1 × 106 cells/ml) after stimulation with LPS and anti-CD40 mAb for 0, 24, 48, or 72 hours. BM DCs were cultured in the presence of GM-CSF and IL-4. (B) IFN-γ levels secreted by LV-transduced, TNF-α–matured WT DCs (1 × 106 cells/ml) 6, 24, or 48 hours after stimulation with recombinant IL-12 (20 ng/ml) from 1 of 3 independent experiments. *P < 0.01 versus SOCS1-siRNA DCs. (C) Levels of IL-12p70 secreted by transduced DCs (1 × 106 cells/ml) derived from WT or IL-12Rβ2–/– mice at different times after stimulation with TLR agonist (LPS, 100 ng/ml) from 1 of 3 independent experiments. All cells were cultured in the presence of GM-CSF and IL-4. *P < 0.01 versus WT SOCS1-siRNA DCs.
Discussion
This study uncovers a novel regulatory mechanism for the control of pathological autoimmune responses by DCs through the restriction of cytokine signaling and the responsiveness to stimuli by SOCS1. We find that optimal immunization with matured WT DCs loaded with high-affinity self peptide (TRP2) plus persistent TLR signaling effectively activate self-reactive T cells but fail to break self tolerance at the host level. In contrast, SOCS1-silenced DCs effectively break tolerance at the host level and cause self antigen–specific, autoimmune pathologies against normal tissues and tumors. These results indicate that the requirement for breaking self tolerance at the cellular level (autoreactive CTL activation) and at the host level (autoimmune pathologies) by DCs is different and that the hardwired signaling inhibitor prevents DCs from overactivating autoreactive CTLs and causing autoimmune pathologies even when potent inflammatory stimuli are persistently present.
The subsequent mechanistic studies reveal that the inability of matured WT DCs plus persistent TLR signals to break self tolerance at the host level is likely due to the restriction of IL-12 and the downstream cytokine cascade signaling by SOCS1. This conclusion is supported by the following observations: (a) The lack of IL-12 production by SOCS1-silenced DCs (derived from IL-12p35–KO mice) abrogates their ability to break self tolerance at the host level; (b) The blockade of IL-12 signaling in SOCS1-silenced DCs (derived from IL-12Rβ2–KO mice) also abrogates their ability to induce autoimmune pathologies and antitumor activities; (c) SOCS1-silenced DCs produce enhanced levels of IL-12p70 and downstream cytokines with a prolonged duration in response to stimulation; (d) In vivo administration of IL-12 as well as TLR agonists more effectively enhance CTL responses induced by SOCS1-silenced DCs than by WT DCs; and (e) WT DCs transduced with Ad–IL-12 to constitutively overexpress IL-12 are unable to induce autoimmune pathologies.
Maturation is known as the control point for DC transition from the immature, tolerogenic state to the activated, immunogenic state (4, 5). Our results demonstrate that proinflammatory cytokine signaling in mature DCs, restricted by SOCS1, critically regulates the magnitude of CTL responses. This indicates that CTL responses are controlled by DCs on at least 2 levels: the well-defined DC maturation required for the initiation of CTL responses and the SOCS1-restricted cytokine signaling of matured DCs for the control of the magnitude and overactivation of CTL responses uncovered in this study. Our results also imply dynamic interactions between DCs and their surrounding environment of various immune cells and stimuli, which collectively determines the magnitude of CTL responses. It has recently been demonstrated that mature DC lifespan is much longer than previously estimated, lasting 2–3 weeks in vivo (39, 40), supporting the necessity of regulating antigen-presenting DCs after maturation.
This study establishes a new principle for breaking self tolerance and inducing pathological autoimmune responses, which is the first requirement for an effective tumor vaccine, by DCs via silencing a signaling inhibitor. The results of this study suggest the requirement of persistent antigen presentation by DCs for inducing autoimmune pathologies and effective antitumor immunity. Importantly, persistent antigen presentation by DCs cannot be achieved by persistent stimulation with proinflammatory stimuli or overproduction of a key proinflammatory cytokine (IL-12), probably because the hardwired signaling inhibitor prevents the establishment of an intricate autocrine/paracrine cytokine signaling network in DCs and permits DCs to stimulate CTLs only in a transient or pulsing fashion, thus limiting pathological autoimmune responses. The requirement of silencing a signaling inhibitor in order to achieve persistent antigen presentation by DCs for inducing autoimmune pathologies is in concept consistent with earlier findings that vaccination with GM-CSF–producing B16 melanoma cells caused vitiligo only when coinjected with anti-CTLA4 blocking antibodies for inhibiting the negative regulator on T cells (28, 41). However, the requirement for breaking tolerance in transgenic mouse models seems less stringent (29, 42). For example, Yang et al. recently showed that persistent in vivo TLR stimulation of WT DCs could break self tolerance at the host level in a transgenic viral antigen model. However, the conclusion drawn from the transgenic mouse models (29, 42) is not supported by the fact that autoimmune diseases are rare even in individuals with chronic infection with pathogens producing various TLR ligands.
Various inhibitory mechanisms exist in T cells to prevent pathological autoimmunity (26, 43, 44). Disabling of 1 of these inhibitory mechanisms, such as the blockade of CTLA4 on T cells or depletion of CD25+ regulatory T cells, is sufficient to break self tolerance at the host level but causes unwanted nonspecific autoimmune pathologies (26, 45, 46). However, inhibition of a signaling inhibitor in antigen-loaded DCs can induce a TAA-specific, pathological autoimmune response against tumor. The results of our study also suggest a new avenue to improve tumor immunotherapy in general by coadministration of an inhibitor of these signaling inhibitors and a cytokine or TLR agonist.
DCs activated by TLR signaling produce a large number of cytokines, including IL-12, TNF-α, IL-6, IFN-α/β, and IFN-γ, most of which are regulated by SOCS1 (17, 47). The importance of cytokines as a third signal for the activation or overactivation of T cells by DCs has been recognized (12, 13, 48). Production and signaling of proinflammatory cytokines such as IL-12 by WT DCs after TLR signaling is transient (31). Recent studies also showed that autocrine and paracrine signaling of cytokines produced by DCs plays a role in determining the outcome of antigen presentation (30, 49). Here we find that unbridled IL-12 and the downstream cytokine signaling play a critical role in the breaking of tolerance and increased CTL responses. Interestingly, SOCS1–/– inflammatory disease is primarily caused by unbridled IFN-γ signaling (17), although IL-12 also plays a role (47), while LPS-induced toxicity in SOCS1–/– mice is linked to unbridled IFN-α/β signaling (32). Hanada et al. recently reported that SOCS1-KO DCs induced more effective antitumor CTL responses probably due to the enhanced expression of IFN-γ (21). Apparently, other cytokines such as IFN-γ (Figure 7), in addition to IL-12, also play a role in the increased CTL responses induced by SOCS1-silenced DCs. Thus, further studies are warranted to delineate the cellular and molecular details of this SOCS1-regulated, complicated autocrine and paracrine cytokine signaling network in controlling the ability of DCs to activate or overactivate autoreactive CTLs in a natural, suppressive environment. In summary, this study contributes to the fundamental understanding of molecular and immunologic regulation of DCs and may lay a foundation for developing more effective tumor vaccines that not only activate autoreactive T cells, but also cause pathological autoimmune antitumor responses.
Methods
Mice.
Approval for performing these mouse experiments was obtained from the institutional review board at Baylor College of Medicine. Four- to 6-week-old female C57BL/6, CD4-KO, CD8-KO, IL-12p35–KO, IFN-γ–KO, and IL-12Rβ2–KO mice were purchased from The Jackson Laboratory and maintained in a pathogen-free mouse facility at Baylor College of Medicine according to institutional guidelines.
Peptides.
H2-Kb–restricted TRP2 peptides, TRP2a (VYDFFVWL) and TRP2b (SVYDFFVWL) (28), were used for this study. The control H2-Kb–restricted peptide was OVA-I (SIINFEKL). All peptides were synthesized and purified by HPLC to greater than 95% purity by Genemed Synthesis Inc. All peptides were dissolved in DMSO before final dilution in endotoxin-free PBS.
Transduction of BM-derived DCs with lentiviral and adenoviral vectors.
Recombinant lentiviral vectors (LV-SOCS1-siRNA and LV-GFP-siRNA) were produced and titrated as described previously (20). A recombinant adenovirus engineered to constitutively express biologically active IL-12 was purchased from InvivoGen and produced according to the manufacturer’s instructions. Mouse BM-derived DCs were prepared as described previously (20). Briefly, mouse BM was flushed from the hind limbs, passed through a nylon mesh, and depleted of red cells with ammonium chloride. After extensive washing with RPMI-1640, cells were cultured with RPMI-1640 supplemented with 10% FBS, recombinant mouse GM-CSF/ml (20 ng/ml; PeproTech), and recombinant mouse IL-4 (20 ng/ml; PeproTech). On days 2 and 4 of culture, the supernatant was removed and replaced with fresh medium containing mGM-CSF and mIL-4. All cultures were incubated at 37°C in 5% humidified CO2. Nonadherent granulocytes were removed after 48 hours of culture, and fresh medium was added. After 7 days of culture, more than 80% of the cells expressed characteristic DC-specific markers as determined by FACS. Transductions of mouse BM-derived DCs (day 5–7 of culture) were performed on 12-well plates with addition of 8 μg/ml Polybrene (Sigma-Aldrich) only for lentivirus-mediated transduction. DCs were washed and plated in 12-well plates at a concentration of 1 × 106 cells/well in 400 μl of RPMI-1640. The cells were exposed to lentiviral or adenoviral vectors with different MOIs. After 8–12 hours of transduction, the cells were washed with PBS and further incubated in fresh tissue culture medium supplemented with GM-CSF and IL-4 as described previously (20, 50).
Cytokine ELISA analysis.
Levels of various cytokines (IL-12p40, IL-12p70, IFN-γ) were quantitated using the supernatant of DC cultures using ELISA analysis (BD Biosciences) according to the manufacturer’s instructions at the time points and with the stimulus as indicated in the figures.
RT-PCR.
Total cellular RNA was isolated from DCs using RNeasy (QIAGEN) according to the manufacturer’s instructions. The expression level of GAPDH was first evaluated as an internal control using serially diluted reverse-transcribed cDNA. The expression levels of the target mRNAs were then assessed using appropriate pairs of sense and antisense primers: GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′; IL-12p35, 5′-AAATGAAGCTCTGCATCCTGC-3′ and 5′-TCACCCTGTTGATGGTCACG-3′; IL-2, 5′-CTTCAAGCTCCACTTCAAGCT-3′ and 5′-CCATCTCCTCAGAAAGTCCACC-3′; IFN-γ, 5′-TCAAGTGGCATAGATGTGGAAGAA-3′ and 5′-TGGCTCTGCAGGATTTTCATG-3′; eYFP, 5′-CACAAGTTCAGCGTGTCCGGC-3′ and 5′-TCCAGCAGGACCATGTGATGC-3′; IRF-1, 5′-CCTGATGACCACAGCAGTTAC-3′ and 5′-CTTCATCTCCGTGAAGACATG-3′; ICSBP, 5′-GATCAAGGAACCTTCTGTGG-3′ and 5′-GAAGCTGATGACCATCTGGG-3′; c-Rel, 5′-TGGCTGACTGACTCACTGACTGACTGACTCGTGCCTTGC-3′ and 5′-CCAACTAAATCATGAGGATGAGGCTTATATGGATCATTC-3′; C/EBP-β, 5′-GCGGCGAGCGCAACAACATCT-3′ and 5′-TGCTTGAACAAGTTCCGCAG-3′. For analysis of in vivo eYFP-expressing DC lifespan and IL-12p35, CD11c+ cells were first purified from the draining LNs using MACS (Miltenyi Biotec). The PCR products were separated by 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
51Cr CTL assays.
CD8+ CTL responses were assessed with a standard chromium release assay, which measures the ability of in vitro–restimulated splenocytes to lyse target cells (20, 50). Splenocytes pooled from immunized mice were restimulated in vitro in RPMI-1640 containing H2-Kb/TRP2 peptide for 4–6 days. TRP2+ target B16 cells, which express TRP2, and control EG.7 cells were labeled with sodium 51Cr chromate solution for 90 minutes at 37°C with shaking. Different numbers of effector cells were incubated with a constant number of target cells (5 × 104/well) in 96-well U-bottomed plates (200 μl/well) for 4 hours at 37°C. The supernatants from triplicate cultures were collected. Percent lysis was calculated as (experimental release – spontaneous release) / (maximum release – spontaneous release) × 100.
IFN- γELISPOT assay.
ELISPOT assays of isolated CD8+ T cells were performed as described in our previous studies (20). H2-Kb/TRP2 peptide was used for CD8+ T cell stimulation. Irrelevant peptide from OVA was also used as a negative control. Splenocytes or CD8+ cells were isolated from splenocytes using MACS CD8 (Ly-2) MicroBeads (Miltenyi Biotec). Ninety-six-well nitrocellulose-base plates (MultiScreen PI; Millipore) were coated overnight with an anti-murine IFN-γ mAb (Mab AN 18, 10 μg/ml; Mabtech). The plates were washed 6 times with PBS and blocked with RPMI-1640 containing 10% FBS at 37°C for 2 hours. The splenocytes or CD8+ cells, followed by irradiated, peptide-pulsed BM-derived DCs, were seeded into wells and incubated for 20 hours at 37°C in 5% CO2. The cells were then removed by 6 washes with PBS 0.5% Tween-20 (Sigma-Aldrich). Biotinylated anti-mouse IFN-γ antibody (Mab R4-6A2 biotin; Mabtech), diluted in PBS containing 0.5% FBS to 1 μg/ml, was added, and the mixture incubated at 25°C for 2 hours. The avidin/biotinylated enzyme complex (ABC; Vector Laboratories) was added for an additional hour. Cytokine-secreting cells were detected after a 4 minute reaction with AEC (3-amino-9-ethylcarbazole; Sigma-Aldrich). The results were evaluated by ZellNet Consulting Inc. with an automated ELISPOT reader system (Zeiss), using KS ELISPOT 4.3 software.
Tetramer staining.
H2-Kb/TRP2 tetramer assays were used to detect TRP2-specific CD8+ T cells. TRP2 tetramers were synthesized at the Baylor College of Medicine Tetramer Core Facility. Splenocytes from immunized mice were double stained with anti–CD8α-FITC and H2-Kb/TRP2-PE tetramers on different days after DC immunization. Tetramer staining was done at 4°C, for 1 hour with 1 μg of anti-CD8α and a 1:100 dilution of TRP2 tetramers per 106 cells, according to the manufacturer’s instructions.
Intracellular cytokine staining.
Splenocytes were harvested from immunized mice and cultured with TRP2 peptide for 8 hours at 37°C. For the final 6 hours of culture, GolgiPlug (BD Biosciences — Pharmingen) was added to the supernatant. After surface staining with anti-CD8, cells were permeabilized and stained for intracellular IFN-γ, with anti–IFN-γ (BD) as we have previously described (51).
Western blotting.
SOCS1-siRNA DCs or GFP-siRNA DCs were continuously stimulated with LPS (Sigma-Aldrich) and plate-immobilized anti-CD40 mAb (BD Biosciences — Pharmingen). Twenty-four, 48, or 72 hours later, the cells were harvested and subjected to SDS-PAGE (20). Following transfer to Hybond-P membrane (Amersham Biosciences), the samples were analyzed by Western blotting with monoclonal anti-pStat4 (Santa Cruz Biotechnology Inc.) or polyclonal anti-Stat4 (Santa Cruz Biotechnology Inc.) antibodies, followed by detection with ECL-Plus reagent (Amersham Biosciences).
DC transfer and tumor mouse study.
BM-derived DCs (day 4–5 of BM culture) were transduced with LV-SOCS1-siRNA or LV-GFP-siRNA at an MOI of 5 or Ad-IL-12 vector at an MOI of 300 or 1,000 (20). DCs were then pulsed with TRP2 peptides for 20 hours, washed with PBS 3 times, stimulated with LPS (100 ng/ml; Sigma-Aldrich) or TNF-α (50 ng/ml; R&D Systems) for 24 hours, washed with PBS 3 times, and then injected into C57BL/6 mice via a rear foot pad. In the therapeutic model, B16 tumor cells (2.5 × 105) were injected s.c. into the right flank of syngeneic mice to establish a tumor model. On day 3 after tumor inoculation, the mice were randomly divided into groups and injected with antigen-pulsed, matured, transduced DCs (1.5 × 106) or PBS control. In some mice, TLR agonist (LPS [30 μg/mouse; Sigma-Aldrich], poly[I:C] [50 μg/mouse; InvivoGen], peptidoglycan [25 μg/mouse; InvivoGen], imiquimod [50 μg/mouse; InvivoGen]) or IL-12 (1 μg/mouse; PeproTech) was administered i.p. after DC vaccination on the days indicated in the figures. Tumor volumes were measured every 2–4 days with an electronic caliper until the experiment was completed.
Statistics.
For statistical analysis, we used 2-tailed Student’s t test, and a 95% confidence limit was taken to be significant, defined as P < 0.05. Results are presented as mean ± SEM.
Acknowledgments
We thank Lisa Rollins, Wenhong Ren, Natasha Lapteva, Andrew Sharabi, Jiali Sun, and Lei Shen for technical assistance and valuable suggestions. This work was supported by grants from the NIH (R01CA90427, R0148480, and R01AI48711), the Leukemia and Lymphoma Society SCOR, and the US Army Prostate Cancer Research Program (to X.F. Huang). K. Evel-Kabler and M. Aldrich were supported by a predoctoral and postdoctoral NIH training grant (T32-AI07495).
Footnotes
Nonstandard abbreviations used: ELISPOT, enzyme-linked immunospot; TAA, tumor-associated antigen; TRP2, tyrosinase-related protein 2.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 6.Gilboa E. The promise of cancer vaccines. Nat. Rev. Cancer. 2004;4:401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 7.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 8.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 10.Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat. Immunol. 2001;2:487–492. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 11.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat. Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 12.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa R, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 15.Kinjyo I, et al. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 16.Hanada T, et al. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 17.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 18.Marine JC, et al. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 19.Alexander WS, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat. Biotechnol. 2004;22:1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 21.Hanada T, et al. Induction of hyper Th1 cell-type immune responses by dendritic cells lacking the suppressor of cytokine signaling-1 gene. J. Immunol. 2005;174:4325–4332. doi: 10.4049/jimmunol.174.7.4325. [DOI] [PubMed] [Google Scholar]
- 22.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 23.Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu. Rev. Med. 1999;50:507–529. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- 24.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu. Rev. Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv. Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 28.van Elsas A, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J. Exp. Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat. Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 30.Gautier G, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 32.Gingras S, Parganas E, de Pauw A, Ihle JN, Murray PJ. Re-examination of the role of suppressor of cytokine signaling 1 (SOCS1) in the regulation of Toll-like receptor signaling. J. Biol. Chem. 2004;279:54702–54707. doi: 10.1074/jbc.M411043200. [DOI] [PubMed] [Google Scholar]
- 33.Chong MM, Metcalf D, Jamieson E, Alexander WS, Kay TW. Suppressor of cytokine signaling-1 in T cells and macrophages is critical for preventing lethal inflammation. Blood. 2005;106:1668–1675. doi: 10.1182/blood-2004-08-3049. [DOI] [PubMed] [Google Scholar]
- 34.Catlett IM, Hedrick SM. Suppressor of cytokine signaling 1 is required for the differentiation of CD4+ T cells. Nat. Immunol. 2005;6:715–721. doi: 10.1038/ni1211. [DOI] [PubMed] [Google Scholar]
- 35.D’Andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor alpha production. J. Exp. Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 37.Barnes BJ, et al. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J. Biol. Chem. 2004;279:45194–45207. doi: 10.1074/jbc.M400726200. [DOI] [PubMed] [Google Scholar]
- 38.Ohteki T, et al. Interleukin 12-dependent interferon gamma production by CD8alpha+ lymphoid dendritic cells. J. Exp. Med. 1999;189:1981–1986. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg S, et al. Genetic tagging shows increased frequency and longevity of antigen-presenting, skin-derived dendritic cells in vivo. Nat. Immunol. 2003;4:907–912. doi: 10.1038/ni962. [DOI] [PubMed] [Google Scholar]
- 40.Machen J, et al. Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J. Immunol. 2004;173:4331–4341. doi: 10.4049/jimmunol.173.7.4331. [DOI] [PubMed] [Google Scholar]
- 41.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waldner H, Collins M, Kuchroo VK. Activation of antigen-presenting cells by microbial products breaks self tolerance and induces autoimmune disease. J. Clin. Invest. 2004;113:990–997. doi:10.1172/JCI200419388. doi: 10.1172/JCI19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan L, Reddi AL, Ghosh A, Dimri M, Band H. The Cbl family and other ubiquitin ligases: destructive forces in control of antigen receptor signaling. Immunity. 2004;21:7–17. doi: 10.1016/j.immuni.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 45.Hodi FS, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eyles JL, Metcalf D, Grusby MJ, Hilton DJ, Starr R. Negative regulation of interleukin-12 signaling by suppressor of cytokine signaling-1. J. Biol. Chem. 2002;277:43735–43740. doi: 10.1074/jbc.M208586200. [DOI] [PubMed] [Google Scholar]
- 48.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–550. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 49.Grohmann U, et al. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315–323. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 50.You Z, et al. Induction of vigorous helper and cytotoxic T cell as well as B cell responses by DCs expressing a modified antigen targeting receptor-mediated internalization pathway. J. Immunol. 2000;165:4581–4592. doi: 10.4049/jimmunol.165.8.4581. [DOI] [PubMed] [Google Scholar]
- 51.Hauser H, Shen L, Gu QL, Krueger S, Chen S-Y. Secretory heat-shock protein as a dendritic cell-targeting molecule: a new strategy to enhance the potency of genetic vaccines. Gene Ther. 2004;11:924–932. doi: 10.1038/sj.gt.3302160. [DOI] [PubMed] [Google Scholar]