Abstract
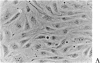
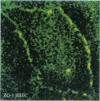
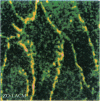
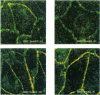
PURPOSES: First, to develop an improved retinal capillary endothelial cell culture system which exhibits some of the physiologic features of the bloodretinal barrier; second, to use this model to determine how histamine and chemical conditions of diabetes effect expression of the tight junction protein, ZO-1; and third, to discuss application of the Henle-Koch postulates to the problem of diabetic retinopathy. METHODS: Bovine retinal capillary endothelial cells were exposed to varying serum and growth factor concentrations, as well as astrocyte-conditioned medium, in order to establish a model of the blood-retinal barrier. Cells were also exposed to varying concentrations of histamine, and of insulin and glucose. The expression of ZO-1 tight junction protein was determined by immunocytochemistry and immunoblotting. RESULTS: Modified concentrations of growth factors reduced endothelial cell proliferation, without reducing viability. Astrocyte conditioned medium increased ZO-1 protein content. Histamine reduced ZO-1 protein content. Both high glucose (20mM) and low insulin (10(-12)M) reduced ZO-1 protein content compared to control conditions (5mM glucose and 10(-9) M insulin). CONCLUSIONS: Control of culture conditions results in a more physiologic in vitro model of the blood-retinal barrier. Soluble factors from astrocytes promote tight junction formation. Both histamine and chemical conditions of diabetes diminish tight junction formation. These factors may mediate blood-retinal barrier breakdown in diabetic retinopathy. Henle-Koch postulates for diabetic retinopathy are presented.
Full text
PDF



















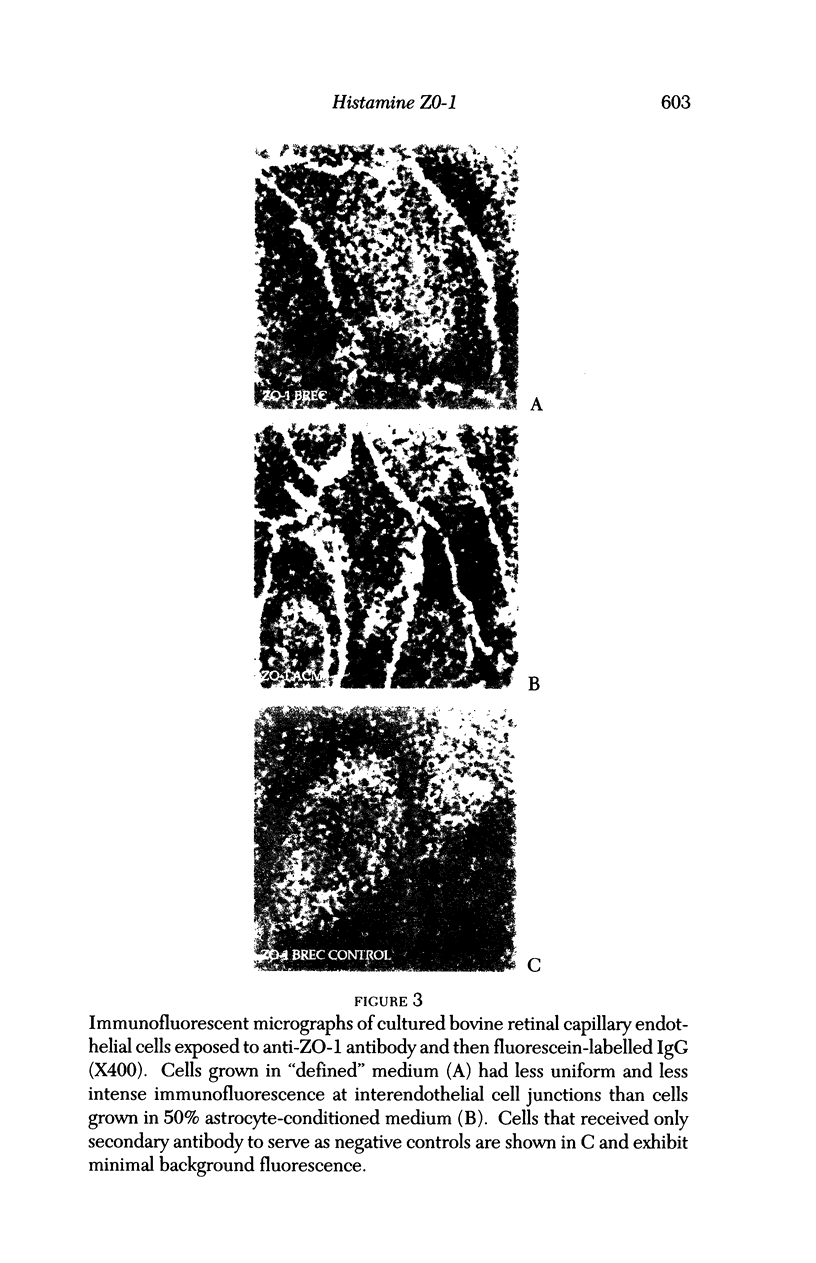
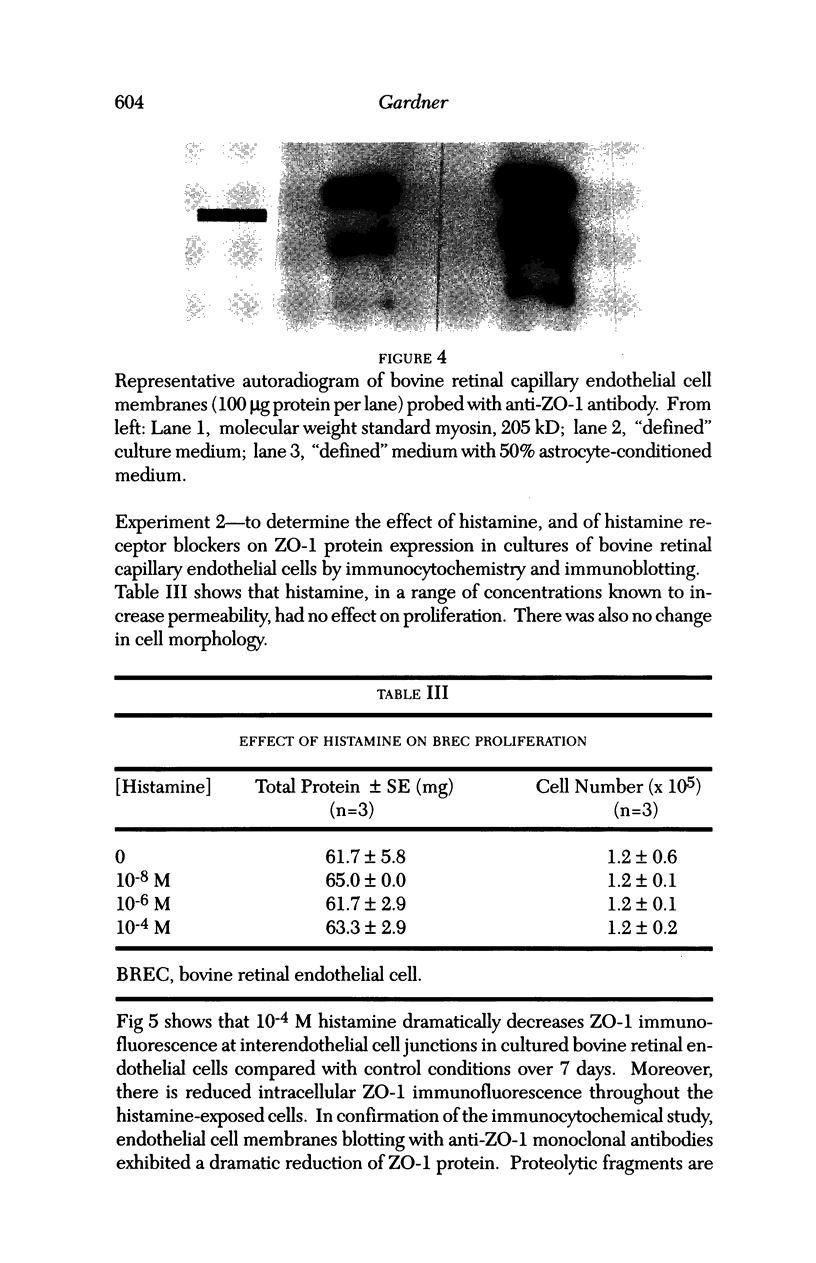
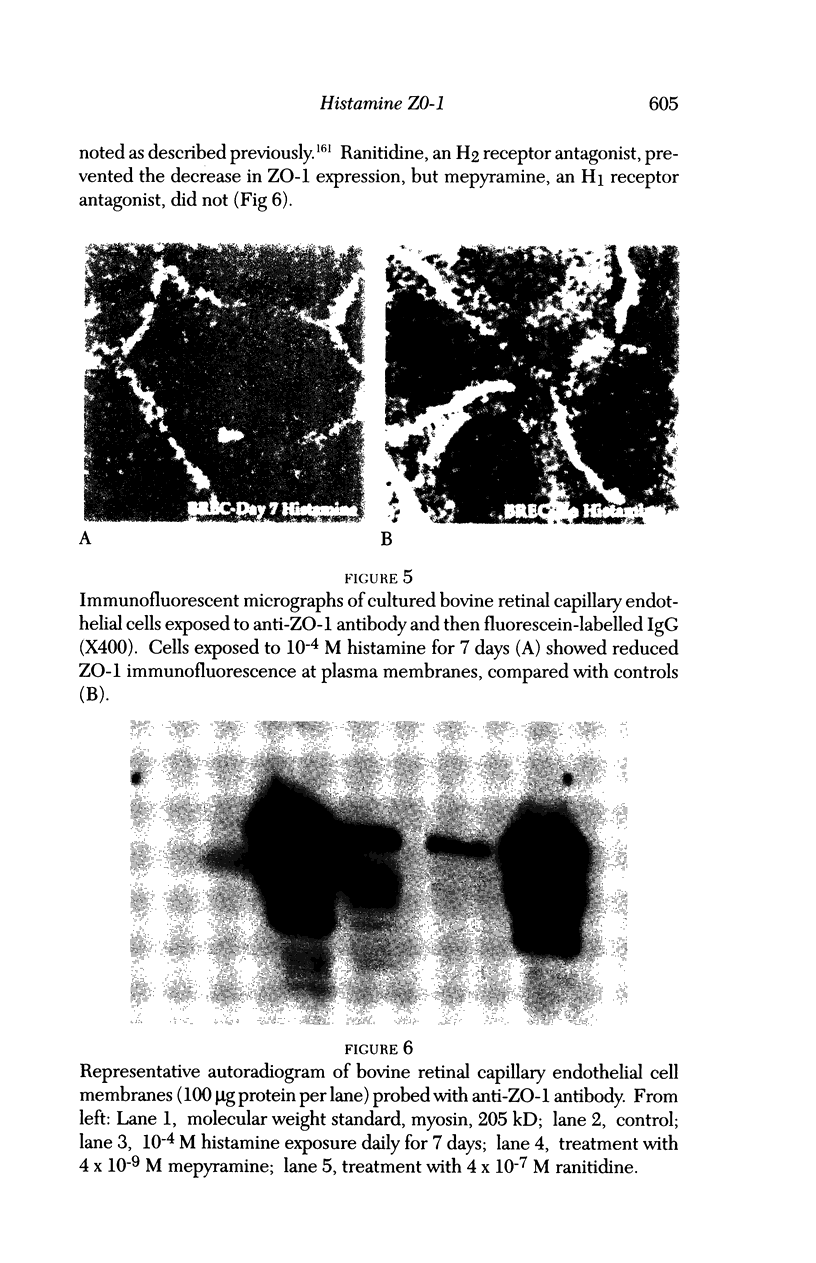















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON N., CUNHA-VAZ J. G. EFFECT OF HISTAMINE ON THE PERMEABILITY OF THE OCULAR VESSELS. Arch Ophthalmol. 1965 Feb;73:211–223. doi: 10.1001/archopht.1965.00970030213014. [DOI] [PubMed] [Google Scholar]
- Abbott N. J., Revest P. A., Romero I. A. Astrocyte-endothelial interaction: physiology and pathology. Neuropathol Appl Neurobiol. 1992 Oct;18(5):424–433. doi: 10.1111/j.1365-2990.1992.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Akagi Y., Kador P. F., Kuwabara T., Kinoshita J. H. Aldose reductase localization in human retinal mural cells. Invest Ophthalmol Vis Sci. 1983 Nov;24(11):1516–1519. [PubMed] [Google Scholar]
- Altura B. M. Contractile responses of microvascular smooth muscle to antihistamines. Am J Physiol. 1970 Apr;218(4):1082–1091. doi: 10.1152/ajplegacy.1970.218.4.1082. [DOI] [PubMed] [Google Scholar]
- Altura B. M. Role of prostaglandins and histamine in reactive hyperemia: in-vivo studies on single mesenteric arterioles. Prostaglandins Med. 1978 Oct;1(4):323–331. doi: 10.1016/0161-4630(78)90052-6. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Stevenson B. R., Jesaitis L. A., Goodenough D. A., Mooseker M. S. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988 Apr;106(4):1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbonés L., Claro E., Picatoste F., García A. [3H]mepyramine binding to histamine H1 receptors in bovine retina. Biochem Biophys Res Commun. 1986 Mar 13;135(2):445–450. doi: 10.1016/0006-291x(86)90014-8. [DOI] [PubMed] [Google Scholar]
- Arthur F. E., Shivers R. R., Bowman P. D. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987 Nov;433(1):155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- BECKER B., MAENGWYN-DAVIES G. D., ROSEN D., FRIEDENWALD J. S., WINTER F. C. The adrenal cortex and B-vitamins in diabetic retinopathy. Diabetes. 1954 May-Jun;3(3):175–187. doi: 10.2337/diab.3.3.175. [DOI] [PubMed] [Google Scholar]
- BEDROSSIAN R. H., POCOCK D. S., HARVEY W. F., Jr, SINDONI A. S. Diabetic retinopathy treated with testosterone. AMA Arch Ophthalmol. 1953 Sep;50(3):277–281. doi: 10.1001/archopht.1953.00920030284002. [DOI] [PubMed] [Google Scholar]
- Beck-Nielsen H., Olesen T., Mogensen C. E., Richelsen B., Olsen H. W., Ehlers N., Charles P., Sørensen N. S. Effect of near normoglycemia for 5 years on progression of early diabetic retinopathy and renal involvement. Diabetes Res. 1990 Dec;15(4):185–190. [PubMed] [Google Scholar]
- Benedito S., Prieto D., Nielsen P. J., Nyborg N. C. Histamine induces endothelium-dependent relaxation of bovine retinal arteries. Invest Ophthalmol Vis Sci. 1991 Jan;32(1):32–38. [PubMed] [Google Scholar]
- Blair N. P., Tso M. O., Dodge J. T. Pathologic studies of the blood--retinal barrier in the spontaneously diabetic BB rat. Invest Ophthalmol Vis Sci. 1984 Mar;25(3):302–311. [PubMed] [Google Scholar]
- Blankenship G. W. Diabetic macular edema and argon laser photocoagulation: a prospective randomized study. Ophthalmology. 1979 Jan;86(1):69–78. doi: 10.1016/s0161-6420(79)35534-8. [DOI] [PubMed] [Google Scholar]
- Blankenship G. W. Fifteen-year argon laser and xenon photocoagulation results of Bascom Palmer Eye Institute's patients participating in the diabetic retinopathy study. Ophthalmology. 1991 Feb;98(2):125–128. doi: 10.1016/s0161-6420(91)32326-1. [DOI] [PubMed] [Google Scholar]
- Blankenship G. W., Machemer R. Long-term diabetic vitrectomy results. Report of 10 year follow-up. Ophthalmology. 1985 Apr;92(4):503–506. doi: 10.1016/s0161-6420(85)34015-0. [DOI] [PubMed] [Google Scholar]
- Boertje S. B., Le Beau D., Williams C. Blockade of histamine-stimulated alterations in cerebrovascular permeability by the H2-receptor antagonist cimetidine. Neuropharmacology. 1989 Jul;28(7):749–752. doi: 10.1016/0028-3908(89)90161-5. [DOI] [PubMed] [Google Scholar]
- Bottaro D., Shepro D., Peterson S., Hechtman H. B. Serotonin, norepinephrine, and histamine mediation of endothelial cell barrier function in vitro. J Cell Physiol. 1986 Aug;128(2):189–194. doi: 10.1002/jcp.1041280208. [DOI] [PubMed] [Google Scholar]
- Brechner R. J., Cowie C. C., Howie L. J., Herman W. H., Will J. C., Harris M. I. Ophthalmic examination among adults with diagnosed diabetes mellitus. JAMA. 1993 Oct 13;270(14):1714–1718. [PubMed] [Google Scholar]
- Bresnick G. H. Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology. 1983 Nov;90(11):1301–1317. doi: 10.1016/s0161-6420(83)34388-8. [DOI] [PubMed] [Google Scholar]
- Bresnick G. H., Palta M. Oscillatory potential amplitudes. Relation to severity of diabetic retinopathy. Arch Ophthalmol. 1987 Jul;105(7):929–933. doi: 10.1001/archopht.1987.01060070065030. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994 Jun;43(6):836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- Butt A. M., Jones H. C. Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain Res. 1992 Jan 8;569(1):100–105. doi: 10.1016/0006-8993(92)90374-i. [DOI] [PubMed] [Google Scholar]
- Buzney S. M., Weiter J. J. Pathogenesis of diabetic retinal angiopathy: proposed mechanisms and current research. Int Ophthalmol Clin. 1984 Winter;24(4):1–12. doi: 10.1097/00004397-198402440-00003. [DOI] [PubMed] [Google Scholar]
- COGAN D. G., TOUSSAINT D., KUWABARA T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961 Sep;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- Carroll W. J., Hollis T. M. Aortic histamine synthesis and aortic albumin accumulation in diabetes: activity-uptake relationships. Exp Mol Pathol. 1985 Jun;42(3):344–352. doi: 10.1016/0014-4800(85)90084-x. [DOI] [PubMed] [Google Scholar]
- Carroll W. J., Hollis T. M., Gardner T. W. Retinal histamine synthesis is increased in experimental diabetes. Invest Ophthalmol Vis Sci. 1988 Aug;29(8):1201–1204. [PubMed] [Google Scholar]
- Chapman L. M., Eddy E. M. A protein associated with the mouse and rat hepatocyte junctional complex. Cell Tissue Res. 1989 Aug;257(2):333–341. doi: 10.1007/BF00261837. [DOI] [PubMed] [Google Scholar]
- Chase H. P., Jackson W. E., Hoops S. L., Cockerham R. S., Archer P. G., O'Brien D. Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA. 1989 Feb 24;261(8):1155–1160. [PubMed] [Google Scholar]
- Citi S., Sabanay H., Jakes R., Geiger B., Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988 May 19;333(6170):272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- D'Antonio J. A., Ellis D., Doft B. H., Becker D. J., Drash A. L., Kuller L. H., Orchard T. J. Diabetes complications and glycemic control. The Pittsburgh Prospective Insulin-Dependent Diabetes Cohort Study Status Report after 5 yr of IDDM. Diabetes Care. 1989 Nov-Dec;12(10):694–670. doi: 10.2337/diacare.12.10.694. [DOI] [PubMed] [Google Scholar]
- Dahl-Jørgensen K., Brinchmann-Hansen O., Hanssen K. F., Ganes T., Kierulf P., Smeland E., Sandvik L., Aagenaes O. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J (Clin Res Ed) 1986 Nov 8;293(6556):1195–1199. doi: 10.1136/bmj.293.6556.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. D. The natural course of diabetic retinopathy. Trans Am Acad Ophthalmol Otolaryngol. 1968 Mar-Apr;72(2):237–240. [PubMed] [Google Scholar]
- Dehouck M. P., Méresse S., Delorme P., Fruchart J. C., Cecchelli R. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J Neurochem. 1990 May;54(5):1798–1801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Duane T. D. Is diabetic retinopathy uncontrollable? Am J Ophthalmol. 1971 Jan;71(1 Pt 2):286–290. doi: 10.1016/0002-9394(71)90400-4. [DOI] [PubMed] [Google Scholar]
- Duncan L. J., Cullen J. F., Ireland J. T., Nolan J., Clarke B. F., Oliver M. F. A three-year trial of atromid therapy in exudative diabetic retinopathy. Diabetes. 1968 Jul;17(7):458–467. doi: 10.2337/diab.17.7.458. [DOI] [PubMed] [Google Scholar]
- Dux E., Joó F. Effects of histamine on brain capillaries. Fine structural and immunohistochemical studies after intracarotid infusion. Exp Brain Res. 1982;47(2):252–258. doi: 10.1007/BF00239384. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Hallengren C. Histamine in the retina. Acta Physiol Scand. 1987 Feb;129(2):263–265. doi: 10.1111/j.1748-1716.1987.tb08067.x. [DOI] [PubMed] [Google Scholar]
- El-Ackad T. M., Brody M. J. Evidence for non-mast cell histamine in the vascular wall. Blood Vessels. 1975;12(3):181–191. doi: 10.1159/000158049. [DOI] [PubMed] [Google Scholar]
- Enea N. A., Hollis T. M., Kern J. A., Gardner T. W. Histamine H1 receptors mediate increased blood-retinal barrier permeability in experimental diabetes. Arch Ophthalmol. 1989 Feb;107(2):270–274. doi: 10.1001/archopht.1989.01070010276036. [DOI] [PubMed] [Google Scholar]
- Engerman R. L., Kern T. S. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987 Jul;36(7):808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- Engerman R., Bloodworth J. M., Jr, Nelson S. Relationship of microvascular disease in diabetes to metabolic control. Diabetes. 1977 Aug;26(8):760–769. doi: 10.2337/diab.26.8.760. [DOI] [PubMed] [Google Scholar]
- Evans A. S. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med. 1976 May;49(2):175–195. [PMC free article] [PubMed] [Google Scholar]
- Falkow S. Molecular Koch's postulates applied to microbial pathogenicity. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S274–S276. doi: 10.1093/cid/10.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- Ferris F. L., 3rd How effective are treatments for diabetic retinopathy? JAMA. 1993 Mar 10;269(10):1290–1291. [PubMed] [Google Scholar]
- Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993 Dec;123(6 Pt 2):1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner T. W., Eller A. W., Friberg T. R., D'Antonio J. A., Hollis T. M. Antihistamines reduce blood-retinal barrier permeability in type I (insulin-dependent) diabetic patients with nonproliferative retinopathy. A pilot study. Retina. 1995;15(2):134–140. [PubMed] [Google Scholar]
- Gill D. S., Barradas M. A., Fonseca V. A., Dandona P. Plasma histamine concentrations are elevated in patients with diabetes mellitus and peripheral vascular disease. Metabolism. 1989 Mar;38(3):243–247. doi: 10.1016/0026-0495(89)90082-6. [DOI] [PubMed] [Google Scholar]
- Gill D. S., Thompson C. S., Dandona P. Increased histamine in plasma and tissues in diabetic rats. Diabetes Res. 1988 Jan;7(1):31–34. [PubMed] [Google Scholar]
- Gordon B., Chang S., Kavanagh M., Berrocal M., Yannuzzi L., Robertson C., Drexler A. The effects of lipid lowering on diabetic retinopathy. Am J Ophthalmol. 1991 Oct 15;112(4):385–391. doi: 10.1016/s0002-9394(14)76244-0. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987 Mar 5;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Grimes P. A., Laties A. M. Early morphological alteration of the pigment epithelium in streptozotocin-induced diabetes: increased surface area of the basal cell membrane. Exp Eye Res. 1980 Jun;30(6):631–639. doi: 10.1016/0014-4835(80)90062-7. [DOI] [PubMed] [Google Scholar]
- Gross P. M., Teasdale G. M., Angerson W. J., Harper A. M. H2-Receptors mediate increases in permeability of the blood-brain barrier during arterial histamine infusion. Brain Res. 1981 Apr 6;210(1-2):396–400. doi: 10.1016/0006-8993(81)90916-1. [DOI] [PubMed] [Google Scholar]
- Gulati A., Dhawan K. N., Shukla R., Srimal R. C., Dhawan B. N. Evidence for the involvement of histamine in the regulation of blood-brain barrier permeability. Pharmacol Res Commun. 1985 Apr;17(4):395–404. doi: 10.1016/0031-6989(85)90019-0. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Lowenkopf T., Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth P. H., Hirabayashi K. The effect of histamine on microvascular permeability in the muscularis externa of rat small intestine. Microvasc Res. 1983 May;25(3):322–332. doi: 10.1016/0026-2862(83)90022-5. [DOI] [PubMed] [Google Scholar]
- Hammes H. P., Martin S., Federlin K., Geisen K., Brownlee M. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11555–11558. doi: 10.1073/pnas.88.24.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrold B. P., Marmion V. J., Gough K. R. A double-blind controlled trial of clofibrate in the treatment of diabetic retinopathy. Diabetes. 1969 May;18(5):285–291. doi: 10.2337/diab.18.5.285. [DOI] [PubMed] [Google Scholar]
- Heltianu C., Simionescu M., Simionescu N. Histamine receptors of the microvascular endothelium revealed in situ with a histamine-ferritin conjugate: characteristic high-affinity binding sites in venules. J Cell Biol. 1982 May;93(2):357–364. doi: 10.1083/jcb.93.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. J. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990 Mar;42(1):45–83. [PubMed] [Google Scholar]
- Hollis T. M., Gallik S. G., Orlidge A., Yost J. C. Aortic endothelial and smooth muscle histamine metabolism. Relationship to aortic 125I-albumin accumulation in experimental diabetes. Arteriosclerosis. 1983 Nov-Dec;3(6):599–606. doi: 10.1161/01.atv.3.6.599. [DOI] [PubMed] [Google Scholar]
- Hollis T. M., Gardner T. W., Vergis G. J., Kirbo B. J., Butler C., Dull R. O., Campos M. J., Enea N. A. Antihistamines reverse blood-ocular barrier breakdown in experimental diabetes. J Diabet Complications. 1988 Jan-Mar;2(1):47–49. doi: 10.1016/0891-6632(88)90029-3. [DOI] [PubMed] [Google Scholar]
- Hollis T. M., Kern J. A., Enea N. A., Cosgarea A. J. Changes in plasma histamine concentration in the streptozotocin-diabetic rat. Exp Mol Pathol. 1985 Aug;43(1):90–96. doi: 10.1016/0014-4800(85)90058-9. [DOI] [PubMed] [Google Scholar]
- Hollis T. M., Rosen L. A. Histidine decarboxylase activity of bovine aortic endothelium and intima-media. Proc Soc Exp Biol Med. 1972 Dec;141(3):978–981. doi: 10.3181/00379727-141-36914. [DOI] [PubMed] [Google Scholar]
- Hollis T. M., Sill H. W., Butler C., Campos M. J., Gardner T. W. Astemizole reduces blood-retinal barrier leakage in experimental diabetes. J Diabetes Complications. 1992 Oct-Dec;6(4):230–235. doi: 10.1016/1056-8727(92)90057-r. [DOI] [PubMed] [Google Scholar]
- Hollis T. M., Strickberger S. A. Inhibition of aortic histamine synthesis by alpha-hydrazinohistidine inhibits increased aortic albumin accumulation in experimental diabetes in the rat. Diabetologia. 1985 May;28(5):282–285. doi: 10.1007/BF00271686. [DOI] [PubMed] [Google Scholar]
- Hori S., Nishida T., Mukai N. Ultrastructural studies on lysosomes in retinal Müller cells of streptozotocin-diabetic rats. Invest Ophthalmol Vis Sci. 1980 Nov;19(11):1295–1300. [PubMed] [Google Scholar]
- Ichikawa I., Brenner B. M. Mechanisms of action of hisamine and histamine antagonists on the glomerular microcirculation in the rat. Circ Res. 1979 Dec;45(6):737–745. doi: 10.1161/01.res.45.6.737. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Tanaka K., Taniguchi Y. Disruption of blood-retinal barrier in experimental diabetic rats: an electron microscopic study. Exp Eye Res. 1980 Apr;30(4):401–410. doi: 10.1016/0014-4835(80)90055-x. [DOI] [PubMed] [Google Scholar]
- Janzer R. C., Raff M. C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987 Jan 15;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- KING R. C., DOBREE J. H., KOK D., FOULDS W. S., DANGERFIELD W. G. EXUDATIVE DIABETIC RETINOPATY. SPONTANEOUS CHANGES AND EFFECTS OF A CORN OIL DIET. Br J Ophthalmol. 1963 Nov;47:666–672. doi: 10.1136/bjo.47.11.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlson G., Rosengren E. New approaches to the physiology of histamine. Physiol Rev. 1968 Jan;48(1):155–196. doi: 10.1152/physrev.1968.48.1.155. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey J. J., Johnston M. G., Movat H. Z. Increased permeability of microcarrier-cultured endothelial monolayers in response to histamine and thrombin. A model for the in vitro study of increased vasopermeability. Am J Pathol. 1986 Jan;122(1):50–61. [PMC free article] [PubMed] [Google Scholar]
- Klein R., Klein B. E., Moss S. E., Davis M. D., DeMets D. L. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA. 1988 Nov 18;260(19):2864–2871. [PubMed] [Google Scholar]
- Kohner E. M., Dollery C. T., Fraser T. R., Bulpitt C. J. Effect of pituitary ablation on diabetic retinopathy studied by fluorescence angiography. Diabetes. 1970 Oct;19(10):703–714. doi: 10.2337/diab.19.10.703. [DOI] [PubMed] [Google Scholar]
- Krause D., Mischeck U., Galla H. J., Dermietzel R. Correlation of zonula occludens ZO-1 antigen expression and transendothelial resistance in porcine and rat cultured cerebral endothelial cells. Neurosci Lett. 1991 Jul 22;128(2):301–304. doi: 10.1016/0304-3940(91)90284-z. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Sirota D. K., Lieberman T. Cryohypophysectomy for diabetic retinopathy. Ophthalmological and endocrine correlation. Ann Intern Med. 1970 Mar;72(3):309–316. doi: 10.7326/0003-4819-72-3-309. [DOI] [PubMed] [Google Scholar]
- Kumari K., Umar S., Bansal V., Sahib M. K. Monoaminoguanidine inhibits aldose reductase. Biochem Pharmacol. 1991 May 15;41(10):1527–1528. doi: 10.1016/0006-2952(91)90571-l. [DOI] [PubMed] [Google Scholar]
- Laterra J., Goldstein G. W. Astroglial-induced in vitro angiogenesis: requirements for RNA and protein synthesis. J Neurochem. 1991 Oct;57(4):1231–1239. doi: 10.1111/j.1471-4159.1991.tb08284.x. [DOI] [PubMed] [Google Scholar]
- Lauritzen T., Frost-Larsen K., Larsen H. W., Deckert T. Two-year experience with continuous subcutaneous insulin infusion in relation to retinopathy and neuropathy. Diabetes. 1985 Aug;34 (Suppl 3):74–79. doi: 10.2337/diab.34.3.s74. [DOI] [PubMed] [Google Scholar]
- Li C. X., Poznansky M. J. Characterization of the ZO-1 protein in endothelial and other cell lines. J Cell Sci. 1990 Oct;97(Pt 2):231–237. doi: 10.1242/jcs.97.2.231. [DOI] [PubMed] [Google Scholar]
- Li W., Shen S., Khatami M., Rockey J. H. Stimulation of retinal capillary pericyte protein and collagen synthesis in culture by high-glucose concentration. Diabetes. 1984 Aug;33(8):785–789. doi: 10.2337/diab.33.8.785. [DOI] [PubMed] [Google Scholar]
- Lipton B. H., Bensch K. G., Karasek M. A. Histamine-modulated transdifferentiation of dermal microvascular endothelial cells. Exp Cell Res. 1992 Apr;199(2):279–291. doi: 10.1016/0014-4827(92)90436-c. [DOI] [PubMed] [Google Scholar]
- Lipton B. H., Bensch K. G., Karasek M. A. Microvessel endothelial cell transdifferentiation: phenotypic characterization. Differentiation. 1991 Mar;46(2):117–133. doi: 10.1111/j.1432-0436.1991.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Little H. L. Alterations in blood elements in the pathogenesis of diabetic retinopathy. Ophthalmology. 1981 Jul;88(7):647–654. doi: 10.1016/s0161-6420(81)34971-9. [DOI] [PubMed] [Google Scholar]
- Little H. L., Rosenthal A. R., Dellaporta A., Jacobson D. R. The effect of pan-retinal photo-coagulation on rubeosis iridis. Am J Ophthalmol. 1976 Jun;81(6):804–809. doi: 10.1016/0002-9394(76)90364-0. [DOI] [PubMed] [Google Scholar]
- Lo W. W., Fan T. P. Histamine stimulates inositol phosphate accumulation via the H1-receptor in cultured human endothelial cells. Biochem Biophys Res Commun. 1987 Oct 14;148(1):47–53. doi: 10.1016/0006-291x(87)91074-6. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Cagliero E. Pathobiology of endothelial and other vascular cells in diabetes mellitus. Call for data. Diabetes. 1991 Jun;40(6):653–659. doi: 10.2337/diab.40.6.653. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Healy D. P., Hawkins R., Printz J. M., Printz M. P. Studies on the permeability of the blood-brain barrier in experimental diabetes. Diabetologia. 1986 Jan;29(1):58–62. doi: 10.1007/BF02427282. [DOI] [PubMed] [Google Scholar]
- Loy A., Lurie K. G., Ghosh A., Wilson J. M., MacGregor L. C., Matschinsky F. M. Diabetes and the myo-inositol paradox. Diabetes. 1990 Oct;39(10):1305–1312. doi: 10.2337/diab.39.10.1305. [DOI] [PubMed] [Google Scholar]
- Lyons T. J., Li W., Wells-Knecht M. C., Jokl R. Toxicity of mildly modified low-density lipoproteins to cultured retinal capillary endothelial cells and pericytes. Diabetes. 1994 Sep;43(9):1090–1095. doi: 10.2337/diab.43.9.1090. [DOI] [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. Altered retinal metabolism in diabetes. II. Measurement of sodium-potassium ATPase and total sodium and potassium in individual retinal layers. J Biol Chem. 1986 Mar 25;261(9):4052–4058. [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandarino L. J. Current hypotheses for the biochemical basis of diabetic retinopathy. Diabetes Care. 1992 Dec;15(12):1892–1901. doi: 10.2337/diacare.15.12.1892. [DOI] [PubMed] [Google Scholar]
- Mandelcorn M. S., Blankenship G., Machemer R. Pars plana vitrectomy for the management of sever diabetic retinopathy. Am J Ophthalmol. 1976 May;81(5):561–570. doi: 10.1016/0002-9394(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Markle R. A., Hollis T. M., Cosgarea A. J. Renal histamine increases in the streptozotocin-diabetic rat. Exp Mol Pathol. 1986 Feb;44(1):21–28. doi: 10.1016/0014-4800(86)90030-4. [DOI] [PubMed] [Google Scholar]
- Martins A. N., Doyle T. F., Wright S. J., Jr, Bass B. G. Response of cerebral circulation to topical histamine. Stroke. 1980 Sep-Oct;11(5):469–476. doi: 10.1161/01.str.11.5.469. [DOI] [PubMed] [Google Scholar]
- McCaleb M. L., McKean M. L., Hohman T. C., Laver N., Robison W. G., Jr Intervention with the aldose reductase inhibitor, tolrestat, in renal and retinal lesions of streptozotocin-diabetic rats. Diabetologia. 1991 Oct;34(10):695–701. doi: 10.1007/BF00401513. [DOI] [PubMed] [Google Scholar]
- McCance D. R., Hadden D. R., Atkinson A. B., Archer D. B., Kennedy L. Long-term glycaemic control and diabetic retinopathy. Lancet. 1989 Oct 7;2(8667):824–828. doi: 10.1016/s0140-6736(89)92996-6. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuskey P. A., McCuskey R. S. In vivo and electron microscopic study of the development of cerebral diabetic microangiography. Microcirc Endothelium Lymphatics. 1984 Apr;1(2):221–244. [PubMed] [Google Scholar]
- Mukai N., Hori S., Pomeroy M. Cerebral lesions in rats with streptozotocin-induced diabetes. Acta Neuropathol. 1980;51(1):79–84. doi: 10.1007/BF00688853. [DOI] [PubMed] [Google Scholar]
- Nagata H., Guth P. H. Effect of histamine on microvascular permeability in the rat stomach. Am J Physiol. 1983 Aug;245(2):G201–G207. doi: 10.1152/ajpgi.1983.245.2.G201. [DOI] [PubMed] [Google Scholar]
- Nowak J. Z., Maslinski C. 3H-mepyramine binding and histamine-stimulated cAMP accumulation in mammalian retina. Agents Actions. 1986 Apr;18(1-2):145–148. doi: 10.1007/BF01988006. [DOI] [PubMed] [Google Scholar]
- Nowak J. Z., Nawrocki J., Maslinski C. Distribution and localization of histamine in bovine and rabbit eye. Agents Actions. 1984 Apr;14(3-4):335–340. doi: 10.1007/BF01973822. [DOI] [PubMed] [Google Scholar]
- Oimomi M., Maeda Y., Baba S., Iga T., Yamamoto M. Relationship between levels of advanced-stage products of the Maillard reaction and the development of diabetic retinopathy. Exp Eye Res. 1989 Aug;49(2):317–320. doi: 10.1016/0014-4835(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Okun E. The effectiveness of photocoagulation in the therapy of proliferative diabetic retinopathy (PDR). (A controlled study in 50 patients). Trans Am Acad Ophthalmol Otolaryngol. 1968 Mar-Apr;72(2):246–252. [PubMed] [Google Scholar]
- Orchard T. J., Dorman J. S., Maser R. E., Becker D. J., Ellis D., LaPorte R. E., Kuller L. H., Wolfson S. K., Jr, Drash A. L. Factors associated with avoidance of severe complications after 25 yr of IDDM. Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes Care. 1990 Jul;13(7):741–747. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- Orlidge A., Hollis T. M. Aortic endothelial and smooth muscle histamine metabolism in experimental diabetes. Arteriosclerosis. 1982 Mar-Apr;2(2):142–150. doi: 10.1161/01.atv.2.2.142. [DOI] [PubMed] [Google Scholar]
- Ou P., Wolff S. P. Aminoguanidine: a drug proposed for prophylaxis in diabetes inhibits catalase and generates hydrogen peroxide in vitro. Biochem Pharmacol. 1993 Oct 5;46(7):1139–1144. doi: 10.1016/0006-2952(93)90461-5. [DOI] [PubMed] [Google Scholar]
- Parving H. H., Viberti G. C., Keen H., Christiansen J. S., Lassen N. A. Hemodynamic factors in the genesis of diabetic microangiopathy. Metabolism. 1983 Sep;32(9):943–949. doi: 10.1016/0026-0495(83)90210-x. [DOI] [PubMed] [Google Scholar]
- Porta M., La Selva M., Molinatti P. A. von Willebrand factor and endothelial abnormalities in diabetic microangiopathy. Diabetes Care. 1991 Feb;14(2):167–172. doi: 10.2337/diacare.14.2.167. [DOI] [PubMed] [Google Scholar]
- Pugliese G., Tilton R. G., Williamson J. R. Glucose-induced metabolic imbalances in the pathogenesis of diabetic vascular disease. Diabetes Metab Rev. 1991 Mar;7(1):35–59. doi: 10.1002/dmr.5610070106. [DOI] [PubMed] [Google Scholar]
- Rand L. I., Krolewski A. S., Aiello L. M., Warram J. H., Baker R. S., Maki T. Multiple factors in the prediction of risk of proliferative diabetic retinopathy. N Engl J Med. 1985 Dec 5;313(23):1433–1438. doi: 10.1056/NEJM198512053132302. [DOI] [PubMed] [Google Scholar]
- Reichard P., Nilsson B. Y., Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993 Jul 29;329(5):304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- Rosenstock J., Friberg T., Raskin P. Effect of glycemic control on microvascular complications in patients with type I diabetes mellitus. Am J Med. 1986 Dec;81(6):1012–1018. doi: 10.1016/0002-9343(86)90398-0. [DOI] [PubMed] [Google Scholar]
- Roth T., Podestá F., Stepp M. A., Boeri D., Lorenzi M. Integrin overexpression induced by high glucose and by human diabetes: potential pathway to cell dysfunction in diabetic microangiopathy. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9640–9644. doi: 10.1073/pnas.90.20.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. L., Hall D. E., Porter S., Barbu K., Cannon C., Horner H. C., Janatpour M., Liaw C. W., Manning K., Morales J. A cell culture model of the blood-brain barrier. J Cell Biol. 1991 Dec;115(6):1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnick M. A., Carey A., Williams S. K. Phenotypic diversity in cultured cerebral microvascular endothelial cells. In Vitro Cell Dev Biol. 1988 May;24(5):435–444. doi: 10.1007/BF02628495. [DOI] [PubMed] [Google Scholar]
- SCHAYER R. W. Evidence that induced histamine is an intrinsic regulator of the microcirculatory system. Am J Physiol. 1962 Jan;202:66–72. doi: 10.1152/ajplegacy.1962.202.1.66. [DOI] [PubMed] [Google Scholar]
- Sawai S., Fukui H., Fukuda M., Wang N. P., Wada H., Manabe R. [3H]mepyramine binding sites, histamine H1-receptors, in bovine retinal blood vessels. Curr Eye Res. 1991 Aug;10(8):713–718. doi: 10.3109/02713689109013865. [DOI] [PubMed] [Google Scholar]
- Sawai S., Wang N. P., Fukui H., Fukuda M., Manabe R., Wada H. Histamine H1-receptor in the retina: species differences. Biochem Biophys Res Commun. 1988 Jan 15;150(1):316–322. doi: 10.1016/0006-291x(88)90522-0. [DOI] [PubMed] [Google Scholar]
- Schayer R. W. Histamine and microcirculation. Life Sci. 1974 Aug 1;15(3):391–401. doi: 10.1016/0024-3205(74)90338-5. [DOI] [PubMed] [Google Scholar]
- Shahinian H. K., Ferguson M. K., Michelassi F. Effect of histamine and histamine receptor antagonists on rat mesenteric microcirculation. J Surg Res. 1987 Jun;42(6):703–707. doi: 10.1016/0022-4804(87)90014-x. [DOI] [PubMed] [Google Scholar]
- Shakib M., Cunha-Vaz J. G. Studies on the permeability of the blood-retinal barrier. IV. Junctional complexes of the retinal vessels and their role in the permeability of the blood-retinal barrier. Exp Eye Res. 1966 Jul;5(3):229–234. doi: 10.1016/s0014-4835(66)80011-8. [DOI] [PubMed] [Google Scholar]
- Sinclair S. H., Grunwald J. E., Riva C. E., Braunstein S. N., Nichols C. W., Schwartz S. S. Retinal vascular autoregulation in diabetes mellitus. Ophthalmology. 1982 Jul;89(7):748–750. doi: 10.1016/s0161-6420(82)34720-x. [DOI] [PubMed] [Google Scholar]
- Sosula L., Beaumont P., Hollows F. C., Jonson K. M., Regtop H. L. Glycogen accumulation in retinal neurons and glial cells of streptozotocin-diabetic rats. Quantitative electron microscopy. Diabetes. 1974 Mar;23(3):221–231. doi: 10.2337/diab.23.3.221. [DOI] [PubMed] [Google Scholar]
- Stauber W. T., Ong S. H., McCuskey R. S. Selective extravascular escape of albumin into the cerebral cortex of the diabetic rat. Diabetes. 1981 Jun;30(6):500–503. doi: 10.2337/diab.30.6.500. [DOI] [PubMed] [Google Scholar]
- Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986 Sep;103(3):755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L. L., Field L. L., Ross S., McArthur R. G. Genetic risk factors in diabetic retinopathy. Diabetologia. 1993 Dec;36(12):1293–1298. doi: 10.1007/BF00400808. [DOI] [PubMed] [Google Scholar]
- Stjernborg L., Persson L. Stabilization of S-adenosylmethionine decarboxylase by aminoguanidine. Biochem Pharmacol. 1993 Mar 9;45(5):1174–1176. doi: 10.1016/0006-2952(93)90266-y. [DOI] [PubMed] [Google Scholar]
- Tomeo A. C., Egan R. W., Durán W. N. Priming interactions between platelet activating factor and histamine in the in vivo microcirculation. FASEB J. 1991 Oct;5(13):2850–2855. doi: 10.1096/fasebj.5.13.1655549. [DOI] [PubMed] [Google Scholar]
- Tout S., Chan-Ling T., Holländer H., Stone J. The role of Müller cells in the formation of the blood-retinal barrier. Neuroscience. 1993 Jul;55(1):291–301. doi: 10.1016/0306-4522(93)90473-s. [DOI] [PubMed] [Google Scholar]
- VAN ECK W. F. The effect of a low fat diet on the serum lipids in diabetes and its significance in diabetic retinopathy. Am J Med. 1959 Aug;27:196–211. doi: 10.1016/0002-9343(59)90340-7. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallow I. H., Engerman R. L. Permeability and patency of retinal blood vessels in experimental diabetes. Invest Ophthalmol Vis Sci. 1977 May;16(5):447–461. [PubMed] [Google Scholar]
- Wallow I. H. Posterior and anterior permeability defects? Morphologic observations on streptozotocin-treated rats. Invest Ophthalmol Vis Sci. 1983 Sep;24(9):1259–1268. [PubMed] [Google Scholar]
- Wang C., Brennan W. A., Jr Rat skeletal muscle, liver and brain have different fetal and adult forms of the glucose transporter. Biochim Biophys Acta. 1988 Dec 8;946(1):11–18. doi: 10.1016/0005-2736(88)90451-8. [DOI] [PubMed] [Google Scholar]
- Wang Z., Dohle C., Friemann J., Green B. S., Gleichmann H. Prevention of high- and low-dose STZ-induced diabetes with D-glucose and 5-thio-D-glucose. Diabetes. 1993 Mar;42(3):420–428. doi: 10.2337/diab.42.3.420. [DOI] [PubMed] [Google Scholar]
- Watson P. M., Anderson J. M., Vanltallie C. M., Doctrow S. R. The tight-junction-specific protein ZO-1 is a component of the human and rat blood-brain barriers. Neurosci Lett. 1991 Aug 5;129(1):6–10. doi: 10.1016/0304-3940(91)90708-2. [DOI] [PubMed] [Google Scholar]
- Welles S. L., Shepro D., Hechtman H. B. Vasoactive amines modulate actin cables (stress fibers) and surface area in cultured bovine endothelium. J Cell Physiol. 1985 Jun;123(3):337–342. doi: 10.1002/jcp.1041230307. [DOI] [PubMed] [Google Scholar]
- Wetzig P. C., Jepson C. N. Treatment of diabetic retinopathy by light coagulation. Am J Ophthalmol. 1966 Sep;62(3):459–465. doi: 10.1016/0002-9394(66)91325-0. [DOI] [PubMed] [Google Scholar]
- Willott E., Balda M. S., Heintzelman M., Jameson B., Anderson J. M. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992 May;262(5 Pt 1):C1119–C1124. doi: 10.1152/ajpcell.1992.262.5.C1119. [DOI] [PubMed] [Google Scholar]
- Willott E., Balda M. S., Heintzelman M., Jameson B., Anderson J. M. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992 May;262(5 Pt 1):C1119–C1124. doi: 10.1152/ajpcell.1992.262.5.C1119. [DOI] [PubMed] [Google Scholar]
- Wolbarsht M. L., Landers M. B., 3rd, Stefansson E. Vasodilation and the etiology of diabetic retinopathy: a new model. Ophthalmic Surg. 1981 Feb;12(2):104–107. [PubMed] [Google Scholar]
- Wong H. C., Boulton M., Marshall J., Clark P. Growth of retinal capillary endothelia using pericyte conditioned medium. Invest Ophthalmol Vis Sci. 1987 Nov;28(11):1767–1775. [PubMed] [Google Scholar]
- Zatz R., Brenner B. M. Pathogenesis of diabetic microangiopathy. The hemodynamic view. Am J Med. 1986 Mar;80(3):443–453. doi: 10.1016/0002-9343(86)90719-9. [DOI] [PubMed] [Google Scholar]
- de Oliveira F. Pericytes in diabetic retinopathy. Br J Ophthalmol. 1966 Mar;50(3):134–143. doi: 10.1136/bjo.50.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]