Abstract
Solid organ transplantation has been, by most measures, a phenomenal success. Nonetheless, the field is plagued by extreme shortages of available organs from a very limited number of donors. One potential solution to this organ availability crisis is the use of animals as organ donors for humans (xenotransplantation). Though the concept remains theoretical, significant advances are being made in the field of genetics and in our understanding of the immunological barriers to xenotransplantation. With these advances also comes increased knowledge about the potential risks of xenotransplants, especially disease transmission. The eventual clinical application of animal-to-human transplants will require a careful, balanced appraisal of these issues.
Organ transplantation has been one of the phenomenal success stories of the latter part of the 20th century. For decades the province of a few bold researchers and clinicians who often captured the public's attention, this field is now solidly entrenched in modern medical therapy. Since the early 1980s, hundreds of thousands of patients have received new kidneys, livers, and hearts (1). Other organs (lung, pancreas, and intestine) are also routinely transplanted, albeit in smaller numbers. The clinical results of these interventions have meant the restoration of meaningful, productive, and active lives to recipients of all organs (2–4).
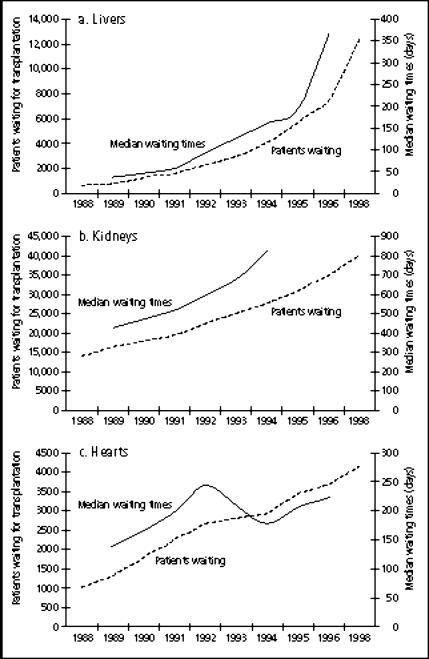
Vexingly, the transplant community has not been able to meet the demand for donor organs that these clinical successes have generated. To be sure, increases in donor organ availability have been noted over the last decade. But these have been explained by an increase in “living” donors (primarily for kidneys but to some extent for livers and lungs) and by the increasing use of cadaver donors that, years ago, would have been deemed unsuitable (the so-called “marginal” or “expanded” donors). The gap between organ need and organ availability continues to widen despite very substantial public education efforts on organ donation (5) (Figure 1). Deaths on the waiting list occur at a rate of 10 patients a day, and patients' waiting times for all major organs continue to grow (1)(Figure 2).
Figure 1.
Numbers of patients waiting and mean waiting times for (a) livers, (b) kidneys, and (c) hearts. Source: United Network for Organ Sharing (1).
Figure 2.
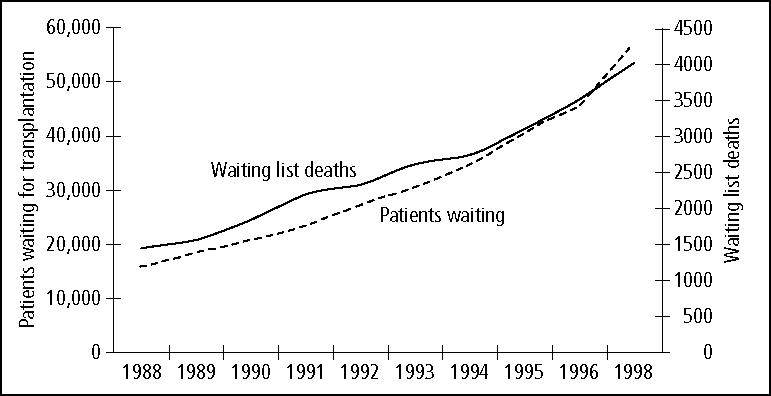
Number of patients waiting for solid organ transplantation and number of waiting list deaths. Source: United Network for Organ Sharing (1).
Against this background of such a pressing need, the case is made for finding an alternative, more plentiful supply of replacement organs for human transplantation. Recent advances in our understanding of the immunologic barriers between humans and animals have brought the field of clinical xenotransplantation (transplantation across species barriers) closer to reality than ever before, yet real and potential obstacles exist and are the subject of this article.
THE “IDEAL” DONOR ANIMAL
A long list emerges when we consider the preferred characteristics of animals appropriate to be organ donors for humans. First, the animal should be of compatible anatomy and physiology for the intended organ to function well in humans. Next, no possibility of cross-species (i.e., animal-to-human) infection should exist. In fact, an ideal animal donor organ should resist human diseases (especially viral) as well. Further, this animal species should be inexpensive to feed and breed, with short gestation times and multiple births per litter to achieve economies of scale. Such an animal should also present no immunologic barriers to transplantation into humans. Finally, use of this animal in this manner should engender little or no ethical controversy.
An animal species meeting all of the above criteria does not exist. Nonhuman primates (apes and monkeys) are most like humans anatomically and physiologically. Further, they may possess resistance to certain human diseases. In fact, this attribute (resistance to HIV and hepatitis B virus) has led to the experimental use of baboon livers as xenografts (6). Nonetheless, the xenotransplant community seems to have abandoned hopes of using nonhuman primates as xenograft donors primarily because of infectious risks to human patients and their contacts. Some monkey viruses—for example, herpes 8—are deadly to humans in a matter of days (7). The costs of raising pathogen-free herds in large enough numbers to satisfy clinical demand are felt to be prohibitive. Finally, the ethical obstacles to using nonhuman primates as organ donors for humans are considerable (8, 9).
The pig, with its large litters (up to 10 littermates), short gestation times (4 months), anatomic/physiologic similarities to humans, widespread use for human consumption (an estimated 90 million pigs consumed yearly in the USA), and long history of providing medicinals (skin, insulin, cardiac prostheses, clotting factors) for humans, has become the most likely candidate for consideration as an organ donor. To be sure, important differences in porcine physiology, including that of the coagulation cascade, may represent significant obstacles (10–12). Immunologic barriers, though increasingly understood, are also far from being overcome.
THE PUBLIC DIALOGUE
Over the past several years, a significant amount of public dialogue on xenotransplantation has taken place. In the USA, this dialogue has been in the form of numerous public meetings held by various regulatory agencies, advisory groups, and quasigovernmental bodies (Table). In the United Kingdom, this debate has often been framed within the context of potential infectious risks to humans and a very public scare over “mad cow disease” (bovine-to-human transmission of Creutzfeldt-Jakob disease).
Table.
Recent public meetings in the field of xenotransplantation
| Meeting | Dates |
| Food and Drug Administration Biologics | December 1994, April 1995, July 1995 |
| National Academy of Sciences/Institute of Medicine | June 1995, June 1996 |
| Public Health Service Workshops | June 1997, January 1998 |
| Food and Drug Administration December Xenotransplantation Subcommittee | December 1997, May 1999 |
| New York Academy of Sciences/Office for Economic Cooperation and Development | March 1998 |
The British government, unlike its American counterpart, has yet to allow clinical research in xenotransplantation (13). In the USA, the Food and Drug Administration is monitoring ongoing clinical trials in both organ and cellular xenotransplantation, including a trial here at Baylor. In the scientific literature, factions both for and against a moratorium on xenotransplant research have developed. The argument for a moratorium relies on the putative risks of infection transmission. Except for one strident voice, the trend seems to support the cautious continuation of this research (14–16). To date, no evidence exists that pigs have transmitted diseases to humans (17–19).
IMMUNOLOGIC BARRIERS AND PRECLINICAL RESULTS
The use of pig organs as xenografts came one step closer to reality with the discovery in humans of naturally occurring antibodies cross-reacting with porcine cells, including, importantly, the porcine vascular endothelium (20, 21). These xenoreactive antibodies are both IgM and IgGs, may exist as the result of crossreactivity with enteric bacteria, and are found in humans and Old World monkeys. They bind in the pig with an α1,3-galactose carbohydrate residue, which morphologically resembles the ABO blood group antigens (22). This antigen is present in very high numbers (107 receptors) on the pig vascular endothelial cell. Unmodulated, perfusion of human blood through pig organs leads to prompt antigen-antibody binding, complement activation, endothelial cell permeability, and capillary fibrin deposition with ischemia—hence, hyperacute rejection.
A number of approaches have been proposed to reduce or eliminate this anti-α-Gal–α1,3-Gal interaction. These include antibody absorption through pretransplant organ (lung or liver) or immunoaffinity column perfusion, the continuous infusion of antibody-depleting (competitive) carbohydrates, the modification of animals lacking (or with greatly reduced) α1,3-Gal antigens, and accommodation (22). These strategies are in various stages of development; none have reached clinical testing.
A more promising and more tested approach has been modifying pigs through microinjection techniques and in vitro fertilization so that they are “humanized” for certain complement-regulatory proteins (23, 24). Since complement activation following antigen-antibody binding is felt to be species-specific, organs from such animals, lacking in porcine complement-regulatory proteins, would upon anti-α-Gal–α1,3-Gal binding not activate complement and not undergo hyperacute rejection. In life-supporting pig-to-primate models using transgenic pigs as kidney donors, this strategy has yielded survivals of up to 35 days after transplant (25). Orthotopic cardiac transplantation has given survivals of up to 3 weeks when used with antibody depletion, lymphoid irradiation, and vigorous immunosuppression (26). Overall, though, these preclinical studies have not made use of state-of-the-art immunosuppressants now commonplace in human allotransplantation (i.e., tacrolimus and mycophenolate mofetil).
Even postulating a greatly diminished or eliminated risk of hyperacute rejection, immunologic barriers to xenotransplantation will probably be more significant than those of allotransplantation. Delayed xenograft rejection (accelerated vascular rejection), probably the result of anti-α-Gal antibodies, looms after several days' exposure to a xenograft (27). The human T cell antiporcine response is probably considerable, mediated by both direct and indirect recognition of xenoantigens. CD4+ and CD8+ T cells probably figure prominently in these processes (28). As with allotransplantation, tolerance is felt to be the sought-after answer to safe, effective immunosuppression for xenotransplantation.
POTENTIAL XENOTRANSPLANTATION-CREATED INFECTIONS
With any proposed species of organ donors for humans, the loudest opposition comes from fears of creating new infections heretofore unknown or poorly known (so-called “xenozoonoses”). As previously mentioned, one of the strongest arguments made thus far against nonhuman primates as potential donors is the difficulty of ensuring a safe, plentiful supply of these animals (7). An almost insurmountable obstacle for advocates of nonhuman primates as xenograft donors has been the recently concluded studies that showed HIV to be just such a zoonosis (29). The Ebola virus may ultimately be found to fall into this category as well (30). These viruses are believed to be nonpathogenic in their natural hosts but devastating to humans.
The recent discovery of a type C endogenous retrovirus in the pig (PoERV) and its ability to infect human cell lines in vitro has brought great scrutiny to that species as well (31–33). Patients parenterally exposed to pig tissue (islet cells, hepatic cells) or whole organs (extracorporeal hepatic and renal support) have failed to show infection by PoERV (17–19), despite repeated testing in some cases. Currently lost in the debate is the risk of other potential xenozoonoses from pigs, including porcine cytomegalovirus and more conventional bacterial pathogens. Some experts in these fields worry that transgenic pig organs, their complement-activating factors no longer porcine in configuration, may be even more susceptible to viral infections, especially in an immunosuppressed patient (34).
SUMMARY
Significant improvements in our understanding of the immunologic barriers between larger animals and humans offer the hope of the clinical application of animal-to-human transplants. More sophisticated genetic engineering of animals, as well as more complex modulation of the animal-to-human antibody and cellular recognition, will probably need to occur for the field to move forward. Porcine organs, and not nonhuman primate ones, are the organs of choice for these endeavors. The concern over potential human infection by animal viruses or nonviral pathogens mandates very close scrutiny of clinical trials as they evolve.
References
- 1.United Network for Organ Sharing (UNOS). 1998 Annual Report Richmond, Va: UNOS, 1999.
- 2.Levy MF, Jennings L, Abouljoud MS, Mulligan DC, Goldstein RM, Husberg BS, Gonwa TA, Klintmalm GB. Quality of life improvements at one, two, and five years after liver transplantation. Transplantation. 1995;59:515–518. [PubMed] [Google Scholar]
- 3.Free MM. Professional and personal aspects of solid organ and hematopoietic stem cell transplantation. BUMC Proceedings. 1995;8(4):29–32. [Google Scholar]
- 4.Simmons RG, Abress L, Anderson CR. Quality of life after kidney transplantation. A prospective, randomized comparison of cyclosporine and conventional immunosuppressive therapy. Transplantation. 1988;45:415–421. doi: 10.1097/00007890-198802000-00034. [DOI] [PubMed] [Google Scholar]
- 5.The Partnership for Organ Donation . The American Public's Attitudes Toward Organ Donation and Transplantation; Gallop Poll Results. Boston, Mass: The Partnership for Organ Donation; 1993. [Google Scholar]
- 6.Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, Doyle H, Zeevi A, Warty V, Michaels M, et al. Baboon-to-human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allan JS. Xenotransplantation at a crossroads: prevention versus progress. Nat Med. 1996;2:18–21. doi: 10.1038/nm0196-18. [DOI] [PubMed] [Google Scholar]
- 8.Vanderpool HY. Critical ethical issues in clinical trials with xenotransplants. Lancet. 1998;351:1347–1350. doi: 10.1016/S0140-6736(97)10354-3. [DOI] [PubMed] [Google Scholar]
- 9.Hughes J. Xenografting: ethical issues. J Med Ethics. 1998;24:18–24. doi: 10.1136/jme.24.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammer C. Physiological obstacles after xenotransplantation. Ann N Y Acad Sci. 1998;862:19–27. doi: 10.1111/j.1749-6632.1998.tb09113.x. [DOI] [PubMed] [Google Scholar]
- 11.Delriviere LD, Havaux X, Gibbs P, Gianello PR. Basic anatomical and physiological differences between species should be considered when choosing combinations for use in models of hepatic xenotransplantation: an investigation of the guinea pig-to-rat combination. Transplantation. 1998;66:112–115. doi: 10.1097/00007890-199807150-00017. [DOI] [PubMed] [Google Scholar]
- 12.Robson SC, Schulte am Esch J, II, Bach FH. Factors in xenograft rejection. Ann N Y Acad Sci. 1999;875:261–276. doi: 10.1111/j.1749-6632.1999.tb08509.x. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy I. Xenotransplantation: ethical acceptability. Transplant Proc. 1997;29:2729–2730. doi: 10.1016/s0041-1345(97)00574-5. [DOI] [PubMed] [Google Scholar]
- 14.Bach FH, Fineberg HV. Call for moratorium on xenotransplants. Nature. 1998;391:326. doi: 10.1038/34766. [DOI] [PubMed] [Google Scholar]
- 15.Sachs DH, Colvin RB, Cosimi AB, Russell PS, Sykes M, McGregor CG, Platt JL. Xenotransplantation—caution, but no moratorium. Nat Med. 1998;4:372–373. doi: 10.1038/nm0498-372a. [DOI] [PubMed] [Google Scholar]
- 16.Salomon DR, Ferguson RM, Helderman JH. Xenotransplants: proceed with caution. Nature. 1998;392:11–12. doi: 10.1038/32023. [DOI] [PubMed] [Google Scholar]
- 17.Allan JS. Cross-species infection: no news is good news? Nat Med. 1998;4:644–645. doi: 10.1038/nm0698-644b. [DOI] [PubMed] [Google Scholar]
- 18.Levy MF, Crippin J, Sutton S, Netto G, McCormack J, Curiel T, Goldstein RM, Newman JT, Gonwa TA, Banchereau J, Diamond LE, Byrne G, Logan J, Klintmalm GB. Liver allotransplantation (OLTX) following extracorporeal hepatic support with transgenic (hCD55/hCD59) porcine livers: clinical results and lack of pig to human transmission of the porcine endogenous retrovirus (PoERV). Transplantation In press. [DOI] [PubMed]
- 19.Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, Chapman LE, Lockey C, Onions D, Otto E. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 20.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alphagalactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 21.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper DK, Thall AD. Xenoantigens and xenoantibodies: their modification. World J Surg. 1997;21:901–906. doi: 10.1007/s002689900324. [DOI] [PubMed] [Google Scholar]
- 23.Platt JL, Vercellotti GM, Dalmasso AP, Matas AJ, Bolman RM, Najarian JS, Bach FH. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990;11:450–456. doi: 10.1016/0167-5699(90)90174-8. discussion 456–457. [DOI] [PubMed] [Google Scholar]
- 24.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 25.Zaidi A, Schmoeckel M, Bhatti F, Waterworth P, Tolan M, Cozzi E, Chavez G, Langford G, Thiru S, Wallwork J, White D, Friend P. Life-supporting pig-to-primate renal xenotransplantation using genetically modified donors. Transplantation. 1998;65:1584–1590. doi: 10.1097/00007890-199806270-00008. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Gundry SR, Hancock WW, Matsumiya G, Zuppan CW, Morimoto T, Slater J, Bailey LL. Prolonged discordant xenograft survival and delayed xenograft rejection in a pig-to-baboon orthotopic cardiac xenograft model. J Thorac Cardiovasc Surg. 1998;115:1342–1349. doi: 10.1016/S0022-5223(98)70218-1. [DOI] [PubMed] [Google Scholar]
- 27.Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC. Delayed xenograft rejection. Immunol Today. 1996;17:379–384. doi: 10.1016/0167-5699(96)10024-4. [DOI] [PubMed] [Google Scholar]
- 28.Dorling A, Lechler RI. T cell-mediated xenograft rejection: specific tolerance is probably required for long term xenograft survival. Xenotransplantation. 1998;5:234–245. doi: 10.1111/j.1399-3089.1998.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 29.Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Cummins LB, Arthur LO, Peeters M, Shaw GM, Sharp PM, Hahn BH. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 30.Peters CJ, LeDuc JW. An introduction to Ebola: the virus and the disease. J Infect Dis. 1999;179(Suppl 1):ix–xvi. doi: 10.1086/514322. [DOI] [PubMed] [Google Scholar]
- 31.Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 1997;389:681–682. doi: 10.1038/39489. [DOI] [PubMed] [Google Scholar]
- 32.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 33.Wilson CA, Wong S, Muller J, Davidson CE, Rose TM, Burd P. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol. 1998;72:3082–3087. doi: 10.1128/jvi.72.4.3082-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss RA. Transgenic pigs and virus adaptation. Nature. 1998;391:327–328. doi: 10.1038/34772. [DOI] [PubMed] [Google Scholar]