Abstract
Recent international reports have suggested that an abnormal pancreatic and bile duct junction can influence the degree of pancreatic fluid regurgitation, resulting in an increased incidence of biliary tract malignancy. To confirm these reports, we retrospectively examined the anatomic relation at the pancreaticobiliary junction in all patients diagnosed with cholangiocarcinoma or gallbladder cancer at Baylor University Medical Center (BUMC) over a 10-year period. From 1989 to 1998, 82 patients with bile duct cancer were treated at BUMC. Adequate visualization of the pancreaticobiliary junction was accomplished in 29 patients (35%). Among these patients, an abnormal junction, with a common channel length of 8 to >15 mm, was noted in 13 patients (45%). Thus, this study confirms previous reports regarding the high incidence of an abnormal pancreaticobiliary junction in patients with bile duct cancer. A prospective effort to examine this anatomy and the length of the common channel should be encouraged to identify a potential high-risk group.
Malignancies of the biliary tract are not common, with >8000 cases reported annually in the USA. Approximately two thirds of these arise in the gallbladder, while the remainder originate from the bile ducts and periampullary region. Biliary tract malignancy is often at an advanced stage by the time symptoms develop. The prognosis is generally poor, with a median survival of 12 to 14 months for patients undergoing resection and 6 months for patients treated with palliative stenting (3). The 5-year survival rate is directly related to disease stage, with a range of 0% survival for stage IV disease to 80% survival for stage I disease. Survival is also related to tumor location: patients with distal bile duct carcinomas do better than patients with mid, proximal, or gallbladder tumors (2).
Recent studies from Japan have suggested that anatomical anomalies of the pancreaticobiliary tree are associated with biliary tract cancer. The junction of the common bile duct and pancreatic duct is crucial for sphincteric control of bile and pancreatic juice drainage, with bidirectional regurgitation occurring if the union is above Oddi's sphincter. An abnormal pancreaticobiliary junction is a junction of the common bile duct and the main pancreatic duct outside the wall of the duodenum that forms a long common channel (<8 mm) (3). According to Kimura, the mode of abnormal pancreaticobiliary junction can be classified into 2 types: type I in which the main pancreatic duct enters the common bile duct and type II in which the common bile duct enters the pancreatic duct (Figure 1) (4). Abnormal pancreaticobiliary junction can also be found in association with various pancreaticobiliary diseases, including choledochal cyst, gallbladder adenomyomatosis, pancreatitis, and pancreas divisum (5). Various oncogenic mechanisms have been suggested in patients with abnormal pancreaticobiliary junction, such as regurgitation of pancreatic juice, cholestasis and biliary infection, and formation of carcinogens in bile (5–7). We retrospectively reviewed all patients with cholangiocarcinoma or gallbladder cancer treated at Baylor University Medical Center (BUMC) and determined how often the pancreaticobiliary junction was visualized and how often an abnormal junction was identified.
Figure 1.
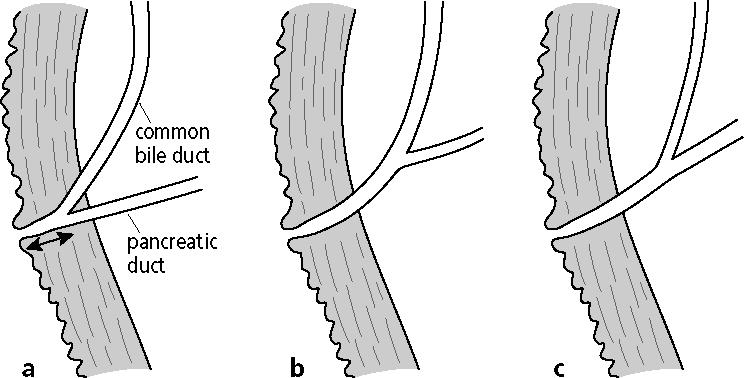
Pancreaticobiliary anatomy. (a) Normal anatomy with a short common channel (arrow) and biliopancreatic junction within the duodenal wall. (b) Abnormal pancreaticobiliary junction type I, with a long common channel and dominant common bile duct. (c) Abnormal pancreaticobiliary junction type II, with a long common channel and dominant pancreatic duct.
METHODS
Patients with cholangiocarcinoma were identified through a retrospective chart review from the pathology, tumor registry, and hospital medical records departments. Appropriate institutional review board approval was obtained.
Cholangiopancreatograms of all patients with cholangiocarcinoma were retrospectively reviewed. Special attention was given to the definition of the anatomy of the pancreaticobiliary junction. The criteria for selection into the study included 1) adequate filling of the common bile duct and the main pancreatic duct and 2) clear identification of the point of entry of either the 2 ducts separately or the common channel into the duodenum. Endoscopic retrograde cholangiopancreatography films were excluded from the study when the distal ends of the 2 ducts were obscured either by the endoscope or by the presence of contrast material in the duodenum or in a duodenal diverticulum. Four to 8 films were available for each patient, and the ducts were considered to be separate if the 2 ducts were seen to open separately into the duodenum. The same observer measured the length of the common channel in all patients. To account for the magnification factor, the distal end of the endoscope in the duodenum was measured and the length of the common duct adjusted accordingly (Figure 2).
Figure 2.
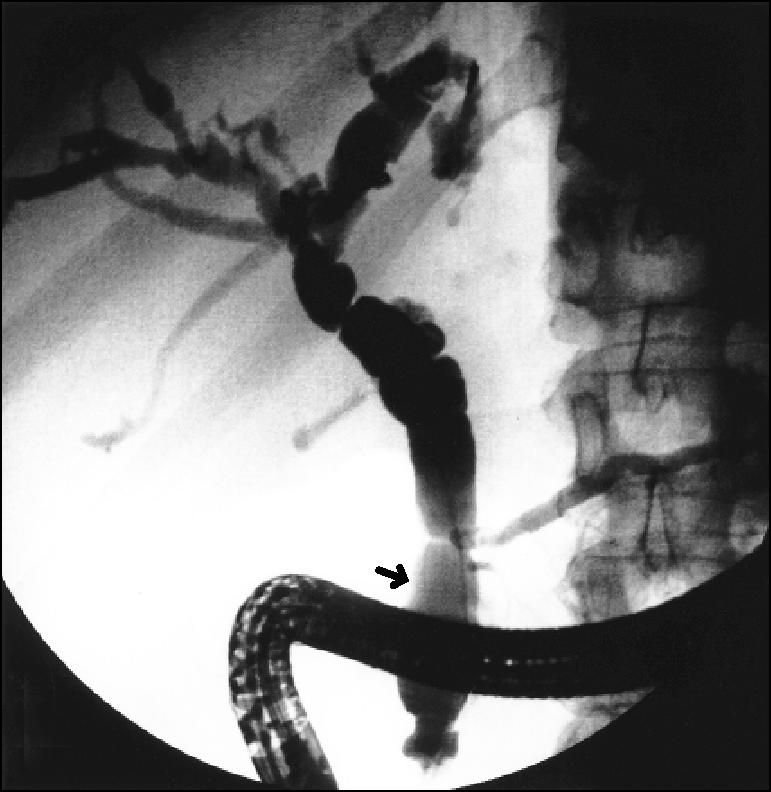
Typical endoscopic retrograde cholangiopancreatography image showing long common channel (arrow).
RESULTS
From 1989 to 1998, 82 patients with biliary tract cancer were identified at BUMC, including 51 patients with common bile duct carcinoma and 31 patients with gallbladder carcinoma. Only 29 patients (35%) were found to have adequate imaging of the pancreaticobiliary junction in order to characterize the anatomy. Sixteen out of the 29 patients had normal pancreaticobiliary junctions, and 13 patients (45%) had abnormal pancreaticobiliary junctions. The mean age of these 5 men and 8 women at the time of diagnosis was 66 years (range, 46 to 82 years). Abnormal pancreaticobiliary junctions included common pancreaticobiliary channel lengths of 8 to 10 mm (n = 5), 10 to 15 mm (n = 2), and >15 mm (n = 6). The mean length of the common channels was 12.5 mm. Nine patients had a type I abnormality, and 4 had a type II abnormality. The 13 patients with malignancy included 11 patients with bile duct carcinoma (84.6%, 4 with stage II disease and 7 with stage IV disease) and 2 patients with gallbladder carcinoma (15.4%, all with stage IV disease). Both patients with gallbladder carcinoma and 2 with common bile duct carcinoma had a type II abnormality. Gallstones were noted in 7 patients, either concurrently or by prior history.
Surgical resection of the tumor was possible in 6 patients. Laparotomy with biopsy and stenting was performed in the remaining 7 patients because of unresectable disease. Five patients had adjuvant chemotherapy and radiotherapy. Mean overall survival of the group was 9.5 months.
DISCUSSION
The pathogenesis of biliary tract malignancy is poorly understood. A variety of associated conditions have been shown to increase the incidence of malignancy. The underlying mechanism has been attributed to stasis, pancreatic enzymes, and bacteria. Nagata et al noted that reflux and stasis of the pancreatic juice into the gallbladder may induce chronic cholecystitis with intestinal metaplasia (8), and this may play an important role in the pathogenesis of well-differentiated adenocarcinoma. Funabiki et al observed high concentrations of deoxycholic acid, lithocholic acid, and unconjugated bile acid in bile obtained from patients with an abnormal pancreaticobiliary junction (9). The importance of the pancreaticobiliary anatomy has recently been identified. This paper is the first to report a US investigation of this anatomic relationship.
The definition of abnormal pancreaticobiliary junction differs among various investigators (4, 11–14) and was revised by the Japanese Study Group on Pancreaticobiliary Maljunction in 1994. In an autopsy study, presumably more accurate in measurement than studies with endoscopic retrograde cholangiopancreatography, the length of the common channel in normal subjects ranged from 1 to 12 mm, with a mean value of 4.5 mm (13). Misra and his colleagues reported that a common channel with a mean length of 4.7 ± 2.5 mm (range, 1.6 to 18.4 mm) was present in 64 (63%) of 102 normal endoscopic retrograde cholangiopancreatography films (3). The reported frequency of abnormal pancreaticobiliary junction ranged from 1.5% to 3.2% in different ethnic populations (4, 11–14). Wang et al found an abnormal pancreaticobiliary junction in 59 (3.4%) of 1752 subjects undergoing endoscopic retrograde cholangiopancreatography (15). The incidence went up to 8.7% in 680 patients when the pancreaticobiliary junction was clearly visualized.
The association of abnormal pancreaticobiliary junction with choledochal cyst was first noted in 1906 (16). In 1973, Babbitt et al proposed abnormal pancreaticobiliary junction as the etiology of choledochal cyst (17). Subsequently, bile duct cancer (16) and gallbladder cancer (10, 11, 16) were found to be in the spectrum of abnormal pancreaticobiliary junction diseases. Kimura et al studied 65 patients with abnormal pancreaticobiliary junction and found that 49 (75.4%) had choledochal cyst alone, 11 (16.9%) had gallbladder cancer alone, and 5 (7.7%) had both choledochal cyst and gallbladder cancer (4). They concluded that the incidence of gallbladder carcinoma in the 65 cases of abnormal pancreaticobiliary junction was 24.5% compared with a 1.9% incidence of this cancer among 635 patients with normal junctions. After characterizing the anatomy of an abnormal pancreaticobiliary junction into 2 types (type I, the pancreatic duct joining the common bile duct; type II, the common bile duct joining the pancreatic duct), they asserted that type I abnormalities carried a higher risk of gallbladder malignancy. Misra et al analyzed endoscopic retrograde cholangiopancreatography films of patients with biliary diseases and found that an abnormal pancreaticobiliary junction (long common channel >8 mm) was seen more frequently in carcinoma of the gallbladder (8 of 21, 38%) compared with normal subjects (3 of 102, 3%) (3). These findings were confirmed by Wang et al, who found that 62.5% (5 of 8) of patients with gallbladder cancer and 33.3% (9 of 27) of patients with common bile duct cancer had an abnormal pancreaticobiliary junction (15).
In the USA, the anatomy of the pancreaticobiliary junction is seldom identified on cholangiopancreatogram reports. Our experience was remarkable in that the junction was not visualized in 65% of all cholangiopancreatograms. A subsequent review of 50 endoscopic retrograde cholangiopancreatograms with nonmalignant disease showed that the junction's anatomy was never reported on hard copy images, even in cases that were clearly visible.
Consistent with previous studies, our results did show a remarkably high prevalence of abnormal pancreaticobiliary junction in patients with biliary tract malignancies. Subtypes of abnormal pancreaticobiliary junction may be correlated with different patterns of reflux and thus indicate different associated diseases. A prospective effort to clearly report this anatomy during every endoscopic retrograde cholangiopancreatography should be encouraged to identify patients at high risk. Close follow-up may allow earlier detection of carcinoma and, thus, curative instead of palliative treatment.
References
- 1.Shimizu M, Miura J, Tanaka T, Itoh H, Saitoh Y. Porcelain gallbladder: relation between its type by ultrasound and incidence of cancer. J Clin Gastroenterol. 1989;11:471–476. [PubMed] [Google Scholar]
- 2.Shinkai H, Kimura W, Muto T. Surgical indications for small polypoid lesions of the gallbladder. Am J Surg. 1998;175:114–117. doi: 10.1016/S0002-9610(97)00262-6. [DOI] [PubMed] [Google Scholar]
- 3.Misra SP, Gulati P, Thorat VK, Vij JC, Anand BS. Pancreaticobiliary ductal union in biliary diseases. An endoscopic retrograde cholangiopancreatographic study. Gastroenterology. 1989;96:907–912. [PubMed] [Google Scholar]
- 4.Kimura K, Ohto M, Saisho H, Unozawa T, Tsuchiya Y, Morita M, Ebara M, Matsutani S, Okuda K. Association of gallbladder carcinoma and anomalous pancreaticobiliary ductal union. Gastroenterology. 1985;89:1258–1265. doi: 10.1016/0016-5085(85)90641-9. [DOI] [PubMed] [Google Scholar]
- 5.Sameshima Y, Muto Y, Uchimura M, Waki S, Hayashi T. Clinicopathological study of the gallbladder in congenital dilatation of the bile duct— with special reference to the association with cancer [in Japanese] Nippon Shokakibyo Gakkai Zasshi. 1982;79:1129–1136. [PubMed] [Google Scholar]
- 6.Komi N, Udaka H, Ogasawara K. Carcinoma arising from congenital dilatation of the bile ducts—a report of two cases. Geka-Shinryo. 1976;158:1266–1274. [Google Scholar]
- 7.Hizawa K, Fukuda F, Akagi G, Kitamura T, Tao S. Carcinoma associated with idiopathic choledochus cyst. Acta Pathol Jpn. 1967;17:101–106. doi: 10.1111/j.1440-1827.1967.tb01205.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagata E, Sakai K, Kinoshita H, Kobayashi Y. The relation between carcinoma of the gallbladder and an anomalous connection between the choledochus and the pancreatic duct. Ann Surg. 1985;202:182–190. doi: 10.1097/00000658-198508000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funabiki T, Sugiue K, Matsubara T, Amano H, Ochiai M. Bile acids and biliary carcinoma in pancreaticobiliary maljunction. Keio J Med. 1991;40:118–122. doi: 10.2302/kjm.40.118. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi S, Koga A, Matsumoto S, Tanaka M, Nakayama F. Anomalous junction of pancreaticobiliary duct without congenital choledochal cyst: a possible risk factor for gallbladder cancer. Am J Gastroenterol. 1987;82:20–24. [PubMed] [Google Scholar]
- 11.Kinoshita H, Nagata E, Hirohashi K, Sakai K, Kobayashi Y. Carcinoma of the gallbladder with an anomalous connection between the choledochus and the pancreatic duct. Report of 10 cases and review of the literature in Japan. Cancer. 1984;54:762–769. doi: 10.1002/1097-0142(1984)54:4<762::aid-cncr2820540429>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.Misra SP, Dwivedi M. Pancreaticobiliary ductal union. Gut. 1990;31:1144–1149. doi: 10.1136/gut.31.10.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowdy GS, Waldron GW, Brown WG. Surgical anatomy of the pancreaticobiliary ductal system. Arch Surg. 1962;84:229–246. doi: 10.1001/archsurg.1962.01300200077006. [DOI] [PubMed] [Google Scholar]
- 14.Kato O, Hattori K, Suzuki T, Tachino F, Yuasa T. Clinical significance of anomalous pancreaticobiliary union. Gastrointest Endosc. 1983;29:94–98. doi: 10.1016/s0016-5107(83)72539-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang HP, Wu MS, Lin CC, Chang LY, Kao AW, Wang HH, Lin JT. Pancreaticobiliary diseases associated with anomalous pancreaticobiliary ductal union. Gastrointest Endosc. 1998;48:184–189. doi: 10.1016/s0016-5107(98)70161-0. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Nagakawa T, Ohta T, Nakano T, Kayahara M, Kanno M, Ueno K, Izumi R, Miyazaki I. Association between gallbladder cancer and anomalous union of the pancreaticobiliary ductal system. Hepatogastroenterology. 1993;40:56–60. [PubMed] [Google Scholar]
- 17.Babbitt DP, Starshak RJ, Clemett AR. Choledochal cyst: a concept of etiology. Am J Roentgenol Radium Ther Nucl Med. 1973;119:57–62. doi: 10.2214/ajr.119.1.57. [DOI] [PubMed] [Google Scholar]


