CASE PRESENTATION
J. Todd Gage, MD: A 32-year-old African American man with AIDS was brought to the emergency department of Baylor University Medical Center. Shortly before breakfast on the day of admission, the patient had abruptly lost consciousness and experienced a tonicclonic seizure, which lasted several minutes and resolved spontaneously. No head trauma, loss of bladder or bowel continence, or tongue biting occurred during the seizure. The patient was alert and oriented on admission but acted somewhat inappropriately and was resistant to questioning. He complained of chronic, nonproductive cough and night sweats. He denied fever, chills, headache, nuchal rigidity, photophobia, diplopia, numbness, weakness, paresthesias, palpitations, chest pain, weight loss, malaise, tobacco use, alcohol use, illicit drug use, tattoos, or prior blood transfusions. He was allergic to no medicines. No recent febrile illnesses, malignancies, or seizure disorders had been in the family.
The patient had been diagnosed with HIV at age 27. His most recent CD4 count had been 4, and his last viral load was 582,240. In September 1999, he had cytomegalovirus (CMV) enteritis, and in October 1999, he had Staphylococcus aureus pneumonia. His medications included hydrocortisone (30 mg daily), lamivudine/zidovudine (300 mg twice daily), abacavir (300 mg twice daily), fluconazole (200 mg daily), epoetin alfa (injection once a week), and lansoprazole (30 mg daily). Double-strength trimethoprim/sulfamethoxazole (3 times a week) and ganciclovir (1 g 3 times a day) were taken prophylactically.
On presentation to the emergency department, the patient's temperature was 37°C (98.7°F); blood pressure, 98/59 mm Hg; heart rate, 120 beats per minute; and respiratory rate, 18 breaths per minute. Examination of his head, eyes, oropharynx, and neck disclosed no abnormalities. His carotid pulses were normal bilaterally without bruits. His lung fields were clear, and precordial examination disclosed no abnormalities. The abdomen was soft and mildly tender in the epigastric region, and bowel sounds were active. He had no hepatosplenomegaly, masses, or abdominal bruits. His extremities showed no clubbing, cyanosis, or edema. His dorsalis pedis pulses were 2+ bilaterally. Neurological examination revealed that cranial nerves II through XII were grossly intact. He had 5+ muscle strength in all 4 extremities. His patellar and biceps reflexes were 2+ bilaterally. He had no decreased sensation to light touch or pinprick. He had normal rapid alternating movements and a normal finger-to-nose test. There was no Babinski sign. He had 2 beats of clonus on the left and 4 beats on the right. Initial laboratory results are summarized in the Table.
Table.
Initial laboratory values
| Sodium | 141 mEq/L | Alanine aminotransferase | 39 U/L |
| Potassium | 4.1 mEq/L | Lactate dehydrogenase | 558 U/L |
| Chloride | 105 mEq/L | White blood cell count | 4.2 × 103/μL |
| Bicarbonate | 28 mEq/L | Differential | 11% bands |
| Blood urea nitrogen | 8 mg/dL | 55% segmented | |
| Creatinine | 0.8 mg/dL | neutrophils | |
| Glucose | 106 mg/dL | 16% lymphocytes | |
| Anion gap | 8 mEq/L | 2% monocytes | |
| Albumin | 3.2 g/dL | 16% eosinophils | |
| Total bilirubin | 0.5 mg/dL | Hemoglobin | 10.3 g/dL |
| Alkaline phosphatase | 74 U/L | Hematocrit | 31% |
| Aspartate aminotransferase | 45 U/L | Platelets | 206 × 103/μL |
A lumbar puncture revealed clear, colorless fluid and a red blood cell count of 6 ×103/μL; white blood cell count, 2 ×103/μL (59% lymphocytes, 13% neutrophils, and 13% monocytes); protein, 6.3 g/dL; and glucose, 48 mg/dL. Toxoplasma IgG and Cryptococcus antigen were negative. Serum Toxoplasma IgG was <1:16; blood cultures were negative; urine cultures were >100,000 CFU/mL (coagulase positive for Staphylococcus); and CMV antigenemia was positive.
IMAGING STUDIES
Peter G. Hildenbrand, MD: Brain computed tomography (CT) without contrast obtained in the emergency department revealed a subtle, discrete mass lesion within the gray-white junction of the right frontal lobe with mild associated mass effect. There was a halo of surrounding vasogenic edema. There were no associated parameningeal inflammatory foci with normal appearance to the paranasal sinuses and mastoids. There was mild ventricular and cortical sulcal prominence consistent with the AIDS diagnosis.
Subsequent infusion magnetic resonance imaging (MRI) evaluation served to confirm the solitary rim-enhancing frontal lobe mass without ependymal or meningeal enhancement. Interestingly, the diffusion-weighted sequences displayed increased signal within the central cystic or necrotic component.
Based upon the imaging finding, the primary differential considerations in this AIDS patient consisted of toxoplasmosis or even pyogenic brain abscess vs lymphoma. Thallium brain imaging was performed in an effort to refine the differential diagnosis. The thallium study was negative for detectable neoplasm.
CASE DISCUSSION
Estil A. Vance, MD: In general terms, the occurrence of central nervous system (CNS) opportunistic infections has changed with the introduction of highly active antiretroviral therapy. The occurrences of HIV dementia, cryptococcal meningitis, toxoplasmosis, and primary CNS lymphoma have fallen off, although the rate of progressive multifocal leukoencephalopathy (PML) infections has stayed the same.
In 1989 Holtzman et al examined patients with HIV to determine causes of seizure (1). AIDS-dementia complex—biopsy proven and suspected—was blamed for 24% to 27% of seizures. In this study, CNS toxoplasmosis caused 28% of seizures; cryptococcal meningitis, 13%. Primary CNS lymphoma (4%) and PML (1%) also caused seizures. The most likely causes for a space-occupying lesion typical of the one seen in our patient would be toxoplasmosis, primary CNS lymphoma, and PML.
In 1994 Gildenberg et al examined the results of 174 brain biopsies in 170 patients with AIDS and space-occupying lesions (2). Their study excluded patients with Toxoplasma and cryptococcal meningitis. PML (51 patients) and non-Hodgkin's lymphoma (50 patients), including primary CNS lymphoma and systemic lymphoma, were present most often at biopsy. Encephalitis was found in 33 patients. Eight biopsies revealed an abscess. In 13 patients, multiple etiologies were found as the source of a space-occupying lesion: HIV encephalopathy, PML, or non- Hodgkin's lymphoma with another complicating event. Six other malignancies were seen. Four patients had no diagnosis. Nonspecific gliosis was seen in 3 patients, and 1 patient had amoebic encephalitis.
Multiple general clinical considerations modify the differential diagnosis in this instance. In a patient with AIDS and a known space-occupying brain lesion, important clinical considerations would include the state of HIV (CD4 count and viral load). Obviously, patients with a high CD4 count would be at risk for different infectious processes than would patients with a very low CD4 count. Prior opportunistic infections are important, as these may sometimes involve the CNS. The use of and compliance with a highly active antiretroviral therapy regimen is important. Compliance with the regimen and reasonable control of the underlying HIV disease may be associated with a likelihood of different processes than those in patients who are not on antiretroviral therapy. Ongoing antibiotics and prophylaxis are important, as these will dramatically impact certain infections. This is certainly true of Toxoplasma and acid-fast bacilli, the occurrence of which may be modified by prophylaxis for Mycobacterium avium complex and Pneumocystis carinii pneumonia. Immune suppression in addition to HIV itself is important, and concurrent neutropenia or ongoing steroids may increase the risk of CNS mycosis. Social history is important, as this may affect the risk of staphylococcal bacteremia (from intravenous drug use) as well as CNS syphilis. Travel history may impact on the risk for endemic mycosis or potential parasitic disease. Prior testing in the setting of a diagnosis of HIV is also important and includes rapid plasma reagin testing, Toxoplasma serology, and prior tuberculin skin test status. Finally, the radiologic appearance of the mass by CT scan, MRI, and nuclear medicine imaging can be useful.
This patient had a very low CD4 count and a high viral load. He had a prior history of CMV enteritis and S. aureus pneumonia. The patient was on a stable 2-drug regimen and was taking trimethoprim/sulfamethoxazole, ganciclovir, and fluconazole. There were no systemic symptoms except night sweats, and he had a concurrent urinary tract infection (S. aureus) as well as prostatitis. His CMV antigen test was positive, Toxoplasma IgG was low, cerebrospinal fluid Toxoplasma IgG was negative, and cerebrospinal fluid cryptococcal antigen was negative. There was no intravenous drug use. The prior history of staphylococcal infection is a risk factor for bacteremia. No extensive foreign travel was reported, decreasing the risk of exotic disease. Radiologic imaging found a single, medium-sized frontal lesion, with edema. No clear central necrosis was present.
There are many potential causes for CNS lesions in patients with HIV: vascular, viral, bacterial, mycobacterial, fungal, parasitic, and neoplastic.
Vascular events, including stroke, can occur in patients with HIV. This patient's presentation would be atypical, and a vascular event is less of a consideration in this instance.
Viral causes for CNS lesions in HIV-positive patients could be CMV encephalitis, PML, or HIV. CMV encephalitis would be considered on the basis of enteritis as well as a positive finding of CMV in the blood and the cerebrospinal fluid. A broad spectrum of CMV infection of the CNS includes polyradiculitis and periventriculitis, as well as mass lesions or abscesses. In this case, we would expect some periventriculitis and/or small mass lesions in the periventricular areas. Trueba et al reviewed MRI and CT scans of autopsy cases of CMV encephalitis (3). Subependymal enhancement on T1 postgadolinium scans was seen in all cases. Other pathology was frequent. Gozlan et al determined that polymerase chain reaction of the cerebrospinal fluid for CMV had positive and negative predictive values of 86% and 97%, respectively (4).
PML is caused by reactivation of latent JC virus in the brain. It is the third major cause of focal CNS abnormalities in AIDS patients. Clinically, there is usually subacute onset of focal neurologic deficits with some accompanying cognitive decline. Seizures are uncommon. It is primarily a demyelinating disorder with a distinctive appearance on MRI (lesions confined to subcortical white matter). Edema and mass effect are unusual, although larger lesions may cavitate. The subacute presentation in combination with the radiologic findings is usually diagnostic. Sometimes the polymerase chain reaction of the spinal fluid for the JC virus can be helpful in making this diagnosis.
Bacterial infections, including embolic or mycotic aneurysms, must be considered in this situation given the patient's history of S. aureus in the lung and the urine.
An anaerobic abscess or S. aureus abscess would be a consideration. Patients are often febrile and may appear toxic, and concurrent bacteremia may be present. Associated vascular events (stroke or hemorrhage) are common with metastatic infection. When present, a hypodense central appearance to the lesion and possible associated hemorrhage would be more suggestive. Despite the absence of intravenous drug use, S. aureus abscess, an opportunist in advanced HIV, is a potential concern in this instance.
Neurosyphilis is a bacterial infection that also must be considered in patients with HIV. HIV may accelerate the development of neurologic sequelae of syphilis, including acute syphilitic meningitis, meningovascular inflammation, and gummatous lesions. A positive rapid plasma reagin result may be higher in HIV-infected patients and is considered reliable. Microhemagglutination–Treponema pallidum and fluorescent treponemal antibody absorption tests are also usually reliable, but, as AIDS progresses, reliability may decline over time. Cerebrospinal fluid VDRL testing may be negative in >20% of patients with CNS disease. Cerebrospinal fluid abnormalities are common (protein >100, pleocytosis). CNS gummata with associated mass effect are unusual.
Mycobacterium tuberculosis also must be considered as a cause of the lesion in our patient. CNS infection can be seen in 5% to 10% of patients with HIV and tuberculosis. Fever occurs in up to 75% of patients. Meningitis is the most common CNS manifestation, although tuberculomas are not uncommon. With CNS tuberculomas, a history of extracerebral disease is usually present (66%) (5). Multiple small ring-enhancing lesions are common. Intravenous drug use as an HIV risk factor is common.
Endemic fungi must be considered. Cryptococcus neoformans is rarely associated with cryptococcoma, seizure, or focal deficit. The absence of a positive test for cryptococcal antigen in cerebrospinal fluid and serum suggests against this in our patient. Histoplasma capsulatum has a 20% incidence of CNS involvement in patients with AIDS (usually meningitis). Histoplasmomas are rare and are usually multiple, small, and ring enhancing. Coccidioides immitis is indigenous to West Texas, with meningitis being the most common manifestation. CNS Coccidioides abscesses are rare and usually accompany dissemination. The concurrent use of fluconazole (despite the relatively low dose) would be expected to reduce, to some degree, the risk of cryptococcal and coccidioidal disease in this instance. Aspergillus is an uncommon but recognized opportunist in advanced HIV (especially with neutropenia, high-dose steroids, chemotherapy, malignancy, or additional immune suppression). Pulmonary disease usually precedes CNS dissemination. Sinus disease with direct extension into the orbits or CNS may occur. Patients usually appear toxic and have poor overall performance status.
The lesion also may be parasitic. Toxoplasmosis is caused by the obligate intracellular protozoan Toxoplasma gondii, which reactivates from latent disease in immune-suppressed states. It is often multifocal with a predilection for the basal ganglia and is ring enhancing with associated edema. Some patients (14% to 27%) have a single disease focus. CT and MRI are not sufficient to distinguish it from primary CNS lymphoma. Porter et al studied 115 patients with HIV and suspected CNS toxoplasmosis (6). Four of 18 patients (22%) with biopsy-proven disease had a negative Toxoplasma serology. Chirianni et al did a prospective evaluation of 123 patients with AIDS (7). For Toxoplasma seropositive patients, the incidence of CNS toxoplasmosis was 41% at 30 months vs 1.9% in seronegative patients. Raffi et al noted that 97% of patients with CNS toxoplasmosis were seropositive by enzyme-linked immunoelectrodiffusion assay (prevalence, 74%) (8). Bands IgG27 and IgG32 by immunoblot were independently associated with increased risk of disease.
I do not think the present patient had toxoplasmosis because the Toxoplasma serology was negative and he was taking trimethoprim/sulfamethoxazole prophylaxis. He also failed to respond to empiric therapy. Luft et al reported that 86% of patients should respond by day 7 and 91% by day 14 (9). It is important to consider where these studies were conducted when interpreting the predictive value of a serology. Europe has a much higher prevalence of Toxoplasma by serology than we do, and this impacts the predictive value of the tests in that population when compared with the US population.
Finally, neoplastic disease must be considered as a cause of the CNS lesion in our patient. Primary CNS lymphoma is the most common CNS neoplasm in patients with AIDS. Systemic lymphoma will frequently involve the nervous system during its course (20% to 50%, often meningitis) and usually accompanies or follows another diagnosis. Glioma (anaplastic astrocytoma) may be increased in frequency in HIV-positive patients. Oligodendroma, ependymoma, and metastatic adenocarcinoma (lung, breast, germ cell) may occur. Primary CNS lymphoma is an opportunistic B-cell neoplasm which may express Epstein-Barr virus antigens and occurs in <5% of patients with advanced AIDS. The incidence may be modified with highly active antiretroviral therapy. Primary CNS lymphoma has a subacute presentation, with symptoms evolving over weeks. Fifty percent of the cases are diagnosed at autopsy. Radiologically, they are ringenhancing, space-occupying masses with associated edema. Although pathologically multicentric, there is often a single dominant radiologic site. Elevated cerebrospinal fluid protein is common. Cytology is usually negative (<5% positive) (10). Epstein-Barr virus polymerase chain reaction of the cerebrospinal fluid is sensitive and specific (>90%) in conjunction with CT and MRI (11).
Standard radiology cannot distinguish between Toxoplasma and primary CNS lymphoma. According to McArthur, “Neither the typographic distribution, multicentricity, nor the pattern of contrast enhancement can reliably distinguish lymphoma from toxoplasmosis” (J McArthur, personal communication).
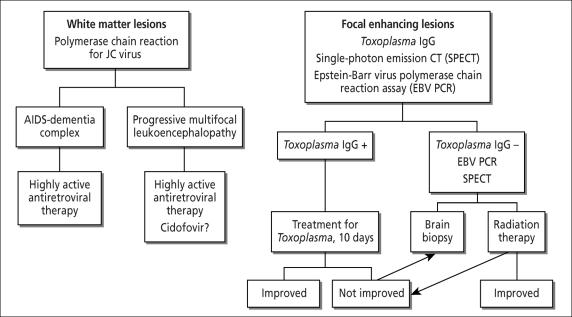
The negative thallium scan is likely the most important study in this case and may be somewhat misleading in this instance. Thallium 201 imaging has sensitivity and specificity of 90% in most studies, depending on the uptake ratios and retention indices chosen. Some argue that the Toxoplasma titer adds little to the thallium scan. Skiest et al examined 30 Dallas patients with HIV and brain lesions (12). Three patients had false-negative single-photon emission CT (SPECT) scans and were Toxoplasma antibody negative. Four patients had false-positive SPECT scans; all had Toxoplasma titers >1:512. No patient with CNS lymphoma had a Toxoplasma titer >1:128. I suggest that the algorithm in Figure 1 may be used to help determine the cause of a CNS lesion in a patient with AIDS.
Figure 1.
An algorithm to determine the cause of a central nervous system lesion in a patient with AIDS. Based on Skiest et al (12).
In my final opinion regarding this patient's diagnosis, I would like to turn to Occam's Razor, which states, “A single explanation that explains all findings is more likely to be true than multiple explanations for the same things.” I would modify that statement: Occam's Razor is true, except in patients with AIDS and those who have received bone marrow transplants. With this in mind, I would like to provide my final differential diagnosis in 2 parts. With regard to the primary process responsible for the radiologic lesion and for his clinical presentation, I think primary CNS lymphoma is most likely. Less likely, but also possible, would be another malignancy (specifically glioma), bacterial abscess, or tuberculoma. Given his extensive history, contributory processes seen on pathology may be present although not responsible for his clinical presentation. Other processes that may be present on pathology in addition to the primary process outlined above would include HIV encephalitis (likely), CMV encephalitis (possible), abscess (possible), and toxoplasmosis (unlikely).
PATHOLOGY
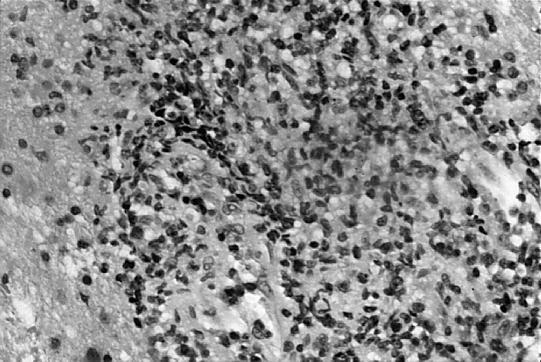
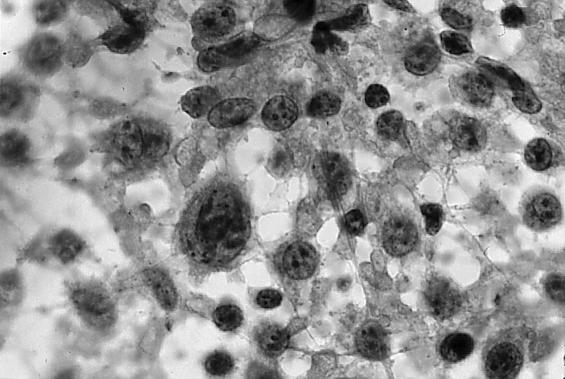
Tanner Mattison, MD: We received several fragments of tissue designated as “brain biopsy.” Histological sections showed diffusely infiltrating cells located in a perivascular pattern (Figure 2). The nuclei of these cells were larger than the nucleus of an endothelial cell. A cytologic preparation was performed to show greater nuclear detail (Figure 3). This slide showed that the cells appeared singly, a characteristic of lymphoma; sheets or clusters of cells would have been more indicative of a carcinoma. These cells were markedly enlarged and very pleomorphic, with enlarged nuclei and prominent nucleoli.
Figure 2.
Histological sections show diffusely infiltrating cells located in a perivascular pattern. The nuclei of these cells were larger than the nucleus of an endothelial cell.
Figure 3.
A cytologic preparation was performed to show greater nuclear detail. It showed that the cells appeared singly and were markedly enlarged and very pleomorphic, with enlarged nuclei and prominent nucleoli.
At this point, highest in the differential was a CNS lymphoma—based on the infiltrating pattern and the dyshesion and morphology of the cells. Somewhat less likely was an undifferentiated carcinoma or a primary CNS tumor. To delineate the etiology, a panel of immunohistochemistry stains was performed. A cytokeratin stain, which will universally stain carcinoma, was negative. A leukocyte common antigen stain showed diffuse positivity of the infiltrating cells; therefore, the cells were of leukocytic origin. A pan B-cell marker, CD20, showed strong positivity in a nodular pattern. T-cell markers CD3 and CD5 showed a reactive pattern of T lymphocytes.
The results were diagnostic of a large B-cell lymphoma. Because of the strong correlation of Epstein-Barr virus and CNS lymphomas in AIDS patients, we performed a pair of stains for Epstein-Barr virus: an immunohistochemical stain and an in situ hybridization. The less sensitive immunohistochemical stain showed scattered positivity among tumor cells while the more sensitive in situ hybridization stain was diffusely positive. Thus our patient had a large cell lymphoma with B-cell phenotype, positive for Epstein-Barr virus.
DISCUSSION
J. Todd Gage, MD: Primary CNS lymphoma is a non- Hodgkin's lymphoma that arises in and is confined to the CNS. It is a fairly rare disorder: only 800 new cases are described in the USA each year. This disease process can occur at any age but is more common in the elderly, particularly in the sixth and seventh decades of life. Primary CNS lymphoma occurs most often in HIV and AIDS populations, occurring in up to 6% of the AIDS population (13). In the immunocompetent population, the male-to-female ratio is 3:2, but in the AIDS population the ratio is 13:1 (13).
The most common presentation is a mass lesion with specific symptoms corresponding to the location of the lesion. Fifty percent of patients present with focal neurological deficits (13). The frontal lobe is the most common area of involvement; therefore, personality changes and an altered level of consciousness are common presenting symptoms.
Lesions are multifocal in 40% of immunocompetent patients. Almost 100% of the AIDS population have multiple lesions. Clinical features often include headaches and symptoms associated with increased intracranial pressure. Because primary CNS lymphoma lesions typically occur in deeper brain structures that are less seizure prone, seizures are a less common presenting symptom than they are in other CNS neoplasms. Seizure is a presenting symptom in about 10% of cases (14).
At the time of diagnosis, the eye is affected in up to 20% of cases (15). The orbit is not actually affected by primary CNS lymphoma but is a metastasis site from systemic non-Hodgkin's lymphoma. People with ocular involvement typically present with blurred vision or floaters, but they can also be clinically sound. Initially, involvement is usually unilateral, but most patients develop bilateral, asymmetric disease.
Primary leptomeningeal involvement without parenchymal brain mass is rare, accounting for 7% of cases. When symptoms occur, they can include leg weakness, urinary incontinence or retention, cranial neuropathies, confusion, or symptoms of increased intracranial pressure. Forty-two percent of patients have evidence of meningeal involvement, but at autopsy 100% have evidence of leptomeningeal involvement. Diagnosis is made by cytology on cerebrospinal fluid examination or by meningeal biopsy. Lumbar puncture invariably indicates an elevated protein, and the lymphocyte count is often >100/mm3(16). The more meningeal involvement there is, the more likely the glucose will be low. One third of patients will have low glucose.
Primary spinal cord involvement is the most rare of primary CNS lymphomas and usually presents as bilateral limb weakness. Sensory symptoms may initially follow a radicular pattern, but eventually a sensory level develops. Cerebrospinal fluid examination is usually normal but may have mildly elevated protein and a few lymphocytes.
Diagnosis of CNS lymphoma is made with MRI, lumbar puncture, and tissue pathologic examination.
MRI is the scanning technique of choice, with 90% of lesions enhancing on contrast (14). However, the lesions of immunosuppressed patients tend to be ring enhanced instead of completely enhanced, perhaps because of a higher incidence of necrosis in the center of the lesion. This makes it difficult to distinguish CNS lymphoma from infections such as toxoplasmosis.
On lumbar puncture, protein is elevated in 85% of cases, although it is rarely more than 150 mg/dL (16). Glucose is usually normal but may be low if florid meningeal involvement exists. More than half of cases have a lymphocytic pleocytosis. Positive cerebrospinal fluid cytology is uncommon but eliminates the need for brain biopsy. Elevated tumor markers—β2-microglobulin, lactic acid dehydrogenase isoenzyme, and β-glucuronidase—provide circumstantial evidence for tumor invasion of the leptomeninges.
Pathology is the gold standard of diagnosis. Most of these tumors are large cell lymphomas or diffuse large cell immunoblastic lymphomas. Macroscopically, they typically present as brown, space-occupying lesions. Histologically, they usually grow in sheets of cells, but a characteristic vasocentric growth pattern with tumor infiltrating the brain between vessels is almost universal. At autopsy, the tumor is always found in multiple regions of the brain.
Epstein-Barr virus is often detected in lymphomatous tissue. More common in AIDS patients, it also occurs in the nonimmunosuppressed. It is believed to be oncogenic. The cerebrospinal fluid polymerase chain reaction for Epstein-Barr virus may become a useful diagnostic tool for noninvasive diagnosis of primary CNS lymphoma.
Staging ensures the lesion is not a secondary non-Hodgkin's lymphoma found first in the CNS. It is suggested that cranial MRI, lumbar puncture, ophthalmologic examination with slit lamp, abdominal CT, bone marrow testing, and chest x-ray be performed.
Treatment modalities include surgery, radiation therapy, steroids, and chemotherapy.
Surgery is important for histologic diagnosis, but it prolongs survival only 3.3 to 5 months compared with 1.8 to 3.3 months with supportive therapy (17). This increase in survival is not significant enough to justify surgery.
Primary CNS lymphoma is exquisitely sensitive to corticosteroids, with 40% of cases showing significant decrease in size of the lesion when examined by MRI. The effects are secondary to direct cytotoxicity. The steroid-induced remissions are usually short lived. Standard care is still combined radiotherapy and steroids. The average survival rate with this regimen in the immunocompromised patient is 12 to 18 months. Primary treatment of ocular disease is radiotherapy of the globe.
It is recommended that chemotherapy be considered in all patients at the time of diagnosis. The optimal chemotherapy regimen in CNS lymphoma has not been determined. The agents must be either lipophilic, such as procarbazine, or given in high enough doses to penetrate the CNS (e.g., methotrexate). Agents should be given prior to radiation therapy. Adjuvant chemotherapy has not been studied adequately, so it is not recommended.
These treatment modalities are less effective and more toxic in the immunosuppressed. Although useful for short-term control of neurological symptoms, steroid use should be undertaken with care, as it can cause further immunosuppression. Chemotherapy has been used sparingly in immunosuppressed patients.
References
- 1.Holtzman DM, Kaku DA, So YT. New-onset seizures associated with human immunodeficiency virus infection: causation and clinical features in 100 cases. Am J Med. 1989;87:173–177. doi: 10.1016/s0002-9343(89)80693-x. [DOI] [PubMed] [Google Scholar]
- 2.Gildenberg PL, Gathe J, Jr, Kim JH. Results of cerebral biopsy in AIDS patients [abstract 230B] International Conference on AIDS. 1994;10:69. [Google Scholar]
- 3.Trueba F, Sivignon F, Baudrimont M, Roullet E, Chatelet F, Girard PM. CMV encephalitis in 10 AIDS patients; computed tomography (CT)/magnetic resonance imaging (MRI) study and pathologic correlation [abstract PO-B16-1760] International Conference on AIDS. 1993;9:428. [Google Scholar]
- 4.Gozlan J, el Amrani M, Baudrimont M, Costagliola D, Salord JM, Duvivier C, Picard O, Meyohas MC, Jacomet C, Schneider-Fauveau V, et al. A prospective evaluation of clinical criteria and polymerase chain reaction assay of cerebrospinal fluid for the diagnosis of cytomegalovirus-related neurological diseases during AIDS. AIDS. 1995;9:253–260. [PubMed] [Google Scholar]
- 5.Lesprit P, Zagdanski AM, de La Blanchardiere A, Rouveau M, Decazes JM, Frija J, Lagrange P, Modai J, Molina JM. Cerebral tuberculosis in patients with the acquired immunodeficiency syndrome (AIDS). Report of 6 cases and review. Medicine (Baltimore) 1997;76:423–431. doi: 10.1097/00005792-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 7.Chirianni A, Tullio CP, Orefice G, et al. Probability of developing cerebral toxoplasmosis in patients with AIDS in relation to serology for anti Toxoplasma gondii [abstract POB 3153]. International Conference on AIDS 1992;8:B112.
- 8.Raffi F, Aboulker JP, Franck J, et al. Contribution of immunoblot to diagnosis of toxoplasma encephalitis: a prospective study of 186 AIDS patients. Fourth Conference on Retro and Opportunistic Infections. 1997;26:128. [Google Scholar]
- 9.Luft BJ, Leport C, Morlat P, et al. Risk factors for development of cerebral toxoplasmosis. National Conference on Human Retrovirus Related Infections. 1993;16:139. [Google Scholar]
- 10.Bonington A, Klapper PE, Strang JK, Wilkins EG. Epstein-Barr virus (EBV) PCR of CSF: is it useful in the diagnosis of primary CNS lymphoma in HIV patients? [abstract 60496] International Conference on AIDS. 1998;12:1091. [Google Scholar]
- 11.De Luca A, Antinori A, Cingolani A, Larocca LM, Linzalone A, Ammassari A, Scerrati M, Roselli R, Tamburrini E, Ortona L. Evaluation of cerebrospinal fluid EBV-DNA and IL-10 as markers for in vivo diagnosis of AIDS- related primary central nervous system lymphoma. Br J Haematol. 1995;90:844–849. doi: 10.1111/j.1365-2141.1995.tb05205.x. [DOI] [PubMed] [Google Scholar]
- 12.Skiest D, Erdman W, Chang W, Fleckenstein J. Use of thallium-201 SPECT scanning and Toxoplasma serology for presumptive diagnosis of CNS mass lesions in patients with AIDS [abstract 60275] International Conference on AIDS. 1998;12:1049. [Google Scholar]
- 13.Fine HA, Loeffler JS. Primary central nervous system lymphoma. In: Canellos GP, Lister TA, Sklar KL, editors. The Lymphomas. Philadelphia: WB Saunders Co; 1999. pp. 481–494. [Google Scholar]
- 14.Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med. 1993;119:1093–1104. doi: 10.7326/0003-4819-119-11-199312010-00007. [DOI] [PubMed] [Google Scholar]
- 15.Herrlinger U. Primary CNS lymphoma: findings outside the brain. J Neurooncol. 1999;43:227–230. doi: 10.1023/a:1006250317940. [DOI] [PubMed] [Google Scholar]
- 16.Jellinger K, Radaskiewicz TH, Slowik F. Primary malignant lymphomas of the central nervous system in man. Acta Neuropathol Suppl (Berl) 1975;6(Suppl):95–102. doi: 10.1007/978-3-662-08456-4_16. [DOI] [PubMed] [Google Scholar]
- 17.Woodman R, Shin K, Pineo G. Primary non-Hodgkin's lymphoma of the brain. A review. Medicine (Baltimore) 1985;64:425–430. doi: 10.1097/00005792-198511000-00006. [DOI] [PubMed] [Google Scholar]