Jaw joint (temporomandibular joint or TMJ) disease is estimated to affect 30 million Americans, with approximately 1 million new patients diagnosed each year (1). Although many of these patients can be managed with nonsurgical therapies, some patients require surgical intervention. The TMJ is a unique joint in that it does not function independently but works in tandem with its contralateral joint. Therefore, disease affecting 1 joint can either directly or indirectly affect the functioning and health of the contralateral joint. When surgical intervention of the TMJ is required, the joint can often be reconstructed with autogenous tissues.
However, certain TMJ conditions and pathology require reconstruction with a total joint prosthesis for predictable treatment outcomes. Some of these conditions include ≥2 previous TMJ surgeries; previous TMJ alloplastic implants containing Proplast/ Teflon (PT), Silastic, acrylic, or bone cements; inflammatory or resorptive TMJ pathology; connective tissue or autoimmune disease (i.e., rheumatoid arthritis, psoriatic arthritis, scleroderma, Sjögren's syndrome, lupus, and ankylosing spondylitis); fibrous or bony ankylosis; absence of TMJ structures due to pathology, trauma, or congenital deformity; and tumors involving the condyle and mandibular ramus area.
Currently, the only TMJ total joint prosthesis approved by the Food and Drug Administration (FDA) is the custom-made device manufactured by TMJ Concepts, Inc. (Camarillo, Calif). The device was manufactured by the same company under the name Techmedica, Inc. from 1989 to 1993.
HISTORICAL CONSIDERATIONS
Although the use of some form of alloplastic TMJ prosthesis dates back to the early 1960s, it was not until the 1980s that TMJ prostheses became popular with the introduction of the Vitek- Kent prosthesis (Vitek, Inc., Houston, Tex) (1, 2). Many other companies subsequently introduced their own TMJ prostheses. The Vitek-Kent prosthesis was the most popular prosthesis used in the 1980s and early 1990s, but PT was one of its main materials.
Early reports on implants with PT were very encouraging, with claims of 91% of 6182 patients having satisfactory results (3). However, continued clinical and radiographic follow-up revealed that most patients developed increasingly severe pain, condylar resorption, malocclusion, and a proliferative foreign body giant cell reaction (FBGCR) to the PT implants (4, 5). The PT implants disintegrated with fragmentation and particularization, creating the FBGCR, which results in severe destruction of local soft and hard tissues (4). This FBGCR continues despite removal of the PT implants and repeated aggressive surgical debridement (3, 4). Numerous clinical complications were reported in the literature, including perforation of the PT implants, unstable occlusion, facial disfigurement, lymphadenopathy, severe osteoarthritis, perforation into the middle cranial fossa, severe pain, headaches, and a multitude of systemic problems such as immunological dysfunction and malnutrition (2). This led the FDA to stop production of TMJ total joint prostheses in 1993.
The only total joint prostheses that were still commercially available were the Christensen and Morgan devices. Although these devices were not FDA approved, they were still available to practitioners as “grandfathered” devices, since they fell into the FDA preamendment of 1976. Both of these devices contained articulating surfaces that are not used in FDA-approved orthopaedic total joint devices because of poor wear properties and subsequent particularization. Another problem with these “off-the-shelf” prosthetic devices was the lack of fit for individual patients. The FDA has recently taken the mandibular components of the Christensen device off the market. The TMJ Concepts total joint prosthesis was granted FDA approval in June 1997.
TECHNICAL CONSIDERATIONS
For a TMJ total joint prosthesis to be successful, the following structural and functional characteristics should be met: 1) biocompatible and functionally compatible materials; 2) low wear, flow, and fatigue coefficients of articulating materials; 3) close adaptability to anatomic structures and function; 4) rigidly stabilized components; 5) corrosion resistant, nonfragmenting, and nontoxic materials; 6) low incidence of hypersensitivity; 7) posterior stop in the fossa component; and 8) close tolerance of the screw and prosthesis hole diameter (6). A prosthesis that meets these criteria is extremely important in the long-term successful outcome of the reconstructive process. The TMJ Concepts custom-made total joint prosthesis was the first TMJ prosthesis that used materials that are well proven in orthopaedics for joint replacement.
The TMJ Concepts total joint prosthesis has 2 basic components: a fossa component and a mandibular component.
The fossa component is made of 2 basic materials. A custom-fitted, commercially pure wrought titanium (CPT) shell conforms to the anatomic contours of the glenoid fossa. This shell is firmly bonded on both sides with 4 layers of CPT mesh. The CPT mesh allows bone and soft tissue ingrowth for long-term stabilization to the glenoid fossa. The articulating surface of the fossa component is made of dense ultra-high-molecular-weight polyethylene (UHMWP) that is bonded to the CPT mesh.
The mandibular component of the prosthesis is also constructed from 2 basic materials. The shaft is made of wrought titanium alloy containing 90% titanium, 6% aluminum, and 4% vanadium. This alloy is extremely hard, very biocompatible, and bend resistant. The articulating surface of the mandibular component is made of a wrought chrome-cobaltmolybdenum alloy that contains approximately 64% cobalt, 28% chromium, 6% molybdenum, and 2% trace elements (nickel, iron, carbon, silicon, manganese, and nitrogen). The functioning surfaces of this prosthesis have low wear, flow, and fatigue coefficients.
PREOPERATIVE CONSIDERATIONS
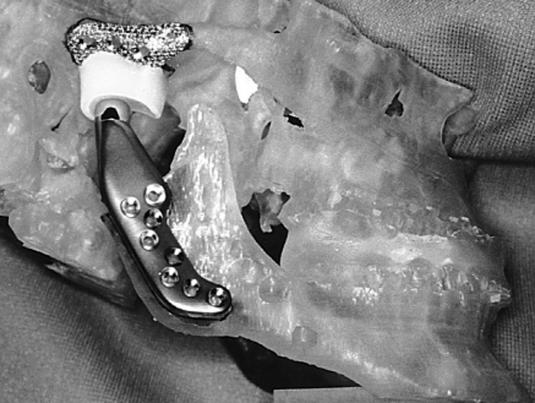
Initially, a preoperative computed tomography (CT) scan of the jaws and jaw joints is obtained using a specific protocol. Using the CT data, a 3- dimensional plastic model of the TMJ and associated jaw structures is made using stereolithographic technology. The mandible is spatially repositioned on the model to correct the functional and aesthetic malalignment problems. The condyle is removed, and any necessary bony recontouring of the fossa and mandibular ramus is completed and marked on the plastic model, since all the alterations on the model must be accurately duplicated on the patient intraoperatively. A custom-made total joint prosthesis conforming to the patient's specific anatomical morphology and jaw interrelationships is then fabricated on the plastic model by the TMJ Concepts engineers working in close collaboration with the surgeon (Figure 1).
Figure 1.
A 3-dimensional plastic model of the TMJs and jaws is manufactured using stereolithographic technology. The TMJ Concepts custom-made total joint prosthesis is constructed on the model.
Many of these complex TMJ patients have associated jaw and facial deformities. Use of a custom-made joint prosthesis allows correction of the facial deformity and reconstruction of the TMJs during the same operation. The surgeon must be able to accurately reposition the mandible on the 3-dimensional plastic model based on preoperative cephalometric surgical treatment objectives (Figure 4b. In these cases, the plastic model, with the mandible placed in its new position, is used to fabricate the custom-made prosthesis as previously described. Prior to surgery, all mandibular movements performed on the plastic model are accurately duplicated on anatomically mounted dental plaster models, from which an intermediate acrylic occlusal splint is constructed for accurate intraoperative repositioning of the mandible.
Figure 4.
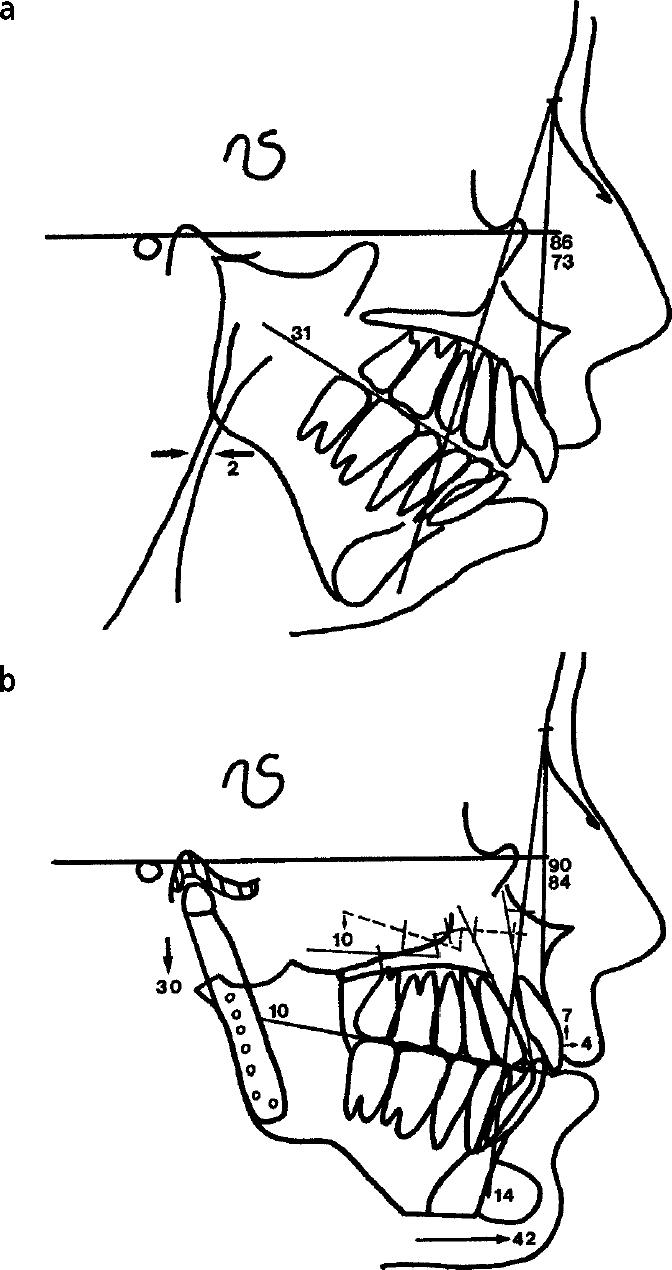
A tracing of the patient's presurgical lateral cephalometric radiograph demonstrates the severity of her jaw and facial deformity. Note the severely decreased oropharyngeal airway of 2 mm (normal dimension is 12 mm). (b) The surgical treatment objective demonstrates the planned surgical movements of the jaws and soft tissue structures.
SURGICAL CONSIDERATIONS
The TMJ is approached via an endaural or preauricular incision, and the mandibular ramus is approached via a submandibular incision. Condylectomy, debridement, and bone recontouring are accomplished as previously determined on the plastic model. Intermaxillary fixation (wiring of the upper and lower jaws together) with the intermediate splint in place is then performed. The fossa component of the prosthesis is inserted through the endaural/ preauricular incision and is stabilized to the zygomatic arch with three to four 2-mm-diameter screws. The mandibular component is inserted via the submandibular incision and fixated to the lateral surface of the ramus with eight to ten 2-mm-diameter screws. Autogenous fat grafts, harvested from the abdomen or buttocks, are packed around the joint prosthesis to prevent postsurgical fibrosis and reactive/heterotropic bone formation (7). Surgical repositioning of the maxilla and other indicated treatment are then performed using standard techniques. At completion of surgery, the intermaxillary fixation is removed and active jaw function is encouraged.
CASE PRESENTATION
A 15-year-old girl with juvenile rheumatoid arthritis had severe destruction of the TMJs, resulting in a facial deformity (Figure 2a, 2b), progressively worsening mandibular retrusion (Figure 3a), and severe sleep apnea symptoms secondary to airway obstruction (Figure 4a). Clinical examination also showed an accompanying excessive vertical growth of the anterior maxilla, resulting in a “gummy smile.” The patient was treated in 1 operation by using the following steps:
Bilateral TMJ reconstruction and mandibular advancement (28 mm) were accomplished using the TMJ Concepts total joint prostheses. Vertical height of the ramus was lengthened 30 mm (Figure 4b).
Multiple maxillary osteotomies were performed with bone plate stabilization and synthetic bone grafting to reposition the anterior maxilla upward (7 mm) and the posterior maxilla downward (10 mm) (Figure 4b).
A 14-mm porous block hydroxyapatite implant was used to augment the chin. The chin point came forward 42 mm as a result of the mandibular advancement and chin augmentation procedures (Figure 4b).
Fat grafts were harvested from the abdomen and placed around the bilateral TMJ prostheses.
Figure 2.
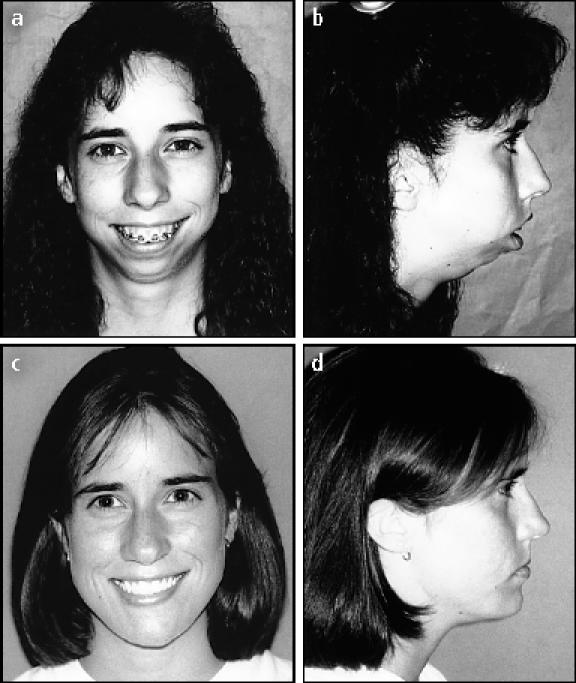
(a, b) A 15-year-old patient is seen with an extensive facial deformity as a result of severe juvenile rheumatoid arthritis. (c, d) The patient is seen 7 years after reconstructive surgery. The TMJ Concepts total joint prosthesis was used to reconstruct the TMJs, advance the mandible (28 mm), and vertically lengthen the ramus (30 mm).
Figure 3.
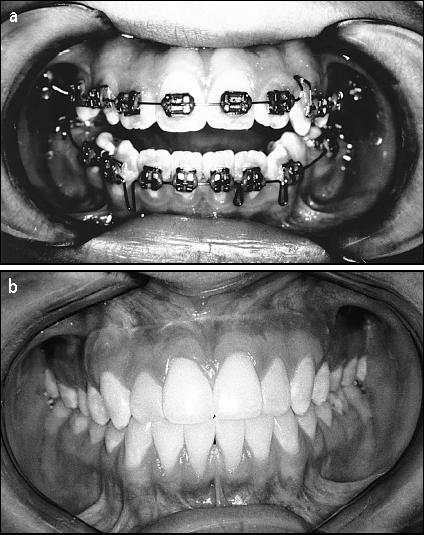
(a) Presurgery, the patient had a very severe bite problem. (b) Seven years after surgery, her bite remains very stable.
Seven years postoperatively the patient demonstrates excellent long-term stability of the TMJ and jaw reconstruction (Figures 2c, 2d, 3b). She is pain free and has a jaw opening of 40 mm. The severe sleep apnea has been completely eliminated.
DISCUSSION
Although the longevity of the TMJ Concepts total joint prosthesis is yet unknown, our clinical experience over the past 10 years shows promising long-term results. Based on material selection and treatment philosophy, it is believed that these devices will provide service life comparable with or longer than that of hip stem devices.
Wolford et al in 1994 reported on 100 reconstructed TMJs in 56 patients using the TMJ Concepts (Techmedica) total joint prosthesis, with an average follow-up of 21/2 years (6). The outcomes were categorized as good, fair, or poor based on clinical and radiographic analysis of stability, function, and pain. They reported that 63% of the patients had good outcomes, 26% had fair outcomes, and 16% had poor outcomes, with irresolvable pain being the prime reason for the poor outcomes. Analyzing a subgroup of those patients who had ≤1 previous TMJ surgery showed that 84% had good outcomes, 16% had fair outcomes, and no patients had poor outcomes. Patients with ≥2 previous TMJ surgeries had relatively poorer outcomes, thereby reinforcing the fact that a greater number of TMJ surgeries results in a higher chance of poor outcomes, mainly due to an inability to reduce postoperative pain levels. Mercuri and Wolford et al in 1995 reported a multicenter study on 215 multiply operated TMJ patients (363 joints) reconstructed with the TMJ Concepts prosthesis (8). The results at 2 years postoperatively showed statistically significant favorable changes in many subjective and objective evaluations, including decrease in pain by 49%, improvement in jaw function by 43%, improvement in dietary intake by 50%, and increase in maximum jaw opening by 31%.
In 1997, Wolford et al presented a 5-year follow-up study on 36 patients with 65 TMJs reconstructed with the TMJ Concepts total joint prosthesis (9). The overall success rate for long-term occlusal and skeletal stability after reconstruction was 90%, and pain reduction was recorded in 89% of patients. This study resulted in FDA approval of the TMJ Concepts device for use as a total joint TMJ replacement prosthesis.
In 1997, Wolford and Karras reported on a technique they developed in which fat grafts harvested from the abdomen were packed around the total joint prosthesis (7). This technique has significantly decreased the postoperative incidence of peri-implant fibrosis, reankylosis, and heterotropic/reactive bone formation. Patients with fat grafts were shown to do better clinically with less pain and increased jaw function compared with similar patients with reconstructions without fat grafts. Prior to the use of fat grafts, approximately 35% of patients required additional surgery to remove heterotropic/reactive bone and dense fibrosis from around the prosthesis. Since developing the fat grafting technique, we have not reoperated on any patient for heterotropic/reactive bone formation in the past 71/2 years.
CONCLUSIONS
Long-term success rates for autogenous reconstruction of the TMJ drop considerably in the multiply operated complex TMJ patient (≥2 previous surgeries), especially for patients with a history of previous alloplastic implants containing PT and Silastic. Many such patients have progressively worsening jaw function, dentofacial deformities, severe chronic pain problems, and other associated systemic, nutritional, and immunological illnesses that cause extreme disability. These patients—and also those with inflammatory diseases, connective tissue/autoimmune diseases, ankylosis, tumors, or absence of TMJ structures—may have the best opportunity of successful treatment with a total joint prosthesis. The only FDA-approved device for total joint TMJ reconstruction is the TMJ Concepts total joint prosthesis. The use of this custom-made prosthesis, made with orthopaedically proven structural materials, in combination with autogenous peri-implant fat grafting has significantly improved the predictability and success rates of treatment for the rehabilitation of complex TMJ patients.
References
- 1.Baird DN, Rea WJ. The temporomandibular joint implant controversy: a review of autogenous/alloplastic materials and their complications. Journal of Nutrition and Environmental Medicine. 1998;8:289–300. [Google Scholar]
- 2.Wolford LM. Temporomandibular joint devices: treatment factors and outcomes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:143–149. doi: 10.1016/s1079-2104(97)90105-0. [DOI] [PubMed] [Google Scholar]
- 3.Vitek, Inc . Survey of TMJ Surgical Results. Houston: Vitek, Inc; 1986. [Google Scholar]
- 4.Henry CH, Wolford LM. Treatment outcomes for temporomandibular joint reconstruction after Proplast-Teflon implant failure. J Oral Maxillofac Surg. 1993;51:352–358. doi: 10.1016/s0278-2391(10)80343-x. [DOI] [PubMed] [Google Scholar]
- 5.Mosby EL, Wagner JD, Robinson RC. Assessment of Teflon-Proplast implants in temporomandibular joint reconstruction following meniscectomy. J Oral Maxillofac Surg. 1986;44(Suppl):13. [Google Scholar]
- 6.Wolford LM, Cottrell DA, Henry CH. Temporomandibular joint reconstruction of the complex patient with the Techmedica custom-made total joint prosthesis. J Oral Maxillofac Surg. 1994;52:2–10. doi: 10.1016/0278-2391(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 7.Wolford LM, Karras SC. Autologous fat transplantation around temporomandibular joint total joint prostheses: preliminary treatment outcomes. J Oral Maxillofac Surg. 1997;55:245–251. doi: 10.1016/s0278-2391(97)90535-8. [DOI] [PubMed] [Google Scholar]
- 8.Mercuri LG, Wolford LM, Sanders B, White RD, Hurder A, Henderson W. Custom CAD/CAM total temporomandibular joint reconstruction system: preliminary multicenter report. J Oral Maxillofac Surg. 1995;53:106–115. doi: 10.1016/0278-2391(95)90381-x. [DOI] [PubMed] [Google Scholar]
- 9.Wolford LM, Riechel O, Franco PF. 5-year follow-up study on the Techmedica custom-made total joint prostheses. J Oral Maxillofac Surg. 1997;55:110–111. [Google Scholar]