Abstract
Bone morphogenetic proteins (BMPs) play important roles at multiple stages of endochondral bone formation. However, the roles of BMP signaling in chondrocytes in vivo are still contentious. In the present study, we overexpressed a constitutively active BMP receptor 1A (caBmpr1a) in chondrocytes by using two systems: caBmpr1a was directly driven by a rat type II collagen promoter in a conventional transgenic system and indirectly driven in a UAS-Gal4 binary system. CaBmpr1a expression caused shortening of the columnar layer of proliferating chondrocytes and up-regulation of maturation markers, suggesting acceleration of differentiation of proliferating chondrocytes toward hypertrophic chondrocytes. In addition to the acceleration of chondrocyte differentiation, conventional transgenic mice showed widening of cartilage elements and morphological alteration of perichondrial cells, possibly due to stimulation of differentiation of prechondrogenic cells. Moreover, bigenic expression of caBmpr1a rescued the differentiation defect of prechondrogenic cells in Bmpr1b-null phalanges. This finding indicates that BMP signaling is necessary for phalangeal prechondrogenic cells to differentiate into chondrocytes and that signaling of BMP receptor 1B in this context is replaceable by that of a constitutively active BMP receptor 1A. These results suggest that BMP signaling in prechondrogenic cells and in growth plate chondrocytes stimulates their chondrocytic differentiation and maturation toward hypertrophy, respectively.
Keywords: bone morphogenetic protein receptor 1A, bone morphogenetic protein receptor 1B, bone morphogenetic protein, chondrocyte differentiation, transgenic
Bone morphogenetic proteins (BMPs) play critical roles at various stages of skeletal development (1, 2). BMPs and their receptors are present in chondrocytes and adjacent perichondrium (3–6) and seem to affect the differentiation of chondrocytes. However, the effects of stimulation of BMP signaling on differentiation of growth-plate chondrocytes are diverse and depend on the model under study. Studies using cell culture systems have shown that BMPs generally stimulate hypertrophic differentiation of chondrocytes (7–12). Studies analyzing the addition of different BMPs to bone explants have yielded variable results, including stimulation of hypertrophy (13), delay in hypertrophy (14), or delay in conversion of early hypertrophic chondrocytes to late hypertrophic chondrocytes (15). Retroviral expression of BMPs, constitutively active or dominant negative BMP receptors, or a BMP inhibitor in developing chicken limbs has shown that increased or decreased BMP action causes abnormal cartilage formation (16–19). In these chicken/retroviral systems, stimulation of BMP signaling generally causes irregular expansion of cartilaginous elements. Overexpression of a constitutively active BMP receptor 1A (caBmpr1a) was reported to inhibit hypertrophic differentiation of chondrocytes (19). Effects of BMPs on the skeletal system also have been studied by using transgenic mice. Mice overexpressing BMP ligands GDF-5 or BMP-4 show an increase in the cartilage mass and expanded hypertrophic regions (20, 21). However, in these models, it is not possible to know the effect of increased BMP activity in chondrocytes, per se, because ligand overexpression likely activates BMP signaling in multiple types of cells. To obtain mice with increased BMP signaling more specific to chondrocytes, we generated transgenic mice overexpressing caBmpr1a in the cartilage using the conventional transgenic method and a UAS-Gal4 bigenic system (22–24). Although these mice show consistent findings on late differentiation of chondrocytes, the two different strategies for overexpressing caBmpr1a in chondrocytes led to substantial differences in early cartilage formation between the models, a result that suggests that the consequences of caBmpr1a expression differ at multiple stages of cartilage development.
Materials and Methods
DNA Constructs and Transgenic Mouse Generation. The cDNA encoding a constitutively active mutant of human BMP receptor-1A (caBmpr1a) has been described (19). The caBmpr1a cDNA released from a viral expression vector RCAS(A) (19) was inserted into the EcoRV site of the UAS expression vector pWEXP3C (23). The prokaryotic sequence was removed by digestion with SalI, and the purified DNA fragment was injected into pronuclei of B6CBAF1/J (C57/6J × CBA/J) zygotes as described (25).
For generation of conventional transgenic embryos carrying the Col2-caBmpr1a transgene, the caBmpr1A cDNA was inserted into the blunt-ended BamHI site of the collagen II expression vector (26). DNA was linearized and subjected to pronuclear injection. Embryos were recovered from host mothers at the indicated ages. The age of transgenic embryos was determined by the duration of the host mother's pregnancy.
Mice. Col2-Gal4 mice have been described (24). UAS-caBmpr1A embryos and mice were genotyped by PCR by using primer 5′-ACG CTT GCC AAG ATG GTT GA-3′ and a primer for the LacZ tag sequence contained in pWEXP3C, 5′-CGG TCA GAC GAT TCA TTG GCA CCAT-3′. Bmpr1b-null mice have been described (6).
Skeletal Preparation and Histology. Alizarin red and/or alcian blue staining was modified after McLeod's method (27). Carcasses were fixed in 95% ethanol, stained with alcian blue and/or alizarin red, cleared in 1% KOH, and kept in 50% glycerol. Embryos were fixed in 4% paraformaldehyde/PBS at 4°C overnight, dissected, processed, and embedded in paraffin. Sections were cut with 5-μm thickness, subjected to hematoxylin/eosin staining, in situ hybridization, and immunostaining.
In Situ Hybridization. In situ hybridization on paraffin sections with radiolabeled antisense RNA probes was performed on paraformaldehyde-fixed tissues as described (28). Probes for Col10a1 (B. Olsen, Harvard Medical School, Boston), Bmpr1b (L. Niswander, Memorial Sloan–Kettering Cancer Center, New York), Noggin (R. Harland, University of California, Berkeley), Sox9 (V. Lefebvre, M.D. Cleveland Clinic, Cleveland), and Cspg2 (29) were labeled by using [35S]UTP. A 1.3-kb Gal4 cDNA fragment was PCR amplified by using primers 5′-AGATGCCGTCACAGATAGATTGGCTTC-3′ and 5′-GGTCTCGTTATTCTCAGCATTCGATT-3′.
Immunohistochemistry. Embryos were fixed in 4% paraformaldehyde for 8 h and transferred to 70% ethanol until paraffin processing. Antibodies against Phospho-Smad-1/5/8 were purchased from Cell Signaling Technology (Beverly, MA). Staining was performed by using the Immunocruz staining system (Santa Cruz Biotechnology) with DAB or TMB as the chromogen (Vector Laboratories) according to the manufacturer's instructions.
Results
Targeted Expression of caBmpr1a in Chondrocytes Using the UAS-Gal4 Bigenic System. Preliminary experiments suggested that expression of caBmpr1a in the murine growth plate using a type II collagen promoter resulted in embryonic lethality, preventing the establishment of transgenic lines by using conventional methodology (L. Niswander, personal communication). To circumvent this problem, we adapted the UAS-Gal4 bigenic system to establish lines. In this system, chondrocytic expression of GAL4 derived from one transgenic parent transactivates a UAS-caBmpr1a transgene from the other parent in doubly transgenic Col2-Gal4:UAS-ca Bmpr1a embryos (bigenic mice) (Fig. 1A). In situ hybridization showed that Gal4 mRNA in Col2-Gal4 mice was exclusively expressed in chondrocytes in embryonic day (E) 13.5 paws and in E17.5 tibiae (Fig. 1B and data not shown). UAS-ca Bmpr1a single transgenic mice of all lines established were phenotypically indistinguishable from wild-type mice. Because the Gal4 transgenic mice themselves had slight shortening of bones, Gal4 single transgenic littermates were used as controls for comparison with Col2-Gal4:UAS-ca Bmpr1a bigenic mice. Of eight founder lines, three lines were selected according to the phenotypes upon cross-mating with Gal4 transgenic mice. All of the lines showed similar gross skeletal phenotypes after crossing with Col2-Gal4 mice. One of the lines was primarily used in the following experiments, and the observations were confirmed in other lines. The effect of caBmpr1a overexpression was determined by immunostaining for phospho-Smad1. In the growth plate of E17.5 tibia of Col2-Gal4:UAS-caBmpr1a bigenic mice, phospho-Smad1 was detected throughout the growth plate, whereas phospho-Smad1 immunoreactivity was principally limited to the prehypertrophic region in control mice (Fig. 1C).
Fig. 1.
Chondrocyte-specific expression of caBmpr1a by the bigenic system. (A) Schematic representations of Col2-Gal4 and UAS-caBmpr1a. Gal4 is driven by a rat type II collagen promoter. Constitutively active mutant cDNA for human BMPR1A (caBmpr1a) is placed downstream of GALl4 responsive UAS sequences and a minimal promoter from the Wnt1 gene. (B) Gal4 mRNA expression in an E13.5 forelimb of the Col2-Gal4 transgenic mouse. The expression is limited to chondrocytes. Gal4 is not expressed in the perichondrium at a detectable level by in situ hybridization. (C) Phospho-Smad1 immunoreactivity in the tibial growth plate of control (Col2-Gal4, Ctrl) and the bigenic (Col2-Gal4:UAS-Bmpr1a, Bg) E17.5 mice. Phospho-Smad1 is predominantly present in the prehypertrophic region in control growth plates, whereas it is diffusely expressed in proliferating chondrocytes in bigenic mice. Staining of the mineralized bone collar is nonspecific.
Overexpression of caBmpr1a in the Growth Plate Causes Perinatal Death and Shortening of Long Bones and Growth Plates. Bigenic mice died a few hours after birth. Milk was never found in the stomach, suggesting a defect in suckling (Fig. 2A). Consistent with this finding, mutant mice had cleft palates (Fig. 2C). Bigenic embryos exhibited shortening of the limbs, tail, and snout (Fig. 2 A and B). Histological analysis of E17.5 embryonic limbs demonstrated that bigenic mice had relatively well organized growth plates, except that the columnar chondrocyte layer was shortened (Fig. 2 D and E). We did not find apparent changes in levels or expression patterns of Col10a1 (Fig. 2F), Col2a1, Ihh, Pthlh (PTHrP), Pthr1 (PTH/PTHrP receptor), Bmp2, Bmp6, Gdf5, Noggin, Chrd (Chordin) or Grem1 (Gremlin) (data not shown). One of the UAS-caBmpr1a lines had fusion of some joints, such as elbow and proximal interphalangeal joints (see Fig. 6B for interphalangeal joints). Other skeletal abnormalities were similar among three lines. We did not find significant differences in rates of proliferation, determined by BrdUrd labeling index, between bigenic mice and controls at E16.5, E17.5, or E18.5 (see Supporting Methods and Fig. 7, which are published as supporting information on the PNAS web site).
Fig. 2.
Skeletal abnormalities of bigenic mice. (A) Bigenic pups exhibit shortening of the limb, tail, and snout at postnatal day (P) 0.5. Milk spots are not seen in bigenic mice (arrows). (B) Skeletal preparation shows shortening of mineralized elements of long bones in bigenic mice. (C) Frontal sections of the head show the presence of cleft palates in bigenic mice, whereas secondary shelves of the palate are already fused in controls (arrows). (D) Shortening of long bones of bigenic mice. The femur of bigenic embryos at E17.5 is shortened with shortening of growth plates. (E) Histological analysis of the distal femoral growth plate shows shortening of the columnar layer (double-head arrows) in bigenic mice. (F) In situ hybridization for Col10a1 mRNA shows that the hypertrophic layer in bigenic mice is located nearer to the articular surface than in control mice. Ctrl, control mice; Bg, bigenic mice.
Fig. 6.
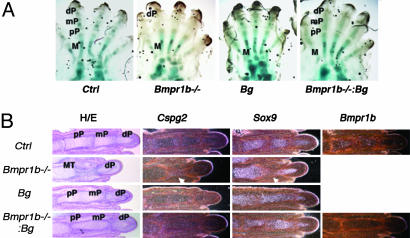
Rescue of the digit defect of Bmpr1b–/– mice by chondrocytic expression of caBmpr1a. (A) Whole-mount alcian blue staining of E17.5 hind paws. Bmpr1b–/– mice fail to form proximal and middle phalanges. Bigenic expression caBmpr1a restores phalangeal formation in Bmpr1b–/– mice. (B) Histological analysis shows that prospective Bmpr1b–/– proximal and middle phalanges remain rudimentary and express higher levels of Cspg2 and lower levels of Sox9. Digits of compound mutants, Bmpr1b–/–:Bg were essentially the same as those in Bg mice. The absence of Bmpr1b transcripts in rescued mice was confirmed by in situ hybridization. In this UAS-caBmpr1a line, proximal interphalangeal joints are fused. Ctrl, wild-type control; Bg, bigenic mice (Col2-Gal4:UAS-caBmpr1a); M, metatarsal; pP, proximal phalange; mP, middle phalange; dP, distal phalange. Arrows indicate the site of the missing mP digit in the Bmpr1b–/– mice.
Conventional Transgenic Mice and Bigenic Mice Develop Different Cartilage Abnormalities. Although bigenic mice showed a lethal skeletal phenotype with clear activation of BMP signaling, we were unable to detect apparent changes in levels of expression of specific molecular markers of differentiation or in BrdUrd labeling assays (noted above). It is possible that expression of caBmpr1a using the bigenic system was not robust enough to cause detectable changes in gene expression or in proliferation. To obtain mice with different expression levels of the caBmpr1a transgene in chondrocytes, we generated transgenic embryos using a conventional construct in which the same caBmpr1a gene was directly driven by the same type II collagen promoter that drove Gal4 in the bigenic system (Fig. 3A). One-cell-stage embryos injected with the linearized construct were returned to host mothers and grown to the ages of E13.5 and E17.5.
Fig. 3.
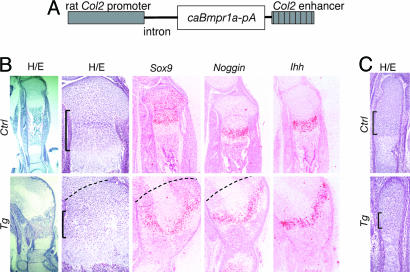
A conventional transgenic embryo at E17.5. (A) CaBmpr1a is directly driven by the same rat collagen type II promoter used in Gal4 transgenic mice. (B) Tibial growth plates of the transgenic mouse (Tg) and a wild-type littermate (Ctrl). Low and high power views of hematoxylin/eosin (H&E) staining, and in situ hybridization for Sox9, Noggin, and Ihh are shown. The transgenic tibial growth plate is enlarged in width and fused with the patella and distal femoral growth plate. Dashed lines indicate putative borders between the tibia and femur. The columnar layer is indicated by brackets. Despite extensive deformity of the cartilage, expression patterns of Sox9, Noggin and Ihh are preserved in the transgenic mouse. Cells in translucent areas in the center of transgenic growth plates do not express chondrocyte markers (data not shown). (C) H&E stained sections of metatarsal growth plates of the transgenic mouse (Tg) and a wild-type littermate (Ctrl). Brackets indicate the columnar layers.
Transgenic embryos that had skeletal phenotypes showed increased phospho-Smad1 immunoreactivity throughout the cartilage, indicating activation of the BMP signaling pathway (data not shown). We found many dead embryos at E17.5, consistent with early embryonic lethality of transgenic mice, as suggested by the previous preliminary experiments. An E17.5 transgenic embryo showed severe skeletal abnormalities; the femur, tibia and patella were fused together, eliminating joint tissues (Fig. 3B). The growth plate cartilage was enlarged in width. However, this widening was not associated with lengthening of the growth plate. The centers of the growth plates contained hypocellular regions that did not express any chondrocyte markers by in situ hybridization (Fig. 3B and data not shown), suggesting quiescence or death of chondrocytes in this region. The columnar layer was shortened in the tibia (Fig. 3B). In metatarsal bones in which bone shapes were relatively preserved, the columnar layer was also shortened (Fig. 3C). Despite these morphological changes, there were not remarkable alterations in expression patterns of molecular markers by in situ hybridization (Fig. 3B); both Sox9 and Noggin mRNAs were diffusely expressed in proliferating chondrocytes and up-regulated in prehypertrophic chondrocytes. Expression patterns of Ihh (Fig. 3B), Pthr1, and Col10a1 mRNAs were preserved (data not shown).
To better understand the cause of the differing phenotypes between bigenic and conventional transgenic mice, we examined embryos at E13.5, an earlier developmental stage. Of six transgenic embryos, three had normal morphology and three had similar cartilage abnormalities. Limb cartilage anlages in transgenic embryos showed irregular borders between the cartilage and surrounding tissues (Fig. 4A). Perichondrial cells in transgenic mice were not organized in flat layers and were wider than those in control (Fig. 5B). Phospho-Smad1/5/8 immunostaining on transgenic bones showed increased BMP signaling in chondrocytes as well as in perichondrial cells with morphological changes (Fig. 4C). These morphological changes of perichondrial cells were also seen in other bones, including basiooccipital bones. The cartilage of basiooccipital and hyoid bones of E13.5 transgenic embryos was irregularly expanded. The surface of the mutant cartilage was irregular, and small clumps of differentiated chondrocytes often protruded in the perichondrial region of the basiooccipital bone (Fig. 4D). Perichondrial cells in transgenic basiooccipital bones were not aligned in orderly layers as in controls. These cells were also spindle-shaped or oval in morphology, different from the typical flat-shaped normal perichondrial cells; this result might suggest differentiation of these cells into chondrocytes (Fig. 5E), although Sox9 mRNA was expressed only weakly in these cells (Fig. 4F). The hyoid body in mutant mice was around three times larger than that of control, whereas there was no apparent change in the proliferation rate determined by BrdUrd labeling (data not shown). This result suggests that the expansion of the chondrocyte mass was not caused by an increase in proliferation of chondrocytes, per se, but instead by increased recruitment of cells into the initial chondrogenic condensation and/or stimulation of differentiation of perichondrial cells into chondrocytes. These changes in perichondrial cells and in the cartilage size were not observed in bigenic mice (Fig. 4B).
Fig. 4.
Morphological changes of perichondrial cells of E13.5 transgenic (Tg) and control (Ctrl) mice. (A) Alcian blue-stained cartilage anlages of the tibia (Tib) and fibula show irregular bone shapes in transgenic mice. (B) Magnified views of boxed areas in A show disorganized perichondrial layers. Perichondrial layers of the tibia from E13.5 bigenic mice (Bg) appear normal. (C) Phospho-Smad 1/5/8 immunostaining shows increased BMP signaling in perichondrial cells with morphological changes of the transgenic tibia. (D) Alcian blue-stained mid-saggital sections show irregularly enlarged hyoid (red arrows), basiooccipital, and vertebral bones of transgenic mice. Small clumps of chondrocytes protrude into the perichondrial region of transgenic mice (black arrows). (E) Magnified views of parts of H&E-stained basiooccipital perichondria corresponding to boxed areas of adjacent sections shown in D. Layers of the perichondrium are disorganized, and perichondrial cells show spindle or oval cell shapes in transgenic mice. PC, perichondrium; C, cartilage; PCo, outer layers of the perichondrium; PCi, inner layers of the perichondrium. (F) Sox9 is weakly expressed in perichondrial cells of transgenic mice.
Fig. 5.
Acceleration of chondrocyte maturation of E13.5 transgenic vertebrae. Saggital sections of conventional transgenic (Tg) and control (Ctrl) vertebrae were subjected to in situ hybridization analysis. A hypertrophic marker, Col10a1 is not expressed in the vertebral bones of either of the mice. Sox9 and Noggin are up-regulated in transgenic vertebrae, suggesting that transgenic chondrocytes are in more advanced stages of chondrocyte maturation. Dotted lines outline cartilage tissues.
Chondrocyte differentiation in early vertebrae of conventional transgenic mice was assessed by in situ hybridization (Fig. 5). A marker of hypertrophic chondrocytes, Col10a1 mRNA, was expressed in basiooccipital bones but not in vertebral bones in both wild-type and transgenic mice. Sox9 and Noggin mRNAs were expressed at greater levels in mutant mice compared with control mice. Because expression levels of Sox9 and Noggin mRNAs increase as proliferating chondrocytes differentiate toward prehypertrophic chondrocytes, this finding suggests that transgenic chondrocytes are in an advanced stage of chondrocyte differentiation.
Bigenic Expression of caBmpr1a in Chondrocytic Cells Reverses the Digit Defect of Bmpr1b–/– Mice. Despite widespread expression of Bmpr1b in the skeletal system, Bmpr1b–/– mice show bone abnormalities only in the digits; the proximal and middle phalanges fail to form, and these prospective phalangeal regions remain rudimentary (3, 6). At E17.5, Bmpr1b–/– cells in this region morphologically resembled those in mesenchymal condensations (data not shown). Compared with chondrocytes in the growth plate, these cells expressed lower levels of Sox9 and higher levels of Cspg2 (Versican), a marker of prechondrogenic mesenchymal cells and early chondrocytes near the articular surface (29) (Fig. 6B). Therefore, it seems that prechondrogenic cells of prospective proximal and middle phalanges are not able to differentiate into chondrocytes in the absence of BMPR1B signaling. Because type II collagen is expressed in prechondrogenic cells of mesenchymal condensations, we expressed caBmpr1a using the bigenic system, which used a type II collagen promoter, in the BMPR1B-null background to determine whether BMP signaling in the chondrocytic lineage was responsible for the Bmpr1b–/– digit phenotype, and whether caBMPR1A could replace BMPR1B signaling. Whole-mount alcian blue staining and histological examination showed that digits of compound mutant mice Col2-Gal4:UAS-Bmpr1a:Bmpr1b–/– (Bmpr1b–/–:Bg) were virtually identical to those of Col2-Gal4:UAS-Bmpr1a:Bmpr+/+ mice (Bg), demonstrating successful rescue of the digit defect of Bmpr1b–/– mice (Fig. 6 A and B).
Discussion
Chondrocyte differentiation in the growth plate is regulated by multiple signaling pathways. The PTHrP/Ihh feedback loop has been well demonstrated to play an important role (30). PTHrP expression depends on and is positively regulated by Ihh. Based on chick/retroviral misexpression experiments, BMP signaling through BMPR1A was proposed to mediate the action of Ihh in PTHrP expression to delay terminal differentiation of chondrocytes (19). However, in mouse and chicken limb organ culture systems, BMP did not stimulate PTHrP expression in the absence of Ihh action (15). In the present study as well, we did not find up-regulation of PTHrP after overexpression of caBmpr1a in either system (data not shown). We also found that expression of a constitutively active PTH/PTHrP receptor (31) in caBmbr1a bigenic growth plates did not rescue the shortening of the growth plate (Fig. 8, which is published as supporting information on the PNAS web site). This finding also supports the hypothesis that the BMP pathway regulates hypertrophic chondrocyte differentiation independently of the PTHrP signaling pathway, because the constitutively active PTH/PTHrP receptor does rescue phenotypic abnormalities in PTHrP-null mice (31) and in mice with decreased numbers of PTH/PTHrP receptors (28).
Previous reports have shown that BMP signaling regulates expression of Ihh in chondrocytes and chondrogenic cells (32, 33). In the present study, we did not obtain evidence that support these previous findings. In a mouse limb culture system as well, BMP treatment did not seem to stimulate Ihh expression (15). This disagreement may have arisen from differences in the system (cell, chicken, or mouse), method used to stimulate BMP signaling (BMP ligands or expression of constitutively active receptors), or robustness of assay systems.
Both bigenic and conventional transgenic mice showed shortening of the columnar layer of proliferating chondrocytes, but no apparent reduction in proliferation was observed in bigenic mice. This combination of findings suggests acceleration of conversion of columnar cells into hypertrophic cells. Together with the similar finding in the conventional transgenic mice, these data suggest that caBmpr1a overexpression accelerates differentiation of proliferating chondrocytes into hypertrophic chondrocytes. Phospho-Smad1 in control growth plates is predominantly present in the prehypertrophic region. Because hypertrophic chondrocytes that express BMP-2 and BMP-6 are major sources for BMP ligands in the growth plate (34), this finding supports the hypothesis that BMPs from the hypertrophic layer stimulate further differentiation of less differentiated chondrocytes (8).
In conventional transgenic mice, the morphology of perichondrial cells was also greatly changed. It is conceivable that expression of Bmpr1a in perichondrial cells or their precursor cells directly alter their differentiation pathway into the chondrocytic lineage. Compared with the conventional transgenic system, the Gal4-UAS bigenic system generally requires more time between promoter activation and expression of the target transgene (23, 35). It is, therefore, possible that the timing of caBmp1a expression in bigenic mice was delayed such that caBmpr1a expression was confined to cells that were developmentally more advanced in bigenic mice, and that this led to the substantial phenotypic differences between mice generated by these systems despite using the same promoter. It is also conceivable that the possible differences in the expression levels of transgenes in these two systems account for the phenotypic variation.
The observation that bigenic caBmpr1a expression was able to restore the digit defect of Bmpr1b–/– mice has several implications. First, this is genetic evidence that the bigenic caBmpr1a system indeed activates BMP signaling. Second, BMP signaling provided by caBmpr1a expression is able to substitute for BMPR1B signaling. This result contrasts with the differing outcomes of caBMR1A and caBMPR1B overexpression in chick limbs (19). Third, a defect in BMP signaling in cells of the chondrocytic lineage, rather than a non-cell autonomous effect, is responsible for the Bmpr1b–/– digit phenotype. Because prospective phalangeal cells of Bmpr1b–/– mice seem to remain at the stage of prechondrogenic mesenchymal condensation, this observation also supports the hypothesis that prechondrogenic mesenchymal cells require BMP signaling to differentiate further (18)
In conclusion, we have demonstrated that overactivity of BMP signaling through caBMPR1A in chondrocytes stimulates chondrocyte maturation toward hypertrophic differentiation. The phenotypic differences between bigenic and conventional transgenic mice and histological analysis of conventional transgenic mice suggest that BMP signaling in perichondrial cells or their precursor cells stimulates their differentiation into chondrocytes. Thus, BMP signaling can stimulate both differentiation of prechondrogenic cells into chondrocytes and differentiation of proliferating chondrocytes toward hypertrophic chondrocytes.
Supplementary Material
Acknowledgments
We thank Dr. Lee Niswander for providing reagents and for sharing valuable unpublished data. We thank Dr. Ung-il Chung for sharing research resources; Mike Rule and Nancy Wu for generation of transgenic mice; and Melissa Knight, David Buzak, and Jennifer Paruch for technical assistance. Work in the H.M.K. and A.P.M. laboratories was supported by National Institutes of Health (NIH) Grant P01 DK56246. K.M.L. is supported by NIH Grant AR44528.
Author contributions: T.K., A.P.M., and H.M.K. designed research; T.K. performed research; T.K., K.M.L., and A.P.M. contributed new reagents/analytic tools; T.K. and K.M.L. analyzed data; and T.K. and H.M.K. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BMP, bone morphogenetic protein; En, embryonic day n.
References
- 1.Hogan, B. L. (1996) Curr. Opin. Genet. Dev. 6, 432–438. [DOI] [PubMed] [Google Scholar]
- 2.Wozney, J. M. & Rosen, V. (1998) Clin. Orthop. Relat. Res. 346, 26–37. [PubMed] [Google Scholar]
- 3.Baur, S. T., Mai, J. J. & Dymecki, S. M. (2000) Development (Cambridge, U.K.) 127, 605–619. [DOI] [PubMed] [Google Scholar]
- 4.Yazaki, Y., Matsunaga, S., Onishi, T., Nagamine, T., Origuchi, N., Yamamoto, T., Ishidou, Y., Imamura, T. & Sakou, T. (1998) Anticancer Res. 18, 2339–2344. [PubMed] [Google Scholar]
- 5.Volk, S. W., D'Angelo, M., Diefenderfer, D. & Leboy, P. S. (2000) J. Bone Miner. Res. 15, 1630–1639. [DOI] [PubMed] [Google Scholar]
- 6.Yi, S. E., Daluiski, A., Pederson, R., Rosen, V. & Lyons, K. M. (2000) Development (Cambridge, U.K.) 127, 621–630. [DOI] [PubMed] [Google Scholar]
- 7.Boskey, A. L., Paschalis. E. P., Binderman, I. & Doty, S. B. (2002) J. Cell Biochem. 84, 509–519. [PubMed] [Google Scholar]
- 8.Grimsrud, C. D., Romano, P. R., D'Souza, M., Puzas, J. E., Reynolds, P. R., Rosier, R. N. & O'Keefe, R. J. (1999) J. Bone Miner. Res. 14, 475–482. [DOI] [PubMed] [Google Scholar]
- 9.Kameda, T., Koike, C., Saitoh, K., Kuroiwa, A. & Iba, H. (2000) Dev. Growth Differ. 42, 229–236. [DOI] [PubMed] [Google Scholar]
- 10.Pateder, D. B., Rosier, R. N., Schwarz, E. M, Reynolds, P. R., Puzas, J. E., D'Souza, M. & O'Keefe, R. J. (2000) Exp. Cell Res. 256, 555–562. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, D., Ferguson, C. M., O'Keefe, R. J., Puzas, J. E., Rosier, R. N. & Reynolds, P. R. (2002) J. Bone Miner. Res. 17, 293–300. [DOI] [PubMed] [Google Scholar]
- 12.Shukunami, C., Ohota, Y., Sakuda, M. & Hiraki, Y. (1998) Exp. Cell Res. 241, 1–11. [DOI] [PubMed] [Google Scholar]
- 13.De Luca, F., Barnes, K. M., Uyeda, J. A., De-Levi, S., Abad, V., Palese, T., Mericq, V. & Baron, J. (2001) Endocrinology 142, 430–436. [DOI] [PubMed] [Google Scholar]
- 14.Haaijman, A., Karperien, M., Lanske, B., Hendriks, J., Lowik, C. W., Bronckers, A. L. & Burger, E. H. (1999) Bone 25, 397–404. [DOI] [PubMed] [Google Scholar]
- 15.Minina, E., Wenzel, H. M., Kreschel, C., Karp, S., Gaffield, W., McMahon, A. P. & Vortkamp, A. (2001) Development (Cambridge, U.K.) 128, 4523–4534. [DOI] [PubMed] [Google Scholar]
- 16.Duprez, D., Bell, E. J., Richardsonv M. K., Archer, C. W., Wolpert, L., Brickell, P. M. & Francis-West, P. H. (1996) Mech. Dev. 57, 145–157. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami, Y., Ishikawa, T., Shimabara, M., Tanda, N., Enomoto-Iwamoto, M., Iwamoto, M., Kuwana, T., Ueki, A., Noji, S. & Nohno, T. (1996) Development (Cambridge, U.K.) 122, 3557–3566. [DOI] [PubMed] [Google Scholar]
- 18.Pizette, S. & Niswander, L. (2000) Dev. Biol. 219, 237–249. [DOI] [PubMed] [Google Scholar]
- 19.Zou, H., Wieser, R., Massague, J. & Niswander, L. (1997) Genes Dev. 11, 2191–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsumaki, N., Nakase, T., Miyaji, T., Kakiuchi, M., Kimura, T., Ochi, T. & Yoshikawa, H. (2002) J. Bone Miner. Res. 17, 898–906. [DOI] [PubMed] [Google Scholar]
- 21.Tsumaki, N., Tanaka, K., Arikawa-Hirasawa, E., Nakase, T., Kimura, T., Thomas, J. T., Ochi, T., Luyten, F. P. & Yamada, Y. (1999) J. Cell Biol. 144, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ornitz, D. M., Moreadith, R. W. & Leder, P. (1991) Proc. Natl. Acad. Sci. USA 88, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowitch, D. H., St-Jaques. B., Lee, S. M., Flax, J. D., Snyder, E. Y. & McMahon, A. P. (1999) J. Neurosci. 19, 8954–8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long, F., Zhang, X. M., Karp, S., Yang, Y. & McMahon, A. P. (2001) Development (Cambridge, U.K.) 128, 5099–5108. [DOI] [PubMed] [Google Scholar]
- 25.Echelard, Y., Vassileva, G. & McMahon, A. P. (1994) Development (Cambridge, U.K.) 120, 2213–2224. [DOI] [PubMed] [Google Scholar]
- 26.Yamada, Y., Miyashita, T., Savagner, P., Horton, W., Brown, K. S., Abramczuk, J., Xie, H. X., Kohno, K., Bolander, M. & Bruggeman, L. (1990) Ann. N. Y. Acad. Sci. 580, 81–87. [DOI] [PubMed] [Google Scholar]
- 27.McLeod, M. J. (1980) Teratology 22, 299–301. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi, T., Chung, U. I., Schipani, E., Starbuck, M., Karsenty, G., Katagiri, T., Goad, D. L., Lanske, B. & Kronenberg, H. M. (2002) Development (Cambridge, U.K.) 129, 2977–2986. [DOI] [PubMed] [Google Scholar]
- 29.Shibata, S., Fukada, K., Imai, H., Abe, T. & Yamashita, Y. (2003) J. Anat. 203, 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kronenberg, H. M. (2003) Nature 423, 332–336. [DOI] [PubMed] [Google Scholar]
- 31.Schipani, E., Lanske, B., Hunzelman, J., Luz, A., Kovacs, C. S., Lee, K., Pirro, A., Kronenberg, H. M. & Juppner, H. (1997) Proc. Natl. Acad. Sci. USA 94, 13689–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, D., Schwartz. E. M., Rosier, R. N., Zuscik, M. J., Puzas, J. E. & O'Keefe, R. J. (2003) J. Bone Miner. Res. 18, 1593–1604. [DOI] [PubMed] [Google Scholar]
- 33.Seki, K. & Hata, A. (2004) J. Biol. Chem. 279, 18544–18549. [DOI] [PubMed] [Google Scholar]
- 34.Chung, U. I., Schipani, E., McMahon, A. P. & Kronenberg, H. M. (2001) J. Clin. Invest. 107, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewandoski, M. (2001) Nat. Rev. Genet. 2, 743–755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.