Abstract
The amyloid β-protein precursor (AβPP) is best known as the parent molecule to the amyloid β-peptide that accumulates in the brains of patients with Alzheimer's disease. Secreted isoforms of AβPP that contain the Kunitz proteinase inhibitor domain are analogous to the previously identified cell-secreted proteinase inhibitor known as protease nexin-2 (PN2). Although PN2/AβPP is enriched in brain and in circulating blood platelets, little is understood of its physiological function and potential role in disease processes outside of amyloid β-peptide generation. We hypothesized that the potent inhibition of certain procoagulant proteinases by PN2/AβPP, coupled with its abundance in platelets and brain, indicate that it may function to regulate cerebral thrombosis. Here we show that specific and modest 2-fold overexpression of PN2/AβPP in circulating platelets of transgenic mice caused a marked inhibition of thrombosis in vivo. In contrast, deletion of PN2/AβPP in AβPP gene knockout mice resulted in a significant increase in thrombosis. Similarly, platelet PN2/AβPP transgenic mice developed larger hematomas in experimental intracerebral hemorrhage, whereas AβPP gene knockout mice exhibited reduced hemorrhage size. These findings indicate that PN2/AβPP plays a significant role in regulating cerebral thrombosis and that modest increases in this protein can profoundly enhance cerebral hemorrhage.
Keywords: Alzheimer's disease, hemorrhage, proteinase inhibitor
The amyloid β-protein precursor (AβPP), a type I transmembrane protein, is most recognized as the precursor to the amyloid β-peptide (Aβ) that accumulates in the brains of patients with Alzheimer's disease and related disorders (1). Aβ is derived through sequential amyloidogenic proteolytic processing by β- and γ-secretase activities (2, 3). Alternatively, AβPP can undergo nonamyloidogenic proteolytic processing by means of a single cleavage through the Aβ domain by α-secretase, resulting in release of the large extracellular domain of the protein into the external environment (4, 5). AβPP can be predominantly derived from three alternatively spliced mRNAs of a single gene located on chromosome 21, giving rise to proteins of 695, 751, and 770 aa; the larger two isoforms contain an additional 56-aa domain that is structurally and functionally related to Kunitz-type serine proteinase inhibitors (KPI) (6, 7). Previously, we showed that secreted KPI domain-containing forms of AβPP are analogous to the cell-secreted proteinase inhibitor known as protease nexin-2 (PN2) (8, 9).
Although much has been learned about the proteolytic processing of AβPP and generation of Aβ peptide, comparatively little is known about the physiological functions of AβPP. Previous in vitro studies have identified a number of biologically active domains on AβPP, including high-affinity binding sites for Cu2+ and Zn2+ (10, 11), cell adhesion (12, 13), growth-promoting activity (14), regulation of Ca2+ homeostasis (15), and intracellular signaling through its C-terminal domain (16, 17). Additionally, we and others have reported that both purified PN2/AβPP and its recombinantly expressed KPI domain are potent, tight-binding inhibitors of certain serine proteinases, most notably several prothrombotic enzymes, including factor XIa, factor IXa, factor Xa, and tissue factor-factor VIIa complex with Ki values in the nanomolar to picomolar range (18-22). Moreover, purified PN2/AβPP and its isolated KPI domain can inhibit the clotting of plasma in vitro and in vivo (20, 23). The potent regulation of the above prothrombotic factors by PN2/AβPP suggests a role for this protein in the maintenance of hemostasis. Further supporting this notion, it has been demonstrated that PN2/AβPP is an abundant platelet α-granule protein that is released upon platelet activation by physiological agonists such as thrombin and collagen (22-25). This finding indicates that platelets provide a rich circulating source of PN2/AβPP that can be delivered to sites of vascular injury on demand. On the basis of these findings, we have hypothesized that released platelet PN2/AβPP has a physiological function in regulating prothrombotic events during vascular injury (26).
Here we show that transgenic mice with modest overexpression of PN2/AβPP in platelets (Tg-rPF4/APP) present with a significantly impaired thrombotic potential that results in increased times to vessel occlusion and more extensive cerebral hemorrhage in in vivo experimental models for carotid artery thrombosis and intracerebral hemorrhage, respectively. In contrast, mice lacking AβPP exhibit increased thrombosis in these cerebral vascular injury models. Together, these findings indicate that PN2/AβPP plays a significant role in regulating cerebral thrombosis and that alterations in the levels of this protein may markedly affect thrombosis in vivo.
Methods
Vector Construction and Generation of Tg-rPF4/APP Transgenic Mice. A pCDNA3 vector containing the 2.1-kb human AβPP (770 isoform) cDNA plus the bovine growth hormone poly(A) was excised from the vector with SpeI and AvrII and cloned into pBluescript KS + vector at SpeI and XbaI sites. The 1.1-kb rat platelet factor 4 (rPF4) promoter was removed from pCDCMV plasmid (a gift from W. Bahou, Stony Brook University) with EcoRI digestion and placed into the human AβPP770/BGH poly(A)/pBluescript KS + construct. The completed transgene cDNA construct was entirely sequenced to confirm its integrity. The 4.3-kb rPF4-human AβPP770-poly(A) tail transgene (Fig. 1) was removed from the vector by digestion with BssHII, electrophoresed on 0.8% agarose gels, and purified. The purified transgene was microinjected into fertilized eggs from inbred FVB/N mice at the Stony Brook Transgenic Mouse Facility. Founder transgenic mice were identified by Southern blot analysis of tail DNA. Transgenic offspring from each line were determined by PCR analysis of tail DNA with the following primers specific for human AβPP: 5′-CCTGATTGATACCAAGGAAGGCATCCTG-3′ and 5′-GTCATCATCGGCTTCTTCTTCTTCCACC-3′ (generating an ≈500-bp product). Transgenic mice were backcrossed for 10 generations onto a pure C57BL/6 background and then were bred to homozygosity for more than 5 generations before their use in these studies. Unless otherwise noted, all of the studies involving Tg-rPF4/APP mice described below used mice that were homozygous for the human AβPP transgene. All work with animals followed National Institutes of Health guidelines and was approved by Stony Brook University Institutional Animal Care and Use Committee.
Fig. 1.
Generation of Tg-rPF4/APP transgenic mice. (a) Schematic of the 4.3-kb transgene construct used to generate the Tg-rPF4/APP transgenic mice containing the rat platelet factor-4 promoter, a cDNA of human APP770, and a poly(A) tail from bovine growth hormone. (b) PCR analysis was performed for human AβPP transgene in DNA isolated from Tg-rPF4/APP mice (lane 2) and wild-type mice (lane 3) as described in Methods. Lane 1 presents a 1-kb ladder. (c) Immunoblot analysis for human PN2/AβPP expression in platelet lysates from wild-type (lane 1), heterozygous Tg-rPF4/APP (lane 2), and homozygous Tg-rPF4/APP (lane 3). The mAb P2-1, which is specific for human AβPP, was used.
AβPP Gene Knockout (KO) Mice. Mice deficient in AβPP were obtained from The Jackson Laboratory. The mice were on a pure C57BL/6 background, the same as the Tg-rPF4/APP described above and the same as the wild-type mice used in the following studies. For genotyping purposes, the wild-type AβPP allele was identified by PCR with sense primer 5′-AGAGCACCGGGAGCAGAG-3′ and antisense primer 5′-AGCAGGAGCAGTGCCAAG-3′, resulting in a 161-bp product. The homozygous AβPP KO offspring are identified by PCR with sense primer 5′-CTTGGGTGGAGAGGCTATTC-3′ and antisense primer 5′-AGGTGAGATGACAGGAGATC-3′, resulting in a 280-bp product.
Platelet Isolation. Mouse blood was collected by terminal cardiac puncture from wild-type or Tg-rPF4/APP mice. Blood was collected in 0.1 vol of 3.8% sodium citrate to prevent coagulation. The citrated blood was centrifuged at 800 × g for 2 min or 5,000 × g for 5 min at 22°C for the preparation of platelet-rich plasma (PRP) or platelet-poor plasma, respectively. PRP was collected and the platelets were isolated by gel filtration through Sepharose 2B columns equilibrated in Hepes-modified Tyrode's platelet buffer [10 mM Hepes/135 mM sodium chloride/5 mM d-glucose/2.7 mM potassium chloride/1 mM magnesium chloride/1 mM sodium citrate/0.5 mM sodium phosphate (pH 7.4) containing 0.1% BSA]. The gel-filtered platelets were collected and counted in a Coulter Z1 particle count and size analyzer. A setting of 5 fl was used to measure mouse platelets to account for the mean platelet volumes (27). The isolated platelets were pelleted by centrifugation at 2,040 × g and adjusted to 3 × 106 per μl. Platelets were lysed in PBS containing 1% SDS, 0.5% Nonidet P-40, complete proteinase inhibitor mixture (Roche Applied Science), and 1 μg/ml chymostatin, and stored at -80°C.
Immunoblot Analysis of AβPP. Isolated mouse platelets or various tissues from perfused mice were homogenized in 10 vol of 50 mM Tris·HCl (pH 7.5) containing 150 mM NaCl, 1% SDS, 0.5% Nonidet P-40, 5 mM EDTA, and proteinase inhibitor mixture. The tissue homogenates were clarified by centrifugation at 14,000 × g for 10 min. Protein concentrations of the resulting supernatants were determined by using the BCA Protein Assay kit (Pierce). The levels of AβPP in the tissue homogenate samples were determined by performing quantitative immunoblotting (28). Briefly, 35 μg of total protein from each sample was electrophoresed in SDS/Tris/glycine/8% polyacrylamide gels and the proteins were transferred onto Hybond nitrocellulose membranes (Amersham Pharmacia). Unoccupied sites on the membranes were blocked overnight with 5% nonfat dry milk in PBS with 0.05% Tween-20. The membranes were probed with either monoclonal antibody (mAb) P2-1 specific for human AβPP (29) or 22C11 (Chemicon International, Temecula, CA) specific for mouse and human AβPP and then incubated with a secondary peroxidase-coupled sheep anti-mouse IgG antibody at a dilution of 1:1,000. The peroxidase activity on the membranes was detected by using a Supersignal Dura West (Pierce). Bands corresponding to AβPP were measured by using a VersaDoc 3000 Imaging System (Bio-Rad) with the manufacturer's quantity one software and compared with standard curves generated from known quantities of purified AβPP.
Platelet Activation Assays. For platelet releasate studies, gel-filtered platelets were pooled from five Tg-rPF4/APP mice, resuspended to 2.2 × 108 per ml in 1 ml of Hepes-modified Tyrode's platelet buffer, and preincubated by slow shaking for 5 min at 37°C. The platelets were then incubated in the absence or presence of 20 μg/ml collagen (Chrono-Log, Havertown, PA) or 10 nM human thrombin (provided by Jolyon Jesty, Stony Brook University) for an additional 10 min at 37°C, followed by centrifugation at 2,040 × g for 10 min at 22°C. The agonist-stimulated platelet releasates were collected, and the platelet pellets were lysed in 70 μl of PBS containing 1% SDS, 0.5% Nonidet P-40, protease inhibitor mixture, and 1 μg/ml chymostatin, and stored at -80°C. Before immunoblot analysis, the platelet pellets were diluted to 1 ml of Hepes-modified Tyrode's platelet buffer, the same volume as the platelet releasates. The platelet releasates and pellets were analyzed for human AβPP by quantitative immunoblotting using mAb P2-1 as described above.
For platelet aggregation studies, gel-filtered platelets of each genotype were adjusted to 1.2 × 108 per ml with Hepes-modified Tyrode's platelet buffer. MgCl2 was added to the final concentration of 5 mM. Platelets were incubated at 37°C for 5 min, then 300 μl of each platelet suspension was placed in aggregation cuvettes with continuous stirring at 1,200 rpm. Aggregation was initiated by the addition of collagen (5-10 μg/ml) or thrombin (0.1-1 nM). Platelet aggregation was measured by using an optical aggregometer (Chrono-Log).
Activated Partial Thromboplastin Time (APTT) Assay. Microtiter plate APTT assays were conducted as described in ref. 30. Briefly, 30 μl of citrated platelet-poor plasma or PRP (containing 5 × 108 platelets per ml) prepared from mice of different genotypes, 30 μl of APTT reagent, and 30 μl of Tris-buffered saline were incubated in triplicate microtiter plate wells for 10 min at 22°C. The APTT assay was initiated by the addition of 30 μl of 25 mM CaCl2, and the time to clot formation was monitored by the change in absorbance at 405 nm in a Vmax kinetic microtiter plate reader (Molecular Devices). The absorbance readings were recorded every 30 sec for 15-25 min.
Carotid Artery Thrombosis Assay. This assay was performed essentially as described by Eitzman et al. (31) and induces a fibrin- and platelet-rich clot. Briefly, mice (8-12 weeks of age) were prepared for surgery and anesthetized by i.p. injection of sodium pentobarbital (70 mg/kg). A midline neck incision was made to expose the common carotid artery, which was then cradled by a miniature flowmeter probe to record blood flow rate and ultimately determining presence of thrombosis. Then 0.1 ml of rose bengal [4,5,6,7-tetrachloro-3′,6′-dihydroxy-2′,4′,5′,7′-tetraiodospiro[isobenzofuran-1(3H),9[9H]xanthen]-3-one dipotassium salt] (50 mg/kg in 0.9% saline) was injected through the tail vein; the dye is activated by laser light (540 nm) to generate a superoxide anion. The superoxide anion leads to endothelial cell damage with a transient thrombus and subsequent neointima formation. When the blood flow ceased for 20 min within the laser specific area, retrospectively, the time to occlusion was documented.
Experimental Intracerebral Hemorrhage. This model was essentially as described by Clark et al. (32). Briefly, mice (8-12 weeks of age) were prepared for surgery and anesthetized by i.p. injection of sodium pentobarbital (70 mg/kg). A sagittal incision was made caudal to rostral, allowing the scalp to be retracted and held in place with microclips. A small hole, 1.0 mm posterior and 3.0 mm lateral of bregma, was drilled to perforate the skull. A 1-μl Hamilton syringe was used to deliver 500 nl of bacterial collagenase (150 units/ml)/saline to the caudate/putamen at a depth of 4.0 mm unilaterally. After the injection of collagenase/saline (≈30 sec), the needle remained in place for another 2 min to prevent reflux of fluid. The surgery was concluded with the closing of the scalp skin by using 4-0 nylon sutures. Twenty-four hours after initiation of hemorrhage, the mice were perfused with PBS, the brains were harvested, and 14-μm sections were prepared by using a cryostat and mounted on glass slides. Sections were stained with hematoxylin, and an Olympus (Melville, NY) BX60 upright systems microscope with a digital camera was used to capture images. The hemorrhage volume was measured by using the stereologer software system. Alternatively, harvested perfused brains were divided midline sagittally, and the hemoglobin levels were determined in the hemorrhage and contralateral hemispheres by using a spectrophotometric assay as a measure of the extent of hemorrhage in the lesioned hemispheres of the mice (32, 33).
Results
To investigate the role of platelet PN2/AβPP in regulating thrombosis in vivo, we generated Tg-rPF4/APP transgenic mice, which express the human AβPP-770 isoform specifically in circulating blood platelets under control of the rPF4 promoter (Fig. 1a). The 770 isoform of AβPP was chosen because this secreted KPI domain-containing form of AβPP, analogous to PN2, is naturally abundant in platelets (24, 34). The Tg-rPF4/APP mice were generated by microinjection of the human APP-770 construct into oocytes of an FVB/N background, which were subsequently backcrossed into a pure C57BL/6 background. The presence of the human AβPP transgene was confirmed by PCR analysis (Fig. 1b).
Tg-rPF4/APP mice showed expression of human PN2/AβPP in platelets, and the level of human PN2/AβPP was ≈2-fold higher in homozygous Tg-rPF4/APP mice compared with mice heterozygous for the transgene (Fig. 1c, lanes 3 and 2, respectively). As expected, no human PN2/AβPP was detected in the nontransgenic wild-type mice (Fig. 1c, lane 1). Although human PN2/AβPP expression was readily found in platelets, there was no detectable expression in other various tissues from perfused Tg-rPF4/APP mice (Fig. 2a). Quantitative immunoblot analysis using an mAb that detects both mouse and human AβPP revealed that expression of transgene-encoded human AβPP in homozygous Tg-rPF4/APP mice was modest and only approximately doubled the total amount of platelet AβPP compared with wild-type mice (190 ± 8 vs. 86 ± 10 ng per 108 platelets) as estimated by quantitative immunoblot analysis (Fig. 2 b and c). The level of transgene-encoded human AβPP expression in platelets did not change over the course of 18 months (data not shown).
Fig. 2.
Platelet-specific expression of transgene-encoded human PN2/AβPP in Tg-rPF4/APP mice. (a) Immunoblot analysis of human PN2/AβPP expression in homogenates prepared from various tissues of Tg-rPF4/APP mice: platelets (lane 1), brain (lane 2), heart (lane 3), kidney (lane 4), liver (lane 5), lung (lane 6), and skeletal muscle (lane 7). (b) Immunoblot analysis of endogenous mouse and transgene-encoded human AβPP in wild-type mouse platelets (lanes 1 and 2) and Tg-rPF4/APP mouse platelets (lanes 3 and 4) homogenates. The mAb 22C11 was used, which detects both human and mouse AβPP. (c) The levels of total AβPP were determined in wild-type mouse platelets (left bar) and Tg-rPF4/APP mouse platelets (right bar) by quantitative immunoblotting measurements as described in Methods. The data presented are the mean ± SD of measurements from n = 3 mice for each group.
Stimulation of platelets with physiological agonists results in the strong release of PN2/AβPP, which is normally stored in the platelet α granules (24, 25, 35). Therefore, we determined whether agonist stimulation of Tg-rPF4/APP platelets similarly caused release of transgene-encoded human PN2/AβPP. In unstimulated control Tg-rPF4/APP platelets, virtually all of the human PN2/AβPP was recovered in the platelet pellet (Fig. 3a, lane 1). However, when Tg-rPF4/APP platelets were stimulated with either collagen or thrombin, ≈90% of the human PN2/AβPP was recovered in the platelet releasates (Fig. 3a, lanes 4 and 6, respectively). These findings indicate that transgene-encoded human PN2/AβPP is properly packaged in the platelet α granule and is released by physiological agonist stimulation of Tg-rPF4/APP mouse platelets.
Fig. 3.
Transgene-encoded human PN2/AβPP is released from Tg-rPF4/APP platelets upon activation and affects clotting in vitro. (a) Platelets isolated from Tg-rPF4 mice were incubated in the absence or presence of 20 μg/ml collagen or 10 nM thrombin. The platelet pellets (P) (lanes 1, 3, and 5) and platelet releasates (R) (lanes 2, 4, and 6) were analyzed for human PN2/AβPP by immunoblotting with mAb P2-1 as described in Methods. (b) Gel-filtered platelets were isolated from PRP prepared from wild-type, Tg-rPF4/APP, and AβPP KO mice and analyzed for aggregation after stimulation with collagen or thrombin as described in Methods. (Scale bar, 4 min.) (c) PRP was prepared from freshly collected blood from wild-type, Tg-rPF4/APP, and AβPP KO mice and then analyzed for in vitro clotting by using an APTT assay as described in Methods. Data shown are mean ± SD of n = 6 mice per genotype. *, P < 0.001.
To begin to determine whether platelet PN2/AβPP altered platelet function and thrombosis, we first performed platelet aggregation assays. Gel-filtered platelets were prepared from wild-type mice and Tg-rPF4/APP mice, and we also included AβPP KO mice in these studies. All mice were on the same pure C57BL/6 background and had very similar platelet counts (wild-type = 8.0 ± 2.0 × 108 per ml, Tg-rPF4/APP = 7.5 ± 0.5 × 108 per ml, and AβPP KO = 7.4 ± 1.3 × 108 per ml), ensuring meaningful comparisons. There were no appreciable differences in collagen- or thrombin-stimulated platelet aggregation between any of the different genotypes (Fig. 3b). Similarly, analysis of in vitro clotting of platelet-poor plasma by using an APTT assay revealed no substantive differences in the time to clot formation among any of the genotypes (data not shown). In contrast, compared with wild-type mice, PRP samples prepared from Tg-rPF4 mice, which release modestly elevated levels of platelet PN2/AβPP, exhibit a highly significant (P < 0.001) near doubling in the time to clot formation (Fig. 3c). However, PRP samples prepared from AβPP KO mice show a highly significant decrease (P < 0.001) in the time to clot formation. These results clearly show that modulating the levels of PN2/AβPP in mouse platelets can significantly affect PRP clotting in vitro and suggested similar findings may be observed in vivo.
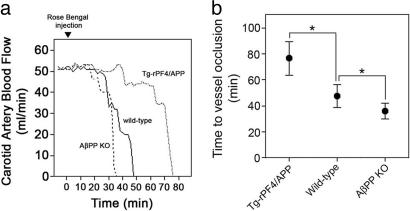
To further investigate this function, we performed experiments to determine the effect of platelet PN2/AβPP on carotid artery thrombosis. Fig. 4a shows representative experiments measuring thrombosis and loss of carotid artery blood flow in mice of the different genotypes. Measurement of carotid artery thrombosis in wild-type mice revealed a mean time of 47.6 ± 9.4 min to cessation of blood flow (Fig. 4b). In contrast, Tg-rPF4/APP mice, with a modest doubling of the total amount of platelet PN2/AβPP, exhibited a pronounced increase in time to vessel occlusion (76.6 ± 13 min, P < 0.00001). However, AβPP KO mice, which lack PN2/AβPP, showed decreased times for vessel occlusion compared with wild-type mice (36.0 ± 6.5 min, P < 0.0002). Together, these in vitro and in vivo thrombosis assays strongly indicate that platelet PN2/AβPP plays a significant role in limiting thrombosis.
Fig. 4.
PN2/AβPP affects thrombosis in vivo.(a) Mice of different genotypes were injected with the photoactivated dye rose bengal at t = 0, and the carotid artery was exposed to a laser light. Blood flow through the carotid artery was monitored with a flow probe. Blood flow ceased because of thrombus formation in the vessel. Representative experiments show wild-type mice (solid line), Tg-rPF4 mice (dotted line), and AβPP KO mice (dashed line). (b) Quantitation of the time to cessation of carotid artery blood flow in mice of different genotypes. Data shown are the mean ± SD from Tg-rPF4/APP mice (n = 11), wild-type (n = 30), and AβPP KO mice (n = 13). *, P < 0.0001.
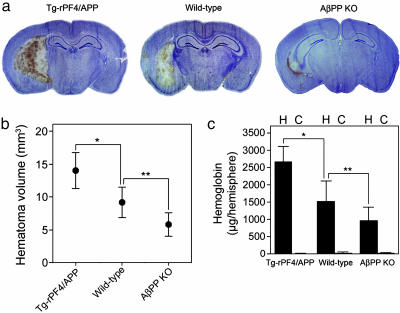
We next determined whether PN2/AβPP might influence cerebral thrombosis during a brain vascular injury event such as intracerebral hemorrhage. Tg-rPF4/APP mice presented with much larger hematomas and AβPP KO with smaller hematomas compared with wild-type mice in experimentally induced intracerebral hemorrhage (Fig. 5a). Quantitative measurement of hematoma volumes showed that, compared with wild-type mice, the hematomas of Tg-rPF4/APP mice were >50% larger (P < 0.0001), whereas the hematomas of AβPP KO mice were nearly 40% smaller (P < 0.002). Similar findings were obtained by using the measurement of hemoglobin levels in hemorrhagic brain tissue as an independent indicator of hemorrhage severity. Hemoglobin levels in hemorrhagic brain hemispheres of Tg-rPF4/APP mice were ≈75% higher when compared with wild-type mice (2,666 ± 445 μg vs. 1,522 ± 607 μg, respectively, P < 0.0005). Again, AβPP KO mice, which are prothrombotic, had nearly 40% lower hemoglobin levels in their hemorrhagic brain hemispheres compared with wild-type mice (980 ± 382 μg, P < 0.02). Together, these data demonstrate that alterations in PN2/AβPP levels have significant effects on cerebral thrombosis during hemorrhagic brain injury.
Fig. 5.
PN2/AβPP affects the severity of intracerebral hemorrhage. (a) Mice were stereotaxically injected with collagenase in the caudate/putamen. Twenty-four hours later, the brain was removed and analyzed for hematoma as described in Methods. Stained brain sections are presented from Tg-rPF4/APP (Left), wild-type (Center), and AβPP KO (Right) mice. (b) Quantitation of hematoma volume from Tg-rPF4/APP mice (n = 12), wild-type mice (n = 14), and AβPP KO mice (n = 7) at 24 h. The data presented are the mean ± SD. *, P < 0.0001; **, P < 0.002. (c) Quantitation of hemoglobin levels in the hemorrhagic (H) and contralateral control (C) hemispheres from Tg-rPF4/APP mice (n = 7), wild-type mice (n = 15), and AβPP KO mice (n = 7) at 24 h. The data presented are the mean ± SD. *, P < 0.0005; **, P < 0.02.
Discussion
In the present study we show that modulating the levels of platelet PN2/AβPP significantly affects prothrombotic events during experimental cerebral vascular injury. Aside from serving as the precursor to Aβ peptide, little is known about the physiological function of AβPP and its regulation of potentially pathological events. Many specific operative domains and functional properties have been identified for AβPP by using in vitro systems, but most of these remain unresolved in vivo. In many cases, the use of transgenic mice that overexpress a given protein, and more so from gene KO mice that lack a given protein, has provided insight into the functions of proteins in complex mammalian organisms. Regarding AβPP, however, gene KO mice have thus far revealed little into functions of this protein. An initial report indicated that AβPP KO mice were viable and fertile with some loss in locomotor activity (36). More recent studies have suggested that AβPP may play a role in maintenance of synaptic function and modulate Cu2+/Zn2+/nitric oxide-catalyzed degradation of glypican-1 (37). The rationale for the present in vivo investigation was based on earlier in vitro studies that identified the secreted KPI-containing forms of AβPP, otherwise known as PN2, as potent inhibitors of several prothrombotic proteinases (18-22). These compelling biochemical data, coupled with the rich investment of PN2/AβPP in platelets and in brain, suggested that AβPP may play a role in regulating cerebral thrombosis. The use of the Tg-rPF4/APP mice described here, in conjunction with AβPP KO mice, allowed us to investigate the inhibition of cerebral thrombosis by PN2/AβPP when mice are challenged with cerebral vascular injury. The present results clearly show that modestly elevated levels of PN2/AβPP in the platelets of Tg-rPF4/APP mice significantly limited thrombosis in cerebral vascular injury.
Several of our findings indicate that the observed gain-of-function effect of platelet PN2/AβPP on inhibiting thrombosis was not merely the result of random artifactual overexpression effects of PN2/AβPP. First, just a minor doubling of the total amount of platelet PN2/AβPP in homozygous Tg-rPF4/APP mice led to a parallel near doubling in time to in vitro clot formation and in vivo vessel occlusion in carotid artery thrombosis (Fig. 4). Second, using heterozygous Tg-rPF4/APP mice, with half the amount of increased platelet PN2/AβPP compared with homozygous mice, showed a corresponding intermediate inhibitory effect on carotid artery thrombosis (data not shown). Last, AβPP KO mice, which lack PN2/AβPP, exhibit the opposite phenotype and are significantly more prothrombotic than wild-type mice both in vitro and in vivo (Figs. 4 and 5, respectively).
Substantial evidence indicates that the primary pathway leading to thrombin formation involves the extrinsic coagulation pathway, which is initiated by tissue factor and includes factor VIIa, factor IXa, and factor Xa as part of multiprotein complexes that assemble on the surfaces of activated and damaged cells (38). Deficiencies in any of these extrinsic coagulation pathway factors in mice result in a strong, and in some cases lethal, hemorrhagic phenotype (39-42). Although the intrinsic coagulation pathway involving factor XIIa and factor XIa appears to be less involved in thrombosis, deficiency of the latter can also be associated with a variable hemorrhagic disorder (43, 44). Regulation of the extrinsic coagulation pathway has been shown to be largely mediated by antithrombin III, activated protein C, and tissue factor pathway inhibitor, which like PN2/AβPP, is a KPI (45-47). During cerebral vascular challenge in Tg-rPF4/APP mice the local activation of platelets causes release of PN2/AβPP (Fig. 3). The suppression of thrombosis in these mice likely results from inhibition at the level of the extrinsic pathway prothrombotic proteinases factor IXa, factor Xa, and tissue factor-factor VIIa, and possibly some contribution through inhibition of factor XIa, by the released PN2/AβPP.
The deletion of the genes for tissue factor pathway inhibitor or protein C in mice results in lethality due to unregulated thrombosis (48, 49). The finding that deletion of the gene for PN2/AβPP in mice is not lethal or did not result in an even more robust prothrombotic phenotype is likely confounded by the presence of endogenous homolog amyloid precursor-like protein-2 (APLP2). The gene for APLP2 was shown to also contain a KPI domain that is 68% identical to the KPI domain contained in PN-2/AβPP (50, 51). It is noteworthy that, similar to PN2/AβPP, mRNA for APLP2 is found in many tissues and is very abundant in brain and in platelets (50, 51). We previously reported that the prothrombotic proteinase inhibitory properties of APLP2 and PN2/AβPP strongly overlapped, and both proteins could inhibit thrombosis in vitro (52). This finding suggests that each of these proteins, abundant in platelets and in brain, may have shared and compensatory activities in regulating thrombosis during cerebral vascular injury. Mice that are deficient for both AβPP and APLP2 are postnatal lethal (53). Although the basis for this lethality in the AβPP/APLP2 double-KO mice is presently unknown, further study is needed to determine whether it involves the overlapping prothrombotic proteinase inhibitory roles or some other redundant function between the two proteins.
Nevertheless, localized increased levels of PN2/AβPP may be associated with antithrombotic pathology. For example, in severe cases of cerebral amyloid angiopathy, as found in hereditary cerebral hemorrhage with amyloidosis Dutch-type, it was shown that there is pathologic accumulation of PN2/AβPP in the amyloid laden cerebral blood vessel walls (54). This accumulation could result in a cerebral vessel wall that possesses an abnormally high antithrombotic potential. Such a localized cerebral thrombotic imbalance could contribute to the hemorrhagic condition that is characteristic of familial cerebral hemorrhagic disorders and in severe cases of cerebral amyloid angiopathy. Similarly, in normal individuals cerebral PN2/AβPP may play an important local role in regulating prothrombotic events during hemorrhagic and ischemic stroke. In any case, the present findings suggest that PN2/AβPP is a significant additional, and perhaps regional, modulator of the complex proteolytic pathways regulating thrombosis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL72553 and NS36645) and the Alzheimer's Association (IIRG-02-3995).
Author contributions: F.X., J.D., and W.E.V.N. designed research; F.X., J.D., J.M., M.L.P., G.R., and K.Z. performed research; F.X., J.M., M.L.P., and W.E.V.N. analyzed data; and F.X., J.D., and W.E.V.N. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: APLP2, amyloid precursor-like protein 2; APTT, activated partial thromboplastin time; Aβ, amyloid β-peptide; AβPP, amyloid β-protein precursor; KO, knockout; KPI, Kunitz-type serine proteinase inhibitor; PN2, protease nexin-2; PRP, platelet-rich plasma; rPF4, rat platelet factor 4.
References
- 1.Selkoe, D. J. (2001) Physiol. Rev. 8, 741-766. [DOI] [PubMed] [Google Scholar]
- 2.Vassar, R., Bennett, B. D., Babu-Khan, S., Khan, S., Mendiaz, E., Denis, P., Teplow, D. B., Ross, S., Amarante, P., Loeloff, R., et al. (1999) Science 286, 735-741. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe, M. S., Xia, W., Ostaszewski, B. L., Diehl, T. S., Kimberly, W. T. & Selkoe, D. J. (1999) Nature 398, 513-517. [DOI] [PubMed] [Google Scholar]
- 4.Esch, F. S., Keim, P. S., Beattie, E. C., Blacher, R. W., Culwell, A. R., Oltersdorf, T., McClure, D. & Ward, P. J. (1990) Science 248, 1122-1124. [DOI] [PubMed] [Google Scholar]
- 5.Wang, R., Meschia, J. F., Cotter, R. J. & Sisodia, S. S. (1991) J. Biol. Chem. 266, 16960-16964. [PubMed] [Google Scholar]
- 6.Ponte, P., Gonzalez-DeWhitt, P., Schilling, J., Miller, J., Hsu, D., Greenberg, B., Davis, K., Wallace, W., Lieberburg, I., Fuller, F. & Cordell, B. (1988) Nature 331, 525-527. [DOI] [PubMed] [Google Scholar]
- 7.Tanzi, R. E., McClatchey, A. I., Lamperti, E. D., Villa-Komaroff, L. L., Gusella, J. F. & Neve, R. L. (1988) Nature 331, 528-530. [DOI] [PubMed] [Google Scholar]
- 8.Van Nostrand, W. E. & Cunningham, D. D. (1987) J. Biol. Chem. 262, 8508-8514. [PubMed] [Google Scholar]
- 9.Van Nostrand, W. E., Wagner, S. L., Suzuki, M., Choi, B. H., Farrow, J. S., Geddes, J. W., Cotman, C. W. & Cunningham, D. D. (1989) Nature 341, 546-549. [DOI] [PubMed] [Google Scholar]
- 10.Multhaup, G., Bush, A. I., Pollwein, P. & Masters, C. L. (1994) FEBS Lett. 355, 151-154. [DOI] [PubMed] [Google Scholar]
- 11.Multhaup, G., Ruppert, T., Schlicksupp, A., Hesse, L., Bill, E., Pipkorn, R., Masters, C. L. & Beyreuther, K. (1998) Biochem. 37, 7224-7230. [DOI] [PubMed] [Google Scholar]
- 12.Schubert, D., Jin, L.-W., Saitoh, T. & Cole, G. (1989) Neuron 3, 689-694. [DOI] [PubMed] [Google Scholar]
- 13.Ghiso, J., Rostagno, A., Gardella, J. E., Liem, L., Gorevic, P. D. & Frangione, B. (1992) Biochem. J. 288, 1053-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitoh, T., Sundsmo, M., Roch, J.-M., Kimura, N., Cole, G., Schubert, D., Oltersdorf, T. & Schenk, D. B. (1989) Cell 58, 615-622. [DOI] [PubMed] [Google Scholar]
- 15.Mattson, M. P., Cheng, B., Culwell, A. R., Esch, F. S., Lieberburg, I. & Rydel, R. E. (1993) Neuron 10, 243-254. [DOI] [PubMed] [Google Scholar]
- 16.Cao, X. & Südhof, T. C. (2001) Science 293, 115-120. [DOI] [PubMed] [Google Scholar]
- 17.Russo, C., Dolcini, V., Salis, S., Venezia, V., Zambrano, N., Russo, T. & Schettini, G. (2002) J. Biol. Chem. 277, 35282-35288. [DOI] [PubMed] [Google Scholar]
- 18.Van Nostrand, W. E., Wagner, S. L., Farrow, J. S. & Cunningham, D. D. (1990) J. Biol. Chem. 265, 9591-9594. [PubMed] [Google Scholar]
- 19.Smith, R. P., Higuchi, D. A. & Broze, G. J. (1990) Science 248, 1126-1128. [DOI] [PubMed] [Google Scholar]
- 20.Schmaier, A. H., Dahl, L. D., Rozemuller, A. J. M., Roos, R. A. C., Wagner, S. L., Chung, R. & Van Nostrand, W. E. (1993) J. Clin. Invest. 92, 2540-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahdi, F., Van Nostrand, W. E. & Schmaier, A. H. (1995) J. Biol. Chem. 270, 23468-23474. [DOI] [PubMed] [Google Scholar]
- 22.Mahdi, F., Rehemtulla, A., Van Nostrand, W. E., Bauer, K. A. & Schmaier, A. H. (2000) Thromb. Res. 99, 267-276. [DOI] [PubMed] [Google Scholar]
- 23.Annich, G., White, T., Damm, D., Zhao, Y., Mahdi, F., Meinhardt, J., Rebello, S., Lucchesi, B., Bartlett, R. H. & Schmaier, A. H. (1999) Thromb. Haemostasis 82, 1474-1481. [PubMed] [Google Scholar]
- 24.Van Nostrand, W. E., Schmaier, A. H., Farrow, J. S. & Cunningham, D. D. (1990) Science 248, 745-748. [DOI] [PubMed] [Google Scholar]
- 25.Bush, A. I., Martins, R. N., Rumble, B., Moir, R., Fuller, S., Milward, E., Currie, J., Ames, D., Weidemann, A., Fischer, P., et al. (1990) J. Biol. Chem. 265, 15977-15983. [PubMed] [Google Scholar]
- 26.Van Nostrand, W. E., Schmaier, A. H., Farrow, J. S. & Cunningham, D. D. (1991) Ann. N.Y. Acad. Sci. 640, 140-144. [DOI] [PubMed] [Google Scholar]
- 27.Tsakiris, D. A., Scudder, L., Hodivala-Dilke, K., Hynes, R. O. & Coller, B. S. (1999) Thromb. Haemostasis 81, 177-188. [PubMed] [Google Scholar]
- 28.Melchor, J. P. & Van Nostrand, W. E. (2000) J. Biol. Chem. 275, 9782-9791. [DOI] [PubMed] [Google Scholar]
- 29.Saporito-Irwin, S. M., Thinakaran, G., Ruffini, L., Sisodia, S. S. & Van Nostrand, W. E. (1997) Amyloid 4, 54-60. [Google Scholar]
- 30.Pratt, C. W. & Monroe, D. M. (1992) BioTechniques 13, 430-433. [PubMed] [Google Scholar]
- 31.Eitzman, D. T., Westrick, R. J., Nabel, E. G. & Ginsburg, D. (2001) Blood 95, 577-580. [PubMed] [Google Scholar]
- 32.Clark, W., Gunion-Rinker, L., Lessov, N. & Hazel, K. (1998) Stroke (Dallas) 29, 2136-2140. [DOI] [PubMed] [Google Scholar]
- 33.Choudhri, T. F., Hoh, B. L., Solomon, R. A., Connolly, E. S., Jr., & Pinsky, D. J. (1997) Stroke (Dallas) 28, 2296-2302. [DOI] [PubMed] [Google Scholar]
- 34.Gardella, J. E., Gorgone, G. A., Munoz, P. C., Ghiso, J., Frangione, B. & Gorevic, P. D. (1992) Lab. Invest. 67, 303-313. [PubMed] [Google Scholar]
- 35.Skrovonsky, D. M., Lee, V. M. & Pratico, D. (2001) J. Biol. Chem. 276, 17036-17043. [DOI] [PubMed] [Google Scholar]
- 36.Zheng, H., Jiang, M., Trumbauer, M. E., Sirinathsinghji, D. J. S., Hopkins, R., Smith, D. W., Heavens, R. P., Dawson, G. R., Boyce, S., Conner, M. W., et al. (1995) Cell 81, 525-531. [DOI] [PubMed] [Google Scholar]
- 37.Cappai, R., Cheng, F., Ciccotosto, G. D., Needham, B. E., Masters, C. L., Multhaup, G., Fransson, L.-A. & Mani, K. (2005) J. Biol. Chem. 280, 13913-13920. [DOI] [PubMed] [Google Scholar]
- 38.Mann, K. G., Butenas, S. & Brummel, K. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 17-25. [DOI] [PubMed] [Google Scholar]
- 39.Toomey, J. R., Kratzer, K. E., Lasky, N. M., Stanton, J. J. & Broze, G. J., Jr. (1996) Blood 88, 1583-1587. [PubMed] [Google Scholar]
- 40.Rosen, E. D., Chan, J. C., Idusogie, E., Clotman, F., Vlasuk, G., Luther, T., Jalbert, L. R., Albrecht, S., Zhong, L., Lissens, A., et al. (1997) Nature 390, 290-294. [DOI] [PubMed] [Google Scholar]
- 41.Kundu, R. K., Sangiorgi, F., Wu, L. Y., Kurachi, K., Anderson, W. F., Maxson, R. & Gordon, E. M. (1998) Blood 92, 168-174. [PubMed] [Google Scholar]
- 42.Dewerchin, M., Liang, Z., Moons, L., Chan, J. C., Carmeliet, P., Collen, D. & Castellino, F. J. (2000) Thromb. Haemostasis 83, 185-190. [PubMed] [Google Scholar]
- 43.Ragni, M. V., Sinha, D., Seaman, F. Lewis, J. H., Spero, J. A. & Walsh, P. N. (1985) Blood 65, 719-724. [PubMed] [Google Scholar]
- 44.Seligsohn, U. (1993) Thromb. Haemostasis 70, 68-71. [PubMed] [Google Scholar]
- 45.Olson, S. T., Bjork, I. & Shore, J. D. (1993) Methods Enzymol. 222, 525-559. [DOI] [PubMed] [Google Scholar]
- 46.Walker, F. J., Sexton, P. W. & Esmon, C. T. (1979) Biochim. Biophys. Acta 571, 333-342. [DOI] [PubMed] [Google Scholar]
- 47.Girard, T. J., Warren, L. A., Novotny, W. F., Likert, K. M., Brown, S. G., Miletich, J. P. & Broze, G. J., Jr. (1989) Nature 338, 518-520. [DOI] [PubMed] [Google Scholar]
- 48.Jalbert, L. R., Rosen, E. D., Moons, L., Chan, J. C., Carmeliet, P., Collen, D. & Castellino, F. J. (1998) J. Clin. Invest. 102, 1481-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang, Z. F., Higuchi, D., Lasky, N. & Broze, G. J., Jr. (1997) Blood 90, 944-951. [PubMed] [Google Scholar]
- 50.Wasco, W., Gurubhagavatula, S., Paradis, M. D., Romano, D. M., Sisodia, S. S., Hyman, B. T., Neve, R. L. & Tanzi, R. E. (1993) Nat. Genet. 5, 95-100. [DOI] [PubMed] [Google Scholar]
- 51.Slunt, H. H., Thinakaran, G., Von Koch, C., Lo, A. C. Y., Tanzi, R. E. & Sisodia, S. S. (1994) J. Biol. Chem. 269, 2637-2644. [PubMed] [Google Scholar]
- 52.Van Nostrand, W. E., Schmaier, A. H., Neiditch, B. R., Siegel, R. S., Raschke, W. C., Sisodia, S. S. & Wagner, S. L. (1994) Biochim. Biophys. Acta 1204, 165-170. [DOI] [PubMed] [Google Scholar]
- 53.von Koch, C. S., Zheng, H., Chen, H., Trumbauer, M., Thinakaran, G., van der Ploeg, L. H., Price, D. L. & Sisodia, S. S. (1997) Neurobiol. Aging 18, 661-669. [DOI] [PubMed] [Google Scholar]
- 54.Rozemuller, A. J. M., Roos, R. A. C., Bots, G. T. A. M., Kamphorst, W., Eikelenboom, P. & Van Nostrand, W. E. (1993) Am. J. Pathol. 142, 1449-1457. [PMC free article] [PubMed] [Google Scholar]