Abstract
Historical introductions of species into new habitats can create rare opportunities to test evolutionary hypotheses, such as the role of natural selection in maintaining traits. This study examines two independent introductions of the African village weaverbird (Ploceus cucullatus) to islands where selection on egg appearance traits is expected to differ markedly from that of the source populations. The color and spotting of village weaver eggs in Africa are highly consistent within clutches, but highly variable between individuals. These two features may be an evolutionary response to brood parasitism. In Africa, weavers are parasitized by each other and by the diederik cuckoo (Chrysococcyx caprius), an egg mimic. African village weavers were introduced one century ago to Mauritius, and over two centuries ago to Hispaniola. Both islands are devoid of egg-mimicking brood parasites. In these two populations, between-individual variation and within-clutch consistency in egg appearance have both decreased, as has the incidence of spotting, relative to the source populations in Africa. These reductions are more pronounced on Hispaniola, the earlier introduction. Such changes support the hypothesis that egg appearance in the African village weaver has been maintained by natural selection as a counteradaptation to cuckoo brood parasitism. These results illustrate that the removal of an agent of selection can sometimes bring about rapid evolutionary consequences.
Keywords: brood parasitism, introduced species, Ploceidae, rapid evolution, trait loss
Species introductions can reveal the operation of natural selection as a mechanism of evolution when environments of source and introduced populations differ in ways that lead to evolutionary predictions (1–3). Evolution in introduced populations can be rapid, even in vertebrates (4), and researchers can use the source population as a control for changes observed in the introduced population (5). This study takes advantage of environmental differences between two source and two introduced populations of the African village weaverbird (Ploceus cucullatus) to test for the role of natural selection in maintaining variation in egg appearance.
Avian brood parasites lay eggs in the nests of other birds. In Africa, the village weaver is a regular host of the diederik cuckoo (Chrysococcyx caprius). The diederik is an obligate brood parasite that has evolved to lay eggs that mimic weaver eggs in appearance. Diederik chicks generally hatch early and remove any other eggs or young from the nest, nullifying the weaver's reproductive attempt (6, 7). Village weavers may also be brood parasites of each other, as indicated by the discovery of mismatched weaver eggs in nests, and more extensive observations in closely related species (8–10). Conspecific brood parasitism is most often observed in species where nests are conspicuous and clustered, as in the village weaver (11). When successfully parasitized by either a cuckoo or a conspecific, a weaver incubates the egg and raises the hatchling to independence.
As a defense against brood parasitism, village weaver females learn the appearance of their own eggs and expel foreign eggs from their nests (12), thereby avoiding much of the cost of parasitism (13). In a test of this egg rejection behavior, Lahti and Lahti (14) switched conspecific eggs among nests of village weavers in West Africa, and observed their responses. Birds rejected eggs in proportion to differences in color and spotting pattern between the foreign eggs and their own.
To maximize effectiveness of egg rejection behavior as a defense against brood parasitism, a village weaver's eggs should possess two features. First, they should be distinctive within the population; weaver eggs should have high between-individual variation, as indeed they do in Africa (9, 15). Although no quantitative comparative study has yet been performed, the level of population variability in egg appearance found in African Ploceus weavers is extreme among birds. The more unusual a weaver egg is in its population, the more likely a parasitic egg laid in its nest will differ from it sufficiently for the host to detect and remove it; whereas a weaver egg of common appearance will be more likely to be matched. This dynamic is expected to maintain a diversity of egg appearance across a village weaver population. Second, a weaver's eggs should be consistent with each other, or have low within-clutch variation (16). High uniformity of eggs within a host clutch minimizes the window of opportunity for the brood parasite: it decreases the number of individuals that could successfully parasitize the host.
Per incidence, cuckoos exert stronger selection for host defenses than conspecifics (17). First, conspecific brood parasitism does not usually ruin the reproductive attempt of the host, because parasitic young usually develop alongside host young. Second, conspecific parasites are also susceptible to parasitism themselves, so individual costs and benefits of parasitism may cancel each other out to some extent. Diederik cuckoos, on the other hand, are expected to coevolve with weavers in egg appearance, maximizing their own reproductive success by matching common weaver eggs, and in turn exerting frequency-dependent selection on weaver egg appearance (18). Likewise, natural selection for egg recognition by hosts is expected to be intense where cuckoo parasitism is a consistent threat (19). Nevertheless, cuckoo (12, 20–22) and conspecific (9, 10) parasitism could both be important agents of selection on village weaver egg appearance and egg rejection behavior in Africa.
The village weaver was introduced from West Africa to the Caribbean island of Hispaniola during the colonial period from before the 1790s (23). A small number of individuals were also introduced from Southern Africa to the island of Mauritius in a single event in 1886 (23). No reports exist of later introductions into either population. On both islands, the weavers are now abundant and presumably experience no gene flow with other populations. Although the diederik cuckoo is a threat to the village weaver throughout the weaver's range in Africa, neither the diederik cuckoo nor any other egg-mimicking brood parasite exists in the two introduced ranges. The only potential interspecific brood parasite of the weaver in either introduced range is the shiny cowbird Molothrus bonariensis, which reached Hispaniola in the 1980s, and whose eggs do not resemble those of Hispaniolan weavers (20).
In this study, I examine these two introduced populations and their source populations to test the hypothesis that variation in egg appearance and uniformity within a clutch have been maintained by natural selection as counteradaptations to cuckoo parasitism. In Africa, where egg-mimicking cuckoo parasitism occurs and perhaps conspecific parasitism, egg color and spotting are used in egg recognition (14). I predicted that, if cuckoo parasitism is an important agent of selection, then after introduction to habitats without cuckoos, variation in weaver egg color and spotting should decrease between individuals and increase within a clutch. Although no direct tests have been performed, there is no reason to suppose that conspecific parasitism is high in Africa and declined precipitously in both introduced populations. Colony sizes and percentages of active nests per colony were similar across all four study sites. On the assumption of similar levels of conspecific parasitism in the source and introduced populations, I predicted no particular changes in egg appearance if conspecific parasitism is an important agent of selection.
Methods
Egg Collection. I collected village weaver clutches 3 days or fewer after clutch completion from the following localities: Janjangbureh Island, The Gambia, West Africa (13°35′ N, 14°40–50′ W; July to August 1999; n = 107); Pietermaritzburg, KwaZulu-Natal, South Africa (29°25–45′ S, 30°25–35′ E; October to December 2000; n = 122); Black River and Rivière du Rempart Divisions, Mauritius (20°00–20'S, 57°20–40′ E; December 2000 to February 2001; n = 52); and Monte Cristi and Valverde Provinces, Dominican Republic, Hispaniola (19°35–45′ N, 71°00–20′ W; April to June 2001; n = 154). Hereafter, Gs and SAs indicate source populations from The Gambia and South Africa, respectively, and Mi and Hi indicate introduced populations from Mauritius and Hispaniola, respectively.
Clutches varied between one and four eggs. One-egg clutches were excluded from within-clutch variation analyses. To prevent site differences in average clutch size from biasing analyses of egg appearance variability, analyses were based on two random eggs (an “effective clutch”) from clutches of more than two eggs.
Measurements. The main methodological objective was to quantify between-female variability and within-clutch uniformity in the four populations. Measurements of egg appearance focused on shape, mass, ground color, and spotting. For each egg characteristic, all measurements across all study sites were performed by a single person. Shells of eggs used in analyses are deposited in the University of Michigan Museum of Zoology.
Length and breadth of each egg was measured with digital calipers to the nearest 0.1 mm. Shape was calculated as the ratio of length to breadth. Mass was measured to the nearest 0.05 with a spring scale.
I matched eggs to color chips in the Villalobos Color Atlas (24) as described (14). I also brought eggshells back to the U.S. and measured eggshell reflectance in the laboratory with an Ocean Optics USB2000 UV-VIS spectrophotometer and ooibase32 software (Ocean Optics, Dunedin, FL). I assessed reflectance at 5-nm intervals over the wavelength range of 300–700 nm with a 200-Hz pulsed xenon light source (Ocean Optics PX-2), and a 400-μ reflection probe (Ocean Optics R400–7) held at a 45° angle 5 mm from the sample. Integration time was set at 250 ms. I standardized measurements with a diffuse tile made of polytetrafluoroethylene that reflects >98% of light over all sampled wavelengths (Ocean Optics WS-1). I performed all measurements under an opaque cloth to avoid an effect of ambient light. A few eggs lacked uniformity in color, in which cases I analyzed the spectra that characterized the largest proportion of the egg's surface area. Reflectance data were reduced by principal components analysis.
I assessed four aspects of eggshell spotting: size, density, and color of spots, and the degree of aggregation at the cap (broad) end of the egg. I ranked each spotted egg in the field into one of three categories for each variable. I later confirmed the consistency of the categorization by obtaining quantitative measurements. For spot size, density, and cap aggregation, 16 representative eggs were measured per level, four from each population. For spot color, reflectance spectra of spots were obtained for five representative eggs per level. In all cases, eggs from different qualitative categories had nonoverlapping quantitative measurements.
Statistical Analysis. Data analysis was performed by using systat (version 10.0, SPSS, Chicago). For continuous variables (relating to egg shape, mass, and color), I compared variation in egg appearance using a variance method and a disparity method. First, I calculated within-clutch and between-individual variances for each population from ANOVA sums of squares. I compared these between source and introduced populations with two-tailed F tests, Bonferroni-adjusted for multiple comparisons. Second, I calculated within-clutch disparity, the difference in value between the two eggs in each effective clutch in a population. I assessed between-individual disparity by calculating the difference between an egg in each clutch and an egg from another clutch in the population, selected at random. I used ANOVA to compare the population means of these disparity values and used the Bonferroni adjustment to obtain significance values for pairwise comparisons. The two methods produced similar results except where noted.
Only the disparity method was appropriate for calculating variation in categorical (egg spotting) variables. I compared population means for within-clutch and between-individual disparity with the Kruskal–Wallis/Mann–Whitney U test. Levels of significance in the Mann–Whitney U test can be distorted when there are too many tied values, which are common in the spotting variables. Therefore, I also recoded spotting disparity within clutches as binary (difference/no difference) and compared populations by a χ2 test for independence. No qualitative differences were found between the two methods, so only Mann–Whitney U results are presented here.
Results
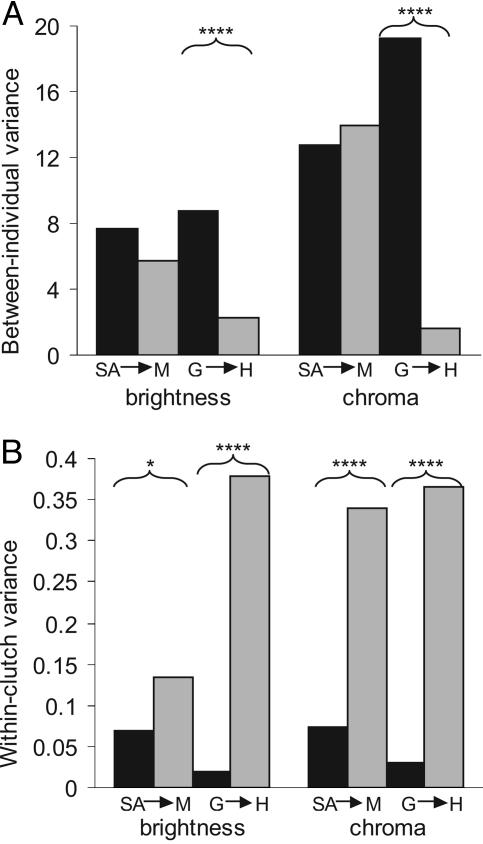
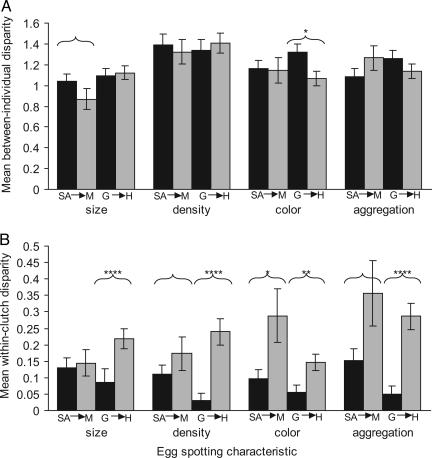
Between-Individual Variation. Principal component analysis reduced the variation in the spectral (color) data to three components. The first factor corresponded closely to spectral brightness, and the second and third factors represented aspects of chromatic variation (as in ref. 25). As predicted, the introduced weaver population on Hispaniola had a lower level of variation between individuals than did the Gambian population in egg color and spotting. Significant differences were found for brightness (color chart variance: F111,143 = 3.83, Fig. 1A; color chart disparity: ANOVA, F3,499 = 12.1, Fig. 2A; spectral variance: PC1, F105,153 = 5.81, P < 0.00001; spectral disparity: ANOVA, F3,433 = 18.5, Fig. 2B), chroma (color chart variance: F111,143 = 11.8, Fig. 1A; color chart disparity: ANOVA, F3,499 = 35.0, Fig. 2C; spectral variance: PC3, F105,153 = 4.51, P < 0.00001; spectral disparity: PC2, F3,431 = 7.5, Fig. 2D), and spot color (Mann–Whitney U = 8,575, Fig. 3A). The introduced population on Mauritius also showed reduced between-clutch variation in comparison to the South African population, but the magnitude of these differences were smaller and most were nonsignificant. A significant difference was found in chroma (spectral variance: PC3, F106,51 = 3.26, P = 0.00003), and there were trends in brightness (spectral disparity: PC1, ANOVA, F3,433 = 18.5, Fig. 2B), chroma (spectral variance: PC2, F106,51 = 3.26), and spot size (U = 3,742, Fig. 3A).
Fig. 1.
Variance in egg color across village weaver populations, calculated from ANOVA sums of squares, based on color charts. SA, South Africa; M, Mauritius; G, The Gambia; H, Hispaniola. Arrows indicate introductions of the species. Significance levels after adjustment to a two-tailed F test and Bonferroni correction: *, P < 0.05; ****, P < 0.00001.
Fig. 2.
Mean disparity in egg color across village weaver populations, based on color charts (left) and spectral analysis (right). Bars indicate SE. Significance levels after Bonferroni correction: ^, P < 0.1; *, P < 0.05; ****, P < 0.00001.
Fig. 3.
Mean disparity in egg spotting characteristics across village weaver populations: size, density, color, and aggregation of spots near the cap (broad) end of the egg. Bars indicate SE. Significance levels after Bonferroni correction: bracket alone, P < 0.1; *, P < 0.05; **, P < 0.01; ****, P < 0.00001.
Within-Clutch Variation. Repeatability of reflectance measurements was low enough (SD of 10 measurements of the same egg was 5.6) to swamp typical within-clutch differences in egg color (mean SD of a clutch in The Gambia = 3.6). Therefore, only color chart data were included in within-clutch analyses. In accordance with predictions, clutches were more variable in the introduced populations than they were in the respective source populations in most measures of egg color and spotting. Significant differences were found in all measures of color and spotting between The Gambia and Hispaniola (brightness disparity: ANOVA, F3,431 = 16.8, Fig. 2E; brightness variance: F143,111 = 19.9, Fig. 1B; chroma disparity: ANOVA, F3,431 = 15.8, Fig. 2F; chroma variance: F143,111 = 11.8, Fig. 1B; spot size: U = 9,514; spot density: U = 9,665; spot color: U = 8,681; spot aggregation: U = 9,657, Fig. 3B). Weavers on Mauritius had less consistent clutches than in South Africa, as predicted, although the differences were again smaller and fewer were significant. Significant differences were found for brightness (variance: F51,121 = 1.93, Fig. 1B), chroma (variance: F51,121 = 4.59, Fig. 1B), and spot color (U = 3,624, Fig. 3B); and two other spotting variables exhibited trends (spot density: U = 3,499; spot aggregation: U = 3,532, Fig. 3B).
Other Measurements. The two African (source) populations did not differ significantly in most egg appearance traits, and directions of differences were not concordant across traits.
Variation in egg shape and mass between individuals did not differ statistically across populations. Within clutches, egg shape did not vary across populations, although variance in egg mass was lower in clutches from The Gambia than in those from Hispaniola (F133,58 = 2.08; P < 0.01).
In analyses of eggs from The Gambia and Hispaniola, I noted instances when an egg appeared inconsistent in color over its surface because of mottling, unpigmented spots, or a darker band in one area of the egg. I identified 16 of 177 eggs (9%) in The Gambia, as compared to 59 of 271 eggs (22%) in Hispaniola (χ2: P = 0.0004) with such inconsistency. This finding affected 14 of 125 clutches (11%) in The Gambia and 33 of 164 clutches (20%) in Hispaniola (χ2: P = 0.042).
The proportion of individuals that laid spotted eggs did not differ between the two African populations, but 16% fewer clutches contained spotted eggs in Mauritius than in South Africa (χ2: P = 0.028), and 14% fewer clutches contained spotted eggs in Hispaniola than in The Gambia (χ2: P = 0.025).
%BI as an Index. The percentage of total variation in an egg appearance characteristic that is between-individual rather than within-clutch (%BI) reflects the effectiveness of that characteristic for egg recognition. In both African (source) populations, almost all (99.1–99.8%) of the variation in egg color (brightness and chroma) was between individuals. For all color and spotting variables, %BI was lower in each introduced population than in its source population. On Hispaniola, %BI was 14–27% lower than in The Gambia for color variables, and 8–16% lower for spotting variables. On Mauritius, %BI was 1–4% lower than in South Africa for color variables, and 3–12% lower for spotting variables.
Discussion
The results support the hypothesis that egg appearance in the African village weaver is maintained by natural selection as a counteradaptation to cuckoo brood parasitism. African populations of the village weaver suffer brood parasitism by the diederik cuckoo and perhaps conspecifics. They lay eggs that vary greatly between individuals but are uniform within a clutch. These features of distinctiveness and consistency in egg appearance permit a female to recognize and eject a foreign egg more readily. In two introduced populations, village weavers are free from cuckoo parasitism, but presumably retain levels of conspecific parasitism similar to their source populations in Africa. In the introduced populations, features of egg appearance that are used in egg recognition are less variable between individuals, and more variable within a clutch, compared to their source populations. Moreover, the incidence of spotting on eggs is also lower in the introduced populations.
Egg Appearance Evolution. Village weavers that were introduced to Mauritius have lost some percentage of the reliability for egg recognition of each egg color and spotting variable. Between-individual variance decreased in spectral chroma, with trends (P < 0.1 after Bonferroni correction) toward increases in brightness and spot size disparity; and within-clutch variation decreased in brightness, chroma, and spot color, with trends toward a decrease in spot density and cap aggregation. The remaining differences in within-clutch and between-individual variation between Mauritius and South Africa, although nonsignificant, were in the predicted direction for all measures of egg color and spotting; the one exception was egg chroma as determined from color charts, its slight change inconsistent with spectral data. As a whole, results from the Mauritian population point to evolution in the direction of lower population variability and higher within-clutch variability, to a small but consistent degree across egg appearance characteristics.
Egg appearance also evolved in Hispaniola, where weavers likewise do not experience the cuckoo parasitism that occurs in West Africa. Between individuals, egg color variation decreased in both brightness and chroma, whether assessed by color charts or spectrophotometry. These differences were readily apparent to a human observer. No white or light-colored eggs were present in Hispaniola, whereas they were common in the other three populations. Variation in spot color also decreased between individuals; changes in the other three spotting variables were not significant, but were in the predicted direction. Within a clutch, variation increased significantly and strongly in all color (brightness and chroma) and spotting variables (size, density, color, and cap aggregation). These changes were obvious to an observer. Unlike in The Gambia, the amount of pigment deposited as ground color in Hispaniola often varied conspicuously within a clutch, and varied twice as often even within an individual egg. However, other aspects of the egg color and spotting pattern were the same across eggs in these clutches indicating that a single female had laid them.
For village weavers, distinctiveness of egg appearance within a population and consistency within an individual's clutch provide cues that enable the recognition of one's own eggs. The utility of these cues is facilitated by between-individual variation in egg appearance, but is hampered by within-clutch variation. Therefore, the observed decline in variation between individuals in the introduced populations is expected if cuckoos had been important in maintaining that variation in Africa. Only two functions for variation in bird eggs have received empirical support: mimicry of host eggs by brood parasites, and egg recognition (12, 26). Likewise, the increase in variation within a clutch in the introduced populations suggests that clutch uniformity is maintained by natural selection for egg recognition. No other function has been demonstrated for uniformity in the appearance of a bird clutch per se, as distinct from uniformity being simply a result of being laid by the same female, or a byproduct of selection for another function such as crypsis (and weaver nests are completely enclosed, concealing the eggs). How the within-clutch variation in color and spotting observed in these populations compares to that of other species is unknown.
Eggs of village weavers in Africa had spots in 74–78% of clutches, but this declined to 58% on Mauritius and to 64% on Hispaniola. This finding raises the possibility that eggshell spotting per se, as distinct from variation among spotting patterns, may be a counteradaptation to brood parasitism. Weavers that lay spotted eggs increase the complexity of their eggs' appearance, which should aid in discriminating their eggs from those of either cuckoos or conspecifics. Moreover, the presence of a few birds in the African populations that lay plain eggs meets an expectation of frequency-dependent selection resulting from cuckoo parasitism: as long as most weavers are laying spotted eggs, most cuckoos should as well, so plain weaver eggs will be distinctive and will permit discrimination against most cuckoo eggs. No evidence exists for any function for egg spotting except parasitic mimicry, crypsis, and egg recognition (27). Because village weavers lay eggs in enclosed nests, only the avoidance of brood parasitism is a likely benefit of spotting.
One unanswered question is why the village weaver has more variable eggs than many other species that are also subject to brood parasitic egg mimicry. Rothstein and Robinson (17) proposed an explanation based on the nest structure of weavers. In most open-nesting birds subject to parasitism, selection for egg appearance is exerted not only by parasites but also by predators. Solar radiation may also cause selection for egg color in these cases (27). Egg variation in open-cup nesters may therefore be constrained by the need for eggs to be cryptic or cool. The enclosed nest of weavers is too opaque for crypsis to be useful, but admits enough light to allow an individual in the nest to recognize eggs (14) and thereby to permit adaptive evolution in egg appearance.
Previous work suggests that the changes in egg color and spotting analyzed in this study are evolutionary. Egg appearance is determined solely by the mother (28), and is consistent throughout her lifetime (21, 29). In experimental studies of other birds, eggshell color and spotting were generally found not to vary with environmental factors, including diet (30, 31). Egg color has been artificially selected during the domestication of poultry (32). Egg color in fowl appears to be under polygenic autosomal control (33), with some color features perhaps inherited through the female line (34).
These findings demonstrate an evolutionary change in egg appearance according to predictions based on the presence of brood parasites. Some collection-based studies have compared variation in egg appearance between populations or species in the presence or absence of brood parasites, with mixed results (35–37). In a few studies, variation in egg recognition has correlated with the presence of brood parasites (e.g., refs 38 and 39), but those differences may be due to plasticity (40, 41). Because the changes in egg appearance observed in this study are likely to be evolutionary, they provide support for the hypothesis that some brood parasites and their hosts are involved in a coevolutionary arms race (42).
Evolutionary Mechanisms. All of the observed changes in egg appearance traits in the introduced populations are predicted by the removal of selection imposed by cuckoo parasitism in the ancestral populations. The changes are more extensive in the older introduction, which is also consistent with this interpretation. Specifically, the changes suggest that the escape from parasitic cuckoos (i) removed the frequency-dependent selection that had resulted in high population variation in egg appearance, (ii) relaxed selection for precise production and deposition of egg pigment that had resulted in high uniformity within a clutch, and (iii) removed selection for spotting.
Viewing the observed evolution of egg appearance as an instance of relaxed selection from the cuckoo does not address the question of what form the evolution took in the introduced populations. In general, three mechanisms may have contributed: (i) bias due to characteristics of the introduced individuals (founder event), (ii) subsequent genetic drift, and (iii) natural selection on egg appearance or correlated traits. Some of the observed changes involve decreases in variability, which is consistent with a founder event. However, other changes, such as the increases in within-clutch variation, are not as readily explicable in such terms. Also, compared to the Hispaniolan population, the Mauritian population descended from a smaller number of individuals more recently, and has always had a smaller population size (23). On this basis, one would predict that a founder event would be more evident on Mauritius than on Hispaniola. On the contrary, the evidence indicates a small degree of change on Mauritius and a much larger degree of change on Hispaniola relative to their source populations.
Subsequent drift and selection are the two other candidate mechanisms for the evolution of egg appearance. Both mechanisms are compatible with the hypothesis that egg appearance changes were made possible by a release from selection by the cuckoo. The cuckoo being absent, the introduced populations would have been able to evolve stochastically (because the populations were small for much of their history) or under another source of selection that had been absent or else superseded by the cuckoo in Africa. Distinguishing between the effects of drift and selection is difficult because clear predictions are not available for either at this time. Weaver eggs in the introduced populations may be under several sources of selection that could have contributed to the observed changes, including the cost of physiological precision in egg pigment production and deposition, any cost of producing or incubating eggs of certain colors, and the cost of producing spots. If these costs exist, they may be similar across populations, but in the presence of the cuckoo the costs are presumably worth the benefits in terms of enhanced egg distinctiveness. In the absence of the cuckoo, selection might reduce these unnecessary expenditures, resulting in decreased clutch uniformity, decreased population variation in egg appearance, and decreased incidence of spotting. When traits such as egg distinctiveness and clutch uniformity fall into disuse, natural selection may operate in another direction or fail to operate on such traits at all, resulting in evolution of the traits away from the states that were functional in a former ecological context.
Acknowledgments
I thank A. Lahti for aiding in the development of the project from the start and performing all field measurements; R. Payne for extensivediscussion and support; R. Alexander, J. Podos, B. Rathcke, B. Hazlett, and two reviewers for comments on the data and the manuscript; and J. Endler and S. Rothstein for helpful input. This work was funded by National Science Foundation Doctoral Dissertation Improvement Grant 0104394, the American Philosophical Society, the University of Michigan Museum of Zoology and Department of Ecology and Evolutionary Biology, the Wilson Ornithological Society, and the American Museum of Natural History.
Author contributions: D.C.L. designed research, performed research, analyzed data, and wrote the paper.
Conflict of interest statement: No conflicts declared.
References
- 1.Johnston, R. F. & Selander, R. K. (1964) Science 144, 548–550. [DOI] [PubMed] [Google Scholar]
- 2.Reznick, D. N., Shaw, F. H., Rodd, F. H. & Shaw, R. G. (1997) Science 275, 1934–1937. [DOI] [PubMed] [Google Scholar]
- 3.Heath, D. D., Heath, J. W., Bryden, C. A., Johnson, R. M. & Fox, C. W. (2003) Science 299, 1738–1740. [DOI] [PubMed] [Google Scholar]
- 4.Berry, R. J. (1964) Evolution (Lawrence, Kans.) 18, 468–483. [Google Scholar]
- 5.Endler, J. A. (1986) Natural Selection in the Wild (Princeton Univ. Press, Princeton).
- 6.Payne, R. B. (2005) Cuckoos (Oxford Univ. Press, Oxford).
- 7.Payne, R. B. (1977) Annu. Rev. Ecol. Syst. 8, 1–28. [Google Scholar]
- 8.Jensen, R. A. C. & Vernon, C. J. (1970) Ostrich 41, 237–246. [Google Scholar]
- 9.Freeman, S. (1988) Ostrich 59, 49–53. [Google Scholar]
- 10.Jackson, W. M. (1992) Auk 109, 435–443. [Google Scholar]
- 11.Rohwer, F. C. & Freeman, S. (1989) Can. J. Zool. 67, 239–253. [Google Scholar]
- 12.Victoria, J. K. (1972) Ibis 114, 367–376. [Google Scholar]
- 13.Payne, R. B. (1997) in Host-Parasite Evolution: General Principles and Avian Models, eds. Clayton, D. H. & Moore, J. (Oxford Univ. Press, Oxford), pp. 338–369.
- 14.Lahti, D. C. & Lahti, A. R. (2002) Anim. Behav. 63, 1135–1142. [Google Scholar]
- 15.Schönwetter, M. (1983) in Handbuch der Oologie, ed. Meise, W. (Academie, Berlin), Vol. 36, pp. 513–549. [Google Scholar]
- 16.Davies, N. B. & Brooke, Mde L. (1989) J. Anim. Ecol. 58, 225–236. [Google Scholar]
- 17.Rothstein, S. I. & Robinson, S. K. (1998) in Parasitic Birds and Their Hosts: Studies in Coevolution, eds. Rothstein, S. I. & Robinson, S. K. (Oxford Univ. Press, New York), pp. 3–56.
- 18.Baker, E. C. S. (1913) Ibis 1, 384–398. [Google Scholar]
- 19.Davies, N. B. (2000) Cuckoos, Cowbirds and Other Cheats (Poyser, London).
- 20.Cruz, A. & Wiley, J. W. (1989) Evolution (Lawrence, Kans.) 43, 55–62. [DOI] [PubMed] [Google Scholar]
- 21.Collias, E. C. (1993) Auk 110, 683–692. [Google Scholar]
- 22.Payne, R. B. (1998) Bioscience 48, 377–386. [Google Scholar]
- 23.Lahti, D. C. (2003) Anim. Biodiv. Conserv. 26, 45–54. [Google Scholar]
- 24.Villalobos-Dominguez, C. & Villalobos, J. (1947) Atlas de Los Colores (Liberia El Ateneo, Buenos Aires, Argentina).
- 25.Endler, J. A. (1990) Biol. J. Linn. Soc. 41, 315–352. [Google Scholar]
- 26.Moksnes, A. (1992) Anim. Behav. 44, 993–995. [Google Scholar]
- 27.Underwood, T. J. & Sealy, S. G. (2002) in Avian Incubation: Behaviour, Environment and Evolution, ed. Deeming, D. C. (Oxford Univ. Press, Oxford), pp. 280–298.
- 28.Lang, M. R. & Wells, J. W. (1987) World's Poultry Sci. J. 43, 238–246. [Google Scholar]
- 29.Collias, E. C. (1984) in Proceedings of the Fifth Pan-African Ornithological Congress (1980), ed. Ledger, J. (South African Ornithol. Soc., Johannesburg), pp. 461–475.
- 30.Goodman, B. R. & Shealy, S. (1977) Poultry Sci. 56, 388–390. [Google Scholar]
- 31.Mikšík, I., Holán, V. & Deyl, Z. (1996) Comp. Biochem. Physiol. B 113, 607–612. [Google Scholar]
- 32.Punnett, R. C. & Bailey, P. G. (1920) J. Genetics 10, 277–292. [Google Scholar]
- 33.Hutt, F. B. (1949) Genetics of the Fowl (McGraw–Hill, New York).
- 34.Punnett, R. C. (1933) Nature 132, 892–893. [Google Scholar]
- 35.Øien, I. J., Moksnes, A. & Røskaft, E. (1995) Behav. Ecol. 6, 166–174. [Google Scholar]
- 36.Soler, J. J. & Møller, A. P. (1996) Behav. Ecol. 7, 89–94. [Google Scholar]
- 37.Aviles, J. M. & Møller, A. P. (2003) Biol. J. Linn. Soc. 79, 543–549. [Google Scholar]
- 38.Davies, N. B. & Brooke, Mde L. (1989) J. Anim. Ecol. 58, 207–224. [Google Scholar]
- 39.Lindholm, A. K. & Thomas, R. J. (2000) Behaviour 137, 25–42. [Google Scholar]
- 40.Brooke, Mde L., Davies, N. B. & Noble, D. G. (1998) Proc. R. Soc. London Ser. B 265, 1277–1282. [Google Scholar]
- 41.Rothstein, S. I. (2001) Anim. Behav. 61, 95–107. [DOI] [PubMed] [Google Scholar]
- 42.Rothstein, S. I. (1990) Annu. Rev. Ecol. Syst. 21, 481–508. [Google Scholar]