Abstract
Biological properties of renal-specific oxidoreductase (RSOR), characteristics of its promoter, and underlying mechanisms regulating its expression in diabetes were analyzed. RSOR expression, normally confined to the renal cortex, was markedly increased and extended into the outer medullary tubules in db/db mice, a model of type 2 diabetes. Exposure of LLCPK cells to d-glucose resulted in a dose-dependent increase in RSOR expression and its enzymatic activity. The latter was related to one of the glycolytic enzymes, myo-inositol oxygenase. The increase in activity was in proportion to serum glucose concentration. The RSOR expression also increased in cells treated with various organic osmolytes, e.g., sorbitol, myoinositol, and glycerolphosphoryl-choline and H2O2. Basal promoter activity was confined to –1,252 bp upstream of ATG, and it increased with the treatment of high glucose and osmolytes. EMSAs indicated an increased binding activity with osmotic-, carbohydrate-, and oxidant-response elements in cells treated with high glucose and was abolished by competitors. Supershifts, detected by anti-nuclear factor of activated T cells, and carbohydrate-response-element-binding protein established the binding specificity. Nuclear factor of activated T cells tonicity-enhancer-binding protein and carbohydrate-response-element-binding protein had increased nuclear expression in cells treated with high glucose. The activity of osmotic-response element exhibited a unique alternate binding pattern, as yet unreported in osmoregulatory genes. Data indicate that RSOR activity is modulated by diverse mechanisms, and it is endowed with dual properties to channel glucose intermediaries, characteristic of hepatic aldehyde reductases, and to maintain osmoregulation, a function of renal medullary genes, e.g., aldose reductase, in diabetes.
Keywords: diabetic nephropathy, hyperglycemia, osmoregulation
Diabetic nephropathy is characterized by hyperplasia/hypertrophy of intrinsic renal cells and increased extracellular matrices (1). These changes are related to the increased cellular flux of glucose intermediaries (2), de novo synthesis of intra- and extracellular advanced glycation end products (3, 4), activation of protein kinase C (5, 6), increased expression of transforming growth factor-β (7), increased activity of GTP-binding and cell-cycle proteins (6, 8), and generation of reactive oxygen species (9, 10), with consequential compromise in renal functions (11, 12). Such complex interrelated cellular signaling events, also involving various forms of MAP/ERK kinases and Smad proteins, have been defined mainly in glomerular cells (13, 14); information relevant to the tubulointerstitial cells, although notably affected, is limited (15, 16). Conceivably, cellular changes in the tubulointerstitium parallel those in the glomerulus, with scarring and thickening of the basement membranes, and they correlate relatively better with the derangements in renal functional parameters (17, 18). Thus, much attention is warranted to the understanding of the pathophysiology of diabetic nephropathy with respect to tubular epithelial biology. A few years ago, we identified a renal tubular protein in mice with streptozotocin-induced diabetes by subtractive suppression hybridization-PCR, and the protein was designated as renal-specific oxidoreductase (RSOR) (19). RSOR is an ≈32-kDa cytosolic protein that is expressed in renal tubules and has an aldoketo reductase (AKR)-3 motif and an NADPH-binding site, as in aldose reductase(AR) and other AKR family members (20). Unlike other AKRs, the RSOR has a potential N-glycosylation site in the AKR-3 motif, suggesting complex structural characteristics and diverse regulatory functions. Interestingly, RSOR has an extensive homology with a glycolytic enzyme known as myoinositol oxygenase (MIOX) (21) that catabolizes myo-inositol (MI) to d-glucuronate, which enters into the pentose phosphate pathway. The MI has a dynamic role for membrane phosphoinositides in providing for the release of second messengers, such as 1,2 diacylglycerol and inositol triphosphate; therefore, the RSOR, normally expressed in the cortex, conceivably modulates phosphoinositide signaling critical for various cellular events of the renal tubular epithelium. Another recently described gene, designated as kidney-specific protein-32 (KSP32), is also homologous to RSOR (22). The KSR-32 gene was discovered serendipitously in rats with acute renal failure. Nevertheless, it seems that RSOR may be largely relevant to glucose metabolism, in view of the original discovery made in the diabetic murine model (19) and the recent data suggesting its biphasic regulation in the kidneys of newborn mice with streptozotocin-induced diabetes (23). This communication describes the RSOR status in db/db mice, a genetic murine model of type 2 diabetes with certain lesions similar to those seen in man (24, 25), and the underlying mechanisms responsible for its gene regulation and MIOX activity.
Materials and Methods
Animals. Six-week-old male diabetic db/db mice (C57BLKs-leprdb/leprdb) and control nondiabetic db/m (C57BLKS/J-leprdb/+) were obtained from The Jackson Laboratory, and CD1 mice, another control genetically unrelated strain, were purchased from Harlan. The mice were killed at 8, 12, and 16 wks of age, their kidneys were used for various studies, and blood was collected for glucose determination.
Expression Studies. Gene and protein expression of RSOR in mouse kidney and cells exposed to glucose and various osmolytes was carried out by Northern and Western blot analyses (19, 23). Spatiotemporal expression of RSOR in the kidneys was assessed by in situ hybridization and immunofluorescence microscopy (19, 23). In addition, expression of carbohydrate-response-element-binding protein (ChREBP) and nuclear factor of activated T cells (NFAT5) in nuclear extract of cells treated with various osmolytes was carried out by immunoblot analyses.
Enzyme Activity Analysis. Kidney cortices were homogenized in a buffer containing 20 mM sodium acetate (pH 6.0), 2.0 mM l-cysteine, 1 mM ferrous ammonium sulfate, 1 mM glutathione, and 1 mM PMSF. The homogenate was centrifuged at 20,000 × g for 30 min, and the supernatant was saved, protein concentration adjusted to 10 mg/ml, and RSOR/MIOX activity measured (26). A typical 1-ml reaction mixture included 50 mM sodium acetate buffer (pH 6.0), 2.0 mM l-cysteine, 1 mM ferrous ammonium sulfate, 60 mM myoinositol, and 100 μl of the supernatant. The reaction was carried out for 15 min at 30°C and terminated by the addition of 0.1 ml of 25% trichloroacetic acid. Precipitated protein was centrifuged, and the amount of d-glucuronic acid in the supernatant was determined.
Cell Culture. The renal proximal tubular epithelial cell line LLCPK was maintained in DMEM (GIBCO BRL) containing 5 mM d-glucose and 10% FBS. The concentration of FBS was reduced to 0.5% when the cells achieved ≈80% confluency for 12 h, and the cells were then treated with d-glucose for 48 h by adjusting its concentration in the medium to 5–50 mM. l-glucose served as control. Cells were also treated with various organic osmolytes, including polyols [(50 mM sorbitol, 50 mM mannitol, 1 mM myo-inositol, and 1 mM chiro-inositol) and tetramethylamines (100 μM glycerophosphorylcholine (GPC) and 50 mM betaine] and 250 mM taurine. The cells were processed for expression studies and MIOX activity. Gene expression in cells treated with 20 μM and 50 μM of H2O2 was assessed by RT-PCR (23). Cells were also processed for preparation of nuclear extract (27) for various EMSAs and expression of translocated transcription factors. Briefly, the nuclear pellet was resuspended in a half-packed nuclear volume of low-salt buffer [20 mM Hepes (pH 7.9)/1.5 mM MgCl2/20 mM KCl/0.2 mM EDTA/25% glycerol (vol/vol)/0.5 mM DTT/0.5 mM PMSF]. An equal volume of high-salt buffer [20 mM Hepes (pH 7.9)/1.5 mM MgCl2/800 mM KCl/0.2 mM EDTA/25% glycerol (vol/vol)/1% Nonidet P-40/0.5 mM DTT/0.5 mM PMSF/4.0 μg/ml leupeptin, aprotinin, and pepstatin] was added. The nuclear suspension was gently shaken for 45 min at 4°C in an orbital shaker, followed by centrifugation at 14,000 × g for 15 min. The supernatant was collected and the protein concentration adjusted to 1 mg/ml.
Isolation of 5′ Flanking Region of RSOR Transcript and Generation of Reporter Constructs. A DNA fragment of ≈1.3 kb flanking the 5′ region was isolated from the mouse Ssp-I genomic library (Clontech) by using adapter AP-2 primer and a RSOR-specific antisense primer (5′-CACATCGACCTTCATCCTGAGGGAGC-3′). The DNA fragment was cloned in pCR 2.1 vector (Invitrogen) and used as a template for generation of serial-deletion PCR products by employing a common antisense primer 5′-GGGGGGGGTACCTGAGGGAGCAGTCAC-3′ and the following sense primers: 5′-GGGGGGGGTACCATTGAGTTATTCCCTT-3′ (–1,252 to –1), 5′-GGGGGTACC CTTCTCTCTCCGAGGGTC-3′ (–858 to –1), 5′-GGGGGTACCTATAGGGAGGGAAG ATTCTA-3′ (–544 to –1), 5′-GGGGGTACCGGTGGGTGAGGTCTGCCT-3′ (–476 to –1), and 5′-GGGGGTACCCTGGCTCTCTAGTGTGGCTC-3′ (–338 to –1). A KpnI site, GGTACC (underlined), was included in the primers. Another construct (–1,252 to –144) was generated by using the above –1,252-bp sense primer and another antisense primer, 5′-GGGGGGGTACCCTCCTTCCTCCTGACCTC-3′. Products were cloned into pCR2.1 and subcloned into pGL3-basic vector (Promega). The inserts were sequenced and their orientation confirmed by using respective vector-specific sense (5′-CTAGCAAAATAGGCTGTCCC-3′) and antisense (5′-CTTTATGTTTTTGGCGTCTTCCA-3′) primers. The upstream 5′ flanking sequence (up to ≈3 kb) of mouse RSOR and its homologues were retrieved from Ensembl bioinformatics (www.ensembl.org). Transcription-factor-binding motifs and promoter predication were searched at the following web sites: www.motif.genome.ad.jp/MOTIF.html, www.genomatix.de, and http://thr.cit.nih.gov/molbio/proscan.
Tansfection of Cells and Luciferase Assays. LLCPK1 (1 × 106 cells) were seeded onto 30-mm dishes in DMEM and maintained to achieve ≈80% confluency. Transfection was carried out by using 10 μl of Lipofectamine (Life Technologies) and 3 μg of reporter plasmid constructs. Cotransfection of 1 μg of pcDNA-rLUC (renilla luciferase) was used as optimized equalization control. Assays for both renilla (rLUC) and firefly luciferase (LUC) activity were performed by using a Dual Luciferase kit (Promega). Basal promoter activity was determined in cells transfected with various reporter constructs and maintained in 5 mM glucose. To assess the effect of organic osmolytes on the promoter activity, the medium was replaced with opti-MEM (Invitrogen) containing various concentrations of osmolytes.
EMSA. Single-stranded sense and antisense oligonucleotides were synthesized, and their sequences are included in Table 1. Double-stranded annealed oligonucleotides were generated and end-labeled with γP32–ATP by using T4-polynucleotide kinase (Promega). Unlabeled oligonucleotides served as control. The binding reaction was carried out in a 20-μl solution of binding buffer [50 mM Tris·HCl (pH 8.0)/750 mM KCl/2.5 mM EDTA/0.5% Triton X-100/62.5% glycerol (vol/vol)/1 mM DTT], 1–3 μg/μl poly-[dI-dC], 1 pmol/μl probe, and 10 μg of nuclear protein for 30 min at 24°C. For competition experiments, a 5- to 50-fold excess of cold unlabeled oligonucleotides was added. For supershift experiments, 1 μg of antibody (anti-ChREBP and -NFAT5) was included in the reaction mixture and analyzed by 7.5% nondenaturing polyacrylamide gels, followed by autoradiography.
Table 1. Oligomers used in electophoretic mobility-shift assay.
| Element | Range | Sequence |
|---|---|---|
| OsRE-851 | -861 to -832 | gccagttgagggaaaccctgtaagtatcaa* |
| OsRE-1,239 | -1,250 to -1,221 | ccctttataagggaaaatcaaacaaacaaa |
| OsRE-1,239 | mutant | ccctttataag---------aacaaacaaac |
| OsRE-1,425 | -1,437 to -1,408 | ctaaaacctctggaaactaacagtgttggc |
| OsRE-1,746 | -1,756 to -1,727 | ttactaagtgggaaaataatgttttgtagg |
| OsRE-2,029 | -2,039 to -2,010 | gtcttaaaaaggaaaatatttgccaggcag |
| OsRE-2,622 | -2,632 to -2,603 | tttaatgcaaggaaactgagctgaaggaac |
| ChRE-2,400 | -2,405 to -2,346 | tgcctcacgtgctaactca |
| ChRE-1,063 | -1,074 to -1,044 | gtttttcaagacacggtttctctgtgtagc |
| ChRE-1,063 | mutant | gtttttcaagacacgggttctctgtgtagc |
| Human ChRE | -1,380 to -1,345 | gagcacgtgacctacccgtgttgggacacgtgagg |
| AP1-1,442 | -1,448 to -1,424 | ataatgaatgactaaaacctctgg |
| Sp1-2,133 | -2,136 to -2,114 | cccccccccgccccccagagct |
| STRE-659 | -665 to -645 | gagggggcaggggaggtcta |
| OxRE-865 | -865 to -843 | taaagccagttgagggaaaccc |
The sequences are written as 5′-3′ in sense orientation. The motifs are underlined, and mutations are in boldface type.
Results
RSOR Expression in db/db Mice. In situ hybridization revealed the RSOR gene localized to outer renal cortical tubules at 8 wks of age in control animals (CD1 and db/m) and did not change up to 16 wks (Fig. 1A, ↔). In db/db mice, the expression was similar to controls, but it increased in its intensity and extended into the outer medulla by 16 wks (Fig. 1B, ↔). Comparable observations were made by immunofluorescence, where protein expression was confined to cortical tubules (Fig. 1C, ↔), whereas, in db/db mice, it spanned into the outer medulla (Fig. 1D, ↔). Northern (Fig. 1E) and Western (Fig. 1F) blots indicated an increase in RSOR expression (arrows) in db/db mice compared with controls, although db/m mice had a higher expression than the genetically unrelated CD1 mice.
Fig. 1.
Expression of RSOR in mice. In situ hybridization (A and B) and immunofluorescence (C and D) indicate RSOR localized to tubules of the outer renal cortex in CD1 or db/m mice (A and C, ↔). In db/db mice, the expression is markedly increased and extends into the medulla (B and D, ↔). (E and F) Northern and Western blots depicting RSOR expression (arrows) in CD1, db/m, and db/db mice at 16 wks. An increase in RSOR expression is seen in db/db mice, whereas β-actin (arrowheads) is unchanged.
RSOR Expression in LLCPK Cells. A basal RSOR expression was observed in LLCPK cells cultured in DMEM containing 5 mM d-glucose. Northern (Fig. 2A) and Western (Fig. 2D) blots revealed a dose-dependent increased RSOR expression up to 30 mM d-glucose (arrows), with no further significant increase at 40 or 50 mM. β-actin expression was unchanged. Under high-glucose (30 mM) ambience, a marked increase in immunofluorescence was observed in cells stained with anti-RSOR antibody (Fig. 2C) compared with control (Fig. 2B).
Fig. 2.
RSOR expression in LLCPK cells treated with 5–30 mM d-glucose. Northern (A) and Western (D) blots indicate a dose-dependent increase in RSOR (arrows). The arrowhead in D represents a sample application site. (B and C) Increased in situ RSOR expression with 30 mM d-glucose.
RSOR/MIOX Activity in Mouse Kidneys and LLCPK Cells. Initially, the MIOX assay was standardized by using mouse recombinant RSOR. Basal enzyme activity in the kidneys of CD1 mice at 8 wks of age was designated as 100%, and the relative percent increase in various strains of mice is described (Fig. 3A). A mild increase in activity was observed with aging in CD1 and db/m mice. The db/db mice had a relatively high activity at 8 wks of age, and it increased remarkably at 12–16 wks. Accentuation in the RSOR/MIOX activity was proportional to the degree of hyperglycemia. Similarly, a dose-dependent increase in expression was also observed in cells treated with d-glucose (Fig. 3B).
Fig. 3.
RSOR/MIOX activity in kidneys and LLCPK cells. (A) Enzyme activity in CD1, db/m, and db/db mice at 8, 12, and 16 wks of age. An increase in activity is observed at 16 wks in db/db mice in proportion to serum glucose levels. A dose-dependent (5–40 mM) increase in enzyme activity is observed in cells treated with d-glucose (B). Basal activity in CD1 mice at 8 wks or cells treated with 5 mM d-glucose is designated as 100%. Data are derived from six animals or experiments.
Isolation of 5′ Flanking Region of Mouse RSOR and Promoter-Activity Analyses. The highest basal promoter activity, defined as a relative luminescence unit (RLU) was observed when the whole intact pGL3–1,252-bp reporter construct was used (Fig. 4A). The remaining deletion constructs yielded variable basal promoter activity. Intriguingly, deletion of the –144-bp region flanking ATG drastically reduced activity. The –1,252-bp construct included GATA 1, 2, and 3 factors, CCAAT-enhancer-binding protein sequences, E-, GC-, and TATA-box motifs, and cap signals for transcription initiation. However, osmotic, carbohydrate-(glucose), stress-, and oxidant-response elements (OsRE, ChRE, STRE, and OxRE, respectively) were localized upstream of –600 bp (Table 1). By using this construct, a 5- to 10-fold increase in RLU was observed in cells exposed to high d-glucose (30 mM) and various organic osmolytes with different biological properties (Fig. 4B). The effect was especially notable with high glucose, myo-inositol, GPC, and betaine. The pGL3–898 and –544 exhibited an only 1- to 2-fold increase in RLU with the above treatments (Fig. 4 C and D). Examination of a 3-kb upstream genomic sequence revealed several OsREs and ChREs and additional binding motifs, i.e., Sp1, AP1, upstream stimulating factor, and NFAT5, the latter two being involved in glucose metabolism and osmoregulation.
Fig. 4.
Effect of osmolytes on RSOR promoter activity. Promoter activity was assessed by luciferase assay and expressed as relative luminescence units (RLU). (A) Data reflecting the effect of various osmolytes on the pGL3-1252 promoter activity, notably (8- to 10-fold) with the treatment of d-glucose, myoinositol, GPC, and betaine. The other two deletion constructs, pGL3-898 and -544, had an only 1- to 2-fold increase in activity (B and C).
Characterization of OsREs by EMSA. NFAT5 is also known as OsRE-binding protein (OsREBP) or tonicity-responsive-enhancer-binding protein, with consensus sequence of binding motif as: NGGAAAWDHMN (the minimal OsRE motif is underlined) (28). Minimal GGAAA motif was present at six positions in the mouse RSOR 5′ flanking sequence (Table 1). Oligonucleotides with this sequence were prepared and used for EMSA. A marked increase in the binding activity of tonicity-binding protein was observed with an oligonucleotide containing OsREs at the –1,239-bp position in cells treated with various osmolytes (Fig. 5A, lanes 2–9, arrow) compared with cells exposed to low 5 mM d-glucose (Fig. 5A, lane 1). The intensity of the shifted band was especially notable in cells treated with high d-glucose (30 mM), myoinositol, GPC, and betaine (Fig. 5A, lanes 2, 6, 7, and 8). The band supershifted to a higher position with anti-NFAT5 antibody included in the binding assay of cells treated with high glucose (Fig. 5A, lane 10). Binding was dose-dependent with increasing concentration of d-glucose (5–50 mM) (Fig. 5B, lanes 1–3). Deletion of motif GGAAA in the –1,239 OsRE mutant abolished the binding activity (Fig. 5C), thus reinforcing the specificity of binding. Examination of all six OsREs, stretching from –851 to –2622 bp (Table 1), revealed that their binding activity is not uniform; rather, it occurs more efficiently at alternate positions, suggesting skipping during transcriptional activation by d-glucose (Fig. 5D, lanes 1, 3, and 5 vs. lanes 2, 4, and 6). This alternate binding-activity pattern is not exclusive to d-glucose (Fig. 5 E vs. F, lanes 1 and 2) but is also observed with myo-inositol treatment (Fig. 5 E and F, lanes 3), when the binding activities of OsREs at positions –1,239 and –851 bp were compared.
Fig. 5.
Characterization of OsREs by EMSA. (A) Effect of osmolytes on binding activity of –1,239-bp OsRE oligonucleotide, seen as a shifted band (arrow). Lanes 1–9 show 5 mM d-glucose, 30 mM d-glucose, 50 mM sorbitol, 50 mM mannitol, 1 mM chiro-inositol, 1 mM myo-inositol, 0.1 mM GPC, 50 mM betaine, and 250 mM taurine. A supershift (SS) band (*) is observed with anti-NFAT5 antibody in cells treated with 30 mM d-glucose (lane 10). (B) Dose-dependency with the treatment of d-glucose (5, 30, and 50 mM, lanes 1–3, respectively). Mutation of –1,239-bp OsRE oligonucleotide abolished the binding activity (C, lanes 1–3). (D) Data of alternate binding-activity pattern of various OsRE oligonucleotides, i.e., –851, –1,239, –1,425, –1,746, –2,029, and –2,622 bp (lanes 1–6). (E and F) Comparative binding data of OsRE-1,239 and -851 oligonucleotides in cells treated with 5 and 50 mM d-glucose (lanes 1 and 2) or 1 mM myo-inositol (lane 3), confirming alternate binding-activity pattern.
Induction of Cytoplasmic RSOR and Nuclear NFAT5 in Cells Treated with Various Osmolytes. Western blot analyses indicated that, compared with low 5 mM d-glucose, the cells exposed to various osmolytes had relatively high expression of RSOR (Fig. 6A), and, like the EMSA results (Fig. 5A, lanes 2–9), a markedly accentuated expression was seen with high 30 mM d-glucose, sorbitol, myo-inositol, and betaine (Fig. 6A, lanes 2, 3, 6, and 8). Concomitant with the up-regulation of RSOR, NFAT5 expression was also increased (Fig. 6C), suggesting a modulation of RSOR by the OsREBP, i.e., NFAT5.
Fig. 6.
Effect of osmolytes on RSOR and NFAT5 expression. (A) Western blot shows increased expression of RSOR (arrow) with the treatment of various osmolytes as described in Fig. 5 (lanes 1–9). A relatively high up-regulation of RSOR is seen with glucose, sorbitol, myo-inositol, and betaine treatment. The arrowhead represents a sample application site. The β-actin expression is unchanged (B). (C) Increased expression of tonicity-responsive-enhancer-binding protein (NFAT5, Mr ≈ 170 kDa) with the treatment of high glucose, sorbitol, mannitol, and myo-inositol (lanes 2–5, arrow) compared with control, 5 mM d-glucose (lane 1).
Characterization of Glucose/ChRE. ChRE motifs conferring glucose/carbohydrate responsiveness are the repeat of two E boxes with consensus CACGGG/CCCGTG or palindromic CACGTG sequences with 5-bp spacing (29). Also, degenerate E-box motif sequences can also confer glucose responsiveness. In mouse RSOR, the CACGTG motif is present at –2,400 bp. In its human homologue, the palindromic E-box motif (CACGTG) separated by 17 bp and a degenerate E-box motif (CCCGTG) motif with 5-bp spacing are present. Another putative ChRE motif CACGGT is present in all species and was used for glucose responsiveness by EMSA (Table 1). When –2,400 ChRE was used, a dose-dependent increase (5–50 mM d-glucose) in binding activity was observed (Fig. 7A, lanes 1–4, arrow). l-glucose treatment also yielded mild activity (Fig. 7B, lanes 1–4). With increasing concentration (5- to 10-fold) of competitor unlabeled probe, the binding efficiency was notably reduced (Fig. 7C), indicating the specificity of ChRE. Binding efficiency was almost identical in cells treated with the same concentration (50 mM) of d-glucose or sorbitol (Fig. 7D, lane 2 vs. lane 3), irrespective of the usage of –1,063-bp ChRE (Fig. 7E). Binding was completely abolished with a 50-fold excess of cold probe (Fig. 7 D and E, lanes 4). Mutation in the –1,063-bp ChRE notably reduced the binding (Fig. 7F), conferring the functionality, in terms of glucose responsiveness, of the motif CACGGT. When the oligonucleotide stretching across E-box motifs was used, results almost similar to those of –2,400- or –1,063-bp ChRE were observed in cells treated with 50 mM d-glucose or sorbitol, although efficiency of binding was less (Fig. 7 G vs. D/E, lanes 2 and 3). Nevertheless, binding was specific, because it was reduced by the competitor (Fig. 7G, lane 4), and a supershift was detected with anti-ChREBP antibody (Fig. 7G, lane 5). Moreover, like the up-regulation of RSOR and NFAT5 by organic osmolytes, an increased expression of ChREBP in cells treated with 50 mM d-glucose, sorbitol, and myo-inositol was observed (Fig. 7H, lanes 2, 3, and 4), authenticating the functionality of ChREs in the RSOR gene.
Fig. 7.
Characterization of ChREs and E box by EMSA. (A) A dose-dependent (5, 15, 30, and 50 mM d-glucose, lanes 1–4, respectively) increase in binding (arrow) with –2,400-bp ChRE oligonucleotide. Mild binding is seen with 15, 30, and 50 mM l-glucose (B, lanes 1–3) and is reduced with a 5- to 10-fold excess of unlabeled oligonucleotide (C, lanes 2 and 3) compared with 30 mM d-glucose (lane 1). Comparative EMSA analyses (D and E) indicate that the –2,400-bp ChRE has a relatively higher binding activity than –1063 ChRE in cells treated with 15 and 50 mM d-glucose and 50 mM sorbitol (D vs. E, lanes 1–3), which is abolished by a 50-fold-excess oligonucleotide (lane 4). Mutation of –1,063 oligonucleotide reduced binding activity in cells treated with glucose and sorbitol (F, lanes 1–4). Analyses of the ChRE E box yielded similar binding activity, as seen in comparative studies (G, lanes 1–4), and the band supershifted to a higher position with anti-ChREBP antibody (lane 5, arrowhead). (H) Increased ChREBP protein (≈95 kDa) expression (arrow) in cells treated with 50 mM d-glucose and sorbitol, and 1 mM myo-inositol (lanes 2–4) compared with control 5 mM d-glucose (lane 1).
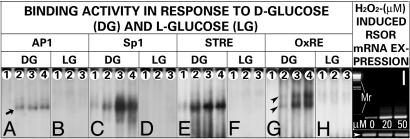
Characterization of AP1, Sp1, STRE, and OxRE. A dose-dependent up-regulation of RSOR mRNA expression in cells exposed to H2O2 was seen (Fig. 8I). In view of this finding, consensus sequences of STRE, OxRE, and relevant transcription factors, e.g., AP1 and Sp1, were used to generate oligonucleotides (Table 1) for EMSA. A dose-dependent binding activity was observed in cells treated with 5–50 mM d-glucose, when incubated with AP1, Sp1, and STRE oligonucleotides (Fig. 8 A, C, and E, lanes 1–4, arrows). No binding activity was observed with cells treated with l-glucose (Fig. 8 B, D, and F). Next, the mechanism(s) directly related to the oxidant stress in the induction of RSOR were investigated. In this regard, TAAAGCCCCTGCGTTTGCTGGG, a sufA operon oxidant stress element, and its consensus AYCCTCA/TRAGAAA, an oxygen-response element, relevant to the biology of redox proteins is described in refs. 30 and 31. So consensus motif TAAAGCCAGTTGAGGGAAACCC, at position –865 in RSOR, was analyzed. A dose-dependent increased binding activity was observed with increased concentrations of d-glucose (Fig. 8G) and was seen as a band of doublets (arrowheads). No activity was seen in cells treated with l-glucose (Fig. 8H), suggesting that oxidant stress is specific to d-glucose.
Fig. 8.
Characterization of AP1, Sp1, STRE, and OxREs by EMSA. A dose-dependent increased binding activity with oligonucleotides for AP1 (A), Sp1 (C), STRE (E), and OxRE (H) is observed with treatment of 5, 15, 30, and 50 mM d-glucose (lanes 1–4, respectively). Cells treated with l-glucose exhibit no binding (B, D, F, and H). Binding with OxRE is seen as a doublet of shifted band (G, arrowheads). (I) Increased mRNA expression of RSOR in cells exposed to 20 and 50 μMH2O2, whereas β-actin is unchanged.
Discussion
Conceivably, the signaling events initiated in response to high-glucose ambience are similar in glomerular and tubular cells (15, 32), e.g., induction of the polyol pathway (2), which is regulated by aldose reductase (AR), an enzyme mostly expressed in the renal medulla, with increased expression in diabetes mellitus (20, 33). Likewise, the expression of RSOR, a cortical enzyme, although bearing no direct relationship with the polyol pathway, was remarkably increased in parallel to the degree of hyperglycemia in db/db mice (Fig. 1). Moreover, exposure of LLCPK cells to high glucose, leading to a dose-dependent increase in the RSOR expression (Fig. 2), would indicate that RSOR is potentially involved in glucose-intermediary metabolism. We demonstrated that RSOR has NADPH-binding activity (19). However, because RSOR has homology to MIOX, and the recombinant RSOR is able to catabolize MI, a byproduct of the glycolytic pathway, the MIOX activity was investigated in vivo in db/db mice and LLCPK cells subjected to high-glucose ambience. Data indicated that MIOX activity increases in proportion to the degree of hyperglycemia in db/db mice and the concentration of glucose in the media (Fig. 3). These observations strengthen the notion that RSOR/MIOX is involved in the glycolytic pathway. In diabetes, the depletion of intracellular myo-inositol pools related to increased synthesis of sorbitol from glucose with accentuated AR activity in the renal medulla and increased urinary excretion of MI to maintain homeostasis inside the cell is described in ref. 34. Thus, the increased RSOR/MIOX activity in the renal cortex in diabetes can be ascribed to the maintenance of homeostasis and osmoregulation in the kidney by catabolizing glucose intermediaries, e.g., myo-inositol. It is well known that osmotic stress occurs mainly in the renal medulla, where genes for transporters of myo-inositol (SMIT), betaine (BGT1) and taurine (TauT), and enzyme AR are expressed (33). However, no such genes are expressed in the cortex. The fact that, in db/db mice, RSOR expression, normally localized to the cortex, extended into the medulla (Fig. 1) lends credence to the notion that this enzyme may be responsive to various osmolytes. The data elucidating increased promoter activity with exposure of LLCPK cells to high d-glucose (30 mM) and various osmolytes, including sorbitol, myoinositol, GPC, betaine, and taurine, indeed suggest that this enzyme is endowed with glycolytic and osmoregulatory properties (Fig. 4). In line with this finding are the observations of concomitantly increased cytosolic/nuclear protein expression of RSOR/MIOX and NFAT5, the most prominent being with the treatment of d-glucose, sorbitol, myoinositol, and betaine (Fig. 6). Here, the question that needs to be addressed is how high-glucose ambience or hyperglycemia modulates that activity or expression.
NFAT5 belongs to the family of transcription factors and is also known as OsREBP or tonicity-responsive-enhancer-binding protein (TonEBP) (28). Upon stimulation by hypertonicity and following phosphorylation, OsREBP and TonEBP rapidly translocate into the nucleus and bind to OsREs in the promoter region of osmoprotective genes to stimulate transcription. Such DNA–protein interactions were readily observed, as reflected by gel-shift assays in nuclear extracts of the cells exposed to osmolytes (Fig. 5A). The specificity of interaction was elucidated by supershift with anti-NFAT5 antibody and dose-dependent exposure of d-glucose and mutation in the consensus sequence of OsREs (Fig. 5 A–C). The RSOR/MIOX gene has six OsREs, and, intriguingly, the binding activities of oligonucleotides were not uniform when their mole-to-mole ratios and the dose-dependence of osmolytes were compared (Fig. 5 D–F). The binding activities were confined to the alternate consensus sequences of OsREs of the RSOR gene. Such a phenomenon has not been described for AR or other osmoprotective genes. This finding may mean that there is a crosstalk among various segments of the gene, so that, when one region is active, the next one is dormant. Alternatively, different transcription factors bind to various OsREs. In addition to the osmoregulatory adaptive response, one can envision that increased degradation of myo-inositol by RSOR/MIOX may be another accessory adjustment to maintain homeostasis and may well be a transcriptional event regulated by ChREBP.
ChREs or glucose-response elements are binding sites for the basic helix–loop–helix leucine zipper family of transcription factors, such as ChREBP; the latter are predominantly expressed in hepatic enzymes involved in glycolysis and lipid metabolism (35). Because RSOR/MIOX seems to be involved in glycolysis, such binding proteins/transcription factors may be present in the kidney, as indicated by immunoblot analyses of nuclear extracts of LLCPK cells (Fig. 7H). Moreover, the fact that its expression increased with the treatment of high glucose or myo-inositol indicates that ChREBP is functionally active. The functionality was also assessed by EMSA using ChREs consensus oligonucleotides localized to the –2,400- and –1,063-bp regions. A dose-dependent binding activity abolished by mutation and competitor authenticated the functionality of carbohydrate-binding sequences in the RSOR/MIOX gene (Figs. 7 A–F). Related to ChRE are the consensus sequences of E boxes recently described in promoters of various genes, e.g., UbA52, ubiquitin ribosomal fusion protein, and TGF-β1, affected by high-glucose ambience (36, 37). When consensus sequences are used, a dose-dependent binding activity in cells exposed to d-glucose or sorbitol/myo-inositol was observed (Fig. 7G, lanes 1–3). The fact that the binding was abolished with the competitor and the band supershifted to a higher molecular weight position (Fig. 7G, lanes 4 and 5) lends support to the theory that the ChREs and E box are relevant to the transcription of the RSOR/MIOX gene involved in the glycolytic pathway.
During glycolysis, the cell undergoes various forms of stresses, when subjected to high-glucose ambience with inductive transcription of a wide variety of genes (38). In addition to glycative and osmotic stress, the oxidative stress related to reactive oxygen species has been well described in the literature (2, 9). Various elements relevant to stress-induced transcription include STRE, AP1, Sp1, OxRE, HNF-4, and upstream stimulating factor. STRE and OxRE are present in the 5′ flanking region of RSOR/MIOX, in addition to AP1 and Sp1. The latter two have been described to be involved in the glucose-induced promoter activation of TGF-β1, a key cytokine responsible for evolution of glomerular lesions in diabetes (7). Consensus sequences for AP1 and Sp1 are also present in RSOR/MIOX, and the fact that a binding activity was detected by EMSA (Fig. 8 A–D) suggests that AP1 and Sp1 modulate the transcription of this enzyme and, thereby, the pathobiology of renal tubules. In regard to STRE, AP1 and Sp1 have been described in genes containing an E box responsive to hyperglycemia, e.g., UbA52 (36), and these promoter elements also seem to be functional in this enzyme, as indicated by DNA–protein interactions (Fig. 8 E and F). The most interesting consensus elements identified in the RSOR/MIOX gene were OxREs relevant to oxidant stress, which is believed to be the common denominator in diabetic pathobiology in target tissues (9). OxREs had a similar consensus sequence recently described in the bacterial sufA operon encoding iron–sulfur assembly proteins induced by superoxide generators and H2O2 (30, 31). Our findings indicated that these OxREs are, indeed, operative in eukaryotic cells subjected to oxidative stress induced by d-glucose (Fig. 8 G and H). Moreover, H2O2-induced increased expression (Fig. 8I) would corroborate the data of d-glucose-induced up-regulation of RSOR/MIOX. Here, it is worth pointing out a potential paradox that a decreased RSOR/MIOX expression has been reported in acute renal failure (39), where tubular cells are under extreme oxidant stress (40). It may be that expression of the enzyme depends on the degree or type of oxidant stress (cytosolic vs. membrane vs. mitochondrial); nevertheless, it seems that OxREs are functionally active in RSOR/MIOX promoter.
In conclusion, RSOR/MIOX gene transcription is modulated by diverse elements localized within its promoter. Although localized in the renal cortex, RSOR/MIOX gene transcription is linked to the biology of osmoregulation, a function of genes expressed in the medulla, e.g., AR. Intriguingly, RSOR/MIOX, a myo-inositol-catabolizing cortical enzyme, undergoes an adaptive response to glucose challenge via ChRE elements described in the genes of hepatic enzymes regulating glycolysis/lipolysis.
Acknowledgments
This work was supported by National Institutes of Health Grants DK28492 and DK60635.
Author contributions: J.W. and Y.S.K. designed research; B.N., P.X., S.A., Q.Y., and L.S. performed research; A.T. analyzed data; and F.R.D., S.S.C., and Y.K. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: AR, aldose reductase; ChRE, carbohydrate-response element; ChREBP, ChRE-binding protein; GPC, glycerophosphorylcholine; MI, myo-inositol; MIOX, MI oxygenase; NFAT, nuclear factor of activated T cells; OsRE, osmotic-response element; OsREBP, OsRE-binding protein; OxRE, oxidant-response element; RSOR, renal-specific oxidoreductase; STRE, stress-response element.
References
- 1.Parving, H.-H., Osterby, R. & Ritz, E. (2000) The Kidney, ed. Brenner, B. M. (Saunder, Philadelphia), pp. 1731–1773.
- 2.LeRoith, D., Taylor, S. I. & Olefsky, J. M. (2004) Diabetes Mellitus: A Fundamental and Clinical Text (Lippincott William & Wilkins, Philadelphia), 3rd Ed.
- 3.Voziyan, P. A., Khalifah, R. G., Thibaudeau, C., Yildiz, A., Jacob, J., Serianni, A. S. & Hudson, B. G. (2003) J. Biol. Chem. 278, 46616–46624. [DOI] [PubMed] [Google Scholar]
- 4.Jakus, V. & Rietbrock, N. (2004) Physiol. Res. 53, 131–142. [PubMed] [Google Scholar]
- 5.Whiteside, C. I. & Dlugosz, J. A. (2002) Am. J. Physiol. 282, F975–F980. [DOI] [PubMed] [Google Scholar]
- 6.Lin, S., Sahai, A., Chugh, S. S., Pan, X., Wallner, E. I., Danesh, F. R., Lomasney, J. W. & Kanwar, Y. S. (2002) J. Biol. Chem. 277, 41725–41735. [DOI] [PubMed] [Google Scholar]
- 7.Ziyadeh, F. N. (2004) J. Am. Soc. Nephrol. 15, S55–S57. [DOI] [PubMed] [Google Scholar]
- 8.Danesh, F. R., Sadeghi, M. M., Amro, N., Philips, C., Zeng, L., Lin, S., Sahai, A. & Kanwar, Y. S. (2002) Proc. Natl. Acad. Sci. USA 99, 8301–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brownlee, M. (2000) Nature 414, 813–820. [DOI] [PubMed] [Google Scholar]
- 10.Schrauwen, P. & Hesselink, M. K. (2004) Diabetes 53, 1412–1417. [DOI] [PubMed] [Google Scholar]
- 11.Kashihara, N., Watanabe, Y., Makino, H., Wallner, E. I. & Kanwar, Y. S. (1992) Proc. Natl. Acad. Sci. USA 89, 6309–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason, R. M. & Wahab, N. A. (2003) J. Am. Soc. Nephrol. 14, 1358–1373. [DOI] [PubMed] [Google Scholar]
- 13.Yang, Y. C., Piek, E., Zavadil, J., Liang, D., Xie, D., Heyer, J., Pavlidis, P., Kucherlapati, R., Roberts, A. B. & Bottinger, E. P. (2003) Proc. Natl. Acad. Sci. USA 100, 10269–10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Runyan, C. E., Schnaper, H. W. & Poncelet, A. C. (2005) J. Biol. Chem. 280, 8300–8308. [DOI] [PubMed] [Google Scholar]
- 15.Ziyadeh, F. N., Simmons, D. A., Snipes, E. R. & Goldfarb, S. (1991) J. Am. Soc. Nephrol. 1, 1220–1229. [DOI] [PubMed] [Google Scholar]
- 16.Phillips, A. O. (2003) Curr. Diabetes Rep. 3, 491–496. [DOI] [PubMed] [Google Scholar]
- 17.Nath, K. A. (1998) Kidney Int. 54, 992–994. [DOI] [PubMed] [Google Scholar]
- 18.Nath, K. A. (1992) Am. J. Kidney Dis. 20, 1–17. [DOI] [PubMed] [Google Scholar]
- 19.Yang, Q., Dixit, B., Wada, J., Tian, Y., Wallner, E. I., Srivastva, S. K. & Kanwar, Y. S. (2000) Proc. Natl. Acad. Sci. USA 97, 9896–9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyndman, D., Bauman, D. R., Heredia, V. V. & Penning, T. M. (2003) Chem. Biol. Interact. 143, 621–631. [DOI] [PubMed] [Google Scholar]
- 21.Arner, R. J., Prabhu, K. S., Thompson, J. T., Hildenbrandt, G. R., Linken, A. D. & Reddy, C. C. (2001) Biochem. J. 300, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, E., Chen, Z., Fredrickson, T., Gellai, M., Jugus, M., Contino, L., Spurr, N., Sims, M., Halsey, W., Van Horn, S., Mao, J., Sathe, G. & Brooks, D. (2000) Am. J. Physiol. 279, F426–F439. [DOI] [PubMed] [Google Scholar]
- 23.Kanwar, Y. S., Akagi, S., Nayak, B., Lin, S., Wada, J., Xie, P., Thakur, A., Chugh, S. S. & Danesh, F. R. (2005) Kidney Int. 68, 1670–1683. [DOI] [PubMed] [Google Scholar]
- 24.Cohen, M. P., Clements, R. S., Hud, E., Cohen, J. A. & Ziyadeh, F. N. (1996) Exp. Nephrol. 4, 166–171. [PubMed] [Google Scholar]
- 25.Sharma, K., McCue, P. & Dunn, S. R. (2003) Am. J. Physiol. 284, F1138–F1144. [DOI] [PubMed] [Google Scholar]
- 26.Charalampous, F. C. & Lyras, C. (1957) J. Biol. Chem. 228, 1–13. [PubMed] [Google Scholar]
- 27.Dignam, J. D., Lebovitz, R. M. & Roeder, R. G. (1983) Nucleic Acids Res. 11, 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Go, W. Y., Liu, X., Roti, M. A., Liu, F. & Ho, S. N. (2004) Proc. Natl. Acad. Sci. USA 101, 10673–10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii, S., Iizuka, K., Miller, B. C. & Uyeda, K. (2004) Proc. Natl. Acad. Sci. USA 101, 15597–15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, J.-H., Yeo, W.-S. & Roe, J.-H. (2004) Mol. Microbiol. 51, 1745–1755. [DOI] [PubMed] [Google Scholar]
- 31.Goldsmith-Fischman, S., Kuzin, A., Edstrom, W., Benach, J., Shastry, R., Xiao, R., Acton, T., Honig, B., Montelione, G. & Hunt, J. F. (2004) J. Mol. Biol. 344, 549–565. [DOI] [PubMed] [Google Scholar]
- 32.Ziyadeh, F. N. (1996) Kidney Int. 54, S10–S13. [PubMed] [Google Scholar]
- 33.Burg, M. (1995) Am. J. Physiol. 268, F983–F996. [DOI] [PubMed] [Google Scholar]
- 34.Kawa, J. M., Przybylaski, R. & Taylor, C. G. (2003) Exp. Biol. Med. 228, 907–914. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita, H., Takenoshita, M., Sakurai, M., Bruick, R. K., Henzel, W. J., Shillinglaw, W., Arnot, D. & Uyeda, K. (2001) Proc. Natl. Acad. Sci. USA 98, 9116–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, L., Pan, X., Wada, J., Haas, C. S., Wuthrich, R. P., Danesh, F. R., Chugh, S. S. & Kanwar, Y. S. (2002) J. Biol. Chem. 277, 29953–29962. [DOI] [PubMed] [Google Scholar]
- 37.Zhu, Y., Casado, M., Vaulont, S. & Sharma, K. (2005) Diabetes 54, 1976–1984. [DOI] [PubMed] [Google Scholar]
- 38.Vaulont, S., Cognet, V. M. & Kahn, A. (2000) J. Biol. Chem. 275, 31555–31558. [DOI] [PubMed] [Google Scholar]
- 39.Hu, E., Chen, Z., Fredrickson, T., Gellai, M., Jugus, M., Contino, L., Spurr, N., Van Horn, S., Mao, J., Sathe, G. & Brooks D. (2000) Am. J. Physiol. 279, F426–F439. [DOI] [PubMed] [Google Scholar]
- 40.Nath, K. A. & Norby, S. M. (2000) Am. J. Med. 109, 665–678. [DOI] [PubMed] [Google Scholar]