Abstract
Increases in subclinical inflammation (C-reactive protein [CRP]) and impaired coagulation have been associated with increased obesity and insulin resistance. Only a few small studies have examined the effect of lifestyle changes, such as weight loss, increased physical activity, and insulin-sensitizing intervention on inflammation and coagulation. The Diabetes Prevention Program (DPP) clinical trial studied the effect of an intensive lifestyle intervention or metformin on progression to diabetes relative to placebo in 3,234 adults with impaired glucose tolerance. The effects of these interventions on CRP and fibrinogen at 12 months are examined in this report. Metformin reduced CRP in women compared with the placebo group. In men, the median changes in CRP from baseline to 1 year were −33% in the lifestyle group, −7% in the metformin group, and +5% in the placebo group. In women, the changes in CRP from baseline to follow-up were −29% in the lifestyle group, −14% in the metformin group, and 0% in the placebo group. In the lifestyle group weight loss rather than increased physical activity seems to account for most of the changes in CRP. Only modest reductions (although significant) were seen in fibrinogen levels in the lifestyle group relative to the metformin and placebo group. Lifestyle intervention reduced levels of nontraditional cardiovascular risk factors relative to both placebo and to a lesser degree to metformin.
Keywords: CRP, C-reactive peptide; DPP, Diabetes Prevention Program; HOMA-IR, homeostasis model assessment for insulin resistance; IGT, impaired glucose tolerance; IRAS, Insulin Resistance Atherosclerosis Study
The risk of cardiovascular disease is markedly increased in patients with type 2 diabetes. In patients with newly diagnosed type 2 diabetes, improved glycemic control is more strongly associated with risk of microvascular than with macrovascular disease (1). One explanation for the relatively stronger association of glycemia with micro- than with macrovascular disease may be the presence of established cardiovascular risk factors (blood pressure, triglycerides, and HDL cholesterol) (2–4) and excess cardiovascular disease (5) before the onset of type 2 diabetes. Individuals with impaired glucose tolerance (IGT) also have increased frequency of traditional cardiovascular risk factors (6,7) and an increased risk of type 2 diabetes (8) and cardiovascular disease (9,10).
Recently much interest has been focused on the role of subclinical inflammation (11) as a nontraditional risk factor for cardiovascular disease. Persons with IGT have increased levels of C-reactive protein (CRP) (12) and fibrinogen (13) in cross-sectional studies. High levels of fibrinogen and CRP are related to obesity and insulin resistance (12–15). Increased levels of CRP (4,16) and fibrinogen (4) have been shown to predict the development of type 2 diabetes in prospective studies. Less information is available on whether lifestyle changes (17–20) or pharmacological interventions (21–23) reduce inflammation. Moreover, many of these studies are small and of short duration.
The mechanisms by which interventions might reduce cardiovascular disease in participants with impaired glucose tolerance are largely unexplored. The Diabetes Prevention Program (DPP) (24) provides an opportunity to examine these issues because it includes a large population of participants with IGT who were randomly assigned to treatment with intensive lifestyle or metformin or placebo therapy. In 2002, the primary results of the DPP study were reported (25). Intensive lifestyle reduced the incidence of type 2 diabetes by 58% and metformin reduced the incidence of type 2 diabetes by 31%. In this report, we examine the effects of intensive lifestyle and metformin on levels of CRP and fibrinogen. We also examine factors that may be related to changes in these inflammatory or coagulation markers, particularly the effect of changes in obesity, physical activity, and insulin resistance.
RESEARCH DESIGN AND METHODS
Details of the DPP protocol have been published (24). The current report includes 3,234 participants seen at baseline. This number includes the three treatment arms investigated in the DPP (i.e., standard lifestyle, intensive lifestyle, and metformin) but not participants from the troglitazone arm, which was discontinued. Individuals were recruited from a variety of sources based on an increased risk for development of diabetes. Written informed consent was obtained from all participants before screening consistent with the Helsinki Declaration and the guidelines of each center’s institutional review board.
Inclusion criteria included a fasting plasma glucose value of 95–125 mg/dl and a 2-h postchallenge glucose value of 140–199 mg/dl after a 75-g glucose load, age ≥ 25 years, and BMI ≥ 24 kg/m2 (≥ 22 kg/m2 for Asian Americans because of differences in body size in this population). Major exclusions included a recent myocardial infarction, symptoms of coronary heart disease, major illness, prior diagnosis of diabetes, use of medications known to impair glucose tolerance or triglyceride level ≥ 600 mg/dl. Standardized interviewer-administered questionnaires were used to obtain data on personal medical history, medications, and diet. Self-reported race/ethnicity was classified according to the question used in the 1990 U.S. Census questionnaire (26). Adiposity was assessed by BMI. Waist circumference was measured in standing participants as midway between the highest point of the iliac crest and the lowest point of the costal margin. Self-reported levels of leisure physical activity were assessed annually with the Modifiable Activity Questionnaire. Physical activity was calculated as the product of the duration and frequency.
Participants were randomly assigned to one of three interventions: metformin at a dose of 850 mg twice a day, placebo, or an intensive program of lifestyle intervention, low-fat diet, and engagement in physical activity of moderate intensity, such as brisk walking, for at least 150 min/week. Details have been published (24).
Laboratory methods
All analytical measurements were performed on blood collected after an overnight fast at the Central Biochemistry Laboratory (Northwest Lipid Research Laboratories, University of Washington, Seattle, WA). Plasma glucose was measured on an autoanalyzer by the glucokinase method. HbA1c was measured by a dedicated ion-exchange high-performance liquid chromatography instrument. Insulin measurements were performed by a polyethylene glycol–accelerated double-antibody radioimmunoassay method developed in the Diabetes Endocrinology Research Center Immunoassay Core Laboratory at the University of Washington. This method is based on the use of an anti-human insulin guinea pig antibody and measures total immunoreactive insulin. The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as follows (27):
High-sensitivity CRP and fibrinogen levels in plasma were measured immunochemically using Dade-Behring reagent on the Behring Nephelometer autoanalyzer, which uses polystyrene particles coated with monoclonal antibodies specific to the ligand. The intra- and interassay coefficients of variation for CRP were 4 and 5%, respectively. The intra- and interassay coefficients of variation for fibrinogen were 3 and 5%, respectively.
Statistical analyses
Participants were followed for an average of 3.2 years from the start of the study in June 1996 through July 2001, a period 4 months longer than previously reported. This period was chosen to maximize the available data that were collected during the masked phase, since unmasking occurred in early August 2001. Baseline characteristics were described using medians with interquartile range and means with SD for quantitative variables as appropriate. Comparisons among groups at baseline were made using ANOVA for quantitative variables and the χ2 test of independence for categorical variables. The nominal P values are listed with no adjustment for multiple comparisons. Partial Spearman correlation and its P value were used to summarize the association between two variables when adjusting for other covariates. Fixed-effects models with the assumption of normally distributed errors (28) were used to assess differences over time in weight, BMI, fasting plasma glucose, HOMA-IR, waist girth, and physical activity among the three treatment groups. The changes from baseline for fibrinogen and CRP are summarized as the percent change from baseline and calculated as [100 × (follow-up value − baseline value)/baseline value]. The differences in the median percent changes at 6 months and year 1 were tested using the nonparametric Wilcoxon’s test.
RESULTS
Table 1 shows baseline characteristics both in the overall population and by treatment group. As might be expected, participants in three treatment groups have similar baseline demographic, anthropometric, metabolic, and inflammatory characteristics. Sixty-eight percent of the participants were women and the average BMI was 34 kg/m2. Participants generally displayed high levels of CRP (5.90 mg/l) and fibrinogen (384 mg/dl) (compared with multiethnic populations such as the Insulin Resistance Atherosclerosis Study [IRAS]) (12,13) compatible with the high rates of obesity and insulin resistance in the DPP cohort.
TABLE 1.
Baseline characteristics by treatment group
| Overall | Placebo | Metformin | Lifestyle | |
|---|---|---|---|---|
| n | 3,234 | 1,082 | 1,073 | 1,079 |
| Age (years) | 50.6 ± 10.7 | 50.3 ± 10.4 | 50.9 ± 10.3 | 50.6 ± 11.3 |
| Sex | ||||
| No. males (%) | 1,043 (32.3) | 335 (31.0) | 363 (33.8) | 345 (32.0) |
| No. females (%) | 2,191 (67.7) | 747 (69.0) | 710 (66.2) | 734 (68.0) |
| Weight (kg) | 94.2 ± 20.3 | 94.3 ± 20.2 | 94.3 ± 19.9 | 94.1 ± 20.8 |
| BMI (kg/m2) | 34.0 ± 6.7 | 34.2 ± 6.7 | 33.9 ± 6.6 | 33.9 ± 6.8 |
| Waist (cm) | 105.1 ± 14.5 | 105.2 ± 14.3 | 104.9 ± 14.4 | 105.1 ± 14.8 |
| Waist-to-hip ratio | 0.92 ± 0.09 | 0.92 ± 0.08 | 0.93 ± 0.09 | 0.93 ± 0.09 |
| Physical activity (MET h/week) | 16.3 ± 25.8 | 17.0 ± 29.0 | 16.4 ± 25.9 | 15.5 ± 22.1 |
| Fasting glucose (mg/dl) | 106.5 ± 8.3 | 106.7 ± 8.4 | 106.5 ± 8.5 | 106.3 ± 8.1 |
| HbA1c (%) | 5.9 ± 0.5 | 5.9 ± 0.5 | 5.9 ± 0.5 | 5.9 ± 0.5 |
| HOMA-IR | ||||
| Mean | 7.1 ± 4.2 | 7.1 ± 4.2 | 7.2 ± 4.1 | 7.0 ± 4.3 |
| Median | 6.2 | 6.2 | 6.2 | 6.0 |
| Fasting insulin (μU/ml) | ||||
| Mean | 26.7 ± 15.2 | 26.7 ± 15.0 | 27.0 ± 14.9 | 26.5 ± 15.5 |
| Median | 24.0 | 24.0 | 24.0 | 23.0 |
| Fibrinogen (mg/dl) | 383.8 ± 85.4 | 386.4 ± 85.7 | 379.7 ± 84.7 | 385.2 ± 85.6 |
| Men | 356.46 ± 77.83 | 357.34 ± 80.96 | 353.00 ± 80.12 | 359.26 ± 72.15 |
| Women | 396.75 ± 85.74 | 399.37 ± 84.59 | 393.40 ± 83.76 | 397.33 ± 88.74 |
| CRP (mg/l) | 5.90 ± 7.38 | 6.06 ± 7.66 | 5.82 ± 7.97 | 5.83 ± 6.44 |
| Men | 3.22 ± 5.32 | 3.07 ± 3.59 | 3.28 ± 7.11 | 3.31 ± 4.47 |
| Women | 7.18 ± 7.87 | 7.40 ± 8.57 | 7.11 ± 8.07 | 7.01 ± 6.87 |
| Median CRP (mg/l) | 3.7 | 3.8 | 3.5 | 3.8 |
| Men | 1.9 | 2.0 | 1.8 | 2.0 |
| Women | 4.9 | 5.1 | 4.7 | 5.0 |
Data are means ± SD unless otherwise indicated.
Table 2 shows baseline correlations for selected variables. Both BMI and waist circumference are significantly correlated with CRP and fibrinogen. The magnitude of the correlations for BMI and waist circumference are very similar, which is not surprising given the very high correlation between BMI and waist circumference in this study (r = 0.86). Fasting glucose and HOMA-IR are also significantly correlated with CRP and fibrinogen although the magnitude of the correlation is weaker than that for BMI and waist circumference.
TABLE 2.
Baseline partial Spearman’s correlation* among inflammation, hemostatic, and metabolic variables
| Baseline correlations | Fasting glucose | HbA1c | HOMA-IR | Fasting insulin | CRP | Fibrinogen | Physical activity | Waist | WHR | BMI |
|---|---|---|---|---|---|---|---|---|---|---|
| Fasting glucose | 1.00 | 0.31 | 0.33 | 0.21 | 0.05 | 0.10 | −0.02 | 0.17 | 0.10 | 0.17 |
| HbA1c | 1.00 | 0.15 | 0.12 | 0.13 | 0.21 | −0.03 | 0.14 | 0.06 | 0.13 | |
| HOMA-IR | 1.00 | 0.99 | 0.19 | 0.18 | −0.10 | 0.44 | 0.27 | 0.42 | ||
| Fasting insulin | 1.00 | 0.20 | 0.18 | −0.10 | 0.43 | 0.27 | 0.42 | |||
| CRP | 1.00 | 0.50 | −0.06 | 0.36 | 0.14 | 0.38 | ||||
| Fibrinogen | 1.00 | −0.06 | 0.29 | 0.09 | 0.32 | |||||
| Physical activity | 1.00 | −0.14 | −0.03 | −0.15 | ||||||
| Waist | 1.00 | 0.56 | 0.86 | |||||||
| WHR | 1.00 | 0.30 | ||||||||
| BMI | 1.00 |
P < 0.01 for absolute Spearman’s correlations of 0.06 or more.
Adjusted for age, sex, and ethnicity. WHR, waist-to-hip ratio.
Table 3 shows the results for changes in weight, physical activity, HOMA-IR, and fasting glucose after 1 year of intervention. The lifestyle group showed greater changes in weight, physical activity, HOMA-IR, and fasting glucose than the placebo or the metformin group. The metformin group had significantly greater reductions in weight, HOMA-IR, fasting insulin, and glucose than the placebo group. The reduction in weight was similar at 6 months and at 1 year for the lifestyle intervention.
TABLE 3.
Mean changes from baseline by treatment group
| Placebo | Metformin | Lifestyle | P* | |
|---|---|---|---|---|
| n | 1082 | 1073 | 1079 | |
| Weight (kg) | ||||
| At 6 months | −0.32 ± 0.14 (−0.3) | −2.26 ± 0.14 (−2.4) | −6.73 ± 0.14 (−7.2) | <0.001 |
| At year 1 | −0.42 ± 0.17 (−0.4) | −2.72 ± 0.17 (−2.9) | −6.76 ± 0.17 (−7.2) | <0.001 |
| BMI (kg/m2) | ||||
| At 6 months | −0.12 ± 0.05 (−0.4) | −0.81 ± 0.05 (−2.4) | −2.41 ± 0.05 (−7.1) | <0.001 |
| At year 1 | −0.15 ± 0.06 (−0.4) | −0.97 ± 0.06 (−2.9) | −2.42 ± 0.06 (−7.1) | <0.001 |
| Fasting glucose (mg/dl) | ||||
| At 6 months | 0.20 ± 0.30 (0.2) | −3.99 ± 0.30 (−3.7) | −4.66 ± 0.30 (−4.4) | <0.001 |
| At year 1 | 0.63 ± 0.36 (0.6) | −4.18 ± 0.36 (−3.9) | −4.94 ± 0.36 (−4.6) | <0.001 |
| HOMA-IR at year 1 | 0.36 ± 0.13 (5.1) | −1.10 ± 0.13 (−15.3) | −1.59 ± 0.12 (−22.7) | <0.001 |
| Waist (cm) at year 1 | −0.69 ± 0.19 (−0.7) | −2.23 ± 0.19 (−2.1) | −6.36 ± 0.19 (−6.1) | <0.001 |
| Waist-to-hip ratio at year 1 | −0.002 ± 0.001 (−0.2) | −0.008 ± 0.001 (−0.9) | −0.021 ± 0.001 (−2.3) | <0.001 |
| Activity at year 1 (MET h/week) | 1.39 ± 0.72 (8.2) | 1.65 ± 0.72 (10.0) | 6.60 ± 0.72 (42.6) | < 0.001 |
Data are means ± SE (% of baseline); % baseline is derived by dividing the mean at baseline with the mean change.
P value represents the mean difference in characteristics among the three treatment groups using ANOVA.
The change in fibrinogen from baseline to 1 year was −2.0% in the lifestyle group, −0.3% in the metformin group, and +0.5% in the placebo group (lifestyle vs. metformin: P = 0.001; lifestyle vs. placebo: P < 0.001; metformin vs. placebo: NS).
CRP was measured at both 6 and 12 months (Fig. 1). Because CRP levels were much higher in women than in men, (as in other studies) (12) sex-specific effects of intervention are shown. In men, the median change in CRP from baseline to 1 year was −33% in the lifestyle group, −7% in the metformin group, and +5% in the placebo group (lifestyle vs. metformin: P < 0.001; lifestyle vs. placebo: P < 0.001; metformin vs. placebo: P = 0.006). In women, the median change in CRP from baseline to 1 year was −29% in the lifestyle group, −14% in the metformin group, and 0% in the placebo group (lifestyle vs. metformin: P < 0.001; lifestyle vs. placebo: P < 0.001; metformin vs. placebo: P < 0.001). The CRP levels continued to decline from 6 months to 1 year in both men and women in the lifestyle group despite no additional weight loss during this interval (Table 3).
FIG. 1.
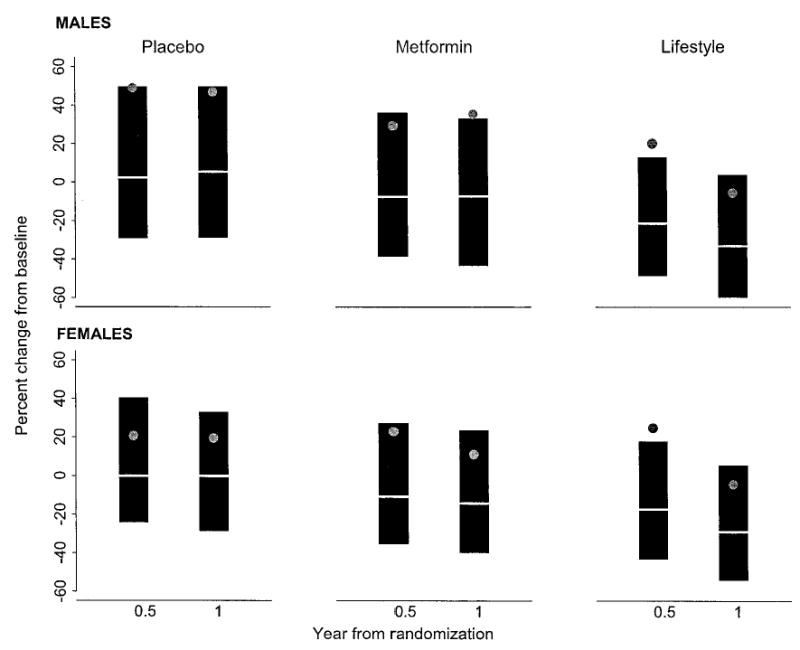
Median percentage of change in CRP from baseline at month 6 and year 1 by sex. The data are presented as mean (gray dot), median (white line), and 25th (top of the gray box) and 75th percentiles (bottom of the gray box). At 6 months and at year 1 after randomization, the Wilcoxon’s test yielded P < 0.001 for intensive lifestyle vs. metformin and intensive lifestyle vs. placebo in both men and women. After 6 months and 1 year of intervention, the P value from the Wilcoxon’s test between metformin vs. placebo was <0.001 for women. Among men, the Wilcoxon’s test for comparing metformin vs. placebo yielded P = 0.01 at 6 months and 0.006 at year 1.
To understand whether the greater changes in the variables were due to changes in weight, physical activity, or insulin resistance, partial Spearman correlations were calculated for the change in CRP and fibrinogen (Tables 4 and 5). In Table 4 (demographic adjustment), CRP was significantly correlated with changes in HOMA-IR, fasting glucose, and weight but not with changes in physical activity. In contrast, fibrinogen was not highly correlated with changes in demographic or metabolic variables. The change in CRP was highly correlated with the change in fibrinogen (r = 0.36). In Table 5, adjustment was made for demographic variables and change in weight. The additional adjustments for change in weight in Table 5 had only modest effects on the correlations with CRP or fibrinogen. In these models, CRP and fibrinogen were not highly correlated with changes in glucose or HOMA-IR. After further adjustment for a change in weight, CRP and fibrinogen remain highly correlated. We also examined whether the changes in CRP and fibrinogen by treatment group (see Fig. 1) were explained by a weight change and a change in physical activity in multiple linear regression analysis. Changes in CRP and fibrinogen in the lifestyle group were partially explained by changes in weight. A change in physical activity was not correlated with a change in CRP and fibrinogen.
TABLE 4.
Partial Spearman’s correlations* of year 1 change from baseline adjusted for demographics and treatment
| Change in characteristic | Fasting glucose | HbA1c | HOMA-IR | Fasting insulin | CRP | Fibrinogen | Physical activity | Waist | WHR | BMI |
|---|---|---|---|---|---|---|---|---|---|---|
| Fasting glucose | 1.00 | 0.23 | 0.51 | 0.39 | 0.09 | 0.05 | −0.04 | 0.24 | 0.11 | 0.31 |
| HbA1c | 1.00 | 0.21 | 0.19 | 0.09 | 0.01 | −0.07 | 0.20 | 0.09 | 0.30 | |
| HOMA-IR | 1.00 | 0.98 | 0.11 | 0.05 | −0.05 | 0.25 | 0.13 | 0.34 | ||
| Fasting insulin | 1.00 | 0.10 | 0.04 | −0.04 | 0.23 | 0.12 | 0.32 | |||
| CRP | 1.00 | 0.36 | −0.03 | 0.14 | 0.06 | 0.19 | ||||
| Fibrinogen | 1.00 | −0.01 | 0.03 | 0.03 | 0.02 | |||||
| Physical activity | 1.00 | −0.10 | −0.05 | −0.12 | ||||||
| Waist | 1.00 | 0.62 | 0.58 | |||||||
| WHR | 1.00 | 0.19 | ||||||||
| BMI | 1.00 |
Adjusted for age at randomization, sex, and race plus treatment status. P < 0.01 for absolute Spearman’s correlations of 0.05 or more. WHR, waist-to-hip ratio.
TABLE 5.
Partial Spearman’s correlations* of year 1 change from baseline adjusted for demographics, treatment, and BMI
| Change in characteristic | Fasting glucose | HbA1c | HOMA-IR | Fasting insulin | CRP | Fibrinogen | Physical activity | Waist | WHR |
|---|---|---|---|---|---|---|---|---|---|
| Fasting glucose | 1.00 | 0.23 | 0.51 | 0.38 | 0.09 | 0.05 | −0.04 | 0.22 | 0.10 |
| HbA1c | 1.00 | 0.21 | 0.19 | 0.09 | 0.01 | −0.07 | 0.20 | 0.09 | |
| HOMA-IR | 1.00 | 0.98 | 0.11 | 0.05 | −0.04 | 0.24 | 0.12 | ||
| Fasting insulin | 1.00 | 0.10 | 0.04 | −0.04 | 0.23 | 0.12 | |||
| CRP | 1.00 | 0.36 | −0.03 | 0.14 | 0.06 | ||||
| Fibrinogen | 1.00 | −0.01 | 0.03 | 0.03 | |||||
| Physical activity | 1.00 | −0.09 | −0.05 | ||||||
| Waist | 1.00 | 0.62 | |||||||
| WHR | 1.00 |
Adjusted for age at randomization, sex, and race plus treatment status and BMI. P < 0.01 for absolute Spearman’s correlations of 0.05 or more. WHR, waist-to-hip ratio.
DISCUSSION
In this report, we demonstrate the beneficial effects of an intensive lifestyle intervention on concentrations of CRP and fibrinogen in adults with IGT. Previous studies of lifestyle interventions and CRP involved less than 50 participants (principally women) (17,18,29). In the current report, we show an ~30% reduction in CRP levels in both sexes. This reduction in CRP occurred despite only a 7% decline in weight achieved at 6 months and is comparable to the 25% reduction in CRP levels reported with starting doses of statins (pravastatin 40 mg, simvastatin 20 mg, and atorvastatin 10 mg) (30). The current study shows very high CRP levels compared with previous studies (12,16,18,31), which probably reflects the nearly universal overweight and insulin resistance in the DPP cohort.
Baseline waist and BMI were strongly correlated with HOMA-IR, CRP, and fibrinogen (Table 2). However at baseline, physical activity was only modestly inversely correlated with HOMA-IR, CRP, and fibrinogen (although significant at P < 0.01). The analysis based on change between baseline and 1 year presented in Tables 4 and 5 also suggests that the reduction in CRP is more closely related to weight loss than to increases in physical activity. However, this result needs to be interpreted with caution because the DPP intensive lifestyle intervention combined both weight loss and increased physical activity and because weight loss is more easily and precisely measured than the amount of exercise (which was based on self-report from three questionnaires).
Although baseline HOMA-IR (a surrogate for insulin resistance) and CRP levels were moderately correlated (r = 0.19) (Table 2), a change in HOMA-IR was only modestly correlated with a change in CRP (r = 0.11, P < 0.001) in Tables 4 and 5. Previous cross-sectional analyses have suggested that high levels of CRP are strongly related to insulin concentrations (15) and insulin resistance (12). Indeed, a recent article from the IRAS in prediabetic participants (the majority of whom have IGT) reported that elevated levels of CRP were more closely related to insulin resistance than to obesity (32). The IRAS used the frequently sampled intravenous glucose tolerance test, a direct measure of insulin resistance, not the HOMA-IR, to distinguish high and low insulin sensitivity. It has been reported that insulin sensitizers such as thiazolidinedione may lower CRP by 20–60% (21–23) despite no weight change or perhaps even a small weight increase. Therefore, it is not clear why changes in insulin resistance did not strongly predict changes in CRP in the DPP study, but it may be partially a measurement issue related to the use of the HOMA-IR or perhaps to the severity of obesity in the DPP population; thus, a truncated distribution of obesity and insulin resistance may be responsible for the attenuation in the relation of CRP with insulin resistance and obesity.
In this report, we showed a larger reduction in CRP levels with lifestyle change than with metformin. In fact, the reduction in CRP levels at 1 year with metformin (−7%) was statistically significant compared with placebo (+5%) in men (P = 0.006). No previous trials have compared the effect of metformin versus intensive lifestyle. The suggestion from this report is that metformin may have only a modest effect on CRP reductions. In a small report that compared metformin and troglitazone in type 2 diabetic patients, the reduction in CRP was greater with troglitazone than with metformin (23).
Consistent with earlier reports, we also observed reductions in fibrinogen with intensive lifestyle interventions (19,20). The reduction in fibrinogen with lifestyle was modest. The correlation of baseline HOMA-IR and fibrinogen was moderate (r = 0.18, P < 0.001). Additionally, the change in HOMA-IR was only modestly correlated with change in fibrinogen (r = 0.05) (Table 5).
The role of subclinical inflammation as a predictor of cardiovascular disease has been widely accepted (11,31). However, it is not clear whether CRP represents a risk factor for cardiovascular disease or is a risk marker. Several lines of evidence suggest that CRP may be etiologically involved in the development of cardiovascular disease, including inhibition of nitric oxide synthesis (33), increases in monocyte chemoattractant protein 1 (34), impairment in endothelial dysfunction (35), and increases in plasminogen activator inhibitor 1 expression in human aortic endothelial cells (36). If the reduction in CRP precedes reductions in cardiovascular disease, over the long term, lifestyle intervention may be associated with a significant reduction in cardiovascular disease as appears to be the case with other interventions such as hydroxym-ethylglutaryl-CoA reductase inhibitors. Over the 2.8 years of the DPP active intervention, participants had too few cardiovascular events to test this hypothesis (25). A 5-year follow up of the DPP study is currently underway.
In conclusion, intensive lifestyle intervention reduced levels of nontraditional cardiovascular risk factors both relative to placebo and to a lesser degree relative to metformin. These significant reductions were achieved despite a relatively modest weight loss of ~6–7% over the 1st year with most participants still being obese at the end of 1 year. The DPP study suggests that not only does intensive lifestyle intervention reduce the risk of developing type 2 diabetes but also it has effects on risk markers that may eventually reduce the risk of cardiovascular disease.
Acknowledgments
Funding was provided by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the Office of Women’s Health, and the National Institute on Aging. In addition, the Indian Health Service, the Centers for Disease Control and Prevention, the American Diabetes Association, and two pharmaceutical companies, Bristol-Myers Squibb and Parke-Davis, contributed support. The General Clinical Research Center Program, National Center for Research Resources, supported many of the clinical centers. Support to the clinical centers and the Coordinating Center was provided by the National Institute of Diabetes and Digestive and Kidney Diseases through a Cooperative Agreement, except for the Southwestern American Indian Centers, which were supported directly by the National Institute of Diabetes and Digestive and Kidney Diseases and the Indian Health Service.
We thank the thousands of volunteers in this program for their devotion to the goal of diabetes prevention. LifeScan, Health O Meter, Hoechst Marion Roussel, Merck-Medco Managed Care, Merck and Co., Nike Sports Marketing, Slim Fast Foods, and Quaker Oats donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices, Matthews Media Group, and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center.
Footnotes
This manuscript was prepared by a writing and review committee consisting of Steven Haffner (Chair), Marinella Temprosa, Jill Crandall, Sarah Fowler, Ronald Goldberg, Edward Horton, Santica Marcovina, Kieren Mather, Trevor Orchard, Robert Ratner, and Elizabeth Barrett-Connor.
References
- 1.UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals: does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 3.Fagot-Campagna A, Narayan KM, Hanson RL, Imperatore G, Howard BV, Nelson RG, Pettitt DJ, Knowler WC. Plasma lipoproteins and incidence of non-insulin-dependent diabetes mellitus in Pima Indians: protective effect of HDL cholesterol in women. Atherosclerosis. 1997;128:113–119. doi: 10.1016/s0021-9150(96)05978-3. [DOI] [PubMed] [Google Scholar]
- 4.Festa A, D′ Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 5.Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25:1129–1134. doi: 10.2337/diacare.25.7.1129. [DOI] [PubMed] [Google Scholar]
- 6.Mykkänen L, Laakso M, Penttilä I, Pyörälä K. Asymptomatic hyperglycemia and cardiovascular risk factors in the elderly. Atherosclerosis. 1991;88:153–161. doi: 10.1016/0021-9150(91)90077-g. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez BL, Curb JD, Burchfiel CM, Huang B, Sharp DS, Lu GY, Fujimoto W, Yano K. Impaired glucose tolerance, diabetes, and cardiovascular risk factor profiles in the elderly: the Honolulu Heart Program. Diabetes Care. 1996;19:587–590. doi: 10.2337/diacare.19.6.587. [DOI] [PubMed] [Google Scholar]
- 8.Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, Haffner SM, Pettitt DJ, Sorkin JD, Muller DC, Hamman RF. Predictors of progression from impaired glucose tolerance to non-insulin dependent diabetes: an analysis of six prospective studies. Diabetes. 1997;46:701–710. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 10.Haffner SM. Impaired glucose tolerance: is it relevant for cardiovascular disease? Diabetologia. 1997;40 (Suppl 2):S138–S140. doi: 10.1007/s001250051430. [DOI] [PubMed] [Google Scholar]
- 11.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 12.Festa A, D′Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome. Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 13.Festa A, D’Agostino R, Jr, Mykkanen L, Tracy RP, Zaccaro DJ, Hales CN, Haffner SM. Relative contribution of insulin and its precursors to fibrinogen and PAI-1 in a large population with different states of glucose tolerance: the Insulin Resistance Atherosclerosis Study (IRAS) Arterioscler Thromb Vasc Biol. 1999;19:562–568. doi: 10.1161/01.atv.19.3.562. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Bouter L, McQuillan G, Wener M, Harris T. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 15.Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 16.Pradhan AD, Manson JE, Rifai N, Mykkanen L, Tracy RP, Haffner SM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 17.Bastard J-P, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 18.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D’Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 19.Marckmann P, Toubro S, Astrup A. Sustained improvement in blood lipids, coagulation, and fibrinolysis after major weight loss in obese subjects. Eur J Clin Nutr. 1998;52:329–333. doi: 10.1038/sj.ejcn.1600558. [DOI] [PubMed] [Google Scholar]
- 20.Van Den Burg PJM, Hospers JEH, Van Vliet M, Mosterd WL, Bouma BN, Huisveld IA. Effect of endurance training and seasonal fluctuation on coagulation and fibrinolysis in young sedentary men. J Appl Physiol. 1997;82:613–620. doi: 10.1152/jappl.1997.82.2.613. [DOI] [PubMed] [Google Scholar]
- 21.Haffner SM, Greenberg A, Weston W, Chen H, Williams K, Freed M. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–684. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 22.Mohanty P, Aljada A, Ghanim H, Tripathy D, Syed T, Hofmeyer D. Rosiglitazone improves vascular reactivity, inhibits reactive oxygen species (ROS) generation, reduces p47phox subunit expression in mononuclear cells (MNC) and reduces C-reactive protein (CRP) and monocyte chemo-tactic protein-1 (MCP-1): evidence of a potent anti-inflammatory effect (Abstract) Diabetes. 2001;50 (Suppl 2):A68. [Google Scholar]
- 23.Chu NV, Kong APS, Kim DD, Armstrong D, Baxi S, Deutsch R, Caulfield M, Mudaliar SR, Reitz R, Henry RR, Reaven PD. Differential effects of metformin and troglitazone on cardiovascular risk factors in patients with type 2 diabetes. Diabetes Care. 2002;25:542–549. doi: 10.2337/diacare.25.3.542. [DOI] [PubMed] [Google Scholar]
- 24.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Diabetes Prevention Program Research Group. reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;246:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bureau of the Census: Census of the Population, 1990 Washington, DC, U.S. Govt. Printing Office, 1990 (OMB 0607-0628)
- 27.Matthews DP, Hosker JP, Rudenski AS, Naylor GA, Treacher DF, Turner RL. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Diggle PJ, Liang K-Y, Zeger SL. Analysis of Longitudinal Data New York, Oxford Univ. Press, 1994
- 29.Tchernof A, Nolan A, Sites C, Aders P, Poehlman E. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–569. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- 30.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–1935. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 31.Ridker P, Rifai N, Rose L, Buring J, Cook N. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 32.Festa A, Hanley AJG, Tracy RP, D’Agostino R, Haffner SM. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003;108:1822–1830. doi: 10.1161/01.CIR.0000091339.70120.53. [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Wang C-H, Li S-H, Dumont AS, Fedak PWM, Badiwala MV, Dhillon B, Weisel RD, Li R-K, Mickle DAG, Stewart DJ. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 34.Pasceri V, Chang J, Willerson JT, Yeh ETH. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–2534. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- 35.Cleland SJ, Sattar N, Petrie JR, Forouhi NG, Elliott HL, Connell JMC. Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin Sci. 2000;98:531–535. [PubMed] [Google Scholar]
- 36.Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]


