Abstract
OBJECTIVE
The Diabetes Prevention Program (DPP) was a large, multicenter, randomized clinical trial testing interventions to prevent or delay type 2 diabetes. A major challenge was to identify eligible high-risk adults, defined by DPP as having both impaired glucose tolerance (IGT) (2-h glucose 140–199 mg/dl) and elevated fasting plasma glucose (EFG) (95–125 mg/dl).
RESEARCH DESIGN AND METHODS
We analyzed how screening yields would be affected by the presence of established risk factors such as age, sex, ethnicity, BMI, and family history of diabetes, and how much yields would be enhanced by preselecting individuals with elevated capillary blood glucose levels. Of 158,177 contacted adults, 79,190 were potentially eligible (no history of diabetes, age 25 years and older, BMI ≥ 24 kg/m2). We focus on the 30,383 participants who completed an oral glucose tolerance test (OGTT).
RESULTS
Based on OGTT, 27% had IGT with EFG, meeting DPP eligibility criteria for being at high risk of diabetes, and 13% had previously undiagnosed diabetes based on OGTT. Older age and higher BMI increased yield of high-risk individuals and those with newly discovered diabetes in most ethnic groups (whites, African Americans, Hispanics, and American Indians). In Asian Americans, age but not BMI predicted high risk and diabetes. Independent of age and BMI, the preliminary fasting capillary glucose predicted screening yield in all ethnic groups, with an inverted-U pattern defining DPP eligibility alone (IGT-EFG) and a steep curvilinear pattern defining either IGT-EFG or newly discovered diabetes. Fasting capillary glucose did not attenuate the affects of other participant characteristics in predicting IGT-EFG or the combination of IGT-EFG and newly discovered diabetes.
CONCLUSIONS
The DPP screening approach identified adults with or at high risk for type 2 diabetes across various ethnic groups and provided guidance to more efficient use of OGTTs. Fasting capillary glucose is a useful adjunct in screening programs combined with data on age and adiposity.
Abbreviations: ADA, American Diabetes Association; CBL, Central Biochemistry Laboratory; DPP, Diabetes Prevention Program; EFG, elevated fasting glucose; IGT, impaired glucose tolerance; OGTT, oral glucose tolerance test
Type 2 diabetes imposes a large and growing burden on public health in the U.S. and worldwide (1,2). Although many treatments are available for adults with diabetes, as used in routine clinical practice such treatments are only partially effective in reducing the risk of serious complications (3). Therefore, primary prevention of type 2 diabetes represents an attractive strategy for reducing diabetes-related morbidity and mortality. The Diabetes Prevention Program (DPP), a trial of primary prevention, showed that among adults at high risk for developing type 2 diabetes, diabetes can be prevented or postponed by a regimen of weight loss and regular physical activity or by daily use of metformin. The trial’s design (4), recruitment (5), baseline characteristics of the participants (6), and main results (7) have been published.
A major challenge in planning the DPP was to quickly and efficiently identify adults at high risk for diabetes. Based on data from six cohort studies (8), individuals with both impaired glucose tolerance (IGT; 2-h glucose of 140–199 mg/dl after a 75-g oral glucose challenge) and elevated fasting plasma glucose (EFG, plasma glucose of 95–125 mg/dl after a 10-h overnight fast) were at highest risk for type 2 diabetes, and this combination of IGT and EFG was adopted as the key eligibility criterion for DPP.
It was anticipated that recruiting the planned DPP number of participants would require screening tens of thousands of adults using oral glucose tolerance tests (OGTTs), which, while safe and noninvasive, would be a significant burden for staff and participants. This burden raised two key questions: 1) How would screening yields be affected by the presence of established risk factors such as age, sex, ethnicity, BMI, and family history of diabetes? 2) How much could yields be enhanced by preselecting individuals with elevated capillary blood glucose levels? We report here data from the screening phase of DPP to determine the yield of various screening approaches to guide the development of future screening programs.
RESEARCH DESIGN AND METHODS
Description of DPP screening approach
The goal of DPP screening was to efficiently identify and recruit a cohort of adults at high risk for the development of type 2 diabetes (having both IGT and EFG) who were likely to adhere to the DPP protocol, had no contraindications to DPP interventions, and had no conditions that might confound interpretation of DPP results (4,5). Some criteria could be assessed by telephone interview (e.g., age, medical history, and medication use). In contrast, at least one clinic visit was required for physiologic measurements, medical examination, or assessment of complex behaviors. Assessment of eligibility criteria was therefore divided into steps (Fig. 1). Step 1 included a brief interview and a blood glucose measurement. Glucose measurements were most often made on capillary blood using a meter (One Touch II, Johnson & Johnson, Milpitas, CA); however, they were sometimes made on the same day as the OGTT using venous blood, depending on individual clinic schedules. Many were made 1–90 days before the OGTT. The local step 1 glucose criteria used to progress to step 2 can be found in online appendix Table 1 (available at http://care.diabetesjournals.org). Measurements made ≥ 8 h after the last meal were classified as “fasting.”
Figure 1.
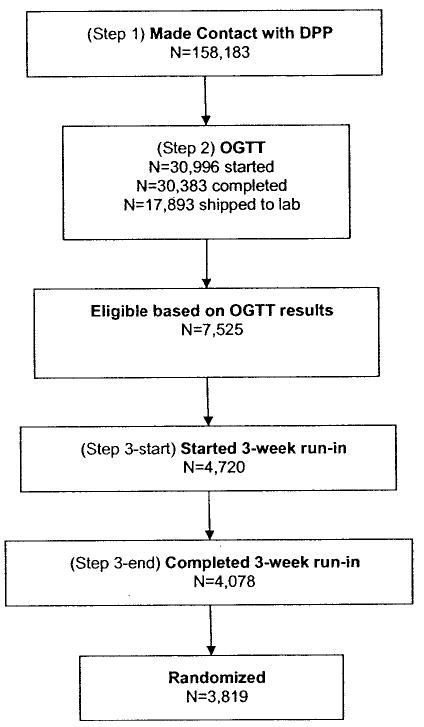
Steps used to identify DPP participants at high risk of diabetes.
Potentially eligible individuals were invited to undergo a standard 75-g OGTT (step 2). Individuals who met eligibility criteria for fasting and 2-h glucose (see below) were invited to a medical examination and a 3-week run-in phase (step 3), following which eligible individuals could be randomized (5) after final informed consent.
Glucose-related eligibility: exceptions and modifications
When DPP began in 1996, the American Diabetes Association (ADA) and the World Health Organization fasting criterion for diabetes was a fasting plasma glucose ≥ 140 mg/dl. Accordingly, DPP set the fasting plasma glucose eligibility window at 100–139 mg/dl (excepting American-Indian centers, where EFG was not required) (4). When the ADA lowered the fasting plasma glucose criterion for diabetes to ≥ 126 mg/dl in 1997 (5), DPP modified the fasting glucose eligibility to 95–125 mg/dl. Descriptions of the flow-through screening are based on the criteria in effect when participants were screened, and the final contacts with an individual were used because some individuals (< 5%) initiated two or more contacts with DPP following a temporary exclusion (such as use of a thiazide, which was subsequently discontinued). For the remainder of this analysis we were interested in drawing inferences about the hypothetical performance of the screening approach. Therefore, the criteria in effect at the time were ignored, and more recent criteria were applied without regard to the calendar time or center. For this analysis, only the first contact with an individual was used.
OGTT classification
Based on a single OGTT, we classified individuals into three categories: 1) either normal fasting glucose (< 95 mg/dl) or normal glucose tolerance (2-h glucose < 140 mg/dl) but not diabetes; 2) diabetes by ADA criteria (9) (fasting glucose ≥ 126 mg/dl or 2-h glucose ≥ 200 mg/dl); 3) EFG from 95–125 mg/dl and IGT (2-h glucose 140 –199 mg/dl). This third group met the glycemic criteria for DPP eligibility (IGT-EFG) and was at high risk for incident diabetes (8).
Locally versus centrally analyzed OGTT
Glucose results from the Central Biochemistry Laboratory (CBL) were used for eligibility. Clinics were directed to not ship blood to the CBL if the locally determined fasting glucose was < 80 mg/dl or > 140 mg/dl or if the locally determined 2-h glucose was < 120 mg/dl or > 220 mg/dl. Minor interclinic differences aside, the effect of these shipping rules was to bias the CBL sample toward IGT and away from normal glucose tolerance and diabetes. To address this, we predicted CBL values for the 12,490 (41%) people being screened who had a complete OGTT but whose blood specimens were not shipped to the CBL. Using data from the 17,893 (59%) people screened who underwent both local and CBL glucose measurements, prediction equations were developed from linear regression models in which CBL glucose was the dependent variable and local glucose was the independent variable. Actual and predicted CBL glucose determinations were then used to categorize the people screened according to glucose tolerance as described above. These prediction equations are shown in online appendix Table 2.
Statistical analysis
Analyses focused on two main outcomes of the OGTT: 1) eligibility for DPP (presence of both IGT and EFG) and 2) previously undiagnosed type 2 diabetes. Stratum-specific screening yields (Yi) were defined as Yi = ni/Ni , where Ni is the number of individuals who underwent an OGTT within the ith stratum, and ni is the number who had IGT-EFG, newly discovered diabetes, or both. Stratification variables included age (10-year groups), ethnicity (white, African American, Hispanic, American Indian, and Asian/Pacific Islander), sex, BMI (5 kg/m2 groups), family history of diabetes (present versus absent), and step 1 glucose measurements, in increments of 5 mg/dl. Comparisons of yield were made using χ2 tests. Screening yield is estimated as if all OGTT samples had been shipped to the CBL.
RESULTS
Screening yield in DPP
Figure 1 summarizes the actual flow of participants through the screening process. Table 1 shows the yield of the OGTT with regard to IGT-EFG and other states of abnormal glucose tolerance using the first OGTT performed for each participant. Of the 30,383 completed oral glucose tolerance tests, 12,490 (41.1%) produced local results that did not meet criteria for shipping to the CBL. Regression analyses described in the research design and methods section were used to convert local results into CBL-equivalent results. The other 17,893 (58.9%) produced local results that met shipping criteria, allowing CBL results to be used for classification.
Table 1.
Glucose tolerance status in 30,383 people screened by the DPP who underwent OGTT by source of data
| Glucose tolerance* | Local glucose data only† | CBL results available | Total |
|---|---|---|---|
| Low risk | 10,342 (82.8) | 7,846 (43.9) | 18,188 (59.8) |
| High risk (IGT − EFG) | 966 (7.7) | 7,294 (40.8) | 8,260 (27.2) |
| Diabetes | 118 (9.5) | 2,753 (15.4) | 3,935 (13.0) |
| Total | 12,490 (100.0) | 17,893 (100.0) | 30,383 (100.0)‡ |
Data are n (%). Results limited to initial OGTTs only.
“Low risk” indicates fasting glucose < 95 mg/dl or 2-h glucose > 140 mg/dl. “High risk” indicates fasting glucose 95–125 and 2-h glucose 140–199 mg/dl, i.e., IGT − EFG. “Diabetes” indicates fasting plasma glucose ≥ 126 mg/dl or a 2-h plasma glucose ≥ 200 mg/dl.
Based on linear prediction models developed from the group with CBL results. See online appendix Table 2 for regression details.
The total number of OGTTs shown here (30,383) is slightly smaller than the total shown in Fig. 1 (30,996). The difference (613) is related to the exclusion from this table of incomplete OGTTs (e.g., test begun, but no 2-h sample obtained).
As planned, the majority of OGTTs with local results only were classified as low risk (82.8%) or diabetic (9.5%). However, an estimated 7.7% of tests with local results only would have met DPP glucose eligibility criteria if they had been sent to the CBL. Although imperfect, these rules produced an enriched sample for shipment to the CBL, because 40.8% met glucose eligibility criteria for DPP and 15.4% had newly discovered diabetes (Table 1, column 3). We then pooled glucose tolerance data from these two groups to determine overall OGTT yields. After pooling, 27.2% had IGT-EFG and 13.0% had newly discovered diabetes.
The relation of screening yield to baseline characteristics
Figure 2 summarizes the relationships of selected baseline characteristics to screening yields. There was no sex difference in the overall yield of IGT-EFG or newly discovered diabetes. Both age and BMI were strongly and positively associated with the yield of IGT-EFG and with yield of IGT-EFG or newly discovered diabetes. Family history of diabetes was not associated with higher yields; in fact, the yield of IGT-EFG or of the combination of IGT-EFG or newly discovered diabetes was slightly lower in those who reported a family history than in those who reported no such history. Screening yields were similar in whites, African Americans, and Hispanics but were significantly higher in Asian/Pacific Islanders and lower in American Indians. Similar patterns were observed when risk factors were stratified by ethnic group (data not shown). In whites, African Americans, and Hispanics, age and BMI showed strong positive associations. In Asian/Pacific Islanders, age also showed a strong association with yield, but the relationship with BMI was markedly attenuated. In American Indians, there was virtually no association of age or BMI with screening yield, but these analyses were limited by smaller numbers.
Figure 2.
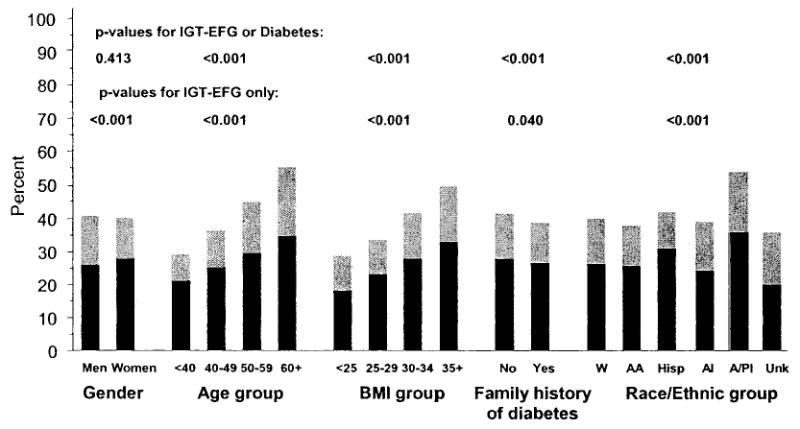
Yield of abnormal glucose tolerance in 30,383 people screened by the DPP who underwent OGTT by sex, age, BMI, family history of diabetes, and ethnicity. Black bars indicate IGT-EFG (fasting glucose 95–125 mg/dl and 2-h glucose 140–199 mg/dl). Gray bars indicate newly discovered diabetes (fasting glucose ≥ 126 mg/dl or 2-h glucose ≥ 200 mg/dl). P values for nonordinal variables are based on Pearson’s χ2 tests and for ordinal variables on Mantel-Haenszel χ2 tests.
Step 1 glucose as a predictor of yield
Figure 3 shows yield of IGT-EFG alone and IGT-EFG or newly discovered diabetes by step 1 glucose after stratification for method and timing of the measurement. Fasting venous glucose as measured by glucose analyzer was strongly associated with the yield of IGT-EFG (rising linearly from 12% to a maximum of 54% as step 1 glucose varied from 80 mg/dl to 129 mg/dl) and with the yield of IGT-EFG or newly discovered diabetes (rising almost linearly from 12 to 100% (Fig. 3A). Fasting capillary glucose performed nearly as well. For fasting capillary glucose measured on the same day as the OGTT, the yield of IGT-EFG reached a maximum of 42% at a step 1 glucose of 105–109 mg/dl, and yield of IGT-EFG or newly discovered diabetes rose linearly from 14 to 96% as step 1 glucose varied from 80 to 124 mg/dl (Fig. 3B). Nearly identical yields were observed for fasting capillary glucose measured 1–90 days before the OGTT (Fig. 3C). There were insufficient nonfasting capillary glucose data for analysis.
Figure 3.
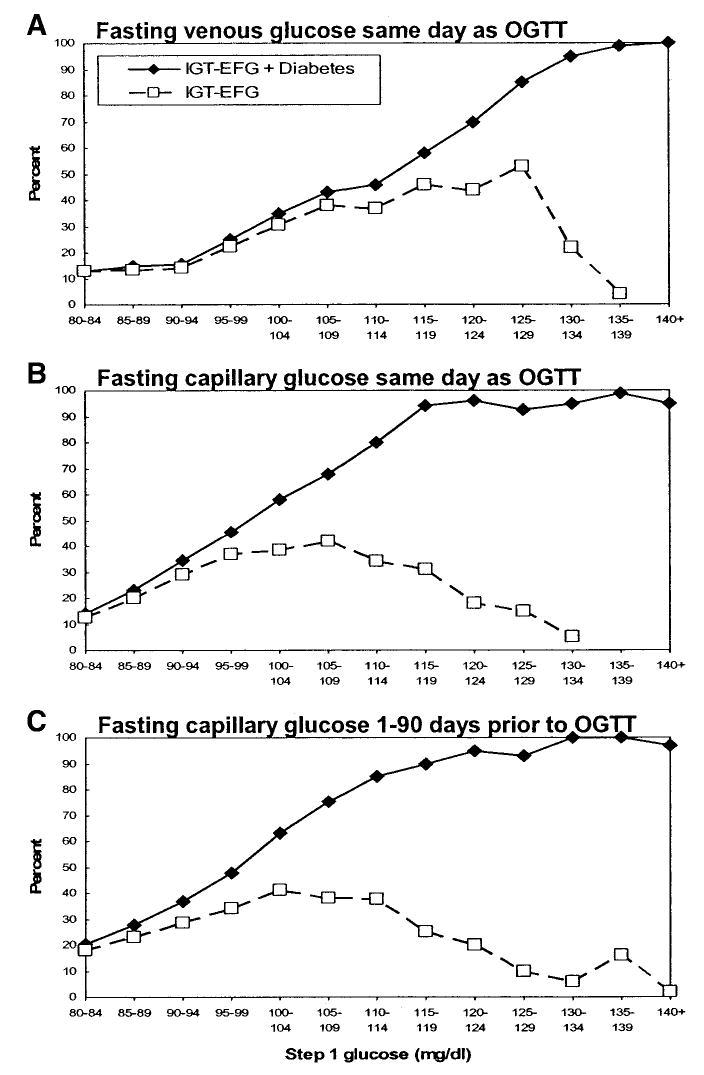
Yield of abnormal glucose tolerance by fasting step 1 glucose. □ , IGT-EFG (see Fig. 2 legend for definition). ♦, IGT-EFG plus newly diagnosed diabetes. A: Fasting venous glucose (analyzer) on the same day as the OGTT. B: Fasting capillary glucose (monitor) on the same day as the OGTT. C: Fasting capillary glucose (monitor) 1–90 days before the OGTT.
The three strongest correlates of yield were age, BMI, and step 1 glucose. We explored screening yield stratified by age or BMI using fasting capillary glucose measured 1–90 days before the OGTT, because this approach might be the most attractive for prescreening in populations. At levels of step 1 glucose below 100–105 mg/dl, both older age and higher BMIs were associated with an approximate doubling of yield of IGT-EFG and IGT-EFG or newly diagnosed diabetes. However, at levels exceeding 105 mg/dl, neither age nor BMI added predictive value beyond step 1 glucose (data not shown).
Multivariate analyses of yield
Finally, we determined the independent associations of the full range of step 1 variables with screening yield among the 5,276 participants who had a fasting capillary glucose measured 1–90 days before the OGTT, using multiple logistic regression (Table 2). In models without capillary glucose, age was strongly related to IGT-EFG. Compared with individuals aged < 40 years, those aged 60 and older were about twice as likely to have IGT-EFG. The relationship of age with IGT-EFG or diabetes was even stronger: compared with individuals aged < 40 years, those aged 60 and older were over four times more likely to have either condition. These associations were independent of sex, ethnicity, BMI, and family history and were only slightly attenuated by additional adjustment for step 1 capillary glucose measured 1–90 days before the OGTT. BMI showed a similarly strong, graded, independent association with both outcomes.
Table 2.
Adjusted relative odds (95% CI) of IGT-EFG and IGT-EFG or diabetes in 5,276 people screened by the DPP by selected characteristics, with and without fasting capillary glucose obtained 1–90 days prior to OGTT as an independent variable
| IGT-EFG
|
IGT-EFG or diabetes
|
|||
|---|---|---|---|---|
| Without capillary glucose | With capillary glucose | Without capillary glucose | With capillary glucose | |
| Men (vs. women) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 1.0 (0.9–1.1) | 0.8 (0.7–0.9) |
| Age (years) | ||||
| < 40 (ref.) | 1.0 | 1.0 | 1.0 | 1.0 |
| 40–49 | 1.2 (1.0–1.5) | 1.2 (1.0–1.4) | 1.6 (1.3–1.9) | 1.4 (1.2–1.7) |
| 50–59 | 1.6 (1.3–1.9) | 1.6 (1.3–1.9) | 2.4 (2.0–2.9) | 2.1 (1.7–2.6) |
| ≥ 60 | 2.0 (1.6–2.5) | 1.9 (1.6–2.4) | 4.4 (3.6–5.3) | 3.5 (2.8–4.3) |
| BMI (kg/m2) | ||||
| < 25 (ref.) | 1.0 | 1.0 | 1.0 | 1.0 |
| 25–29.9 | 1.7 (1.2–2.2) | 1.7 (1.2–2.3) | 1.5 (1.1–1.9) | 1.6 (1.2–2.2) |
| 30–34.9 | 2.1 (1.5–2.8) | 2.1 (1.5–2.8) | 2.4 (1.8–3.1) | 2.3 (1.7–3.1) |
| ≥ 35 | 3.0 (2.2–4.1) | 3.0 (2.2–4.1) | 4.1 (3.1–5.3) | 3.3 (2.5–4.4) |
| Ethnicity | ||||
| White (ref.)* | 1.0 | 1.0 | 1.0 | 1.0 |
| African American | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 1.0 (0.9–1.1) | 0.9 (0.8–1.1) |
| Hispanic | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 0.9 (0.7–1.2) |
| American Indian | 0.9 (0.4–2.0) | 1.0 (0.5–2.2) | 1.2 (0.6–2.4) | 1.0 (0.5–2.3) |
| Asian/Pacific Islander | 1.7 (1.1–2.5) | 1.7 (1.1–2.7) | 4.3 (2.8–6.5) | 3.8 (2.4–6.0) |
| Other | 0.8 (0.4–1.6) | 0.9 (0.5–1.7) | 1.3 (0.7–2.3) | 1.3 (0.7–2.4) |
| Family history of diabetes (vs. none) | 1.2 (1.0–1.3) | 1.1 (1.0–1.3) | 1.3 (1.1–1.5) | 1.3 (1.1–1.4) |
| Fasting glucose (mg/dl) | ||||
| 0–89 (ref.) | 1.0 | 1.0 | ||
| 90–99 | 1.5 (1.3–1.7) | 1.8 (1.6–2.1) | ||
| 100–109 | 2.1 (1.7–2.5) | 5.2 (4.4–6.3) | ||
| 110–119 | 1.4 (1.1–1.8) | 17 (12–24) | ||
| 120–129 | 0.5 (0.3–0.7) | 57 (26–122) | ||
| ≥ 130 | 0.2 (0.1–0.5) | 250 (35– > 999) | ||
All models adjust simultaneously for sex, age, ethnicity, BMI, and family history.
White indicate non-Hispanic white; family history indicates history of diabetes in a first-degree relative; glucose indicates fasting capillary glucose obtained 1–90 days prior to OGTT.
Only Asian/Pacific Islanders had a significantly different pattern of screening yields, with a 70–80% greater odds of IGT-EFG, but over fourfold greater odds of IGT-EFG or diabetes, adjusted for other risk factors. Yields in African Americans, Hispanics, American Indians, and individuals of other ethnic groups were otherwise similar to yields in whites.
Family history of diabetes was weakly associated with both outcomes adjusted for other risk factors. Sex was not a consistent predictive factor.
Step 1 capillary glucose measured 1–90 days before the OGTT showed a powerful association with IGT-EFG or newly discovered diabetes. Compared with participants with step 1 glucose of 80–89 mg/dl, those with step 1 glucose of 120–129 mg/dl were 50 times as likely to have either IGT-EFG or diabetes. Despite the strength of this association, introduction of step 1 glucose into the combined outcome model produced little or no attenuation of the predictive value of other readily assessed characteristics.
The relationship of step 1 capillary glucose with IGT-EFG was more complex. Compared with participants with step 1 glucose of 80–89 mg/dl, those in the 100- to 109-mg/dl range were about twice as likely to have IGT-EFG, but those ≥ 130 mg/dl were only one-fifth as likely to have IGT-EFG, because a much higher proportion of these participants met criteria for diabetes. Again, introduction of step 1 glucose into the IGT-EFG models produced little or no attenuation of the predictive value of other characteristics.
CONCLUSIONS
The DPP recruitment and eligibility approaches were successful in enriching the OGTT-tested sample and discovered 27% of tested adults to have IGT-EFG. By restricting the sample to only those samples analyzed at the CBL, the yield was increased to 40.8% with IGT-EFG (Table 1). Age, BMI, and fasting step 1 glucose were strong, independent predictors of screening yields, even when the step 1 glucose was measured on capillary blood using a handheld monitor up to 90 days before the OGTT. Strengths of the DPP that lend weight to these conclusions include its large size, nationwide scope, ethnic diversity, and attention to standardized documentation of the screening process.
Several recent studies have investigated approaches to identify individuals at high risk for type 2 diabetes without recourse to formal OGTTs (10–17). In these studies, useful non-OGTT data included age, sex, ethnicity, family history of diabetes, self-reported physical activity, smoking history, BMI, medication use (e.g., corticosteroids), blood pressure (or history of hypertension), presence of certain symptoms (e.g., thirst, pain, and exertional dyspnea), and, in women, having given birth to a large infant (> 4 kg). Although nonfasting capillary glucose appeared useful in one study conducted in Egypt (16), we are unaware of other published data on the utility of fasting capillary glucose to identify individuals at high risk for type 2 diabetes.
The goal of the DPP screening process was to identify individuals at high risk for type 2 diabetes for the purposes of enrollment in a primary prevention study. The DPP’s successful demonstration that lifestyle modifications and metformin therapy reduce the risk of developing type 2 diabetes (7) supports the potential value of the identification of such high-risk individuals as a desirable goal in public health policy and clinical practice. Moreover, newly discovered diabetes is a potentially useful target in its own right, because early treatment of clinically diagnosed type 2 diabetes has been shown to reduce the incidence of diabetes-related complications (3). Therefore, yields and analyses related to the combined outcome of IGT-EFG or newly discovered diabetes are probably the most relevant to public health translation.
The similar pattern of yields across most ethnic groups was unexpected. A vast previous literature documents the excess risk of IGT and type 2 diabetes in ethnic minorities in the U.S. (18). However, in the DPP screening process, only Asian/Pacific Islanders displayed a significantly higher yield. The most likely explanation is that the leveling effect arose from the selection process before the OGTT. This included participant self-selection in recruitment (which called for people who were overweight, had a history of gestational diabetes, or had a family history of diabetes) and clinic-driven selection at step 1 (based on BMI and step 1 glucose). The consequent elimination of low-risk white individuals could explain the equalization of yields in whites with those in African Americans, Hispanics, and American Indians but would not explain the persistently greater yields in Asian/Pacific Islanders. The screening approach devised by the DPP identified individuals at high risk for type 2 diabetes and should be useful in many clinical and public health settings. Age, BMI, and fasting capillary glucose appear to be key elements for any future screening program. This information should be helpful in translating the successful strategies used in the DPP and other recent diabetes prevention trials (19 –21) to population-based programs for the primary prevention of type 2 diabetes in high-risk adults.
Acknowledgments
Support to the clinical centers and the Coordinating Center was provided by the National Institute of Diabetes and Digestive and Kidney Diseases through a Cooperative Agreement. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources supported many of the clinical centers. Funding was also provided by the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, the American Diabetes Association, Bristol-Myers Squibb, and Parke-Davis.
We gratefully acknowledge the thousands of participants willing to be screened for the DPP, as well as the commitment and dedication of the randomized DPP participants.
Footnotes
A complete list of members of the Diabetes Prevention Program Research Group appears in New Engl J Med 346:393–403, 2002.
Additional information for this article can be found in an online appendix available at http://care.diabetesjournals.org.
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
References
- 1.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025 prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 4.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin RR, Fujimoto WY, Marrero DG, Brenneman T, Charleston JB, Edelstein SL, Fisher EB, Jordan R, Knowler WC, Lichterman LC, Prince M, Rowe PM DPP Research Group. The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials. 2002;23:157–171. doi: 10.1016/s0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Program Research Group. The diabetes prevention program: baseline characteristics of the randomized cohort. Diabetes Care. 2000;23:1619–1629. doi: 10.2337/diacare.23.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor E, Dowse GK, Haffner SM, Pettitt DJ, Sorkin JD, Muller DC, Collins VR, Hamman RF. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701–710. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 10.Stern MP, Williams K, Haffner SM. Identification of individuals at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med. 2002;136:575–581. doi: 10.7326/0003-4819-136-8-200204160-00006. [DOI] [PubMed] [Google Scholar]
- 11.Park PJ, Griffin SJ, Sargeant L, Wareham NJ. The performance of a risk score in predicting undiagnosed hyperglycemia. Diabetes Care. 2202;25:984–988. doi: 10.2337/diacare.25.6.984. [DOI] [PubMed] [Google Scholar]
- 12.Griffin SJ, Little PS, Hales CN, Kinmonth AL, Wareham NJ. Diabetes risk score: towards earlier detection of type 2 diabetes in general practice. Diabetes Metab Res Rev. 2000;16:164–171. doi: 10.1002/1520-7560(200005/06)16:3<164::aid-dmrr103>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Baan C, Ruige JB, Stolk RP, Witteman JC, Dekker JM, Heine RJ, Feskens EJ. Performance of a predictive model to identify undiagnosed diabetes in a health care setting. Diabetes Care. 1999;22:213–219. doi: 10.2337/diacare.22.2.213. [DOI] [PubMed] [Google Scholar]
- 14.Ruige JB, de Neeling JN, Kostense PJ, Bouter LM, Heine RJ. Performance of an NIDDM screening questionnaire based on symptoms and risk factors. Diabetes. 1997;20:491–496. doi: 10.2337/diacare.20.4.491. [DOI] [PubMed] [Google Scholar]
- 15.Herman WH, Smith PJ, Thompson TJ, Engelgau MM, Aubert RE. A new and simple questionnaire to identify people at increased risk for undiagnosed diabetes. Diabetes Care. 1995;18:382–387. doi: 10.2337/diacare.18.3.382. [DOI] [PubMed] [Google Scholar]
- 16.Engelgau MM, Thompson TJ, Smith PJ, Herman WH, Aubert RE, Gunter EW, Wetterhall SF, Sous ES, Ali MA. Screening for diabetes mellitus in adults: the utility of random capillary glucose measurements. Diabetes Care. 1995;18:463–466. doi: 10.2337/diacare.18.4.463. [DOI] [PubMed] [Google Scholar]
- 17.Rolka DB, Narayan KMV, Thompson TJ, Goldman D, Lindental D, Alich K, Bacall D, Benjamin EM, Lamb B, Stuart DO, Engelgau MM. Performance of recommended screening tests for undiagnosed diabetes and dysglycemia. Diabetes Care. 2001;24:1899–1903. doi: 10.2337/diacare.24.11.1899. [DOI] [PubMed] [Google Scholar]
- 18.National Diabetes Data Group:Diabetes in America Harris MI, Ed. Bethesda, MD, NIH, NIDDK, 1995 (NIH publ. no. 95-1468)
- 19.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 20.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M STOP-NIDDM Trial Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 21.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]


