Abstract
Purpose of review
There have been many recent advancements in our understanding of cochlear function within the past ten years. In particular, several mechanisms that underlie the sensitivity and sharpness of mammalian tuning have been discovered. This review focuses on these issues.
Recent findings
The cochlear amplifier is essentially a positive feedback loop within the cochlea that amplifies the traveling wave. Thus, vibrations within the organ of Corti are sensed and then force is generated in synchrony to increase the vibrations. Mechanisms that generate force within the cochlea include outer hair cell electromotility and stereociliary active bundle movements. These processes can be modulated by the intracellular ionic composition, the lipid constituents of the outer hair cell plasma membrane, and the structure of the outer hair cell cytoskeleton.
Summary
A thorough understanding of the cochlear amplifier has tremendous implications to improve human hearing. Sensorineural hearing loss is a common clinical problem and a common site of initial pathology is the outer hair cell. Loss of outer hair cells causes loss of the cochlear amplifier, resulting in progressive sensorineural hearing loss.
Keywords: cochlea, cochlear amplifier, outer hair cell, hearing, hearing loss, otoacoustic emissions
Introduction
One important survival advantage mammals have over other vertebrates is a better sense of hearing. This includes an improved sensitivity to quiet sounds, the ability to hear higher frequency sounds, and better frequency discrimination to distinguish between two tones of nearly the same frequency. These advancements enhance the ability of mammals to hear important environmental cues, such as the sound of an approaching predator. Additionally, they underlie the ability of humans to hear nuances associated with speech. While many of the fundamental mechanisms function similarly between the mammalian and the nonmammalian cochlea, mammalian hearing is better because of the cochlear amplifier. The cochlear amplifier is essentially a positive feedback loop within the cochlea that amplifies the traveling wave. Thus, vibrations within the organ of Corti are sensed and then force is generated in synchrony to increase the vibrations. In this review, the role of force production within the cochlea to produce cochlear amplification and the micromechanical mechanisms that underlie it are discussed.
Organ of Corti
The organ of Corti (Fig. 1) is a highly organized sensory structure. There is a single row of inner hair cells and three rows of outer hair cells that are positioned on top of the basilar membrane by various supporting cells. There are tight junctions between the apex of the hair cells and the surrounding supporting cells. This forms a barrier between the endolymph and the perilymph called the reticular lamina. Outer hair cell stereocilia insert into the overlying tectorial membrane whereas inner hair cell stereocilia do not.
Figure 1. Organ of Corti.
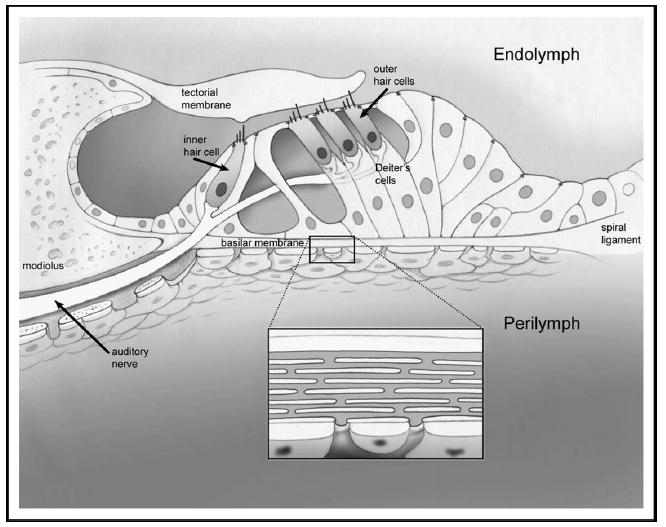
There is a single row of inner hair cells and three parallel rows of outer hair cells. Outer hair cells are supported by Deiter cells. The hair cells and all supporting cells sit on the basilar membrane. A section of the basilar membrane is enlarged to demonstrate the radial arrays of collagen filaments within it. The basilar membrane and the tectorial membrane are fixed at different locations to the modiolus. When sound vibrations cause the organ of Corti to move up and down, a shearing force is created, which deflects the hair cell stereocilia.
The entire organ of Corti vibrates up and down in response to sound pressure waves. However, because the insertion points of the basilar membrane and the tectorial membrane onto the osseous spiral lamina are at slightly different radial positions, a transverse shearing movement develops between the reticular lamina and the tectorial membrane. This directly deflects outer hair cell stereocilia, whereas the fluid movement in the space between the tectorial membrane and the reticular lamina is believed to indirectly deflect inner hair cell stereocilia [1].
Passive cochlear mechanics
The cochlea has both passive and active mechanical properties. The passive properties are found even in a postmortem cochlea, and define its tonotopic organization. Mechanically, the cochlea can be modeled as a series of radial sections ranging from the base to the apex. The resonant frequency of each section is based on the average mass, stiffness, and damping of the basilar membrane at that section. There are systematic differences in these physical properties along the length of the cochlea that determine the frequency response at any specific location [2]. Thus, the basilar membrane at the base of the cochlea has a lower mass and a higher stiffness and vibrates maximally in response to high-frequency sounds. In contrast, the basilar membrane at the apex of the cochlea has a higher mass and a lower stiffness and vibrates maximally in response to low-frequency sounds.
Sound pressure waves are transmitted to the perilymph of scala vestibuli by the stapes footplate at the base of the cochlea. The pressure waves then begin propagating up the length of the cochlear duct towards the apex (Fig. 2A). Basilar membrane vibration is maximal at the point where the frequency of the incoming sound matches the characteristic frequency of the basilar membrane. At that point, the pressure wave crosses the organ of Corti, enters scala tympani, and travels back down scala tympani to the round window.
Figure 2. Pressure waves begin propagating up the length of the cochlear duct towards the apex.
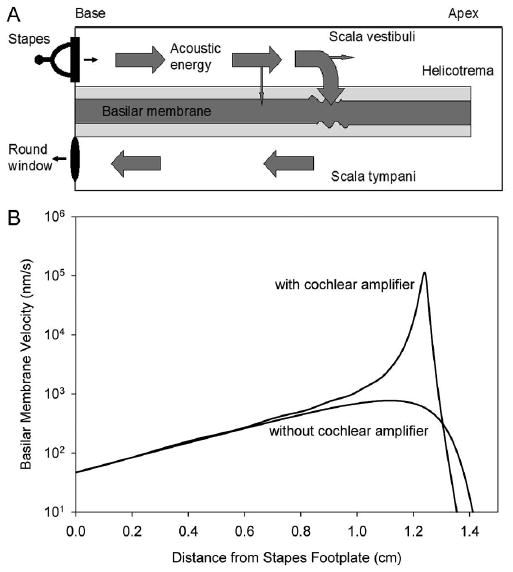
(A) Acoustic energy propagation down the cochlea. Sound pressure waves enter the scala vestibuli through movement of the stapes footplate. The acoustic energy propagates down the perilymph, traversing the basilar membrane predominantly at the region of its characteristic frequency. This creates the classical “traveling wave” motion of the basilar membrane. The region of the characteristic frequency is the area of maximal vibrations of the basilar membrane. The energy then propagates back to the round window through scala tympani. (B) Peak amplitudes of basilar membrane motion with and without the cochlear amplifier. These plots are based on simulations of the cochlear traveling wave as it propagates down the cochlea from the stapes to the helicotrema when a single frequency tone is played into the ear. The peak amplitude of the traveling wave is plotted. Without the cochlear amplifier, the traveling wave gradually reaches a peak, and then rapidly declines. With the cochlear amplifier, there is a large increase in basilar membrane motion. Also, note that there is sharpening of the peak with the cochlear amplifier. This permits improved frequency discrimination.
Cochlear amplifier
Experimental measurements of basilar membrane vibrations within the postmortem cochlea (ie, without a cochlear amplifier) demonstrate that increasing sound pressure level linearly increases basilar membrane motion. However, these passive mechanical properties can not explain the exquisite sensitivity and frequency selectivity of mammalian hearing. An active mechanism within the cochlea was first proposed by Thomas Gold [3]. The concept that a source of mechanical energy exists in the cochlea appeared validated when it was discovered that sounds can be produced by the inner ear, called otoacoustic emissions [4]. Now, it has been well proven that mammalian hearing is enhanced by some type of amplification process within the cochlea.
Measurements of basilar membrane vibration in the living cochlea demonstrate dramatically improved sensitivity and sharpness of tuning [5–8] (Fig. 2B). In other words, the basilar membrane vibrates more in a living cochlea than in a dead cochlea, particularly in response to quiet sounds. This property is called the cochlear amplifier. Positive feedback occurs locally along the length of the cochlea, to amplify vibrations of the basilar membrane on a cycle-by-cycle basis. These precise cochlear tuning characteristics are conveyed to auditory nerve fibers via synaptic transmission from the inner hair cells [9–11].
Force of the cochlear amplifier is generated by outer hair cells
Differential movements between the tectorial membrane and basilar membrane deflect hair cell stereocilia. Mechanoelectrical transduction channels are present at the tips of the stereocilia (Fig. 3). Deflecting the stereocilia bundle one way opens the channels and deflecting the bundle the other way closes the channels [12]. Because of the positive endolymphatic potential (approximately +80 mV) and the high cation (K+ and Ca+2) concentrations in the endolymph, opening the transduction channels causes an influx of current, depolarizing the hair cell. While the normal resting potential of a hair cell is around −60 mV, it can vary by up to several millivolts with high-intensity sound stimuli [13,14]. This variation of the intracellular voltage caused by mechanoelectrical transduction is called the receptor potential.
Figure 3. Stereotypical hair cell.
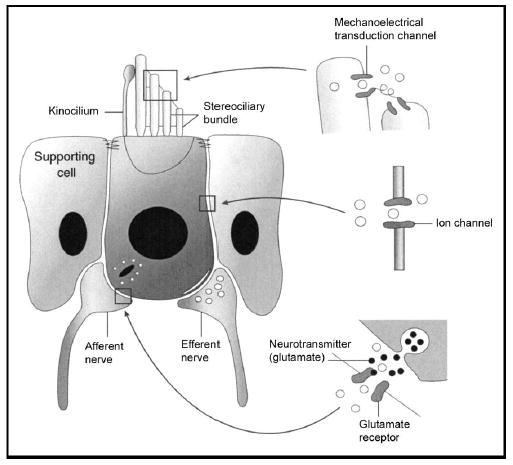
The boundary between the endolymph and the perilymph is the apical tight junctions between hair cells and supporting cells (the reticular lamina). There are three major functions within all hair cells. (1) Mechanoelectrical transduction occurs at the tips of the stereocilia, allowing ions from the endolymph to enter the cell. (2) Efferent nerve terminals and other ion channels modulate the intracellular voltage inside the hair cell and permit potassium exit from the cell for recycling. (3) Depolarization of the hair cell causes synaptic transmission of the afferent auditory nerve terminals. The neurotransmitter is glutamate. Figure derived from Eatock (1997) [84].
Current influx triggers processes within the outer hair cell (OHC) to generate force and amplify the sound pressure wave. A low endolymphatic potential diminishes the cochlear amplifier [15•]. The force produced by the cochlear amplifier does not act to amplify sounds equally at all frequencies. It is tuned to generate maximal force only at the characteristic frequency [16•]. This provides a sharpening of the tuning curve and improves frequency selectivity, the ability to distinguish between two tones of nearly the same frequency. If there was no frequency dependence of the cochlear amplifier, frequency sensitivity (the ability to hear quiet sounds) would be improved, but not frequency selectivity.
The relative contributions of the passive and the active properties of the organ of Corti in defining the tonotopic frequency map of the cochlea are poorly understood. In nonmammalian species, frequency specificity occurs at the level of the hair cell, and some phylogenetic remnant of this property probably remains in the mammalian cochlea. Certainly, hair cell morphology changes along the length of the cochlear duct, with longer OHCs at the base and shorter OHCs at the apex. Additionally, there are spatial variations in the mechanosensitive stereociliary bundles that might determine frequency selectivity. However, a distinct role for hair cell tuning within the mammalian cochlea has not been identified.
It is possible that hair cells at any position along the length of the mammalian cochlea could be made to function at any frequency simply by changing the passive properties of the basilar membrane. One hypothesis of how the cochlear amplifier is tuned is based upon the angle of the OHCs relative to the basilar membrane. The stereocilia are closer to the stapes than is the base of the cell. Thus, the force created by an OHC in response to having its stereocilia deflected acts in a “feed-forward” mechanism to deliver energy to a more apical section of the cochlea, slightly ahead of the traveling wave [17–19].
Outer hair cell electromotility
The cellular basis behind the cochlear amplifier is thought to be OHC electromotility [20–23•]. Electromotility is a process unique to the OHC in which its length changes with intracellular voltage (Fig. 4). Electromotility can occur at frequencies up to 100 kHz and does not require ATP or calcium. Thus, OHCs elongate and contract at acoustic frequencies, acting to amplify the natural vibrations of the traveling wave. No other hair cell (nor any other kind of cell) is able to change its length so rapidly in response to electrical stimulation. Human OHC electromotility functions similarly to that of other mammals [24,25].
Figure 4. Outer hair cell electromotility.
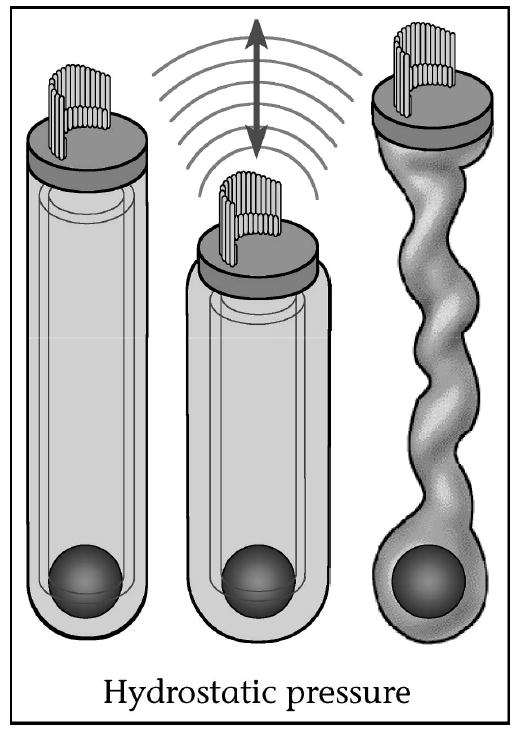
Outer hair cell electromotility. Outer hair cells contract and elongate with each cycle of sound as their intracellular voltage changes. This amplifies the vibration of the organ of Corti, permitting exquisite hearing sensitivity and frequency selectivity. OHCs have an intracellular turgor pressure to help maintain their shape. Loss of OHC turgor pressure causes the cell to constrict so that it can no longer produce electromotile force. Figure derived from Brownell (1999) [85].
Outer hair cells have a cylindrical shape. They vary in length from approximately 12 μm at the basal end of the cochlea to approximately 90 μm at the apical end. Their diameter is approximately 9 μm. Each region of the OHC has a specific function. The stereocilia at the apex of the cell are responsible for converting the mechanical energy of the traveling wave into electrical energy. Synaptic structures are found at the base of the hair cell and they are responsible for converting electrical energy into chemical energy by modulating the release of neurotransmitters. These two regions provide functions that are common to all hair cells (inner, outer, and vestibular).
The central portion of the OHC is different than all other cells because this is where electrical energy is converted into mechanical energy (electromotility). Most cells have a central cytoskeleton to maintain their shape. Because such an internal skeleton would impede electromotility, a specialized cytoskeleton is found just inside the plasma membrane, which acts to permit electromotile cell length changes (Fig. 5) [26]. The lateral wall has a unique trilaminate structure composed of a plasma membrane, a cytoskeleton, and a membranous organelle called the subsurface cisternae. The plasma membrane is a phospholipid bilayer that holds many particles between the inner and outer leaflets. The cytoskeleton contains parallel actin filaments crosslinked with spectrin, associated with Protein 4.1 [27–29]. Pillars of unknown composition tether the actin filaments to the plasma membrane [28,30]. The subsurface cisterna is an intracellular organelle, similar to endoplasmic reticulum or Golgi apparatus, which lines the inside of the cytoskeleton.
Figure 5. Diagram of OHC with detail of lateral wall components.
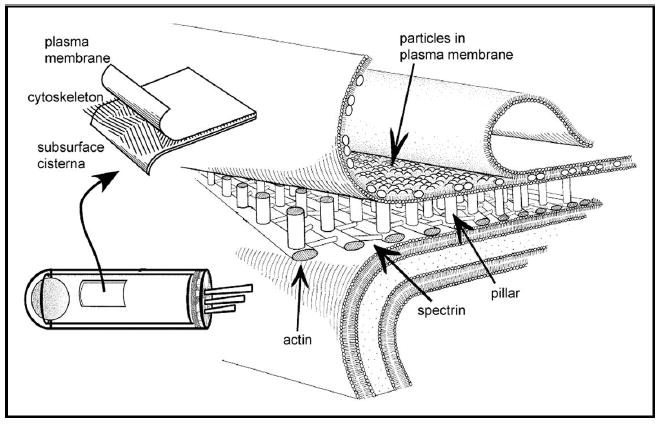
The lateral wall is composed of the plasma membrane, the cytoskeleton, and the subsurface cisterna. The cytoskeleton contains actin, spectrin, and pillar molecules. Some (perhaps most) of the particles in the plasma membrane are prestin proteins. Figure derived from Oghalai et al. (1998) [26].
Electromotility originates within the lateral wall of the OHC. A complete understanding of the mechanisms behind force generation within the lateral wall is lacking. However, the prestin protein within the plasma membrane is clearly central to this process [31–35]. This protein is not expressed in other hair cells, and likely works in concert with associated proteins and lipids of the lateral wall to form “motor complexes.” Each motor complex senses the intracellular voltage and individually generates force by changing its surface area [36–44]. The motor mechanism is a biologic form of piezoelectricity [45–49•]. Intracellular anions can modulate prestin function [50,51•]. Changing the intracellular anion content inhibits electromotility and decreases the longitudinal stiffness of OHCs [52••].
The lipid component of the OHC lateral wall plasma membrane is important to the generation of electromotility. The fluidity of the plasma membrane of the OHC lateral wall is similar to that of other eukaryotic cells [53]. But, changing the voltage across the membrane changes the ability of lipids to diffuse within the membrane [54]. This may be due to membrane curvature changes or interactions with prestin proteins. Further evidence supporting the importance of the lipid component of the plasma membrane for normal electromotility is that chlorpromazine, a drug that modulates membrane curvature, affects OHC electromotility and reduces cochlear function [55,56•].
The forces generated by each of the individual motors are coupled together through the lateral wall plasma membrane and cytoskeleton to achieve a net change in cell length. Because the OHC is fixed apically to the reticular lamina and basally to the cup of a Deiter’s cell, electromotile shape changes can modify the vibration of the cochlear partition [57]. The OHC is pressurized to be strong enough to transmit force to the rest of the organ of Corti. Calcium may modulate OHC stiffness as well [58•]. Recently a prestin knockout mouse has been generated that has been found to have sensorineural hearing loss, supporting the importance of prestin protein for normal hearing [59].
Active stereociliary bundle movements
Electromotility might not be the only force generating process within the OHC. Recently, a negative stiffness has been identified in the mechanoelectrical transduction process that is probably responsible for otoacoustic emissions found in nonmammalian species. In simple terms, this means that after deflecting the stereocilia a certain distance, a force produced within the stereocilia causes them to deflect further. This process may also be important for normal functioning of the mammalian cochlear amplifier [60–62]. Active bundle movements, called fast adaptation, are triggered by the entry of cations through the mechanoelectrical transducer channel [63••–67].
Other, active processes within the stereocilia associated with mechanoelectrical transduction also produce nonlinearities that might modulate the cochlear amplifier. These processes are thought to be much slower than either electromotility or active bundle movements, and can not function at the higher frequency range of mammalian hearing. This includes slow adaptation of mechanoelectrical transduction, a process by which actin-myosin interactions function to tighten tip links between adjacent stereocilia to bias the mechanoelectrical transduction channels in an operating region of maximal sensitivity [68,69]. Also, in the nonmammalian cochlea, electrical resonance is derived from the interplay between voltage-dependent ion channels within an individual hair cell [70,71]. Although electrical resonance has not been identified in mammalian cochlear hair cells, voltage-gated ion channels are certainly important in shaping the receptor potential [72]. In the OHC, this directly impacts the electromotile response.
Efferent modulation of the cochlear amplifier
The gain of the cochlear amplifier appears to be regulated by the medial olivocochlear bundle. These efferent nerve fibers originate within the brainstem and project to the OHCs, releasing acetylcholine as their neurotransmitter. OHCs have a specialized α9 acetylcholine receptor on their synaptic pole that permits calcium influx from outside the cells and calcium release from intracellular stores [73]. This triggers the opening of potassium channels and causes cell hyperpolarization. There is also a slow effect of acetylcholine, which may produce changes in the OHC cytoskeleton via second messenger systems [74–76•]. Acetylcholine has been shown to increase the amplitude of electromotility in isolated OHCs by decreasing cell stiffness [77]. In vivo, stimulation of the efferent nerve bundle elevates cochlear thresholds [78,79] and reduces motion of the cochlear partition, protecting the cochlea from acoustic overstimulation [80]. Thus, the central nervous system actively controls cochlear function by changing OHC electromotility. This may be important clinically as an innate mechanism that reduces hair cell damage from loud noise exposure [81,82]. Additionally, the efferent system may play a role in filtering out background noise, improving the ability of humans to understand speech in noisy environments [1].
Otoacoustic emissions
One consequence of having an active system is that oscillations can occur even when no energy is coming into the system from the outside. In the cochlea, these are called spontaneous otoacoustic emissions. Other types of otoacoustic emissions can be measured as well, including distortion product otoacoustic emissions and transient evoked otoacoustic emissions. These can be triggered by playing certain types of sound stimuli into the ear, and so are more useful clinically than the measurement of spontaneous otoacoustic emissions. For otoacoustic emissions to be generated, most of the peripheral auditory pathway must be functioning, with the exception of inner hair cells and the auditory nerve. Specifically, otoacoustic emissions test for nonlinearities caused by OHC force production, and thus test the cochlear amplifier [83•]. The measurement of otoacoustic emissions has become an important nondiagnostic tool to test cochlear function, particularly in newborn hearing screening.
Conclusion
A thorough understanding of the cochlear amplifier has tremendous implications to improve human hearing. Sensorineural hearing loss is a common clinical problem, and can be caused by many different etiologies including noise exposure, ototoxicity, and age-related hearing loss (presbyacusis). The common site of pathology for all of these conditions within the inner ear is the OHC. The attachments of OHC stereocilia to the tectorial membrane can be broken even with mild noise exposure. This reduces the ability of electromotility to provide positive feedback, leading to a temporary hearing loss. With further damage, the actin core of the stereocilia can fracture. With enough trauma, hair cell death occurs and a permanent hearing loss results because mammalian cochlear hair cells do not regenerate. The inner ear sensory epithelia are among the smallest organs in the body, containing less than 20,000 sensory cells. The small number of cells in the hearing organ means that the loss of even a small number affects hearing. After OHCs begin to degenerate, additional structures within the cochlea begin to sustain damage as well, including inner hair cells, supporting cells, and auditory nerve cells.
Sensorineural hearing loss is a common disease and there are no effective treatments. Clinically, it is heartbreakingly common to see a small elevation in high-frequency thresholds lead to a dramatic worsening of a patient’s speech discrimination ability, particularly in the presence of background noise. This occurs because frequencies above 2 kHz contain the formants of consonants. Also, a significant amount of information regarding sound source directionality is contained within these higher frequencies. While hearing aid technology continues to improve, they function basically to compress and amplify incoming sounds. A hearing aid cannot make up for loss of the cochlear amplifier because it cannot improve frequency discrimination. New techniques of treating sensorineural hearing loss will likely need to recreate the mechanical properties of the cochlear amplifier. This may require the development of technologies based on implantable micro- and/or nano-scale machines.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
• Of special interest
•• Of outstanding interest
- 1.Geisler CD:From Sound to Synapse: Physiology of the Mammalian Ear. New York: Oxford University Press; 1998.
- 2.von Bekesy G: Experiments in Hearing. McGraw-Hill; 1960.
- 3.Gold T. Hearing II. The physiological basis of the action of the cochlea. Proc R Soc Edinb. 1948;135:492–498. [Google Scholar]
- 4.Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- 5.Rhode WS. Observations of the vibration of the basilar membrane in squirrel monkeys using the Mossbauer technique. J Acoust Soc Am. 1971;49(suppl 2):1218. doi: 10.1121/1.1912485. [DOI] [PubMed] [Google Scholar]
- 6.Khanna SM, Leonard DG. Basilar membrane tuning in the cat cochlea. Science. 1982;215:305–306. doi: 10.1126/science.7053580. [DOI] [PubMed] [Google Scholar]
- 7.Cooper NP, Rhode WS. Basilar membrane mechanics in the hook region of cat and guinea-pig cochleae: sharp tuning and nonlinearity in the absence of baseline position shifts. Hear Res. 1992;63:163–190. doi: 10.1016/0378-5955(92)90083-y. [DOI] [PubMed] [Google Scholar]
- 8.Cooper NP, Rhode WS. Basilar membrane tonotopicity in the hook region of the cat cochlea. Hear Res. 1992;63:191–196. doi: 10.1016/0378-5955(92)90084-z. [DOI] [PubMed] [Google Scholar]
- 9.Narayan SS, Temchin AN, Recio A, et al. Frequency tuning of basilar membrane and auditory nerve fibers in the same cochleae. Science. 1998;282:1882–1884. doi: 10.1126/science.282.5395.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggero MA, Rich NC, Robles L, et al. Middle-ear response in the chinchilla and its relationship to mechanics at the base of the cochlea. J Acoust Soc Am. 1990;87:1612–1629. doi: 10.1121/1.399409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggero MA, Robles L, Rich NC, et al. Basilar membrane responses to two-tone and broadband stimuli. Philos Trans Lond B Biol Sci. 1992;336:307–314. doi: 10.1098/rstb.1992.0063. discussion 314–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 13.Preyer S, Hemmert W, Pfister M, et al. Frequency response of mature guinea-pig outer hair cells to stereociliary displacement. Hear Res. 1994;77:116–124. doi: 10.1016/0378-5955(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 14.Sellick PM, Patuzzi R, Johnstone BM. Comparison between the tuning properties of inner hair cells and basilar membrane motion. Hear Res. 1983;10:93–100. doi: 10.1016/0378-5955(83)90019-9. [DOI] [PubMed] [Google Scholar]
- 15•.Mills DM, Schmiedt RA. Metabolic presbycusis: differential changes in auditory brainstem and otoacoustic emission responses with chronic furosemide application in the gerbil. J Assoc Res Otolaryngol. 2004;5:1–10. doi: 10.1007/s10162-003-4004-3. This paper demonstrates the electrophysiologic changes associated with chronic lowering of the endocochlear potential. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Olson ES. Harmonic distortion in intracochlear pressure and its analysis to explore the cochlear amplifier. J Acoust Soc Am. 2004;115:1230–1241. doi: 10.1121/1.1645611. This paper measures changes in perilymphatic pressure across the cochlear partition and relates this to force production by the cochlear amplifier. [DOI] [PubMed] [Google Scholar]
- 17.Steele CR, Lim KM. Cochlear model with three-dimensional fluid, inner sulcus and feed-forward mechanism. Audiol Neurootol. 1999;4:197–203. doi: 10.1159/000013841. [DOI] [PubMed] [Google Scholar]
- 18.Steele CR. Toward three-dimensional analysis of cochlear structure. ORL J Otorhinolaryngol Relat Spec. 1999;61:238–251. doi: 10.1159/000027681. [DOI] [PubMed] [Google Scholar]
- 19.Geisler CD, Sang C. A cochlear model using feed-forward outer-hair-cell forces. Hear Res. 1995;86:132–146. doi: 10.1016/0378-5955(95)00064-b. [DOI] [PubMed] [Google Scholar]
- 20.Ruggero MA, Rich NC. Furosemide alters organ of corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci. 1991;11:1057–1067. doi: 10.1523/JNEUROSCI.11-04-01057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallos P, Corey ME. The role of outer hair cell motility in cochlear tuning. Curr Opin Neurobiol. 1991;1:215–220. doi: 10.1016/0959-4388(91)90081-h. [DOI] [PubMed] [Google Scholar]
- 22.Brownell WE, Bader CR, Bertrand D, et al. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- 23•.Ospeck M, Dong XX, Iwasa KH. Limiting frequency of the cochlear amplifier based on electromotility of outer hair cells. Biophys J. 2003;84:739–749. doi: 10.1016/S0006-3495(03)74893-0. This paper models the low-pass filter characteristics of the outer hair cell and addresses how this might affect the function of the cochlear amplifier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oghalai JS, Holt JR, Nakagawa T, et al. Ionic currents and electromotility in inner ear hair cells from humans. J Neurophysiol. 1998;79:2235–2239. doi: 10.1152/jn.1998.79.4.2235. [DOI] [PubMed] [Google Scholar]
- 25.Oghalai JS, Holt JR, Nakagawa T, et al. Harvesting human hair cells. Ann Otol Rhinol Laryngol. 2000;109:9–16. doi: 10.1177/000348940010900102. [DOI] [PubMed] [Google Scholar]
- 26.Oghalai JS, Patel AA, Nakagawa T, et al. Fluorescence-imaged microdeformation of the outer hair cell lateral wall. J Neurosci. 1998;18:48–58. doi: 10.1523/JNEUROSCI.18-01-00048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holley MC, Ashmore JF. A cytoskeletal spring in cochlear outer hair cells. Nature. 1988;335:635–637. doi: 10.1038/335635a0. [DOI] [PubMed] [Google Scholar]
- 28.Flock A, Flock B, Ulfendahl M. Mechanisms of movement in outer hair cells and a possible structural basis. Arch Otorhinolaryngol. 1986;243:83–90. doi: 10.1007/BF00453755. [DOI] [PubMed] [Google Scholar]
- 29.Drenckhahn D, Schafer T, Prinz M: Actin, myosin, and associated proteins in the vertebrate auditory and vestibular organs: immunocytochemical and biochemical studies. In Auditory Biochemistry. Edited by Thomas CC. Springfield, Il: D.G. Dreschers; 1985:317–335.
- 30.Arima T, Kuraoka A, Toriya R, et al. Quick-freeze, deep-etch visualization of the “cytoskeletal spring” of cochlear outer hair cells. Cell Tissue Res. 1991;263:91–97. doi: 10.1007/BF00318403. [DOI] [PubMed] [Google Scholar]
- 31.Dallos P, Evans BN, Hallworth R. Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature. 1991;350:155–157. doi: 10.1038/350155a0. [DOI] [PubMed] [Google Scholar]
- 32.Huang G, Santos-Sacchi J. Motility voltage sensor of the outer hair cell resides within the lateral plasma membrane. Proc Natl Acad Sci U S A. 1994;91:12268–12272. doi: 10.1073/pnas.91.25.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forge A. Structural features of the lateral walls in mammalian cochlear outer hair cells. Cell & Tissue Research. 1991;265:473–483. doi: 10.1007/BF00340870. [DOI] [PubMed] [Google Scholar]
- 34.Gulley RL, Reese TS. Regional specialization of the hair cell plasmalemma in the organ of corti. Anat Rec. 1977;189:109–123. doi: 10.1002/ar.1091890108. [DOI] [PubMed] [Google Scholar]
- 35.Zheng J, Shen W, He DZ, et al. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- 36.Kalinec F, Holley MC, Iwasa KH, et al. A membrane-based force generation mechanism in auditory sensory cells. Proc Natl Acad Sci U S A. 1992;89:8671–8675. doi: 10.1073/pnas.89.18.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashmore JF: Mammalian hearing and the cellular mechanisms of the cochlear amplifier. In Sensory Transduction. Edited by Corey DP, Roper SD. New York: Rockefeller University Press; 1992:396–412. [PubMed]
- 38.Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasa KH, Chadwick RS. Elasticity and active force generation of cochlear outer hair cells. J Acoust Soc Am. 1992;92:3169–3173. doi: 10.1121/1.404194. [DOI] [PubMed] [Google Scholar]
- 40.Iwasa KH. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J. 1993;65:492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwasa KH. A membrane motor model for the fast motility of the outer hair cell. J Acoust Soc Am. 1994;96:2216–2224. doi: 10.1121/1.410094. [DOI] [PubMed] [Google Scholar]
- 42.Gale JE, Ashmore JF. Charge displacement induced by rapid stretch in the basolateral membrane of the guinea-pig outer hair cell. Proc R Soc Lond B Biol Sci. 1994;255:243–249. doi: 10.1098/rspb.1994.0035. [DOI] [PubMed] [Google Scholar]
- 43.Kakehata S, Santos-Sacchi J. Membrane tension directly shifts voltage dependence of outer hair cell motility and associated gating charge. Biophys J. 1995;68:2190–2197. doi: 10.1016/S0006-3495(95)80401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kakehata S, Santos-Sacchi J. Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J Neurosci. 1996;16:4881–4889. doi: 10.1523/JNEUROSCI.16-16-04881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong XX, Ospeck M, Iwasa KH. Piezoelectric reciprocal relationship of the membrane motor in the cochlear outer hair cell. Biophys J. 2002;82:1254–1259. doi: 10.1016/S0006-3495(02)75481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwasa KH. A two-state piezoelectric model for outer hair cell motility. Biophys J. 2001;81:2495–2506. doi: 10.1016/S0006-3495(01)75895-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brownell WE, Spector AA, Raphael RM, et al. Micro- and nanomechanics of the cochlear outer hair cell. Annu Rev Biomed Eng. 2001;3:169–194. doi: 10.1146/annurev.bioeng.3.1.169. [DOI] [PubMed] [Google Scholar]
- 48.Spector AA, Ameen M, Popel AS. Simulation of motor-driven cochlear outer hair cell electromotility. Biophys J. 2001;81:11–24. doi: 10.1016/S0006-3495(01)75676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Spector AA, Brownell WE, Popel AS. Effect of outer hair cell piezoelectricity on high-frequency receptor potentials. J Acoust Soc Am. 2003;113:453–461. doi: 10.1121/1.1526493. This paper models the outer hair cell as a piezoelectric device and estimates how electromotility could produce force at high frequencies. [DOI] [PubMed] [Google Scholar]
- 50.Oliver D, He DZ, Klocker N, et al. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- 51•.Rybalchenko V, Santos-Sacchi J: Cl-flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J Physiol 2003. This paper measures chloride movement through the plasma membrane of the outer hair cell, and suggests that this might regulate prestin protein function. [DOI] [PMC free article] [PubMed]
- 52••.He DZ, Jia S, Dallos P. Prestin and the dynamic stiffness of cochlear outer hair cells. J Neurosci. 2003;23:9089–9096. doi: 10.1523/JNEUROSCI.23-27-09089.2003. The stiffness of the outer hair cell changes with electromotility, and is modulated by chloride and acetylcholine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oghalai JS, Tran TD, Raphael RM, et al. Transverse and lateral mobility in outer hair cell lateral wall membranes. Hear Res. 1999;135:19–28. doi: 10.1016/s0378-5955(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 54.Oghalai JS, Zhao HB, Kutz JW, et al. Voltage- and tension-dependent lipid mobility in the outer hair cell plasma membrane. Science. 2000;287:658–661. doi: 10.1126/science.287.5453.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lue AJ, Zhao HB, Brownell WE. Chlorpromazine alters outer hair cell electromotility. Otolaryngol Head Neck Surg. 2001;125:71–76. doi: 10.1067/mhn.2001.116446. [DOI] [PubMed] [Google Scholar]
- 56•.Oghalai JS: The effect of chlorpromazine on cochlear function in guinea pigs. Hear Res 2003. Chlorpromazine perfused through the perilymphatic space of the guinea pig cochlea reduces cochlear function.
- 57.Mammano F, Ashmore JF. Reverse transduction measured in the isolated cochlea by laser Michelson interferometry. Nature. 1993;365:838–841. doi: 10.1038/365838a0. [DOI] [PubMed] [Google Scholar]
- 58•.Frolenkov GI, Mammano F, Kachar B. Regulation of outer hair cell cytoskeletal stiffness by intracellular Ca2+: underlying mechanism and implications for cochlear mechanics. Cell Calcium. 2003;33:185–195. doi: 10.1016/s0143-4160(02)00228-2. Outer hair cell cytoskeletal stiffness is modulated by calcium and this may set the operating point of the cochlear amplifier. [DOI] [PubMed] [Google Scholar]
- 59.Liberman MC, Gao J, He DZ, et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- 60.Martin P, Hudspeth AJ, Julicher F. Comparison of a hair bundle’s spontaneous oscillations with its response to mechanical stimulation reveals the underlying active process. Proc Natl Acad Sci U S A. 2001;98:14380–14385. doi: 10.1073/pnas.251530598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin P, Mehta AD, Hudspeth AJ. Negative hair-bundle stiffness betrays a mechanism for mechanical amplification by the hair cell. Proc Natl Acad Sci U S A. 2000;97:12026–12031. doi: 10.1073/pnas.210389497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart CE, Hudspeth AJ. Effects of salicylates and aminoglycosides on spontaneous otoacoustic emissions in the Tokay gecko. Proc Natl Acad Sci U S A. 2000;97:454–459. doi: 10.1073/pnas.97.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Kennedy HJ, Evans MG, Crawford AC, et al. Fast adaptation of mechano-electrical transducer channels in mammalian cochlear hair cells. Nat Neurosci. 2003;6:832–836. doi: 10.1038/nn1089. Mammalian outer hair cells produce active force in their stereocilia similar to hair cells from nonmammalian species. [DOI] [PubMed] [Google Scholar]
- 64.Wu YC, Ricci AJ, Fettiplace R. Two components of transducer adaptation in auditory hair cells. J Neurophysiol. 1999;82:2171–2181. doi: 10.1152/jn.1999.82.5.2171. [DOI] [PubMed] [Google Scholar]
- 65.Ricci AJ, Crawford AC, Fettiplace R. Mechanisms of active hair bundle motion in auditory hair cells. J Neurosci. 2002;22:44–52. doi: 10.1523/JNEUROSCI.22-01-00044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fettiplace R, Ricci AJ, Hackney CM. Clues to the cochlear amplifier from the turtle ear. Trends Neurosci. 2001;24:169–175. doi: 10.1016/s0166-2236(00)01740-9. [DOI] [PubMed] [Google Scholar]
- 67.Ricci AJ, Fettiplace R. The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J Physiol. 1997;501:111–124. doi: 10.1111/j.1469-7793.1997.111bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hudspeth AJ, Gillespie PG. Pulling springs to tune transduction: adaptation by hair cells. Neuron. 1994;12:1–9. doi: 10.1016/0896-6273(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 69.Eatock RA, Corey DP, Hudspeth AJ. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog’s sacculus. J Neurosci. 1987;7:2821–2836. doi: 10.1523/JNEUROSCI.07-09-02821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crawford AC, Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol. 1981;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Navaratnam DS, Bell TJ, Tu TD, et al. Differential distribution of Ca2+-activated K+ channel splice variants among hair cells along the tonotopic axis of the chick cochlea. Neuron. 1997;19:1077–1085. doi: 10.1016/s0896-6273(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 72.Eatock RA, Hurley KM, Vollrath MA. Mechanoelectrical and voltage-gated ion channels in mammalian vestibular hair cells. Audiol Neurootol. 2002;7:31–35. doi: 10.1159/000046860. [DOI] [PubMed] [Google Scholar]
- 73.Elgoyhen AB, Johnson DS, Boulter J, et al. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 74.Kakehata S, Nakagawa T, Takasaka T, et al. Cellular mechanism of acetylcholine-induced response in dissociated outer hair cells of guinea-pig cochlea. J Physiol (Lond) 1993;463:227–244. doi: 10.1113/jphysiol.1993.sp019592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frolenkov GI, Mammano F, Belyantseva IA, et al. Two distinct Ca(2+)-dependent signaling pathways regulate the motor output of cochlear outer hair cells. J Neurosci. 2000;20:5940–5948. doi: 10.1523/JNEUROSCI.20-16-05940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76•.Zhang M, Kalinec GM, Urrutia R, et al. ROCK-dependent and ROCK-independent control of cochlear outer hair cell electromotility. J Biol Chem. 2003;278:35644–35650. doi: 10.1074/jbc.M301668200. A rho GTPase-based second messenger system may regulate outer hair cell cyto-skeletal stiffness. [DOI] [PubMed] [Google Scholar]
- 77.Dallos P, He DZ, Lin X, et al. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci. 1997;17:2212–2226. doi: 10.1523/JNEUROSCI.17-06-02212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiederhold ML, Kiang NY. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am. 1970;48:950–965. doi: 10.1121/1.1912234. [DOI] [PubMed] [Google Scholar]
- 79.Galambos R. Suppression of auditory activity by stimulation of the efferent fibers to the cochlea. J Neurophysiol. 1956;19:424–437. doi: 10.1152/jn.1956.19.5.424. [DOI] [PubMed] [Google Scholar]
- 80.Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci. 1996;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci. 2000;20:4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puel JL, Bobbin RP, Fallon M. An ipsilateral cochlear efferent loop protects the cochlea during intense sound exposure. Hear Res. 1988;37:65–69. doi: 10.1016/0378-5955(88)90078-0. [DOI] [PubMed] [Google Scholar]
- 83•.Nobili R, Vetesnik A, Turicchia L, et al. Otoacoustic emissions from residual oscillations of the cochlear basilar membrane in a human ear model. J Assoc Res Otolaryngol. 2003;4:478–494. doi: 10.1007/s10162-002-3055-1. A mathematical model predicts subtle effects of the cochlear amplifier on otoacoustic emissions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eatock RA, Rusch A. Developmental changes in the physiology of hair cells. Semin Cell Dev Biol. 1997;8:265–275. doi: 10.1006/scdb.1997.0142. [DOI] [PubMed] [Google Scholar]
- 85.Brownell WE. How the ear works–nature’s solutions for listening. Volta Review. 1999;99:9–28. [PMC free article] [PubMed] [Google Scholar]


