Abstract
Rationale
Exposure to extreme stress has been suggested to produce long-term, detrimental alterations in the hypothalamic–pituitary–adrenal (HPA) axis leading to the development of mental disorders such as depression. Therefore, compounds that block the effects of stress hormones were investigated as potential therapeutics for depression.
Objectives
In the present study, we compared the potential antidepressant-like effects of four CRF antagonists, antalarmin, CP154,526, R121919, and LWH234 (at 3, 10, and 30 mg/kg i.p., 60 min prior to the forced swim test) and the corresponding effect on swim-induced HPA activation to better elucidate the relation between HPA activity and antidepressant activity.
Methods
The antidepressant-like effects of the CRF antagonists and known antidepressants were determined in the rat forced swim test, and blood samples were obtained before and after swimming for the evaluation of adrenocorticotropin-releasing hormone (ACTH) levels.
Results
Antalarmin, CP154,526, and R121919 did not produce antidepressant-like effects in the forced swim test although these compounds decreased swim-induced increases in ACTH to various extents. In contrast, LWH234 reduced immobility in the forced swim test, without altering the swim-stress-induced ACTH response. However, this compound antagonized restraint-induced ACTH release.
Conclusions
These data suggest that reducing stress-induced increases in HPA activity alone may not be sufficient to produce antidepressant-like activity; however, reductions in HPA activity may contribute to antidepressant actions of some treatments. In addition, it is proposed that CRF antagonists may alter differentially the HPA axis depending on the type of stressor used or behavioral measure evaluated.
Keywords: CRF antagonists, Forced swim test, Rats, ACTH
Introduction
Following acute exposure to a stressful event, the hypothalamic–pituitary–adrenal (HPA) axis is activated, releasing stress hormones into circulation that alter many physiological and behavioral processes. More specifically, cells in the paraventricular nucleus of the hypothalamus secrete corticotropic-releasing factor (CRF) into portal blood, activating CRF receptors in the pituitary and causing the release of adrenocorticotropin-releasing hormone (ACTH) into the bloodstream. ACTH acts at the adrenal glands to release glucocorticoids, cortisol (in humans and primates) or corticosterone (in rodents). Circulating glucocorticoids can act in the brain and at the pituitary to dampen HPA axis activation, termed negative feedback. These stress responses are required for fight-and-flight responses that are essential for survival. Many current theories suggest that, in some individuals, continued exposure to extreme stress produces detrimental alterations in the HPA axis participating in the development of mental disorders such as anxiety and depression (e.g., Holsboer 1999, 2000; McEwen 2000).
In clinical studies, it has been observed that some depressed patients demonstrate a hyperactivity of the HPA axis as well as an impaired negative feedback system (for review, see Holsboer and Barden 1996). However, this hyperactivity, although not observed in all patients with depression (Watson et al. 2002), has been identified as a useful diagnostic tool. It has been suggested that a successful treatment with a variety of antidepressant medications and therapies reverses HPA hyperactivity. For example, in depressed patients, fluoxetine reduced CRF in the CSF and improved the depression scores on the Hamilton Depression Rating Scale (De Bellis et al. 1993). Therefore, potential therapeutics that selectively block the HPA axis may prove useful as novel antidepressant treatments. To investigate the potential therapeutic effect of CRF antagonists, a preliminary study with the CRF receptor 1 (CRF-R1) antagonist R121919 in 20 depressed patients demonstrated that R121919 reduced depression scores; however, in these patients, it did not block HPA activity following CRF challenge (Zobel et al. 2000).
The use of CRF receptor 1 (CRF-R1) antagonists as potential antidepressant therapies has been studied in animal models. The CRF-R1 antagonist CP154,526 demonstrated antidepressant-like effects in the learned helplessness model of depression (Mansbach et al. 1997). Also, the CRF-R1 antagonists antalarmin and SSR125543A decreased the duration of immobility in the forced swim test in rats, indicating an antidepressant-like effect with these compounds (Griebel et al. 2002a). Conversely, HPA activity was elevated in a number of animal strains that have inherent depressive-like behaviors and are used as models of depression (Rittenhouse et al. 2002; Urani and Gass 2003; Keck et al. 2003).
Although CRF antagonists produced antidepressant-like effects in some studies, few reports directly compared anti-depressant activity with the effects on the HPA axis within the same experimental conditions. Some previous studies demonstrated that CRH antagonists might not antagonize stress-induced endocrinological changes in a similar manner (Deak et al. 1999; Broadbear et al. 2004). Therefore, in the present study we compared the potential antidepressant-like effects of four CRF antagonists and the corresponding effect on swim-induced HPA activation to better elucidate the relation between HPA activity and antidepressant activity. Four CRF antagonists, antalarmin, LWH234, CP154,526, and R121919 (Fig. 1), are compared with two known antidepressants, desipramine and fluoxetine, in the forced swim test in rats. In addition, the blood serum levels of rats exposed to the forced swim test were tested for levels of ACTH to determine if behavioral changes correlated with inhibition of swim-induced increases in ACTH. Based on previous findings, it was hypothesized that all of the CRF antagonists tested would have similar effects on swim-induced increases in ACTH and similar antidepressant properties, as measured in the forced swim test in Sprague–Dawley rats. In the current studies, antalarmin, CP154,526, and R121919 did not produce antidepressant-like effects in the forced swim test although these compounds reduced swim-induced increases in ACTH to different extents. However, the CRF antagonist LWH234 significantly decreased immobility in the forced swim test without reducing the HPA axis response. In general, these data suggest that altering HPA activity alone through CRF-R1 receptor occupation may not be sufficient to produce antidepressant effects.
Fig. 1.
Structures of antalarmin (a), LWH234 (b), CP154,526 (c), and R121919 (d)
Materials and methods
Subjects
Male Sprague–Dawley rats, weighing 250–300 g, were used in these studies (Harlan Sprague Dawley, Indianapolis, IN). Upon arrival, groups of three rats were housed in clear acrylic cages located in a climate-controlled room with 12-h light/dark cycle (lights on at 0600). Food and water were available ad libitum. Rats were allowed to habituate to the environment for at least 5 days before they were used for study. In forced swim test experiments, rats were randomly assigned to either vehicle or drug treatment groups. In restraint experiments, rats were singly housed for 7 days after the initial acclimation period before testing commenced. Studies were performed in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The experimental protocols were approved by the University of Michigan University Committee on the Use and Care of Animals.
Procedures
Blood sampling
Rat blood samples were collected from a nick at the tip of tail. Without restraining the rat, the tail was gently massaged to increase blood collection. For each sample, 240 μl of blood was collected in heparinized microhematocrit capillary tubes (Fisher Scientific, Chicago, IL). Blood samples were stored on ice (less than 15 min) until centrifuged (4,100 rpm), and blood plasma was removed and stored at −80°C. Blood samples were collected between 1300 and 1700 h.
Forced swim test
Rats swam in a clear acrylic container [46 cm (height)×20 cm (diameter)] filled with 30 cm of 25°C (±1°C) water. Two swimming sessions were conducted: one 15-min swim exposure (habituation, day 1), followed by a 5-min swim 24 h later (test, day 2). The 5-min test swim was videotaped from above the cylinder and scored at a later time. Cylinder water was changed after every other rat. After each swim period, the rats were removed from the water, towel-dried, and placed in a heated cage for 15 min. Rat tail blood samples were taken immediately before and after each swim session.
Videotaped 5-min test swims were scored for immobility, swimming, and climbing behaviors (Detke et al. 1995). These behaviors are defined as follows: immobility—floating in the water without struggling and using only small movements to keep the head above water; swimming —moving limbs in an active manner (more than required to keep head above water) causing movement around the cylinder; climbing—making active movements with the forepaws in and out of the water, usually directed against the wall. Every 5 s, a trained observer who was blind to the treatment conditions categorized the subject’s behavior as one of the three behaviors listed previously. The total counts of each behavior during the 5-min test swim were summed for each rat and averaged within each treatment group.
Restraint
Baseline blood samples were obtained from each rat as previously described prior to drug injection. Following baseline blood collection, rats were injected with either vehicle or drug (time 0). Sixty minutes after the injection, rats were placed headfirst into 8.57×21.59-cm clear plastic cylinders (Braintree Scientific, Braintree, MA) for 15 min. The restraint cylinder allowed limited movement of the head and limbs; however, the tail was exposed to allow for easy blood collection. Blood samples were then collected at 15, 30, 60, 90 min after restraint initiation (75, 90, 120, 180, 240 min post injection). Between blood collections, rats were returned to their home cage.
ACTH measurements
Blood plasma samples were assayed using radioimmunoassay kits for adrenocorticotropin (ACTH; Nichols Institute Diagnostics, San Juan Capistrano, CA). The ACTH assay kit measures the amount of intact ACTH molecules that contain both N-terminal and C-terminal regions, and the assay has a sensitivity of 1.0 pg/ml. In these experiments, only ACTH was measured because the time points of blood collection were not optimal for measuring corticosterone levels in blood plasma. Blood samples were collected immediately before and after swim (either 5 or 15 min after swim exposure) at time points prior to maximal corticosterone release.
Drug treatments
In the forced swim test, drugs were administered three times prior to day 2 swim, such that intraperitoneal (i.p.) injections were administered 23.5, 5, and 1 h prior to the 5-min swim test. Rats (N=6–8 per treatment) were administered three injections of the same dose of either desipramine (1, 3, or 10 mg/kg), fluoxetine (1, 3, or 10 mg/kg), antalarmin (3, 10, or 30 mg/kg), CP154,526 (3, 10, or 30 mg/kg), LWH234 (3, 10, or 30 mg/kg), R121919 (3, 10, or 30 mg/kg), or vehicle. Desipramine and fluoxetine were dissolved in sterile saline. Antalarmin, CP154,526, and LWH234 were dissolved in a 1:1:9 solution of ethanol, emulphor (oil), and sterile water, respectively. R121919 was dissolved in ~10% of a 0.1 M tartaric acid solution and 90% sterile water. Vehicle injections consisted of the appropriate solvent. In the restraint experiment, rats (N=6) were administered a single injection (i.p.) of either vehicle, 10, or 30 mg/kg LWH234 60 min before restraint initiation. Injection volumes were 1 to 1.5 ml/kg.
Statistical analysis
Each behavior in the forced swim test was compared among groups by using a one-way factorial analysis of variance (ANOVA). Dunnett’s post hoc comparison was used to compare control vehicle groups to groups treated with drugs. ACTH values for post 5-min swim were compared using one-way factorial analysis and Dunnett’s post hoc comparisons were used to determine statistical significant differences between treatment groups.
Results
Forced swim test
Immobility scores associated with administration of the vehicle solution were relatively high between 33 and 39 counts (Fig. 2). Swimming and climbing scores were low in rats treated with vehicle, approximately eight to 18 counts of either activity. Desipramine produced a significant, dose-dependent decrease in immobility (F3,28=3.97; p=0.02) and produced a trend to increase climbing (F3,28=2.87; p=0.06) (Fig. 2a). Desipramine had no effect on swimming at any dose tested. Fluoxetine significantly decreased immobility (F3,23=4.84; p=0.01) at 3 mg/kg (p<0.05) and at 10 mg/kg (p<0.01), and produced a nonsignificant increase in swimming at the highest dose (Fig. 2b). However, fluoxetine did not significantly or dose-dependently alter climbing.
Fig. 2.
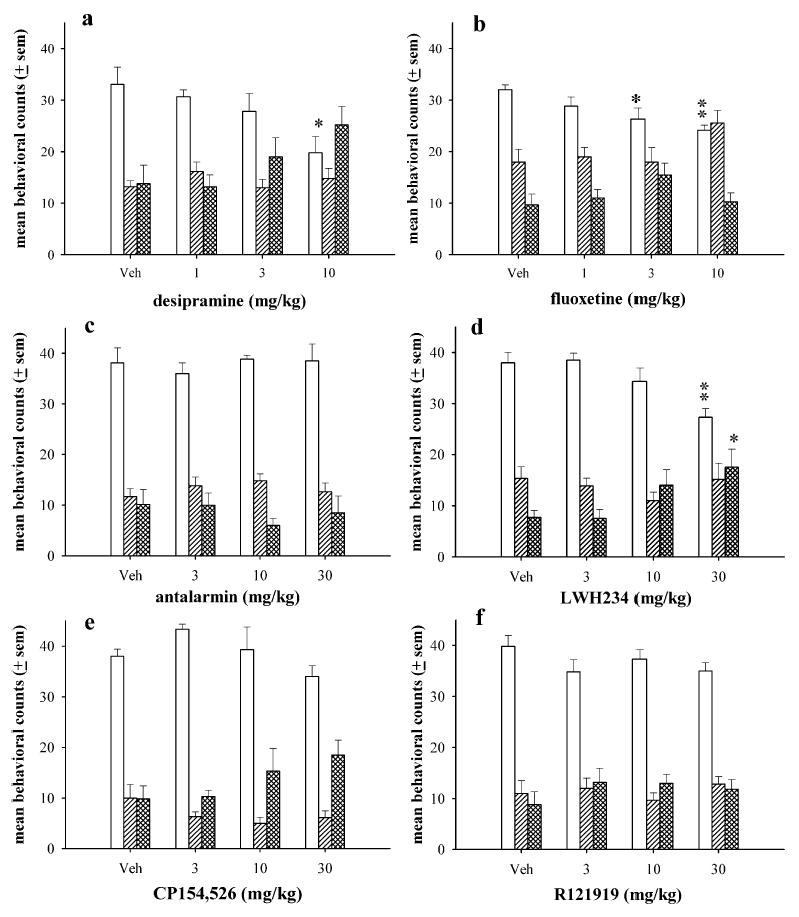
The effects of desipramine (a), fluoxetine (b), antalarmin (c), LWH234 (d), CP154,526 (e), and R121919 (e) in the forced swim test in rats. Rats were administered sub-chronic injections of vehicle or a single dose of either drug 23.5, 5, and 1 h prior to day 2 swim test (N=6–8 per dose). The bars and vertical lines above each bar represent the mean and standard error of the mean (SEM) for immobility (open bars), swimming (single-hatched bars), and climbing counts (double-hatched bars). *p<0.05, **p<0.01
In the forced swim test, the CRF antagonists antalarmin, CP154,526, and R121919 did not alter immobility, swimming, or climbing at any dose tested (Fig. 2c, e, f, respectively). However, the CRF antagonist LWH234 produced a significant decrease in immobility (F3,27=6.65; p=0.002) at 30 mg/kg (p<0.01) and an increase in climbing (F3,23=4.20; p=0.02) at 30 mg/kg (p<0.05), but did not alter swimming (Fig. 2d).
ACTH measurements
Blood samples collected from tail cuts before and after each swim period were assayed for ACTH. Although tail sampling might be stressful for the rats, the effects on ACTH were minimal as demonstrated by the low levels of baseline ACTH and ACTH measure in the vehicle groups. On day 1 swim (habituation) prior to any drug treatments, all rat groups had similar baseline and swim stress-induced levels of ACTH (Fig. 3). Prior to swimming, ACTH levels were ~50 pg/ml or below for all treatment groups. Fifteen minutes of swim exposure increased ACTH levels to 200–340 pg/ml, a four- to sevenfold increase above basal measurements. Between day 1 and day 2 swim, rats received three injections of either vehicle or drug at 23.5, 5, and 1 h before day 2 swim test. One hour after the final injection of vehicle or drug and immediately prior to day 2 swim (test session), all treatment groups had ACTH levels between 15 and 67 pg/ml, similar to levels measured before day 1 swim.
Fig. 3.
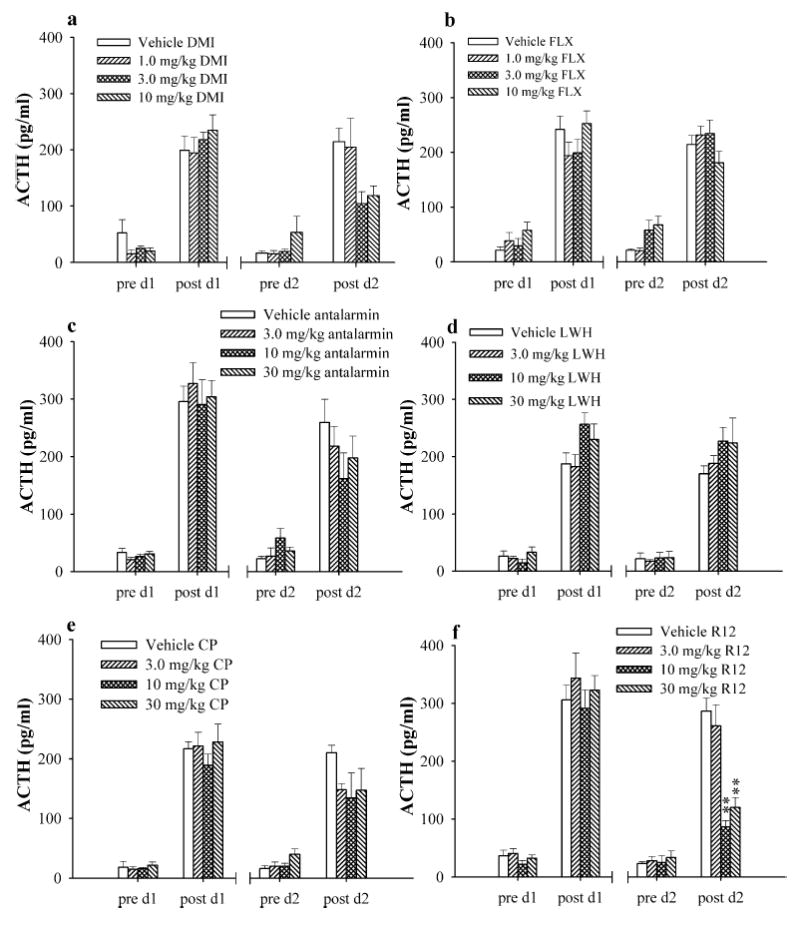
The effects of desipramine (DMI) (a), and fluoxetine (FLX) (b), antalarmin (c), LWH234 (LWH) (d), CP154,526 (CP) (e), and R121919 (R12) (f) on swim-induced increase in ACTH. Blood samples were taken before (pre d1) and after (post d1) day 1 swim and before (pre d2) and after (post d2) day 2 swim. **p<0.01
After the 5-min test swim, ACTH levels in rats that received vehicle injections were relatively similar to day 1 post swim levels of ACTH (Fig. 3). Subchronic administration of desipramine significantly decreased swim-induced increases in ACTH (F3,23=3.23; p=0.04) (Fig. 3a). Although post hoc analysis did not identify a dose that produced a significant decrease, 3 and 10 mg/kg desipramine appeared to have the greatest effect on decreasing ACTH levels. Fluoxetine did not alter ACTH levels measured following the day 2 test swim (Fig. 3b).
After subchronic treatment, antalarmin produced a non-significant and nonconsistent reduction in ACTH; however, there was a large degree of variability (Fig. 3c). Similarly, CP154,526 slightly reduced ACTH at all doses tested; however, the effect was not statistically significant (Fig. 3e). R121919 significantly decreased swim-induced increases in ACTH levels (F3,23=17.98; p<0.0001) at 10 and 30 mg/kg (p<0.01 for both doses) (Fig. 3f). In contrast, the CRF antagonist LWH234 did not reduce swim-induced elevations in ACTH to any extent following day 2 swim (Fig. 3d).
Restraint experiment
The effects of LWH234 on restraint-induced increases in ACTH were also measured to evaluate this compound following exposure to different stressors. Baseline ACTH levels prior to restraint were relatively low, about 50 pg/ml or lower (Fig. 4). Restraint alone increased ACTH levels to ~250 pg/ml, a fivefold increase over baseline values. With a 10-mg/kg LWH234 pretreatment, restraint produced a modest increase in ACTH to 115 pg/ml, on average. At 30 mg/kg, LWH234 produced a complete blockade of restraint-induced increases in ACTH.
Fig. 4.
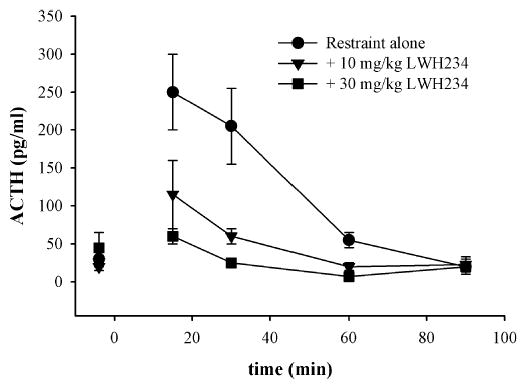
The effects of LWH234 on restraint-induced increases in ACTH. Baseline bloods were collected before drug injection, and LWH234 was injected 60 min prior to restraint initiation. Blood samples were collected immediately after restraint (15 min) and 30, 60, and 90 min following restraint termination
Discussion
As previously described, CRF antagonists demonstrated antidepressant-like effects in several behavioral assays. In the current study, a number of CRF antagonists were compared with known antidepressants in the same behavioral procedure while we measured the effects of these compounds on the HPA axis. In summary, the two known antidepressants, desipramine and fluoxetine, decreased immobility in the forced swim test, indicating an antidepressant-like effect. Interestingly, desipramine, but not fluoxetine, lowered the swim-induced increase in ACTH. These results support previous findings that antidepressant treatments can alter HPA activity, which may contribute to their antidepressant effects. However, as demonstrated with fluoxetine, antidepressant activity can also be observed without changes in HPA axis reactivity.
The CRF-R1 antagonists, antalarmin and CP154-526, were previously shown to block stress-induced increases in ACTH. In the current study, these two compounds produced minor, nonsignificant decreases in ACTH. Alternatively, the CRF-R1 antagonist, R121919, produced profound reductions in swim-induced increases in ACTH. Although these compounds had varying degrees of effect on the HPA axis, none of these compounds produced antidepressant-like effects in the forced swim test. One explanation for these finding is that these compounds suppressed ACTH release at the levels of the pituitary, but did not enter the CNS and, therefore, did not produce centrally mediated antidepressant activity in the forced swim test. Similarly, previous studies demonstrated that peripheral blockade of the HPA axis with dexamethasone did not alter behavioral effects produced by central administration of CRF (Britton et al. 1986a,b). However, this theory seems unlikely as it was previously demonstrated that orally administered R121919 occupied brain CRF1 receptors (Heinrichs et al. 2002) and decreased anxiety responses in rats (Gutman et al. 2003) and that CP154,526 and antalarmin had antidepressant-like effects in other animal models of depression, suggesting that these compounds enter the CNS to produce these centrally mediated behavioral effects. Therefore, it was expected that these compounds crossed the blood–brain barrier, but failed to produce antidepressant-like activity in the current study.
It is possible that antalarmin, CP154,526, and R121919 did not decrease immobility in the forced swim test in the current experiment because these compounds have some immobilizing or behavior-suppressing effects in rats. This behavioral suppression might have masked the antidepressant-like effect, thus producing a false negative result. However, based on observations in the present study, the behavioral suppression produced by these compounds immediately after injection dissipated prior to testing in the forced swim test. Likewise, an increase in immobility was not observed with these compounds as is frequently observed with immobilizing or anxiogenic compounds (Skrebuhhova et al. 1999).
Overall, these present results suggest that blocking stress-induced increases in ACTH alone does not produce antidepressant-like effects in the forced swim test. It is interesting to note that none of the CRF antagonists completely blocked swim-induced increases in ACTH, such that ACTH levels never returned to baseline values after CRF antagonist treatment. Similarly, a previous study demonstrated that the synthetic glucocorticoid, dexamethasone, failed to completely suppress swim-stress-induced ACTH increases (Jiang et al. 2004). These findings suggest that other factors or stress-related neuropeptides may be continuing to increase ACTH during swimming. In addition, the failure to completely block swim-induced increases in ACTH may contribute to the lack of effect observed in the forced swim test. However, these findings do not negate the fact that changes in HPA activity may contribute to or participate in the actions of some antidepressants, as observed with desipramine.
Other research also demonstrated that blocking the HPA axis alone might not be sufficient to produce antidepressant activity in animal models. For example, chronic infusion of CRF-R1 antisense did not alter immobility in the forced swim test, suggesting that the CRF-R1 receptor may not play a role in depressive mood states (Liebsch et al. 1999). In contrast to the expectation that blocking stress hormones might have antidepressant properties, intracerebroventricular administration of CRF decreased immobility in the forced swim test in rats, indicating antidepressant-like activity (Garcia-Lecumberri and Ambrosio 2000). In addition, administration of ACTH fragments enhanced the therapeutic effects of antidepressants in the forced swim test (Zebrowska-Lupina et al. 1997). These findings suggest that the involvement of stress hormones in antidepressant activity in animal models might be more complex than originally proposed. In addition, CRF antagonists were demonstrated to produce inconsistent antidepressant effects across animal models. For example, the CRF-R1 receptor antagonist R278995/CRA0450 produced antidepressant-like effects in some behavioral assays (learned helplessness and olfactory bulbectomy), but not in others (mouse tail suspension and the forced swim test) (Chaki et al. 2004). Nielsen et al. (2004) made similar observations in mice to those reported in this work. They found that R121919, but not antalarmin, decreased immobility in the mouse tail suspension test; however, none of the CRF antagonists tested demonstrated antidepressant activity in the mouse forced swim test. It seems unlikely that the lack of consistency across behavioral assays will be resolved in the near future; too many methodological possibilities exist among studies.
It has also been suggested that changes in the HPA axis (ACTH measurements in blood plasma) may not be representative of central CRF systems involved in affective regulation (Heinrichs and Koob 2004). Thus, the compounds tested in the current study may have different effects on the HPA axis as compared to central CRF systems. Although this is a fascinating hypothesis, it presents a new challenge of demonstrating that compounds are actually acting on central CRF systems. All of these findings suggest that the role of the HPA axis or CRF in depression as well as its role in therapeutic treatments for depression is very complicated and not well understood.
In the present study, the CRF antagonist LWH234 produced antidepressant-like effects in the forced swim test without reducing swim-induced increases in ACTH. LWH234 was also the only CRF antagonist tested that did not decrease swim-induced increases in ACTH to any extent; however, this compound did block restraint induced increases in ACTH. Although restraint and swimming produced similar elevations in ACTH, LWH234 reduced only restraint-induced increases in ACTH. Therefore, these results suggest that the antidepressant action of LWH234 in the forced swim test was independent of its effect on the HPA axis, but may be produced by central CRF systems or other neurotransmitter systems. These data also indicate that CRF antagonist-induced endocrinological or behavioral changes may be dependent on the drug or stress paradigm used.
In addition to the effects of CRF on stress-induced behavioral and endocrinological changes, other neuropeptides released in response to stress may play a role in these measurements. For example, arginine vasopressin (AVP) and CRF are coreleased into portal circulation following stress exposure and have synergistic effects on ACTH secretion. The release of these peptides was reported to be differently regulated by different stressors (Plotsky 1987). Likewise, forced swim stress differentially altered CRF and AVP transcription (Jiang et al. 2004), and the ACTH response in AVP-deficient rats as compared to control rats was not altered by hypertonic saline stress, but was diminished after the 10-min swim stress (Makara et al. 2004). Interestingly, vasopressin was proposed as a potential target of antidepressant therapy, and vasopressin antagonists were shown to have antidepressant activity in preclinical models (for review, see Scott and Dinan, 2002; Griebel et al. 2002a,b; Alonso et al. 2004). Therefore, some of the CRF antagonists, such as LWH234, evaluated in the present may have different effects on ACTH release or the synergistic actions of CRF and AVP depending on the type of stressor used. Based on the current data, the effects of LWH234 on stress hormones, in general, obviously differed from the effects of antalarmin, CP154,526, and R121919. For future studies, coadministration of a CRF and AVP antagonist may produce antidepressant effects under the current experimental design.
Previous research has also demonstrated that CRF antagonists have different effects depending on the type of measure evaluated. For example, CRF antagonists astressin, d-PheCRF12–41, and α-helical CRF9–41 altered some CRF-induced behavioral and physiological effects, but not others (Jones et al. 1999; Spina et al. 2000). In one study, astressin, d-PheCRF12–41, and α-helical CRF9–41 blocked the effects of CRF (i.c.v.) on food intake, but only astressin and α-helical CRF9–41 blocked CRF-induced locomotor activity (Jones et al. 1999). In contrast, Spina et al. (2000) demonstrated that d-PheCRF12–41 and α-helical CRF9–41, but not astressin, blocked CRF-induced locomotor activity. Similarly, the CRF-R1 antagonist antalarmin produced behavioral effects (induction and expression of conditioned fear) without altering the stress-induced ACTH and corticosterone responses (Deak et al. 1999). In monkeys, astressin B antagonized the effects of CRF on ACTH and cortisol, but antalarmin blocked only CRF-induced ACTH release (Broadbear et al. 2004). These data present an interesting hypothesis that CRF-R1 receptor antagonists may have unique profiles of action depending on the behavior or measure being studied.
Another explanation for the lack of effect of these CRF antagonists in the current forced swim test experiments was that the compounds were administered to relatively “normal” rats. Potential antidepressant activity of CRF antagonists may be best evaluated in subjects with altered HPA axis responsivity. Studies have found that prior exposure to stress or that genetic differences can greatly alter the behavioral profiles of antidepressant drugs and of CRF antagonists in animal models of depression (Borsini et al. 1989; Overstreet and Rezvani 1996; López-Rubalcava and Lucki 2000; Overstreet et al. 2004). In the forced swim test, initial exposure to the swim tank on day 1 supposedly induces a state of “behavioral despair,” such that these rats have altered emotional reactivity. The current data might suggest that depressive behaviors that develop between day 1 and day 2 swim were not sufficient to observe changes sensitive to CRF antagonist treatments; therefore, other animal models than the forced swim test might be required to evaluate the antidepressant properties of CRF antagonists.
In conclusion, the CRF antagonists antalarmin, CP154,526, and R121919 did not produce antidepressant-like effects in the forced swim test although these compounds reduced swim-induced elevations in ACTH to different extents. The CRF antagonist LWH234 demonstrated antidepressant-like effects without altering HPA activity in the forced swim test. These data suggest that reducing stress-induced increases in HPA activity alone may not produce antidepressant-like activity; however, reductions in HPA activity may contribute to antidepressant actions of some treatments, as demonstrated with the known antidepressant desipramine. In addition, these studies propose that CRF antagonists may have different profiles of action endocrinologically and behaviorally depending on the type of stressor or paradigm used. The differences among stressors and the actions of CRF antagonists under various stress conditions should be studied in order to better understand the HPA axis and the effects of CRF antagonists.
Acknowledgments
This research supported by USPHS grants DA00254, DA14349, GM07767, and DA07267.
Contributor Information
H. Houshyar, Department of Physiology, University of California San Francisco, San Francisco, CA, 94143-0444, USA
L.-W. Hsin, School of Pharmacy, College of Medicine, National Taiwan University, No.1 Jen-Ai Road, Section 1, Room 1336, Taipei, 10018, Taiwan
K. C. Rice, Laboratory of Medicinal Chemistry, NIDDK, National Institutes of Health, Bethesda, MD, USA
References
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrié P. Blockade of CRF1 and V1b receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry. 2004;9:278–286. doi: 10.1038/sj.mp.4001464. [DOI] [PubMed] [Google Scholar]
- Borsini F, Lecci A, Sessarego A, Frassine R, Meli A. Discovery of antidepressant activity by forced swimming test may depend on pre-exposure of rats to a stressful situation. Psychopharmacology. 1989;97:183–188. doi: 10.1007/BF00442247. [DOI] [PubMed] [Google Scholar]
- Britton DR, Varela M, Garcia A, Rosenthal M. Dexamethasone suppresses pituitary–adrenal but not behavioral effects of centrally administered CRF. Life Sci. 1986a;38(3):211–216. doi: 10.1016/0024-3205(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Britton DR, Lee G, Dana R, Risch SC, Koob GF. Activating and ‘anxiogenic’ effects of corticotropin releasing factor are not inhibited by blockade of the pituitary–adrenal system with dexamethasone. Life Sci. 1986b;39(14):1281–1286. doi: 10.1016/0024-3205(86)90189-x. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rivier JE, Rice KC, Woods JH. Corticotropin-releasing hormone antagonists, astressin B and antalarmin: differing profiles of activity in rhesus monkeys. Neuropsychopharmacology. 2004;29:1112–1121. doi: 10.1038/sj.npp.1300410. [DOI] [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, Vinken P, Ashton D, Langlois X, Steckler T. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur J Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong M-L, Chrousos GP, Webster E, Gold PW. The impact of the nonpeptidic corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140(1):79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Gold PW, Geracioti TD, Listwak SJ, Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry. 1993;150(4):656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Garcia-Lecumberri C, Ambrosio E. Differential effect of low doses of intracerebroventricular corticotropin-releasing factor in forced swimming test. Pharmacol Biochem Behav. 2000;67:519–525. doi: 10.1016/s0091-3057(00)00384-1. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand J-P, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl] 5-methyl-N-(2-propynl)-1,3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor1 receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002a;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simland J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand J-P, Soubrié P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002b;99(9):6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff C. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther. 2003;304:874–880. doi: 10.1124/jpet.102.042788. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type 1 receptor selective antagonist. Neuropsychophar-macology. 2002;27(2):194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRF-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Barden N. Antidepressants and hypothalamic–pituitary–adrenocortical regulation. Endocr Rev. 1996;17(2):187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- Jiang Y-Q, Kawashima H, Iwasaki Y, Uchida K, Sugimoto K, Itoi K. Differential effects of forced swim-stress on the corticotropin-releasing hormone and vasopressin gene transcription in the parvocellular division of the paraventricular nucleus of rat hypothalamus. Neurosci Lett. 2004;358:201–204. doi: 10.1016/j.neulet.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Jones DNC, Kortekaas R, Hatcher PD, Middlemiss DN, White A, Hagan JJ. Influence of peptide CRF receptor antagonists upon the behavioural effects of human/rat CRF. Eur J Pharmacol. 1999;373:141–145. doi: 10.1016/s0014-2999(99)00287-3. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Müller MB, Uhr M, Ohl M, Ohl F, Wigger A, Toschi N, Holsboer F, Landgraf R. Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychopharmacology. 2003;28:235–243. doi: 10.1038/sj.npp.1300040. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Landgraf R, Engelmann M, Lorscher P, Holsboer F. Differential behavioural effects of chronic infusion of CRF 1 and CRF 2 receptor antisense oligonucleotides in the rat brain. J Psychiatr Res. 1999;33:153–163. doi: 10.1016/s0022-3956(98)80047-2. [DOI] [PubMed] [Google Scholar]
- López-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22(2):191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Makara GB, Mergl Z, Zelena D. The role of vasopressin in hypothalamo–pituitary–adrenal axis activation during stress: an assessment of the evidence. Ann NY Acad Sci. 2004;1018:151–161. doi: 10.1196/annals.1296.018. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur J Pharmacol. 1997;323:21–26. doi: 10.1016/s0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Nielsen DM, Carey GJ, Gold LH. Antidepressant-like activity of corticotropin-releasing factor receptor antagonists in mice. Eur J Pharmacol. 2004;499:135–146. doi: 10.1016/j.ejphar.2004.07.091. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH. Behavioral differences between two inbred strains of Fawn-Hooded rat: a model of serotonin dysfunction. Psychopharmacology. 1996;128:328–330. doi: 10.1007/s002130050141. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Keeney A, Hogg S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. Eur J Pharmacol. 2004;492:195–201. doi: 10.1016/j.ejphar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Regulation of hypophysiotropic factors mediating ACTH secretion. Ann NY Acad Sci. 1987;512:205–217. doi: 10.1111/j.1749-6632.1987.tb24962.x. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, López-Rubalacava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar–Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Scott LV, Dinan TG. Vasopressin as a target for antidepressant development: an assessment of the available evidence. J Affect Disord. 2002;72:113–124. doi: 10.1016/s0165-0327(02)00026-5. [DOI] [PubMed] [Google Scholar]
- Skrebuhhova T, Allikmets L, Matto V. Effects of anxiogenic drugs in rat forced swimming test. Methods Find Exp Clin Pharmacol. 1999;21(3):173–178. doi: 10.1358/mf.1999.21.3.534826. [DOI] [PubMed] [Google Scholar]
- Spina MG, Basso AM, Zorrilla EP, Heyser CJ, Rivier J, Vale W, Merlo-Pich E, Koob GF. Behavioral effects of central administration of the novel CRF antagonist astressin in rats. Neuropsychopharmacology. 2000;22(3):230–239. doi: 10.1016/S0893-133X(99)00108-6. [DOI] [PubMed] [Google Scholar]
- Urani A, Gass P. Corticosteroid receptor transgenic mice models for depression? Ann NY Acad Sci. 2003;1007:379–393. doi: 10.1196/annals.1286.037. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Del-Estal D, Hearn A, Ferrier IN, Young AH. Hypothalamic–pituitary–adrenal axis function in patients with chronic depression. Psychol Med. 2002;32:1021–1028. doi: 10.1017/s0033291702005998. [DOI] [PubMed] [Google Scholar]
- Zebrowska-Lupina I, Pietrasiewicz T, Ossowska G, Lupina T, Klenk-Majewska B. ACTH 4–9 analogue facilitates the antiimmobility effect of antidepressants and dopamine agonists in swimming rats. J Physiol Pharmacol. 1997;48(2):263–275. [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Künzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]


