Abstract
The mechanisms subserving hypoandrogenemia and relative hypogonadotropism in older men are not known. The present study tests the clinical hypothesis that aging impairs hypothalamopituitary adaptations to feedback withdrawal induced by antagonism of estrogen biosynthesis. To this end, we appraised gonadal axis responses to estrogen depletion induced by anastrozole (a potent and selective aromatase inhibitor) in nine older and 11 young men vs. placebo in 17 other older and eight young men. The study design comprised a prospectively randomized, double-blind, parallel-cohort intervention. To monitor LH release, blood was sampled every 10 min for 24 h; LH concentrations were assayed by two-site monoclonal immunoradiometric assay; pulsatile LH release quantitated by a model-free discrete peak-detection technique (Cluster); feedback-dependent orderliness of LH secretion via the approximate entropy statistic; and 24-h rhythmicity of LH concentrations by cosine analysis. At baseline, older men had comparable estradiol and testosterone but lower LH concentrations than young controls. Exposure to anastrozole reduced (24-h pooled) serum estradiol concentrations by 50% (P < 0.001) and elevated mean LH concentrations by 2.1-fold (P < 0.001) in both the young and older cohorts. However, older men failed to achieve young adult augmentation of the following: 1) total testosterone concentrations (P < 0.01) or molar testosterone to SHBG ratios (P < 0.01); 2) incremental LH pulse amplitude (P < 0.001) and LH peak area (P < 0.01); 3) mean LH pulse frequency (P = 0.0044); and 4) quantifiable irregularity (approximate entropy) of LH release patterns (P < 0.001). FSH concentrations became comparable in the two age cohorts.
In summary, administration of a potent and selective aromatase antagonist reduces estradiol and elevates mean LH concentrations equivalently in young and older men. The low estrogen-feedback state in elderly men unmasks diminished incremental LH pulse amplitude and area; absence of further acceleration of LH pulse frequency; impaired regulation of the orderliness of LH release; and reduced testosterone to SHBG ratios. Thus, aging alters expected hypothalamopituitary-gonadal adaptations to short-term partial estrogen depletion in healthy men.
Abbreviations: ApEn, Approximate entropy; IRMA, immunoradiometric assay
Healthy older men exhibit a 30–50% reduction in testosterone bioavailability without a consistent reciprocal elevation in LH concentrations (1–9). Although the foregoing disparity is consistent with hypogonadotropic hypogonadism, the mechanistic basis of hypoandrogenemia is not known (3, 5, 10–14). Plausible considerations include deficient hypothalamic GnRH drive, impaired Leydig cell responsiveness to LH, and/or heightened feedback repression by endogenous sex steroid hormones. Available studies of the last issue are incomplete or conflicting. First, concentration-dependent negative feedback by circulating estrogen and testosterone has not been studied rigorously in the aged male. Second, exogenous testosterone and 5α-dihydrotestosterone reportedly suppress LH concentrations to variously greater, equivalent, or lesser degrees in older than young adults (12–17). And, third, nonsteroidal antagonists of the androgen or estrogen receptor stimulate pulsatile LH secretion inconsistently in elderly men (1, 12, 18, 19). The precise basis for such discrepant data is not known. Relevant considerations would include differing selection criteria, the limited size of most study cohorts, variable metabolic transformation of testosterone and 5α-dihydrotestosterone to active and inactive steroids, and confounding technical factors, such as nonuniform sampling schedules, LH immunoassays, and pulse-analysis methods (10, 11).
Rare male patients harboring a loss-of-function mutation of the estrogen receptor-α or aromatase enzyme gene maintain higher mean LH and testosterone concentrations than controls (20–22). Pharmacological estrogen receptor antagonists, such as clomiphene citrate and tamoxifen HCL, augment LH and testosterone secretion in healthy men (1, 18, 23–29). These agents are now known to be weak estrogen agonists as well (30). δ-1 Testolactone, a microbially synthesized progesterone derivative, inhibits the aromatase enzyme and stimulates LH output in young adults (31–35). However, this drug has antiandrogenic properties (31). Anastrozole, a highly selective aromatase inhibitor, reduces systemic estradiol concentrations by 30–50% and amplifies pulsatile LH secretion by 2- to 3-fold in eugonadal young men (32, 36). Anastrozole offers a relevant probe of estrogen-dependent negative feedback because the aromatase enzyme uniquely catalyzes estrogen biosynthesis (37).
The present study tests the clinical hypothesis that aging disrupts expected hypothalamopituitary-gonadal adaptations induced by partial withdrawal of endogenous estrogen-driven negative feedback. To this end, we compared pulsatile, entropic (pattern-regularity), and nyctohemeral (24-h) LH release in healthy young and older men randomized to receive placebo or anastrozole for 5 d.
Patients and Methods
Clinical protocol
Fifty-one healthy, ambulatory, community-dwelling, unmedicated men participated (31 individuals aged 18–33 yr and 20 volunteers aged 60–76 yr). Each volunteer provided written informed consent, as approved by the institutional review board and reviewed by the U.S. Food and Drug Administration under an investigator-initiated new drug file. Subjects were reimbursed for the time committed to participate. The following were normal at screening: medical history (including libido and potentia) and physical examination (including male habitus, virilization, and testis size); biochemical measurements of hematologic, hepatic, renal, and metabolic function; and fasting concentrations of T4, TSH, testosterone, estradiol, IGF-I, LH, FSH, and prolactin. Exclusion criteria included substance abuse, exposure to psychotropic or neuroactive drugs within 8 biological half-lives, use of glucocorticoids or sex hormones, anemia (hemoglobin < 13 gm/dl), recent weight loss or gain (> 2 kg change in 1 month), transmeridian travel (more than three time zones traversed in the preceding 7 d), shift work, untreated prostatic disease, acute or chronic systemic illness, and unwillingness to provide written informed consent.
The study design was a prospectively randomized, double-blind, placebo-controlled, parallel-cohort intervention. Placebo or anastrozole (five 1-mg tablets twice daily) was administered orally for 5 d beginning at 0800 h clock time on d 1 (38, 39). The foregoing and lesser doses of this aromatase inhibitor reduce total estradiol concentrations by 35–50% in young men (32, 36, 37, 40).
Sampling procedure
Subjects were admitted to the General Clinical Research Center (GCRC) on the fourth evening of intervention to allow overnight adaptation to the unit and placement of a forearm venous catheter. Blood samples (1.25 ml) were withdrawn every 10 min for 24 h beginning at 0800 h the next morning. Placebo or drug was continued. Meals were provided at 0800, 1200, and 1700 h. Ambulation was permitted to the lavatory. Smoking, caffeinated beverages, daytime sleep, and vigorous exercise were disallowed during the sampling interval.
Hormone assays
Serum LH concentrations were assayed in duplicate by two-site monoclonal immunoradiometric assay (IRMA) (Nichols Diagnostics Institute, San Juan Capistrano, CA) via an automated pipetting, bead-washing, and data-reduction system (2, 6, 12, 36). Sensitivity is 0.2 IU/liter (First International Reference Preparation) and cross-reactivity less than 0.3% for FSH, free alpha, LH β-subunits, and TSH. Median coefficients of variation in the present study were 5.1 (intraassay) and 6.3% (interassay). LH immunoreactivity by IRMA correlates well (r = +0.975) with that quantitated by in vitro rat Leydig cell bioassay (6, 12). Other hormones were measured in 24-h pooled sera (50 μl/sample × 145) as follows: testosterone and estradiol by coated-tube and double-antibody RIA, and SHBG, FSH, prolactin, and TSH by IRMA, respectively (6, 36, 41).
Cluster analysis
Cluster analysis was used as a validated, model-free method of discrete peak detection (42). The intention was to limit uncertainty in pulse detection due to unknown potential waveform changes under estrogen-feedback depletion. Conservative (< 5.0% false-positive rate) pulse-analysis criteria included test cluster sizes of 2 for the putative nadir and 1 for the presumptive peak and a (pooled-variance) t-statistic threshold of 2.0 to identify significant pulse onset and offset times (10, 43, 44). Definitionally, peak maximum denotes the highest (absolute) concentration attained in the pulse; nadir, the prepeak (mean) hormone concentration; incremental peak amplitude, the algebraic difference between the maximum and preceding nadir; incremental peak area, the trapezoidally integrated hormone concentration above the mean of the pre- and postpeak nadir values; pulse frequency, the number of significant peaks identified per 24 h; and interpeak interval, the mean time (minutes) separating consecutive peak maxima.
Cluster analysis was validated earlier by quantitating the concordance of identified LH peaks with the following: 1) discrete GnRH pulses in hypothalamopituitary portal venous blood in sheep; 2) mediobasal hypothalamic multiunit electrophysiological activity in the Rhesus monkey; 3) true-positive LH pulses induced by bolus iv infusion of GnRH or LH in men with hypogonadotropism; and 4) mathematically simulated LH pulse trains (10, 42–47). These analyses establish nominal discriminative indices for LH peak detection (sensitivity, specificity, negative and positive predictive accuracy) of 85–93%.
Approximate entropy analysis
The approximate entropy (ApEn) statistic provides a model-free measure of within-axis feedback changes, monitored by the serial regularity or orderliness of hormone release patterns (48–51). Technically ApEn is computed as the sum of the negative logarithms of the conditional probabilities that patterns of vector length m in a series of N data points recur on next (m+ 1) incremental comparison within a given tolerance range r (52). Earlier analyses demonstrated the statistical validity, sensitivity, and replicability of ApEn for neurohormone profiles comprising 30–300 samples under parameter choices of m = 1 (test pattern size) and r = 20% (normalized threshold to corroborate pattern recurrence, given as a percentage of the overall time-series sd). Normalized ApEn provides a translation (additivity)- and scale (concentration)-independent discriminative measure of the regularity of patterns. To normalize ApEn against interassay differences, we computed the mean ratio of individual ApEn to each of 1000 randomly shuffled (null) versions of the cognate series and the number of sds (z score) separating observed from mean random ApEn (50).
Cosine regression analysis
The 24-h rhythmicity of LH concentration profiles was quantitated by cosinor analysis, as described earlier. This procedure entails unweighted nonlinear regression of a cosine function of 1440-min periodicity on the hormone time series. The 95% statistical confidence intervals were determined for the fitted amplitude (50% of the nadir-zenith difference), mesor (24 h rhythmic mean), and acrophase (clock time of maximal diurnal value) (53).
Statistical analysis
One-way ANOVA was used to contrast (log-transformed) measures of LH pulsatility, ApEn, and 24-h rhythmicity among the four independent study groups. Data are presented as the mean ± sem. P < 0.05 was construed as statistically significant.
Results
Figure 1 illustrates LH concentration profiles derived by frequent (10-min) and extended (24-h) blood sampling in two young and two older volunteers studied on the fifth day of placebo or anastrozole administration. Peaks identified by computer-assisted (Cluster) analysis are marked for visual comparison. Figure 2 summarizes mean and integrated (24-h) serum LH concentrations for all four study cohorts. In the placebo context, older subjects exhibited somewhat lower LH concentrations than young controls but comparable concentrations of (total) testosterone and estradiol (Fig. 3). In the absence of active drug, older men maintained higher (24-h pooled) SHBG and FSH concentrations, a lower mean prolactin concentration, and a reduced molar ratio of testosterone to SHBG, compared with young men (Table 1 and Fig. 3).
Fig. 1.
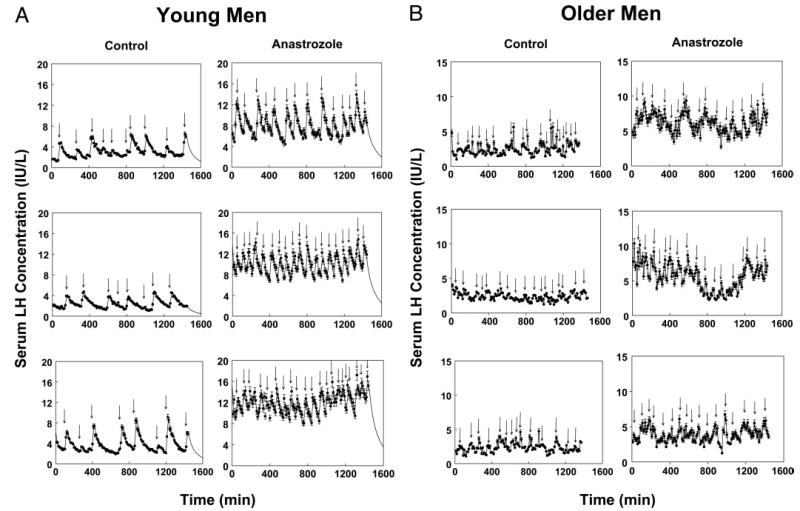
Illustrative serum LH concentration profiles in two young and two older men. Subjects underwent blood sampling every 10 min for 24 h on the fifth day of exposure to placebo or anastrozole. LH concentrations were measured via automated, double-monoclonal, LH β-subunit-directed IRMA. Vertical bars associated with each data point denote the within-assay concentration-dependent sd. Arrows mark statistically significant LH pulses detected by model-free Cluster analysis (see Patients and Methods).
Fig. 2.
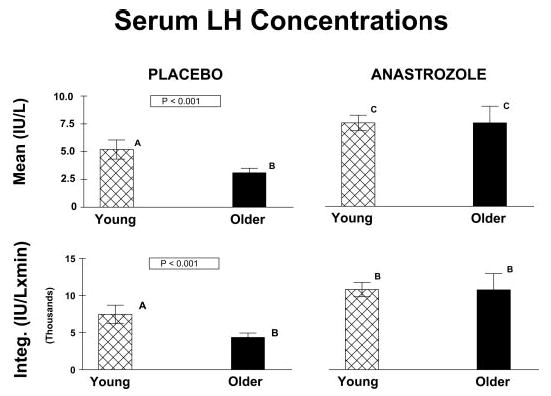
Mean and integrated (24-h) LH concentrations in young and older men administered either placebo or anastrozole for 5 d. Values are the mean ± sem. P values were computed by ANOVA. Means with unique (unshared) alphabetic superscripts A, B, and C differ significantly.
Fig. 3.
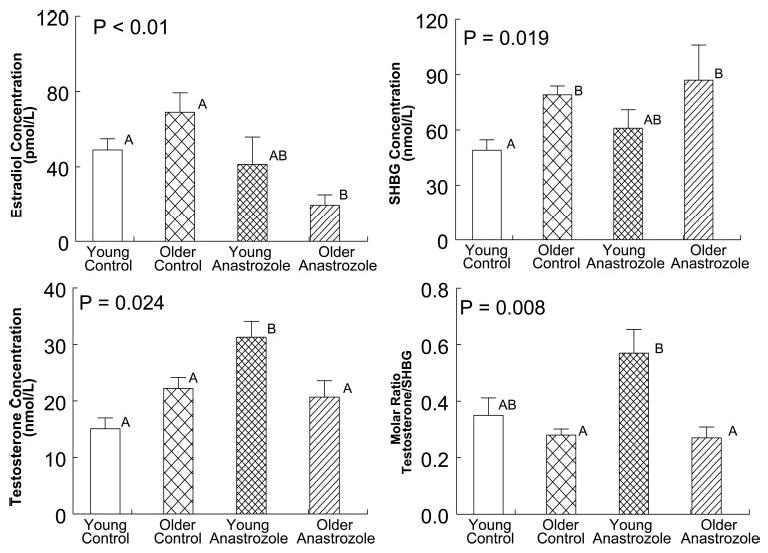
Serum testosterone, estradiol, and SHBG concentrations and the molar ratio of testosterone to SHBG concentrations in young and older men studied on the fifth day of exposure to either placebo or anastrozole. See legend of Fig. 2 for format of data presentation.
TABLE 1.
Serum hormone concentrations in young and older men administered placebo or anastrozole orally for 5 d
| Placebo
|
Anastrozole
|
||||
|---|---|---|---|---|---|
| Hormone | Young (n = 15) | Older (n = 16) | Young (n = 11) | Older (n = 9) | P |
| FSH (IU/liter) | 2.6 ± 0.4a | 6.9 ± 0.7b | 11 ± 2.5c | 15 ± 3.9c | <0.01 |
| Prolactin (μg/liter) | 8.0 ± 1.1a | 4.5 ± 0.8b | 11 ± 2.0a | 6.8 ± 1.3a,b | <0.05 |
| TSH (mIU/liter) | 2.3 ± 0.5 | 2.0 ± 0.6 | 1.9 ± 0.3 | 1.8 ± 0.5 | NS |
Data are (24-h pooled serum) determinations, presented as the mean ± sem (n denotes group size). Means with unique (unshared) alphabetic superscripts are significantly different within a row. P values were estimated by ANOVA. NS denotes P > 0.05.
Exposure to anastrozole, compared with placebo for 5 d, increased 24-h mean LH concentrations significantly and equivalently in young and older men (both P < 0.001) (Fig. 2). However, administration of the aromatase inhibitor failed to elevate (24-h pooled) total testosterone concentrations to the same degree in older as young men (P < 0.001) (Fig. 3). In confirmation of this age-related contrast, anastrozole block did not augment the molar ratio of testosterone to SHBG concentrations equivalently in older and young men (P < 0.001). Mean (24-h pooled) FSH concentrations (albeit higher in older than young men given placebo) were comparable by age in subjects receiving anastrozole (Table 1).
Discrete peak-detection analysis was used to quantitate feedback adaptations in pulsatile LH release. In the placebo setting, older males exhibited a higher mean LH pulse frequency (P = 0.0046) and shorter interpeak interval (P = 0.0044) than young controls (Fig. 4A). Anastrozole increased LH peak frequency and decreased LH interpulse intervals in young but not older men, thus yielding comparable stimulated values. In this setting, LH peak maxima and interpeak nadir LH concentrations were similar in the two age groups (P < 0.001 each vs. placebo; P = NS by age; not shown). In contrast, under pharmacological estrogen deprivation, mean incremental LH peak amplitude (P < 0.001) and incremental LH peak area (P = 0.001) were significantly lower in older than young men (Fig. 4B).
Fig. 4.
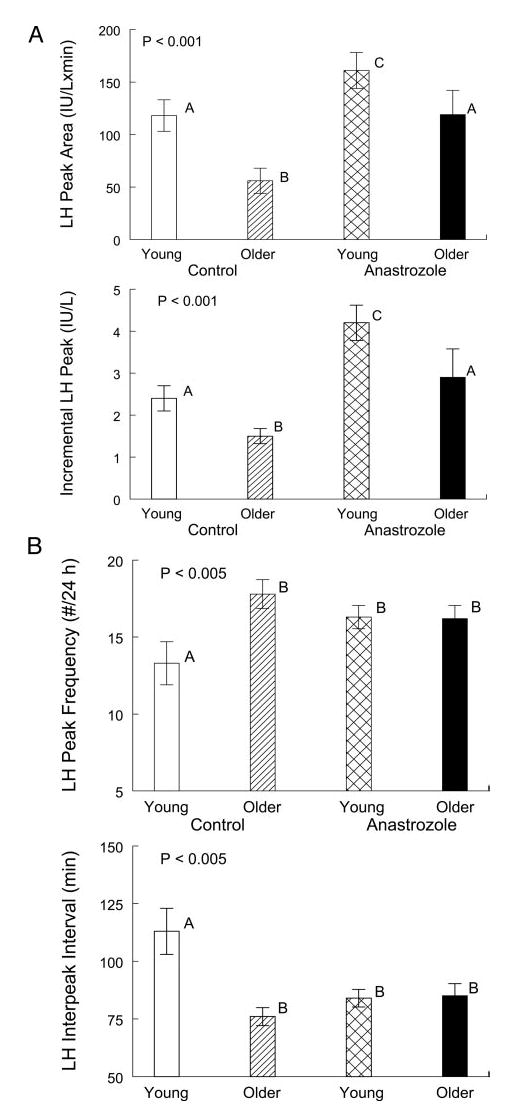
Measures of pulsatile LH release in healthy young and older men administered either placebo or anastrozole for 5 d. A, Incremental LH peak amplitude and area. B, LH peak frequency (minutes) and LH interpeak interval (number of pulses per 24 h). See legend of Fig. 2 for data format.
The ApEn statistic was applied to monitor the regularity of LH release. In the placebo intervention, ApEn was higher in older than young men (P = 0.0022), denoting more disorderly secretory patterns (Fig. 5, top panel). Administration of anastrozole elevated LH ApEn in young but not older individuals. Accordingly, stimulated LH ApEn did not differ in the two cohorts. The foregoing interventional contrasts were corroborated by computing the mean ratio of observed-to-random ApEn and the number of sds separating observed from random ApEn (defined by 1000 random shuffles of each LH time series) (Fig. 5, middle and bottom panels).
Fig. 5.
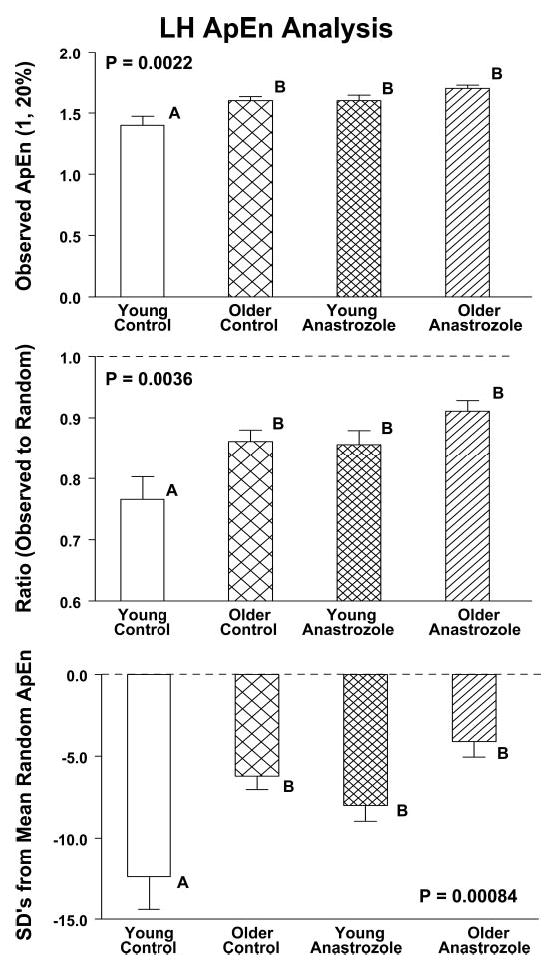
ApEn of LH concentration time series in young and older men administered either placebo (control) or anastrozole for 5 d. The panels show observed LH ApEn (top panel) and the ApEn ratio (middle panel). A higher value of either ApEn measure denotes greater irregularity (reduced orderliness) of the LH release process. Conversely, a lesser absolute number of sds separating observed from mean random ApEn (lower panel) signifies greater irregularity. Data are presented as described in the legend of Fig. 2.
Cosine regression analysis was used to quantitate the 24-h rhythmicity of LH concentrations. In the placebo context, older men maintained a lower mesor (daily rhythmic mean) than young men (Fig. 6). Administration of anastrozole amplified the LH mesor significantly and equivalently by age. Neither age nor active drug influenced the nyctohemeral amplitude (50% of the zenith-to-nadir difference) or acrophase (timing of the diurnal maximum) of LH release.
Fig. 6.
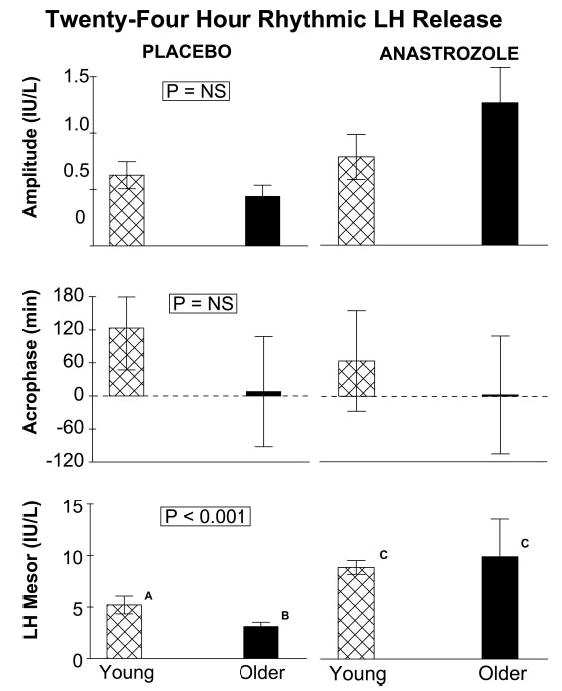
Measures of 24-h rhythmicity of LH concentrations. Panels depict (from top to bottom) the cosine amplitude (half the zenith-to-nadir difference), acrophase (clock time of the diurnal maximum), and mesor (daily rhythmic mean). See legend of Fig. 2 for data presentation.
Discussion
Healthy older men may exhibit normal, low, or elevated LH concentrations in the face of normal total testosterone and estradiol concentrations. Elevated LH secretion putatively reflects a feedback response to partial failure of Leydig cell steroidogenesis (10, 54, 55). Normal and low LH levels would point toward relative GnRH and/or LH deficiency. Assuming that gonadotrope secretory responses to injected GnRH are normal or enhanced in older individuals (7), a plausible working hypothesis is that hypothalamic GnRH outflow is reduced in this setting. According to this perspective, some elderly men manifest incipient features of hypogonadotropic hypogonadism (54, 55). We study such individuals here, as defined provisionally by normal concentrations of total testosterone and estradiol, reduced molar ratios of testosterone to SHBG and decreased mean LH concentrations.
The present clinical investigation tests the hypothesis that aging in healthy men disrupts endogenous, estrogen-dependent negative-feedback control of pulsatile, entropic (pattern-sensitive), and daily rhythmic LH release. To examine this postulate, we quantitated LH release during placebo and selective aromatase-enzyme inhibition in healthy young and older individuals. Model-free discrete peak-detection analysis was applied to obviate possible confounding by changes in LH pulse waveform in the low-estrogen milieu (56). Statistical comparisons disclosed that older (unlike young) volunteers exposed to anastrozole evince no detectable adaptations in either LH pulse frequency or the quantifiable regularity of LH release. In addition, compared with young men also given the aromatase inhibitor, elderly males maintain significantly lower incremental LH peak amplitudes and areas and molar ratios of testosterone to SHBG concentrations. The foregoing ensemble distinctions point to selective mechanisms of regulatory disruption in aging men.
Age did not influence the ability of anastrozole to depress (24-h pooled) estradiol concentrations by 50% (compared with placebo) and elevate mean LH concentrations by 2-fold. Nonetheless, under pharmacologically equivalent estrogen withdrawal, older subjects failed to achieve normal young adult augmentation of total testosterone concentrations or molar testosterone to SHBG ratios (Fig. 3). Given statistically comparable integrated LH concentrations, impoverished testosterone output could reflect a less effectual pattern of LH release, attenuated LH bioactivity, diminished LH uptake by the testis, and/or impaired gonadal steroidogenesis in the aged individual. In the last regard, testosterone concentrations do not increase maximally in elderly men after administration of a mixed estrogen receptor antagonist/agonist, pharmacological amounts of human chorionic gonadotropin, and near-physiological pulses of GnRH or recombinant human LH (1, 3, 6, 8, 45, 54). In conjunction with the current data and reduced rather than accentuated metabolic clearance of testosterone in older men (10, 54), we interpret the foregoing collective observations as indicative of an age-related defect in Leydig cell steroidogenesis.
Aromatase-enzyme blockade and attendant relative hypoestrogenemia elevated incremental LH pulse amplitude and LH peak area significantly in young but not older men. From a mechanistic vantage, these two measures of LH pulse amplitude are proportionate to the amount of LH (international units) secreted per unit distribution volume (liters) per burst (57). Peak height depends jointly on hypothalamic GnRH release, the sensitivity of gonadotrope cells to GnRH, and the elimination kinetics of LH (58, 59). Whereas central neuronal GnRH release cannot be monitored directly in the human, recent detailed dose-response analyses show that submaximally effective pulses of GnRH stimulate LH secretion more in older than young whether androgenic-negative feedback is withdrawn experimentally (60, 61). Other studies based on the iv infusion of midphysiological amounts of recombinant LH under leuprolide blockade indicate that LH kinetics do not differ in young and older men (45, 62). Based on such outcomes, we postulate that endogenous GnRH stimulus strength is reduced in aging men and that partial relief of estrogenic-negative feedback by anastrozole heightens detection of the putative defect.
The exact basis for inferred attenuation of hypothalamic GnRH drive is not known but could include diminished pulsatile GnRH release; a less effective waveform of GnRH secretion; and/or impaired access of GnRH to anterior-pituitary gonadotrope cells. The fact that mean testosterone to SHBG ratios were concomitantly lower in older men reinforces the inference of reduced hypothalamic GnRH stimulus strength, inasmuch as relative hypoandrogenemia provides an additional stimulus to GnRH production and potentiates GnRH action (see introductory text). The present interpretation of depressed GnRH outflow would be concordant with both in vitro and in vivo experimental data in the aged male rodent (4, 5, 9, 63–67).
Quantitation of the orderliness of 24-h LH release patterns revealed marked irregularity in older men, in consonance with earlier findings (6, 9, 49). On analytical grounds, disorderly LH secretion signifies relative disruption of coordinate control of feedback and feed-forward signaling within the interlinked hypothalamopituitary-gonadal axis (49, 50, 52). Integrative failure could arise by way of altered feed-forward (agonistic) and/or feedback (inhibitory) connections. In this regard, impaired feed-forward drive may include diminished GnRH signaling strength (see above) and reduced responsiveness of Leydig cells (present data and Refs. 9, 68, 69). The nature of putative feedback defects is not clear but plausibly could reflect reduced availability and/or attenuated actions of testosterone (6, 9, 18, 45, 68–70). In this regard, short-term hypoandrogenemia in young men elicits frequent, low-incremental amplitude and irregular LH pulses, akin to the patterns observed here in older men (41, 50, 70).
In summary, administration of a selective aromatase antagonist lowers (24-h mean) concentrations of estradiol by 50% and elevates LH concentrations by 100% in young and older healthy men. Negative-feedback adaptations to partial estrogen withdrawal differ significantly by age. In particular, relative estrogen depletion in older, unlike young, men fails to evoke (further) augmentation of the following: 1) incremental LH peak amplitude and LH pulse area; 2) daily LH pulse frequency; 3) LH secretory-pattern irregularity; and 4) the molar ratio of testosterone to SHBG concentrations. These outcomes extend concepts of aging-associated regulatory defects in the male by hypothalmopituitary gonadal axis to include estrogen-dependent feedback control.
Acknowledgments
We thank Kandace Bradford for preparation of the manuscript; Paula P. Azimi for assistance in statistical analysis, data management, and graphics; Brenda Grisso for performance of the immunoassays; Zeneca Laboratories (Wilmington, DE) for donation of anastrozole (Arimidex) tablets; and the nursing staff in the GCRC for protocol implementation.
Footnotes
This work was supported in part by GCRC Awards MO1 RR00847 and RR00585 to the University of Virginia and Mayo Clinic, respectively, from the National Center for Research Resources (Rockville, MD), a Veterans Affairs Merit Review grant (Washington, DC), and R01 AG23133 from the National Institutes of Health (Bethesda, MD).
References
- 1.Urban RJ, Veldhuis JD, Blizzard RM, Dufau ML. Attenuated release of biologically active luteinizing hormone in healthy aging men. J Clin Invest. 1988;81:1020–1029. doi: 10.1172/JCI113412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veldhuis JD, Urban RJ, Lizarralde G, Johnson ML, Iranmanesh A. Attenuation of luteinizing hormone secretory burst amplitude is a proximate basis for the hypoandrogenism of healthy aging in men. J Clin Endocrinol Metab. 1992;75:52–58. doi: 10.1210/jcem.75.3.1517359. [DOI] [PubMed] [Google Scholar]
- 3.Veldhuis JD. Recent insights into neuroendocrine mechanisms of aging of the human male hypothalamopituitary-gonadal axis. J Androl. 1999;20:1–17. [PubMed] [Google Scholar]
- 4.Mitchell R, Hollis S, Rothwell C, Robertson WR. Age-related changes in the pituitary-testicular axis in normal men; lower serum testosterone results from decreased bioactive LH drive. Clin Endocrinol (Oxf) 1995;42:501–507. doi: 10.1111/j.1365-2265.1995.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen A, Deslypere JP, De Meirleir K. A new look at the andro-pause: altered function of the gonadotrophs. J Steroid Biochem. 1989;32:163–165. doi: 10.1016/0022-4731(89)90158-1. [DOI] [PubMed] [Google Scholar]
- 6.Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol. 1999;141:257–266. doi: 10.1530/eje.0.1410257. [DOI] [PubMed] [Google Scholar]
- 7.Zwart AD, Urban RJ, Odell WD, Veldhuis JD. Contrasts in the gonadotropin-releasing dose-response relationships for luteinizing hormone, follicle-stimulating hormone, and α-subunit release in young versus older men: appraisal with high-specificity immunoradiometric assay and deconvolution analysis. Eur J Endocrinol. 1996;135:399–406. doi: 10.1530/eje.0.1350399. [DOI] [PubMed] [Google Scholar]
- 8.Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Amplified nocturnal luteinizing hormone (LH) secretory burst frequency with selective attenuation of pulsatile (but not basal) testosterone secretion in healthy aged men: possible Leydig cell desensitization to endogenous LH signaling—a clinical research center study. J Clin Endocrinol Metab. 1995;80:3025–3031. doi: 10.1210/jcem.80.10.7559891. [DOI] [PubMed] [Google Scholar]
- 9.Keenan DM, Veldhuis JD. Hypothesis testing of the aging male gonadal axis via a biomathematical construct. Am J Physiol. 2001;280:R1755–R1771. doi: 10.1152/ajpregu.2001.280.6.R1755. [DOI] [PubMed] [Google Scholar]
- 10.Urban RJ, Evans WS, Rogol AD, Kaiser DL, Johnson ML, Veldhuis JD. Contemporary aspects of discrete peak detection algorithms. I. The paradigm of the luteinizing hormone pulse signal in men. Endocr Rev. 1988;9:3–37. doi: 10.1210/edrv-9-1-3. [DOI] [PubMed] [Google Scholar]
- 11.Veldhuis JD 1999 Male hypothalamicpituitary-gonadal axis. In: Yen SSC, Jaffe RB, Barbieri RL, eds. Reproductive endocrinology. 4th ed. Philadelphia: W. B. Saunders Co.; 622–631
- 12.Veldhuis JD, Urban RJ, Dufau ML. Differential responses of biologically active LH secretion in older versus young men to interruption of androgen negative feedback. J Clin Endocrinol Metab. 1994;79:1763–1770. doi: 10.1210/jcem.79.6.7989483. [DOI] [PubMed] [Google Scholar]
- 13.Deslypere JP, Kaufman JM, Vermeulen T, Vogelaers D, Vandalem JL, Vermeulen A. Influence of age on pulsatile luteinizing hormone release and responsiveness of the gonadotrophs to sex hormone feedback in men. J Clin Endocrinol Metab. 1987;64:68–73. doi: 10.1210/jcem-64-1-68. [DOI] [PubMed] [Google Scholar]
- 14.Winters SJ, Atkinson L. Serum LH concentrations in hypogonadal men during transdermal testosterone replacement through scrotal skin: further evidence that aging enhances testosterone negative feedback. Clin Endocrinol (Oxf) 1997;47:317–322. doi: 10.1046/j.1365-2265.1997.2551065.x. [DOI] [PubMed] [Google Scholar]
- 15.Winters SJ, Sherins RJ, Troen P. The gonadotropin-suppressive activity of androgen is increased in elderly men. Metabolism. 1984;33:1052–1059. doi: 10.1016/0026-0495(84)90237-3. [DOI] [PubMed] [Google Scholar]
- 16.Baker HWG, Burger HG, de Kretser DM, Hudson B, O’Connor S, Wang C, Mirovics A, Court J, Dunlop M, Rennie GC. Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf) 1976;5:349–372. doi: 10.1111/j.1365-2265.1976.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 17.Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. J Clin Endocrinol Metab. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. [DOI] [PubMed] [Google Scholar]
- 18.Tenover JS, Matsumoto AM, Plymate SR, Bremner WJ. The effects of aging in normal men on bioavailable testosterone and luteinizing hormone secretion: response to clomiphene citrate. J Clin Endocrinol Metab. 1987;65:1118–1125. doi: 10.1210/jcem-65-6-1118. [DOI] [PubMed] [Google Scholar]
- 19.Veldhuis JD, Urban RJ, Beitins I, Blizzard RM, Johnson ML, Dufau ML. Pathophysiological features of the pulsatile secretion of biologically active luteinizing hormone in man. J Steroid Biochem. 1989;33:739–750. doi: 10.1016/0022-4731(89)90486-x. [DOI] [PubMed] [Google Scholar]
- 20.Mullis PE, Yoshimura N, Kuhlmann B, Lippuner K, Jaeger P, Harada H. Aromatase deficiency in a female who is compound heterozygote for two new point mutations in the P450arom gene: impact of estrogens on hypergonadotropic hypogonadism, multicystic ovaries, and bone densitometry in childhood. J Clin Endocrinol Metab. 1997;82:1739–1745. doi: 10.1210/jcem.82.6.3994. [DOI] [PubMed] [Google Scholar]
- 21.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female sibling caused by a novel mutation and the physiological role of estrogen. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 22.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 23.Ronnberg L. The effect of clomiphene citrate on different sperm parameters and serum hormone levels in preselected infertile men: a controlled double-blind cross-over study. Int J Androl. 1980;3:479–486. doi: 10.1111/j.1365-2605.1980.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 24.Spijkstra JJ, Spinder T, Gooren L, van Kessel H. Divergent effects of the antiestrogen tamoxifen and of estrogens on luteinizing hormone (LH) pulse frequency, but not on basal LH levels and LH pulse amplitude in men. J Clin Endocrinol Metab. 1988;66:355–360. doi: 10.1210/jcem-66-2-355. [DOI] [PubMed] [Google Scholar]
- 25.Veldhuis JD, Dufau ML. Estradiol modulates the pulsatile secretion of biologically active luteinizing hormone in man. J Clin Invest. 1987;80:631–638. doi: 10.1172/JCI113115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winters SJ, Janick JJ, Loriaux LD, Sherins RJ. Studies on the role of sex steroids in the feedback control of gonadotropin concentrations in men. II. Use of the estrogen antagonist, clomiphene citrate. J Clin Endocrinol Metab. 1979;48:222–227. doi: 10.1210/jcem-48-2-222. [DOI] [PubMed] [Google Scholar]
- 27.Gooren LJ, Van der Veen EA, van Kessel H, Harmsen-Louman W. Estrogens in the feedback regulation of gonadotropin secretion in men: effects of administration of estrogen to agonadal subjects and the antiestrogen tamoxifen and the aromatase inhibitor delta′-testolactone to eugonadal subjects. Andrologia. 1984;16:568–577. doi: 10.1111/j.1439-0272.1984.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 28.Winters SJ, Troen P. Evidence for a role of endogenous estrogen in the hypothalamic control of gonadotropin secretion in men. J Clin Endocrinol Metab. 1985;61:842–845. doi: 10.1210/jcem-61-5-842. [DOI] [PubMed] [Google Scholar]
- 29.Urban RJ, Veldhuis JD, Dufau ML. Estrogen regulates the gonadotropin-releasing-hormone stimulated secretion of biologically active luteinizing hormone in man. J Clin Endocrinol Metab. 1991;72:660–668. doi: 10.1210/jcem-72-3-660. [DOI] [PubMed] [Google Scholar]
- 30.Brodie AM. Aromatase, its inhibitors and their use in breast cancer treatment. Pharmacol Ther. 1993;60:501–515. doi: 10.1016/0163-7258(93)90033-a. [DOI] [PubMed] [Google Scholar]
- 31.Vigersky RA, Mozingo D, Eil C, Purohit V, Bruton J. The antiandrogenic effects of Δ1-testolactone (Teslac) in vivo in rats and in vitro in human cultured fibroblasts, rat mammary carcinoma cells, and rat prostate cytosol. Endocrinology. 1982;110:214–219. doi: 10.1210/endo-110-1-214. [DOI] [PubMed] [Google Scholar]
- 32.Bhatnagar AS, Muller P, Schenkel L, Trunet PF, Beh I, Schieweck K. Inhibition of estrogen biosynthesis and its consequences on gonadotrophin secretion in the male. J Steroid Biochem Mol Biol. 1992;41:437–443. doi: 10.1016/0960-0760(92)90369-t. [DOI] [PubMed] [Google Scholar]
- 33.D’Agata R, Vicari E, Aliffi A, Gulizia S, Palumbo G. Direct evidence in men for a role of endogenous oestrogens on gonadotrophin release. Acta Endocrinol (Copenh) 1981;97:145–149. doi: 10.1530/acta.0.0970145. [DOI] [PubMed] [Google Scholar]
- 34.Marynick SP, Loriaux LD, Sherins RJ, Pita JC, Lipsett MB. Evidence that testosterone can suppress pituitary gonadotropin secretion independently of peripheral aromatization. J Clin Endocrinol Metab. 1979;49:396–398. doi: 10.1210/jcem-49-3-396. [DOI] [PubMed] [Google Scholar]
- 35.Bagatell CJ, Dahl KD, Bremner WJ. The direct pituitary effect of testosterone to inhibit gonadotropin secretion in men is partially mediated by aromatization to estradiol. J Androl. 1994;15:15–21. [PubMed] [Google Scholar]
- 36.Schnorr JA, Bray MJ, Veldhuis JD. Aromatization mediates testosterone’s short-term feedback restraint of 24-hour endogenously driven and acute exogenous GnRH-stimulated LH and FSH secretion in young men. J Clin Endocrinol Metab. 2001;86:2600–2606. doi: 10.1210/jcem.86.6.7520. [DOI] [PubMed] [Google Scholar]
- 37.Mauras N, Klein KO, Hayes V. Estrogen suppression in males: metabolic effects. J Clin Endocrinol Metab. 2001;85:2370–2377. doi: 10.1210/jcem.85.7.6676. [DOI] [PubMed] [Google Scholar]
- 38.Plourde P, Martin D, Dowsett M, Demers L, Yates R, Webster A. Anastrazole: a new oral, once-a-day aromatase inhibitor. J Ster Biochem Molec Biol. 1995;53:175–179. doi: 10.1016/0960-0760(95)00045-2. [DOI] [PubMed] [Google Scholar]
- 39.Plourde P, Martin D, Dukes M. Anastrazole: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30:103–111. doi: 10.1007/BF00682745. [DOI] [PubMed] [Google Scholar]
- 40.Hayes FJ, Seminara SB, DeCruz S, Boepple PA, Crowley Jr WF. Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab. 2000;85:3027–3035. doi: 10.1210/jcem.85.9.6795. [DOI] [PubMed] [Google Scholar]
- 41.Veldhuis JD, Zwart AD, Iranmanesh A. Neuroendocrine mechanisms by which selective Leydig-cell castration unleashes increased pulsatile LH release in the human: an experimental paradigm of short-term ketoconazole-induced hypoandrogenemia and deconvolution-estimated LH secretory enhancement. Am J Physiol. 1997;272:R464–R474. doi: 10.1152/ajpregu.1997.272.2.R464. [DOI] [PubMed] [Google Scholar]
- 42.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 43.Urban RJ, Kaiser DL, Van Cauter E, Johnson ML, Veldhuis JD. Comparative assessments of objective peak-detection algorithms: II. Studies in men. Am J Physiol. 1988;254:E113–E119. doi: 10.1152/ajpendo.1988.254.1.E113. [DOI] [PubMed] [Google Scholar]
- 44.Urban RJ, Johnson ML, Veldhuis JD. In vivo biological validation and biophysical modeling of the sensitivity and positive accuracy of endocrine peak detection: I. The LH pulse signal. Endocrinology. 1989;124:2541–2547. doi: 10.1210/endo-124-5-2541. [DOI] [PubMed] [Google Scholar]
- 45.Mulligan T, Iranmanesh A, Veldhuis JD. Pulsatile iv infusion of recombinant human LH in leuprolide-suppressed men unmasks impoverished Leydig-cell secretory responsiveness to midphysiological LH drive in the aging male. J Clin Endocrinol Metab. 2001;86:5547–5553. doi: 10.1210/jcem.86.11.8004. [DOI] [PubMed] [Google Scholar]
- 46.Partsch C-J, Abrahams S, Herholz N, Peter M, Veldhuis JD, Sippell WG. Variability of pulsatile LH secretion in young male volunteers. Eur J Endocrinol. 1994;131:263–272. doi: 10.1530/eje.0.1310263. [DOI] [PubMed] [Google Scholar]
- 47.Urban RJ, Johnson ML, Veldhuis JD. Biophysical modeling of the sensitivity and positive accuracy of detecting episodic endocrine signals. Am J Physiol. 1989;257:E88–E94. doi: 10.1152/ajpendo.1989.257.1.E88. [DOI] [PubMed] [Google Scholar]
- 48.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci USA. 1996;93:14100–14105. doi: 10.1073/pnas.93.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veldhuis JD, Straume M, Iranmanesh A, Mulligan T, Jaffe CA, Barkan A, Johnson ML, Pincus SM. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol. 2001;280:R721–R729. doi: 10.1152/ajpregu.2001.280.3.R721. [DOI] [PubMed] [Google Scholar]
- 51.Veldhuis JD, Johnson ML, Veldhuis OL, Straume M, Pincus S. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol. 2001;281:R1975–R1985. doi: 10.1152/ajpregu.2001.281.6.R1975. [DOI] [PubMed] [Google Scholar]
- 52.Pincus SM. Greater signal regularity may indicate increased system isolation. Math Biosci. 1994;122:161–181. doi: 10.1016/0025-5564(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 53.Veldhuis JD, Iranmanesh A, Johnson ML, Lizarralde G. Twenty-four hour rhythms in plasma concentrations of adenohypophyseal hormones are generated by distinct amplitude and/or frequency modulation of underlying pituitary secretory bursts. J Clin Endocrinol Metab. 1990;71:1616–1623. doi: 10.1210/jcem-71-6-1616. [DOI] [PubMed] [Google Scholar]
- 54.Veldhuis JD, Johnson ML, Keenan D, Iranmanesh A 2003 The ensemble male hypothalamo-pituitary-gonadal axis. In: Timiras PS, ed. Physiological basis of aging and geriatrics. 3rd ed. Boca Raton, FL: CRC Press; 213–231
- 55.Veldhuis JD, Iranmanesh A, Keenan DM 2004 An ensemble perspective of aging-related hypoandrogenemia in men. In: Winters SJ, ed. Male hypogonadism: basic, clinical, and theoretical principles. Totowa, NJ: Humana Press; 261–284
- 56.Veldhuis JD, Evans WS, Johnson ML. Complicating effects of highly correlated model variables on nonlinear least-squares estimates of unique parameter values and their statistical confidence intervals: estimating basal secretion and neurohormone half-life by deconvolution analysis. Meth Neurosci. 1995;28:130–138. [Google Scholar]
- 57.Veldhuis JD, Lassiter AB, Johnson ML. Operating behavior of dual or multiple endocrine pulse generators. Am J Physiol. 1990;259:E351–E361. doi: 10.1152/ajpendo.1990.259.3.E351. [DOI] [PubMed] [Google Scholar]
- 58.Veldhuis JD, O’Dea LS, Johnson ML. The nature of the gonadotropin-releasing hormone stimulus-luteinizing hormone secretory response of human gonadotrophs in vivo. J Clin Endocrinol Metab. 1989;68:661–670. doi: 10.1210/jcem-68-3-661. [DOI] [PubMed] [Google Scholar]
- 59.Keenan DM, Veldhuis JD. A biomathematical model of time-delayed feedback in the human male hypothalamic-pituitary-Leydig cell axis. Am J Physiol. 1998;275:E157–E176. doi: 10.1152/ajpendo.1998.275.1.E157. [DOI] [PubMed] [Google Scholar]
- 60.Mulligan T, Kuno HL, Vij S, Iranmanesh A, Veldhuis JD Healthy older men show heightened LH secretory sensitivity and capacity to GnRH stimulation. Proc Annual Meeting of the American Geriatrics Society, Philadelphia, PA, 1999, p 122 (Abstract 843)
- 61.Mulligan T, Iranmanesh A, Veldhuis JD A novel acute hypoandrogenemic clamp abolishes the age difference in GnRH’s dose-dependent stimulation of LH secretion and orderliness in older men. Program of the 83rd Annual Meeting of The Endocrine Society, Denver, CO, 2001, p 38 (Abstract 170)
- 62.Keenan DM, Sun W, Veldhuis JD. A stochastic biomathematical model of the male reproductive hormone system. SIAM J Appl Math. 2000;61:934–965. [Google Scholar]
- 63.Bonavera JJ, Swerdloff RS, Leung A, Lue YH, Baravarian S, Superlano L, Sinha-hikim AP, Wang C. In the male Brown-Norway (BN) male rat reproductive aging is associated with decreased LH-pulse amplitude and area. J Androl. 1997;18:359–365. [PubMed] [Google Scholar]
- 64.Gruenewald DA, Naai MA, Hess DL, Matsumoto AM. The Brown Norway rat as a model of male reproductive aging: evidence for both primary and secondary testicular failure. J Gerontol. 1994;49:B42–B50. doi: 10.1093/geronj/49.2.b42. [DOI] [PubMed] [Google Scholar]
- 65.Karpas AE, Bremner WJ, Clifton DK, Steiner RA, Dorsa DM. Diminished luteinizing hormone pulse frequency and amplitude with aging in the male rat. Endocrinology. 1983;112:788–791. doi: 10.1210/endo-112-3-788. [DOI] [PubMed] [Google Scholar]
- 66.Huang HH, Kissane JQ, Hawrylewicz EJ. Restoration of sexual function and fertility by fetal hypothalamic transplant in impotent aged male rats. Neurobiol Aging. 1987;8:465–472. doi: 10.1016/0197-4580(87)90042-x. [DOI] [PubMed] [Google Scholar]
- 67.Sortino MA, Aleppo G, Scapagnini U, Canonico PL. Different responses of gonadotropin-releasing hormone (GnRH) release to glutamate receptor agonists during aging. Brain Res Bull. 1996;41:359–362. doi: 10.1016/s0361-9230(96)00199-2. [DOI] [PubMed] [Google Scholar]
- 68.Nankin HR, Lin T, Murono EP. The aging Leydig cell III. Gonadotropin stimulation in men. J Androl. 1981;2:181–186. [Google Scholar]
- 69.Reubens R, Dhondt M, Vermeulen A. Further studies on Leydig cell response to human choriogonadotropin. J Clin Endocrinol Metab. 1976;39:40–45. doi: 10.1210/jcem-39-1-40. [DOI] [PubMed] [Google Scholar]
- 70.Zwart A, Iranmanesh A, Veldhuis JD. Disparate serum free testosterone concentrations and degrees of hypothalamo-pituitary-LH suppression are achieved by continuous versus pulsatile intravenous androgen replacement in men: a clinical experimental model of ketoconazole-induced reversible hypoandrogenemia with controlled testosterone add-back. J Clin Endocrinol Metab. 1997;82:2062–2069. doi: 10.1210/jcem.82.7.4035. [DOI] [PubMed] [Google Scholar]


