Pectic polysaccharides are an essential component of the primary plant cell wall. They are particularly prominent in pollen tubes, where they control the structure and yielding characteristics of the cell wall at the growing apex of these rapidly expanding cells. The properties of pectin meshworks, to a considerable degree, are regulated by postsecretory changes resulting from the activity of cell wall–associated enzymes. For example, the homogalacturonan (HGA) fraction, a major component of pectins, is typically deposited into the apoplasm in highly methylesterified condition. Pectin methylesterases (PMEs; EC 3.1.1.11) in the apoplasm catalyze the demethylesterification of these HGAs, exposing carboxyl residues, which can be cross-linked by calcium. These changes markedly affect the rheological properties of the cell wall as well as its porosity and ionic status. It follows that the tight control of PME activity, both spatially and temporally, occupies a pivotal position in the control of cell wall growth and development. The pollen tube represents an excellent model system for studying PMEs since the wall of the apical growth region consists almost exclusively of a pectinaceous network. This essay focuses on recent findings that underline the significance of PMEs for pollen tube growth and discusses the complex array of mechanisms that exist to regulate PME activity. Although representing a particular system, data obtained for PMEs in pollen tubes are likely to enhance our understanding of the importance of these enzymes in other cell types.
PME ACTION AND ITS CONSEQUENCES
The pectin network consists of three different polysaccharide units, HGA, rhamnogalacturonan-I, and rhamnogalacturonan II, which are thought to be covalently linked to each other (O'Neill et al., 1990). HGAs are linear homopolymers composed of (1,4)-α-d-galacturonic acid (GalA) residues. Current evidence indicates that these polysaccharides are synthesized in the Golgi, where as many as 70 to 80% of the GalA residues are methylesterified before secretion into the wall (O'Neill et al., 1990; Staehelin and Moore, 1995; Mohnen, 1999). PMEs convert the methoxyl groups on the polygalacturonic acid chain into negatively charged carboxyl groups, releasing both protons and methanol. It is generally believed that most plant PMEs remove methyl esters in a block-wise fashion, creating long contiguous stretches of deesterified pectins (Limberg et al., 2000). The negatively charged carboxyl groups from neighboring chains can be cooperatively cross-linked by Ca2+, which results in a stiff three-dimensional pectate network (Grant et al., 1973; Catoire et al., 1998). The strength of the interaction between Ca2+ and pectin increases with decreasing average degree of pectin methylesterification and increasing length of the unsubstituted galacturonan backbone. Therefore, the affinity of highly methylesterified pectins for Ca2+ is generally not high enough to induce the formation of a pectate gel (Tibbits et al., 1998). The observation that HGAs with a more random distribution of methylesters are also abundant in primary cell walls suggests that at least some plant PMEs are able to deesterify pectins in a nonblock-wise fashion, similar to the action pattern of bacterial and fungal PMEs (Willats et al., 2001). These differences in mode of action, block-wise versus nonblock-wise, as well as the degree of methylesterification, determine the mechanical and porosity properties of the cell wall and influence its pH and ion balance (Goldberg et al., 2001). It is important to note that the cell wall properties are also affected by the hydrodynamics of the pectin network. The hydration of the network is influenced by the balance between the osmotic stress exerted on the cell wall by the cell contents and cross-linking of the network, which tend to restrict swelling, and the affinity of the network for water, which drives swelling (Zsivanovits et al., 2004).
In addition to exposing carboxyl residues, the changes brought about by PME activity have further important consequences. For example, the localized reduction in pH, generated by the protons released during the deesterification process, can inhibit some PME isoforms (Moustacas et al., 1991) and at the same time stimulate the activity of cell wall hydrolases, such as polygalacturonases and pectate lyases (Nari et al., 1986). The combined effect can result in cell wall loosening (Wen et al., 1999), which will enhance wall yielding and cell expansion. Yet another factor to consider is the possibility that demethylation mediated by PMEs renders pectins susceptible for degradation by pectinolytic enzymes (Koch and Nevins, 1989). The released oligogalacturonides, which consist of 2 to ∼20 GalA residues, can act as signaling molecules in plant defense responses and plant growth and development (Coté and Hahn, 1994; Ridley et al., 2001). Not surprisingly, PMEs are implicated in a variety of physiological and biochemical processes in plants, including cell wall extension, cell adhesion, fruit maturation and senescence, cambial cell differentiation, and even systemic movement of the Tobacco mosaic virus (Tieman and Handa, 1994; Wen et al., 1999; Micheli et al., 2000; Chen and Citovsky, 2003).
PME REGULATION
Analysis of the Arabidopsis thaliana transcriptome reveals that plants invest extensively in the biosynthesis and modification of pectins. An analysis of the putative PME isoforms encoded in the Arabidopsis genome is presented in Figure 1. Gene expression data from a comparative Arabidopsis transcriptome study using the Affymetrix ATH1 genome array, which contains 60 of the 66 open reading frames that have been annotated as PMEs, show that at least 18 PME isoforms are expressed in Arabidopsis pollen (Pina et al., 2005; Figure 1B). The expression level of some of these isoforms is extremely high compared with the levels in the other tissues analyzed (Bosch et al., 2005). Thus, from a total of 1584 expressed genes in Arabidopsis pollen, 8 PME isoforms are among the 50 highest expressed transcripts, underlining the importance of PMEs for pollen germination and/or pollen tube growth. Here, it needs to be noted that high transcript levels do not automatically translate into high protein levels or, maybe more importantly, high levels of enzymatic activity. Data on expression levels and activity levels of PME isoforms are very limited. Only few putative PME isoforms have been actually tested for PME activity and biochemically characterized.
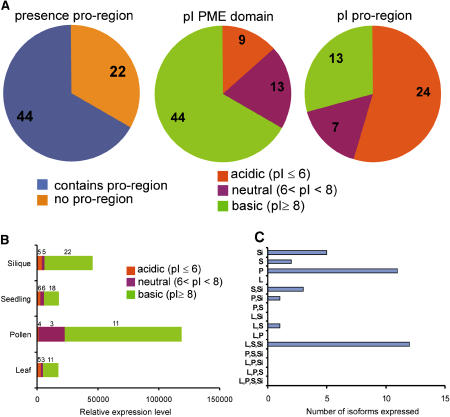
Figure 1.
Analysis of PME Isoforms Encoded in the Arabidopsis Genome.
(A) The number of isoforms with and without a pro-region as well as the number of isoforms of which the pI of the predicted PME domain and pro-region falls within a certain pH interval are depicted.
(B) The total relative expression level of the PME isoforms in a certain organ or cell type. The contribution of acidic, neutral, and basic PME isoforms is depicted. Numbers above the bar segments indicate the number of isoforms that contribute to the expression of the segment. Total PME activity is extremely high in pollen when compared with the other organs/cell types.
(C) Organ and cell-type specificity of PME isoforms. Each isoform was analyzed for its expression in several organs and cell types. The number of isoforms specifically expressed in one organ/cell type or combination of organs/cell types is depicted. Pollen expresses its own specific subset of isoforms. By comparison, other organs/cell types share isoforms as is indicated by the high number of isoforms expressed in leaf, seedling, and silique. Si, silique; S, seedling; P, pollen; L, leaf. Only present calls with expression levels >200 are included in the analysis. Expression data of the PME isoforms are taken from Pina et al. (2005).
Most Arabidopsis isoforms encode a PME domain with a predicted molecular mass between 33 and 43 kD and an alkaline pI. The latter feature explains their tight association with the slightly acidic cell wall and the need for high ionic strength to solubilize these isoforms. The existence of neutral, and especially acidic, isoforms suggests that not all PMEs are necessarily tightly associated with cell wall components. Indeed, highly soluble acidic PME isoforms have been detected in hypocotyls of mung bean (Vigna radiata) and across the cambial region of hybrid aspen (Populus tremula × Populus tremuloides) (Bordenave and Goldberg, 1994; Micheli et al., 2000). The different ionic interactions between PME isoforms and the cell wall are likely to influence PME mobility and enzyme activity (Bordenave and Goldberg, 1994).
While it is clear that PME activity must be tightly regulated to fine-tune properties of the pectin network in specific regions of the cell wall, the precise nature of the control mechanism in vivo is poorly understood. One level by which PME activity is regulated is the differential expression, both spatially and temporally, of PME isoforms. However, multiple PME isoforms have been shown to be expressed in certain tissues at the same time (see Figure 1C). From the action pattern of the few PME isoforms studied so far, it appears that the intrinsic activity of different isoforms is the same but that certain substrate specificities and reaction mechanisms require different environmental conditions (Denès et al., 2000; Limberg et al., 2000; Goldberg et al., 2001). It has been shown that the distribution of carboxyl units along the pectin backbone, and to a lesser extent the methylation degree, are important in controlling PME activity. Free carboxyl groups in the vicinity of the ester linkage increase the affinity of the enzyme for its substrate (Catoire et al., 1998; Goldberg et al., 2001). The immobilization of PMEs at these anionic sites has been reported to either increase or decrease their deesterification rate, possibly due to conformational changes induced by the microenvironment (Bordenave and Goldberg, 1994). As a result, the behavior of PME isoforms in solution often differs from that exhibited by the cell wall–bound isoforms; thus, care should be taken when data on PME activity obtained in solution are extrapolated to the in vivo situation. It is often mentioned that plant PMEs with a basic pI, which represents most isoforms, act in a block-wise fashion, while plant PMEs with an acidic pI act in a nonblock-wise (more random) fashion. However, evidence for this is scarce, and it appears that apoplastic pH rather than the pI is the more important determinant for PME activity. Different isoforms have different pH optima, and the action pattern of certain isoforms has been shown to be pH dependent (Catoire et al., 1998; Denès et al., 2000; Goldberg et al., 2001). Cations also are necessary for PME activity, where they affect the binding of the enzyme to its substrate (Moustacas et al., 1991).
The Arabidopsis PME mutant vanguard1 (vgd1) dramatically affects pollen tube growth (discussed later in this essay), although mutant pollen exhibit only 18% less overall PME activity when compared with the wild type (Jiang et al., 2005). This relatively small reduction in PME activity would not be expected to have such a pronounced impact on pollen tube growth unless the intrinsic enzymatic properties of VGD1 differ from those of other PME isoforms expressed in pollen tubes. These data are in support of the idea that, independent of the environmental conditions, different PME isoforms can exhibit distinct specificities for their pectic substrate. More studies on the action pattern and substrate specificity and affinity of different PME isoforms are needed to test this idea.
In addition to differential expression patterns and environmental conditions, plant PME activity can be regulated by PME inhibitors (PMEIs; Giovane et al., 2004). PMEIs are small proteins that inhibit PME activity through the formation of a reversible 1:1 complex of which the stability is pH dependent, being higher in acidic conditions, typical of the apoplastic environment (D'Avino et al., 2003). Although we know that PMEIs inhibit plant PMEs in vitro and the crystal structure of the complex between a plant PME and PMEI has recently been resolved (Di Matteo et al., 2005), little is known about how PMEIs regulate PME activity in planta. PMEIs, together with invertase inhibitors, constitute a gene family with very high expression levels in Arabidopsis pollen (Bosch et al., 2005; Pina et al., 2005). The high degree of sequence similarity between the family members does not allow classification into functional groups (Hothorn et al., 2004). However, it is known that at least some pollen-expressed members represent functional PMEIs (Wolf et al., 2003). Therefore, the pollen tube system may be favorable for the study of PMEI function and regulation in vivo.
Finally, we draw attention to the recently emerging idea that PME is subject to intramolecular regulation. In addition to the catalytic PME domain, and the N-terminal signal peptide, which is required for protein targeting to the endoplasmic reticulum, most (two-thirds) of the isoforms present in the Arabidopsis genome encode a pro-region downstream of the signal peptide, with a predicted molecular mass of 15 to 25 kD and acidic to alkaline pIs (Figure 1). Only the mature PME, without the pro-region, can be detected with PME antibodies or activity-based staining in cell wall extracts, indicating that the pro-region is cleaved off either during secretion or at some point after secretion (Micheli, 2001). Interestingly, this region shares some homology with PMEIs. Recent localization studies on the tobacco (Nicotiana tabacum) pollen-specific PME, NtPPME1, reveal that the full-length product fused to green fluorescent protein is secreted, whereas NtPPME1 lacking the pro-region is not, suggesting that the pro-region participates in the correct targeting of the mature PME (Bosch et al., 2005). In addition, whereas overexpression of the whole NtPPME1 protein, including its pro-region, does not affect in vitro pollen tube growth, transient expression of only the PME domain reduces the growth rate dramatically. Expression of the PME domain also leads to the accumulation of deesterified pectins in the apical pollen tube wall, which appears to alter the rheological properties of the apical wall concomitant with the cessation of growth (Bosch et al., 2005). Importantly, the inhibitory effect caused by the PME domain can be partly rescued by coexpressing the pro-region. These experiments not only show that the PME domain alone is sufficient for exerting its enzymatic activity but also support the idea that the pro-region acts as an intramolecular inhibitor of PME activity, thereby preventing premature deesterification of pectins prior to secretion (Figure 2C). The predicted structural similarity between the pro-region and PMEIs (Pfam 4043; Bateman et al., 2004) substantiates this idea.
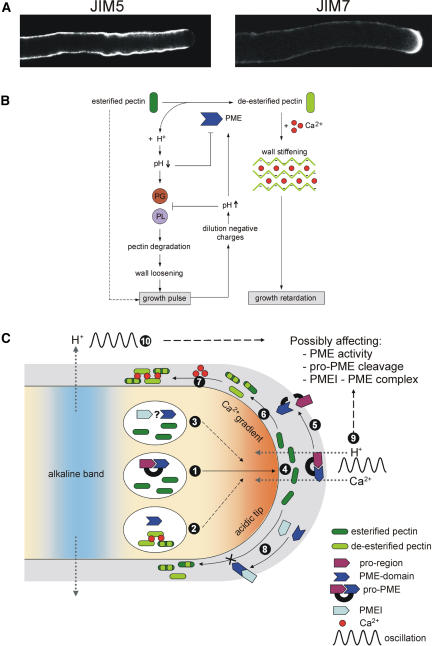
Figure 2.
Regulation of PME Activity in Pollen Tubes.
(A) Example of the distribution of relatively deesterified pectins and esterified pectins in N. tabacum pollen tubes by means of JIM5 and JIM7, respectively, immunolabeling. Notice the sudden transition from apical esterified pectin epitopes to deesterified pectin epitopes.
(B) Potential feedback mechanism for the regulation of apical PME activity in pollen tubes that could contribute to an oscillatory growth pattern. PG, polygalacturonase; PL, pectate lyase.
(C) Regulation of PME activity by its pro-region, PMEIs, and oscillating ion fluxes. (1) The pro-region functions as an intramolecular inhibitor of PME activity, preventing the premature deesterification of pectins prior to secretion. (2) Expression of only the PME domain can lead to premature, intravesicular, demethylesterification of pectins, causing inhibition of tube growth. (3) It remains to be seen if PMEIs can inhibit premature deesterification of PME isoforms lacking a pro-region. (4) Pectins are secreted in a highly methylesterified state. (5) Removal of the pro-region by cleavage of pro-PMEs is necessary for the PME domain to become enzymatically active. (6) The active PME domain catalyzes the demethylesterification of pectins. (7) Ca2+ binds cooperatively to the free carboxyl groups, which causes rigidification of the cell wall. (8) PMEIs are able to inhibit the PME activity in the cell wall by forming a 1:1 reversible complex. (9) The oscillatory influx of H+ and Ca2+ ions, which maintain the apical acidic domain and the tip-focused Cai2+ gradient, respectively, through the apical cell wall might directly influence the PME activity, the conditions necessary for cleavage of pro-PME, and the formation of the pH-dependent PMEI-PME complex. (10) The same is true for the oscillatory efflux of H+ ions maintaining the alkaline band at the base of the clear zone.
APICAL WALL DYNAMICS IN POLLEN TUBES
Pollen tube growth, which is fast and restricted to the extreme apex of the tube, involves a massive secretion and assembly of new plasma membrane and cell wall. Throughout this process, a balance point must be achieved in which the apical cell wall is plastic enough to allow wall stretching and the incorporation of these new wall components, while at the same time rigid enough to withstand the high internal turgor pressure (e.g., 0.2 MPa in Lilium longiflorum pollen tubes; Benkert et al., 1997) and prevent bursting. As noted earlier, the apical tube wall is almost exclusively composed of a pectic network (Ferguson et al., 1998). The traditional load-bearing components, consisting of a cellulose-hemicellulose framework (O'Neill and York, 2003), can only be found in the subapical and more distal parts of the tube wall (Taylor and Hepler, 1997). Consequently, the pectic network represents the load-bearing component at the pollen tube apex. Not surprisingly, PMEs emerge as potential key regulators of pollen tube growth since these enzymes determine the rheological properties of the apical pollen tube wall.
Evidence for a differential distribution of PME activity can be gleaned from examining the localization of methylesterified and demethylesterified pectins along the pollen tube wall. Two monoclonal antibodies, JIM5 and JIM7 (Knox et al., 1990), have been particularly useful in resolving the patterns of esterification. JIM5 binds preferably to at least four contiguous unesterified GalA residues and labels the relatively deesterified pectin epitopes. By contrast, JIM7 binds to methylesterified residues with adjacent or flanking unesterified GalA residues and therefore indicates the presence of relatively high methylesterified pectin epitopes (Clausen et al., 2003). In general, esterified pectin epitopes are most abundant in the apical region, while deesterified pectins are more concentrated in areas away from the tip (Li et al., 1994; Bosch et al., 2005; Parre and Geitmann, 2005). Some variations in labeling pattern may occur, depending on the species and experimental conditions used (Stępka et al., 2000; Parre and Geitmann, 2005). An example for the distribution pattern of pectins in N. tabacum pollen tubes can be seen in Figure 2A, in which the sudden transition from apical esterified pectins to subapical deesterified pectins is most evident.
Further studies supporting a role of PME in pollen tube growth involved the application of pollen tube growth media supplemented with orange peel PME. The results reveal that exogenous PME inhibits in vitro growth (Bosch et al., 2005; Parre and Geitmann, 2005) and causes a significant thickening of the apical cell wall and dissipation of the intracellular tip-focused Ca2+ gradient (Bosch et al., 2005). As can be expected, exposing pollen tubes to such enhanced extracellular PME activities converts apical methylesterified pectins into deesterified pectins (Parre and Geitmann, 2005), which will readily form a stiff calcium-mediated pectate gel at the tip, preventing turgor-driven elongation and instead leading to cell wall thickening. Support for this comes from microindentation experiments showing that treatment with exogenous PME leads to a dramatic increase in the apical cellular stiffness and a decrease in the visco-elastic behavior (Parre and Geitmann, 2005). These data show that the PME-mediated configuration of pectins at the apex is an important component in controlling pollen tube growth.
Genetic proof for the critical role of PME activity in the regulation of pollen tube growth comes from a study in which an Arabidopsis pollen-specific PME, VGD1, was functionally interrupted by the insertion of a transposon (Jiang et al., 2005). VGD1, which is encoded by At2g47040, is a PME isoform with one of the highest levels of expression in pollen as measured on the ATH1 gene chip (Pina et al., 2005). Total PME activity in the mutant pollen was 82% compared with that of wild-type pollen. Most of the mutant tubes burst when grown in vitro, suggesting that the decrease in overall PME activity leads to an imbalance of the apical wall dynamics, resulting in failure to resist the internal turgor pressure. Initial in vivo growth of the mutant tubes on the surface of stigmatic cells is not affected; however, growth is greatly retarded in the style and transmitting tract. Despite this retardation, a few tubes manage to fertilize ovules in the upper part of the silique (Jiang et al., 2005). Mutant tubes may perceive enough structural support from the surrounding female sporophytic tissues, and from each other, to partially overcome the severe phenotype observed in vitro.
The idea that sporophytic tissue, which provides mechanical support, can influence pectin levels is supported by studies on in vitro pollen tube growth. Thus, the total amount of pectin surrounding the pollen tube is dramatically lower in tubes grown in solidified medium compared with those grown in liquid (Parre and Geitmann, 2005). It is plausible, therefore, that structural compensation, which is provided by the stylar environment, diminishes the requirement for pollen tube cell wall pectin. Also, the presence of endogenous PME activity in the transmitting tract might contribute to the less severe phenotype observed in vivo. In an independent study, silencing of N. tabacum pollen-specific PPME1 resulted in a mild but significant decrease of in vivo pollen tube growth, while the overall PME activity in pollen was not significantly decreased (Bosch and Hepler, 2005). This indicates that the existing equilibrium of the apical cell wall dynamics in providing both support and plasticity is very delicate and that a minor disturbance of this equilibrium compromises pollen tube growth.
Although it is apparent that PMEs are important for controlling pollen tube growth, there are still many open questions. For instance, are all the pollen-expressed PME isoforms redundant, or do different isoforms employ different functions? Experiments in which two Arabidopsis pollen-expressed PME isoforms (At2g47030 and At3g62170) with high amino acid identity to VGD1 (85 and 69%, respectively) were placed under the control of the VGD1 promoter showed that only At2g47030 was able to complement the VGD1 mutant phenotype, while no complementation was observed for At3g62170 (Jiang et al., 2005). These data, together with the notion that sequence identities between many different isoforms are likely to be lower than in the above example, suggest that many isoforms are not merely redundant. Rather, the differences in pIs, presence of a pro-region, different pH optima, and different substrate specificities and action mechanism at a certain pH among the different isoforms are all likely to contribute to certain isoform specificities.
A ROLE FOR PECTIN/PME IN OSCILLATORY POLLEN TUBE GROWTH
An interesting property of pollen tube growth is that the rate oscillates both in vivo (Iwano et al., 2004) and in vitro (Holdaway-Clarke and Hepler, 2003). Since changes in the yielding properties of the cell wall could underlie these changes in rate, it is reasonable to imagine that modifications of pectin are a controlling factor (Holdaway-Clarke et al., 1997). One potential mechanism for the regulation of PME activity at the tip involves a negative feedback, in which the local decrease in pH generated by protons released during the deesterification reduces PME activity (Moustacas et al., 1991). A decrease in pH would also be expected to activate enzymes such as polygalacturonases and pectate lyases. The combined effect of inactivating PME and activating polygalacturonase and pectate lyase would loosen the cell wall and facilitate a growth pulse. However, the subsequent dilution of negative charges would increase the pH and cause a reactivation of PME and inactivation of polygalacturonase and pectate lyase, leading toward an apical wall stiffening and a decrease in growth rate. Thus, PME, together with other pectin-associated enzymes, could play a key role in controlling oscillations in pollen tube growth (Figure 2B).
There are different lines of evidence that support the above scenario. First, the apical wall thickness in lily pollen tubes, which has been shown to oscillate with the same frequency as growth, becomes thickened in anticipation of the next growth rate increase (Holdaway-Clarke and Hepler, 2003). Related to this, pectins, predominantly the deesterified configuration, appear as perpendicular periodic rings along the axes of pollen tubes and are deposited in periods of slow growth (Li et al., 1994, 1996). These changes might reflect an oscillatory behavior of the apical wall dynamics mediated by PME activity. Second, polygalacturonase- and pectate lyase–related transcripts are highly expressed in Arabidopsis pollen (Pina et al., 2005), suggesting a function in pollen germination and tube growth. Although genetic evidence for these functions is still lacking, application of a polygalacturonase from Aspergillus niger has been shown to stimulate pollen germination and tube growth (Parre and Geitmann, 2005).
Other factors might include the endogenous oscillations of intracellular H+, which could participate in the regulation of PME activity or vice versa. Pollen tubes possess two domains that oscillate in pH intensity: an acidic domain at the extreme apex and an alkaline band at the base of the clear zone (Feijó et al., 1999; Cardenas et al., 2005), an apical area in which Golgi-derived secretory vesicles accumulate and to which larger organelles are excluded. The acidic tip is thought to be maintained by a proton influx at the apex, while the alkaline band is maintained by a localized proton efflux, presumably under the control of a plasma membrane proton ATPase (Figure 2C). These proton fluxes are likely to affect the pH in specific cell wall regions, which in turn might influence the activity of PMEs in these regions. As mentioned before, the stability of the interaction between PMEs and PMEIs is pH dependent, as might also be the interaction of the pro-region with the PME domain. A local change in pH may even activate specific proteases that process the pro-region and release the active PME domain. It will be interesting to see if the pectin methylesterification pattern, such as seen in Figure 2A, can be attributed to specific pH conditions in the cell wall that are generated by the proton fluxes. However, the rapid turnover of cell wall material at the apex in oscillating tubes makes pH measurements in this region a particular challenge that still needs to be resolved.
Pollen tubes also display an intracellular tip-focused Cai2+ gradient whose magnitude oscillates markedly. This gradient is thought to be maintained by the influx of extracellular Ca2+ across the plasma membrane. It has been suggested that both Ca2+ and H+ enter at the tip through the same stretch-activated cation channels (Holdaway-Clarke and Hepler, 2003). This might set the stage for a cyclic event in which PME-mediated oscillations in apical wall yielding determine the status of stretch-activated channels. The resulting ion fluxes generated by these channels might in turn affect the activities of PMEs and, thus, wall yielding.
FUTURE PROSPECTIVES
It is apparent that PMEs are essential enzymes involved in many plant physiological processes, including, and maybe in particular, pollen tube growth. Many of the players involved in the regulation of PME activity have been identified. However, resolving the full impact of PME activities in planta requires much more attention. It will be important to unravel the respective functions and action patterns of the array of PME isoforms. It also needs to be noted that although many PME isoforms, such as those used for the analysis shown in Figure 1, contain a PME-related domain (Pfam011095; Bateman et al., 2004) and harbor many of the characteristic segments and conserved residues recently described for PMEs (Markovič and Janeček, 2004), conformation of their demethylesterification capacity is needed as is their biochemical characterization. In addition, genetic studies will be necessary in which expression levels of specific isoforms are upregulated or downregulated and consequences for the pectin network are assessed. The local cell wall properties constitute a major determinant for the PME activity profile. Therefore, advanced probing techniques need to be implemented to obtain information about local wall conditions and link them to the corresponding methylesterification patterns observed in these wall areas.
Probably one of the most potent regulators of PME activity is the PMEIs, yet very little is known about the spatial and temporal regulation of these proteins and their interaction with PMEs in vivo. It will be interesting to see if PMEIs, analogous to the action of the pro-region, can prevent premature deesterification of pectins before these are secreted or if complex formation between PMEs and PMEIs is limited to the apoplastic space. A recent model proposed for the interaction between PME and PMEI also allowed convenient intramolecular binding of the PMEI homologous pro-region to the PME domain (Hothorn et al., 2004). Although the importance of the pro-region has been recently established, future studies need to explore their mode of action in more detail. The inhibitory potential of pro-regions needs to be tested in vitro with PME activity assays, while colocalization studies with antibodies against the pro-region and the PME domain, as well as fluorescence resonance energy transfer analysis of pro-PMEs, will provide crucial spatial and temporal information.
Although many questions still need clarification, it is evident that the enzymatic activity of PME plays a central role in the control of cell wall properties and, thus, in cell growth and development. The pollen tube, given its extensive investment in pectins and its rapid growth rates, constitutes a favorable system in which to examine the role of PME in wall dynamics and cell elongation. While we must not ignore the contribution of other factors in determining the cell wall properties, advances made in current research define PMEs as critical enzymes in the control of pectin dynamics. Thus, it is not surprising that a complex regulatory network emerges that carefully orchestrates PME activity. We confidently predict that findings made in the pollen tube system will also apply broadly to other plant cell types.
Acknowledgments
We thank our colleagues for helpful discussions during the preparation of this essay. This work has been supported by Grants MCB-0077599 and MCB-0517852 from the National Science Foundation.
References
- Bateman, A., et al. (2004). The Pfam protein families database. Nucleic Acids Res. 32, D138–D141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkert, R., Obermeyer, G., and Bentrup, F.W. (1997). The turgor pressure of growing lily pollen tubes. Protoplasma 198, 1–8. [Google Scholar]
- Bordenave, M., and Goldberg, R. (1994). Immobilized and free apoplastic pectinmethylesterases in mung bean hypocotyl. Plant Physiol. 106, 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch, M., Cheung, A.Y., and Hepler, P.K. (2005). Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol. 138, 1334–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch, M., and Hepler, P.K. (6October2005). Silencing of the tobacco pollen pectin methylesterase NtPPME1 results in retarded in vivo pollen tube growth. Planta http://dx.doi.org/10.1007/s00425-005-0131-x. [DOI] [PubMed]
- Cardenas, L., Lovy-Wheeler, A., Wilsen, K.L., and Hepler, P.K. (2005). Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motil. Cytoskeleton 61, 112–127. [DOI] [PubMed] [Google Scholar]
- Catoire, L., Pierron, M., Morvan, C., du Penhoat, C.H., and Goldberg, R. (1998). Investigation of the action patterns of pectinmethylesterase isoforms through kinetic analyses and NMR spectroscopy. Implications in cell wall expansion. J. Biol. Chem. 273, 33150–33156. [DOI] [PubMed] [Google Scholar]
- Chen, M.H., and Citovsky, V. (2003). Systemic movement of a tobamovirus requires host cell pectin methylesterase. Plant J. 35, 386–392. [DOI] [PubMed] [Google Scholar]
- Clausen, M.H., Willats, W.G.T., and Knox, J.P. (2003). Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr. Res. 338, 1797–1800. [DOI] [PubMed] [Google Scholar]
- Coté, F., and Hahn, M.G. (1994). Oligosaccharins: Structures and signal transduction. Plant Mol. Biol. 26, 1379–1411. [DOI] [PubMed] [Google Scholar]
- D'Avino, R., Camardella, L., Christensen, T., Giovane, A., and Servillo, L. (2003). Tomato pectin methylesterase: Modeling, fluorescence, and inhibitor interaction studies-comparison with the bacterial (Erwinia chrysanthemi) enzyme. Proteins 53, 830–839. [DOI] [PubMed] [Google Scholar]
- Denès, J.M., Baron, A., Renard, C., Péan, C., and Drilleau, J.F. (2000). Different action patterns for apple pectin methylesterase at pH 7.0 and 4.5. Carbohydr. Res. 327, 385–393. [DOI] [PubMed] [Google Scholar]
- Di Matteo, A., Giovane, A., Raiola, A., Camardella, L., Bonivento, D., De Lorenzo, G., Cervone, F., Bellincampi, D., and Tsernoglou, D. (2005). Structural basis for the interaction between pectin methylesterase and a specific inhibitor protein. Plant Cell 17, 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó, J.A., Sainhas, J., Hackett, G.R., Kunkel, J.G., and Hepler, P.K. (1999). Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. J. Cell Biol. 144, 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, C., Teeri, T.T., Siika, A.M., Read, S.M., and Bacic, A. (1998). Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 206, 452–460. [Google Scholar]
- Giovane, A., Servillo, L., Balestrieri, C., Raiola, A., D'Avino, R., Tamburrini, M., Clardiello, M.A., and Camardella, L. (2004). Pectin methylesterase inhibitor. Biochim. Biophys. Acta 1696, 245–252. [DOI] [PubMed] [Google Scholar]
- Goldberg, R., Pierron, M., Bordenave, M., Breton, C., Morvan, C., and du Penhoat, C.H. (2001). Control of mung bean pectinmethylesterase isoform activities. Influence of pH and carboxyl group distribution along the pectic chains. J. Biol. Chem. 276, 8841–8847. [DOI] [PubMed] [Google Scholar]
- Grant, G.T., Morris, E.R., Rees, D.A., Smith, P.J.C., and Thom, D. (1973). Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 32, 195–198. [Google Scholar]
- Holdaway-Clarke, T.L., Feijó, J.A., Hackett, G.R., Kunkel, J.G., and Hepler, P.K. (1997). Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9, 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke, T.L., and Hepler, P.K. (2003). Control of pollen tube growth: Role of ion gradients and fluxes. New Phytol. 159, 539–563. [DOI] [PubMed] [Google Scholar]
- Hothorn, M., Wolf, S., Aloy, P., Greiner, S., and Scheffzek, K. (2004). Structural insights into the target specificity of plant invertase and pectin methylesterase inhibitory proteins. Plant Cell 16, 3437–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano, M., Shiba, H., Miwa, T., Che, F.S., Takayama, S., Nagai, T., Miyawaki, A., and Isogai, A. (2004). Ca2+ dynamics in a pollen grain and papilla cell during pollination of Arabidopsis. Plant Physiol. 136, 3562–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L.X., Yang, S.L., Xie, L.F., Puah, C.S., Zhang, X.Q., Yang, W.C., Sundaresan, V., and Ye, D. (2005). VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17, 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox, J.P., Linstead, P.J., King, J., Cooper, C., and Roberts, K. (1990). Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181, 512–521. [DOI] [PubMed] [Google Scholar]
- Koch, J.L., and Nevins, D.J. (1989). Tomato fruit cell-wall. 1. Use of purified tomato polygalacturonase and pectinmethylesterase to identify developmental-changes in pectins. Plant Physiol. 91, 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.Q., Chen, F., Linskens, H.F., and Cresti, M. (1994). Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex. Plant Reprod. 7, 145–152. [Google Scholar]
- Li, Y.Q., Zhang, H.Q., Pierson, E.S., Huang, F.Y., Linskens, H.F., Hepler, P.K., and Cresti, M. (1996). Enforced growth-rate fluctuation causes pectin ring formation in the cell wall of Lilium longiflorum pollen tubes. Planta 200, 41–49. [Google Scholar]
- Limberg, G., Korner, R., Buchholt, H.C., Christensen, T., Roepstorff, P., and Mikkelsen, J.D. (2000). Analysis of different de-esterification mechanisms for pectin by enzymatic fingerprinting using endopectin lyase and endopolygalacturonase II from A. niger. Carbohydr. Res. 327, 293–307. [DOI] [PubMed] [Google Scholar]
- Markovič, O., and Janeček, S. (2004). Pectin methylesterases: Sequence-structural features and phylogenetic relationships. Carbohydr. Res. 339, 2281–2295. [DOI] [PubMed] [Google Scholar]
- Micheli, F. (2001). Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 6, 414–419. [DOI] [PubMed] [Google Scholar]
- Micheli, F., Sundberg, B., Goldberg, R., and Richard, L. (2000). Radial distribution pattern of pectin methylesterases across the cambial region of hybrid aspen at activity and dormancy. Plant Physiol. 124, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen, D. (1999). Biosynthesis of pectins and galactomannans. In Comprehensive Natural Products Chemistry, Vol. 3: Carbohydrates and Their Derivatives Including Tannins, Cellulose and Related Lignins, D. Baron and K. Nakanishi, eds (Amsterdam: Elsevier Science), pp. 497–527.
- Moustacas, A.M., Nari, J., Borel, M., Noat, G., and Ricard, J. (1991). Pectin methylesterase, metal ions and plant cell-wall extension. The role of metal ions in plant cell-wall extension. Biochem. J. 279, 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nari, J., Noat, G., Diamantidis, G., Woudstra, M., and Ricard, J. (1986). Electrostatic effects and the dynamics of enzyme reactions at the surface of plant cells. 3. Interplay between limited cell-wall autolysis, pectin methyl esterase-activity and electrostatic effects in soybean cell walls. Eur. J. Biochem. 155, 199–202. [DOI] [PubMed] [Google Scholar]
- O'Neill, M., Albersheim, P., and Darvill, A.G. (1990). The pectic polysaccharides of primary cell walls. In Methods in Plant Biochemistry, Carbohydrates, P.M. Dey and J.B. Harborne, eds (London: Academic Press), pp. 415–441.
- O'Neill, M., and York, W. (2003). The composition and structure of primary cell walls. In Annual Plant Reviews, Vol. 8: The Plant Cell Wall, J. Rose, ed (Oxford/Boca Raton, FL: Blackwell Publishing/CRC Press), pp. 1–54.
- Parre, E., and Geitmann, A. (2005). Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220, 582–592. [DOI] [PubMed] [Google Scholar]
- Pina, C., Pinto, F., Feijó, J.A., and Becker, J.D. (2005). Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control and gene expression regulation. Plant Physiol. 138, 744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, B.L., O'Neill, M.A., and Mohnen, D.A. (2001). Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57, 929–967. [DOI] [PubMed] [Google Scholar]
- Staehelin, L.A., and Moore, I. (1995). The plant Golgi apparatus: Structure, functional organization and trafficking mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 261–288. [Google Scholar]
- Stępka, M., Ciampolini, F., Charzynska, M., and Cresti, M. (2000). Localization of pectins in the pollen tube wall of Ornithogalum virens L. Does the pattern of pectin distribution depend on the growth rate of the pollen tube? Planta 210, 630–635. [DOI] [PubMed] [Google Scholar]
- Taylor, L.P., and Hepler, P.K. (1997). Pollen germination and tube growth. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 461–491. [DOI] [PubMed] [Google Scholar]
- Tibbits, C.W., MacDougall, A.J., and Ring, S.G. (1998). Calcium binding and swelling behaviour of a high methoxyl pectin gel. Carbohydr. Res. 310, 101–107. [Google Scholar]
- Tieman, D.M., and Handa, A.K. (1994). Reduction in pectin methylesterase activity modifies tissue integrity and cation levels in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiol. 106, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, F.S., Zhu, Y.M., and Hawes, M.C. (1999). Effect of pectin methylesterase gene expression on pea root development. Plant Cell 11, 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats, W.G.T., Orfila, C., Limberg, G., Buchholt, H.C., van Alebeek, G., Voragen, A.G.J., Marcus, S.E., Christensen, T., Mikkelsen, J.D., Murray, B.S., and Knox, J.P. (2001). Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 276, 19404–19413. [DOI] [PubMed] [Google Scholar]
- Wolf, S., Grsic-Rausch, S., Rausch, T., and Greiner, S. (2003). Identification of pollen-expressed pectin methylesterase inhibitors in Arabidopsis. FEBS Lett. 555, 551–555. [DOI] [PubMed] [Google Scholar]
- Zsivanovits, G., MacDougall, A.J., Smith, A.C., and Ring, S.G. (2004). Material properties of concentrated pectin networks. Carbohydr. Res. 339, 1317–1322. [DOI] [PubMed] [Google Scholar]