Short abstract
This article is part of a series examining the cost effectiveness of strategies to achieve the millennium development goals for health
Abstract
Objective To assess the costs and health effects of a range of interventions for preventing the spread of HIV and for treating people with HIV/AIDS in the context of the millennium development goal for combating HIV/AIDS.
Design Cost effectiveness analysis based on an epidemiological model.
Setting Analyses undertaken for two regions classified using the WHO epidemiological grouping—Afr-E, countries in sub-Saharan Africa with very high adult and high child mortality, and Sear-D, countries in South East Asia with high adult and high child mortality.
Data sources Biological and behavioural parameters from clinical and observational studies and population based surveys. Intervention effects and resource inputs based on published reports, expert opinion, and the WHO-CHOICE database.
Main outcome measures Costs per disability adjusted life year (DALY) averted in 2000 international dollars ($Int).
Results In both regions interventions focused on mass media, education and treatment of sexually transmitted infections for female sex workers, and treatment of sexually transmitted infections in the general population cost < $Int150 per DALY averted. Voluntary counselling and testing costs < $Int350 per DALY averted in both regions, while prevention of mother to child transmission costs < $Int50 per DALY averted in Afr-E but around $Int850 per DALY in Sear-D. School based education strategies and various antiretroviral treatment strategies cost between $Int500 and $Int5000 per DALY averted.
Conclusions Reducing HIV transmission could be done most efficiently through mass media campaigns, interventions for sex workers and treatment of sexually transmitted infections where resources are most scarce. However, prevention of mother to child transmission, voluntary counselling and testing, and school based education would yield further health gains at higher budget levels and would be regarded as cost effective or highly cost effective based on standard international benchmarks. Antiretroviral therapy is at least as cost effective in improving population health as some of these interventions.
Introduction
The sixth millennium development goal, adopted by the United Nations in 2000, aims to halt by 2015 and begin to reverse the spread of HIV/AIDS. Since the millennium development goals were set, the incidence of HIV infection and associated mortality have continued to climb in low and middle income countries, reaching levels of about five million new infections and three millions deaths worldwide in 2004.1 The HIV/AIDS pandemic now threatens the viability of health infrastructure, social systems, and economic growth in many resource-poor countries.2,3
Most countries face uncertain prospects of attaining the HIV related target expressed in goal six. Shortage of resources is one important reason for slow progress; the projected funding gap for the year 2007 is estimated at around 50% of the need.4 In this study we focus on two related issues—whether resources currently available are achieving as much as they could and how best to use any new resources that become available.
Since the adoption of the millennium development goals, studies have examined the total costs of a comprehensive package of interventions against HIV/AIDS,5 projected the benefits of implementing this package,6,7 and compiled cost effectiveness estimates for different interventions.8-10 These studies provide useful inputs into policy debates, but comparisons across independent evaluations of particular interventions can provide ambiguous information for policy makers, who need to know what would occur when multiple interventions are implemented concurrently; conversely, evaluating only a comprehensive package may not offer flexible guidance for priority setting under different levels of resource constraint. Therefore, a comprehensive and standardised analysis of available interventions, singly and in different combinations, is needed.
The millennium development goals were defined when antiretroviral drugs were widely regarded as being prohibitively expensive. The goal for HIV/AIDS therefore focused on reducing transmission. Since then, the annual cost of first line antiretrovirals has fallen from more than US$10 000 per patient to as low as $140.11 While halting the spread of HIV infection remains a critical—and unfulfilled—objective, there is also an urgent need to assess the extent to which treatment improves population health and is consistent with the intent of the goals. With the recent price reductions, re-evaluation of the cost effectiveness of treatment is essential.
This paper assesses the effectiveness and costs of a variety of interventions for preventing and treating HIV/AIDS, individually and in combinations that incorporate interactions between interventions in both costs and health impacts. The analyses focus on two particular regions with high HIV/AIDS burdens, classified using the World Health Organization epidemiological grouping—Afr-E, which includes those countries in sub-Saharan Africa with very high adult and high child mortality, and Sear-D, which includes those countries in South East Asia with high adult and high child mortality.
Methods
Another article in this series provides details on the standardised methods used in all analyses in the series.12 In this section we provide additional detail on methods exclusive to this paper.
Strategies for HIV/AIDS control
We considered the range of available interventions for preventing the spread of HIV infection in generalised epidemics and various strategies for treating people with HIV/AIDS. Our choice of interventions (see box) was limited by available data13 and restricted to strategies that are most relevant to the epidemics in sub-Saharan Africa and South East Asia, where transmission occurs mostly through heterosexual contact. Most interventions were evaluated at coverage levels of 50%, 80%, and 95%. For treatment of sexually transmitted infections, we considered current coverage, enhanced coverage (defined as the current coverage level for antenatal care), and 95%. For prevention of mother to child transmission and treatment with highly active antiretroviral therapy (HAART), we used a coverage level equal to current coverage of antenatal care—assumed to be an achievable target in the short term.
Model
We adapted an existing model of the transmission and natural course of HIV/AIDS that was used previously to assess the potential impact of preventive interventions.6 The model includes underlying regional demography, acquisition of HIV and other sexually transmitted infections, and progression from HIV infection to AIDS and death. Heterosexual transmission occurs among five interacting risk groups: single men, married men, female sex workers, single women, and married women. The probability of transmitting HIV in a single unprotected sexual contact between serodiscordant partners depends on the stage of disease of the infected partner14,15 and is magnified by the presence of other sexually transmitted infections. A description of the model has been published elsewhere,7 and details are provided in the appendix on bmj.com.
We used baseline prevalence projections from the Joint United Nations Programme on HIV/AIDS and the World Health Organization to calibrate key parameter values for Afr-E and Sear-D. We ran multiple simulations of the model using parameter values sampled randomly from ranges defined by available information (see appendix for details). Each simulation was compared against baseline projections of prevalence by sex. Point estimates were based on the best fitting set of parameter values, with a wider range of parameter sets considered in uncertainty analyses.
Modelling intervention effects
We evaluated interventions and their combinations incrementally against a “no intervention” scenario (defined by setting condom use, treatment for sexually transmitted infections, and use of antiretroviral drugs to zero). Impacts of preventive interventions were incorporated as changes in condom use, seeking treatment for sexually transmitted infections, numbers of sexual partners, probabilities of males visiting female sex workers, and ages of sexual debut, derived from a previous synthesis of the published literature.6,13 Prevention of mother to child transmission was modelled directly based on HIV prevalence among women of childbearing age and the effectiveness of nevirapine. For HAART strategies, we assumed that treatment begins at the onset of AIDS and that it prolongs median survival by 3-8 years, depending on the range of available drugs and intensity of monitoring (see appendix).
We used the estimated differences in incidence and mortality between the “no intervention” and various intervention strategies to calculate the number of disability adjusted life years (DALYs) averted by each strategy.
Estimating costs
We followed the standardised approach to estimating programme and patient costs applied in other papers in this series.12 Total costs are a combination of resource inputs and unit costs (see table A on bmj.com). Quantities were determined by demographic and epidemiological outputs from the model, combined with assumptions about coverage levels and uptake of the interventions.
Sensitivity and uncertainty analyses
In our sensitivity analyses we varied uncertain assumptions about the input costs and behavioural impacts of interventions. To allow for uncertainty around baseline behaviours and biological assumptions, we also recomputed results using the 10 best fitting parameter sets identified during the calibration of the model to epidemiological projections (see appendix).
Interventions for HIV/AIDS considered in this analysis
Mass media—Includes television and radio episodes and inserts in key newspapers, repeated every two years; development and administration costs included; effectiveness scaled by proportion of population reporting weekly exposure to radio, television, or newspapers
Voluntary counselling and testing—Performed in primary care clinics for anyone requesting the services; includes training of health workers; based on rapid test; number of tests over five year period assumed to be twice average annual prevalence
Peer education for sex workers—Training of selected sex workers by social workers to undertake peer education; provision of condoms
Peer education and treatment of sexually transmitted infections for sex workers—In addition to training of sex workers for peer education, referrals made for testing and possible treatment of sexually transmitted infections
School based education—Targeted at youths aged 10-18 years; sessions provided during regular lessons to all students, to promote prevention of HIV and other sexually transmitted infections; includes training of selected teachers at each school
Treatment of sexually transmitted infections (general population)—Provided in primary care facilities, available to anyone who requests it; includes visits, drugs, counselling, advice on protection, and condom distribution if requested; effectiveness scaled by access and likelihood of using the services
Prevention of mother to child transmission—Information provided to women seeking antenatal care on benefits and risks of nevirapine for prophylaxis; pre-test counselling offered; single dose provided to women who accept, and single dose provided to child if delivered in a healthcare facility
Highly active antiretroviral therapy (HAART)—Standard HAART involves monthly visits to healthcare providers, while intensive monitoring involves weekly contact; either first line drugs only or first line drugs plus second line drugs when required
See appendix for further details
Results
Intervention effects
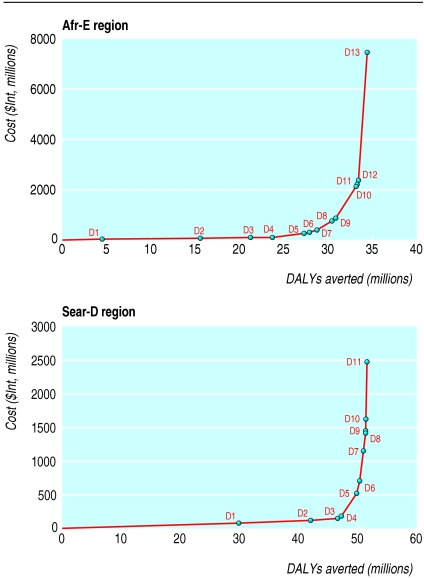
Tables 1 and 2 provide the total annual health effects for single interventions expressed in infections and DALYs averted compared with the “no intervention” scenario, along with the total annual costs for each intervention in Afr-E and Sear-D. The size of intervention benefits reported here should not be compared with current epidemiological estimates since the “no intervention” comparator subtracts current levels of condom use, treatment of sexually transmitted infections, and antiretroviral treatment. In both regions the largest number of DALYs are averted through education and treatment of sexually transmitted infections for sex workers, while the smallest gains are from school based education. Tables 3 and 4 report on incremental cost effectiveness ratios for interventions, listed in the order that they would be added with increasing budgets if cost effectiveness were the only consideration. The figure shows this expansion path graphically, with the slope of the line joining any two points indicating the incremental cost effectiveness ratio for the more costly option (see related article by Evans et al12 for further details).
Table 1.
Annual costs in international dollars ($Int),* infections averted, DALYs averted, and average cost effectiveness for various single interventions to control HIV/AIDS in Afr-E region, compared with no intervention†
| Intervention | Coverage level | Yearly costs ($Int, millions) | Yearly infections averted (millions)† | Average cost effectiveness ratio ($Int/infection averted) | Yearly DALYs averted (millions)† | Average cost effectiveness ratio ($Int/DALY averted) |
|---|---|---|---|---|---|---|
| Mass media | 100% | 16 | 0.27 | 58 | 4.5 | 3 |
| Peer education for sex workers | 50%
|
40
|
0.57
|
70
|
9.2
|
4
|
| 80%
|
61
|
0.89
|
68
|
14.3
|
4
|
|
| 95% | 70 | 1.04 | 68 | 16.7 | 4 | |
| Peer education and treatment of sexually transmitted infections for sex workers
|
50%
|
42
|
0.72
|
58
|
11.6
|
4
|
| 80%
|
63
|
1.09
|
58
|
17.5
|
4
|
|
| 95%
|
74
|
1.26
|
59
|
20.2
|
4
|
|
| School based education | 50%
|
58
|
0.01
|
9448
|
0.1
|
530
|
| 80%
|
77
|
0.01
|
7908
|
0.2
|
444
|
|
| 95% | 77 | 0.01 | 6704 | 0.2 | 376 | |
| Voluntary counselling and testing | 95% | 406 | 0.31 | 1315 | 5.0 | 82 |
| Prevention of mother to child transmission | ANC | 161 | 0.19 | 847 | 4.7 | 34 |
| Treatment of sexually transmitted infections
|
Current
|
43
|
0.14
|
304
|
2.3
|
19
|
| ANC
|
111
|
0.34
|
328
|
5.4
|
21
|
|
| 95%
|
229
|
0.45
|
514
|
7.1
|
32
|
|
| Antiretroviral therapy: | ||||||
| No intensive monitoring, first line drugs only | ANC | 1350 | 0.05 | 28 038 | 2.4 | 556 |
| Intensive monitoring, first line drugs only | ANC | 1507 | 0.04 | 34 825 | 2.5 | 596 |
| No intensive monitoring, first and second line drugs | ANC | 6434 | 0.04 | 181 099 | 3.2 | 2010 |
| Intensive monitoring, first and second line drugs | ANC | 6945 | 0.04 | 185 396 | 3.5 | 1977 |
ANC=antenatal care coverage level.
International dollars are a hypothetical unit of currency that has the same purchasing power that the US$ has in the United States at a given point in time. Details of this approach are discussed elsewhere.12
Intervention benefits are not comparable with current epidemiological estimates because results in this analysis are computed in relation to a “no intervention” comparator, which subtracts current levels of condom use, treatment of sexually transmitted infections, and antiretroviral treatment.
Table 2.
Annual costs in international dollars ($Int),* infections averted, DALYs averted, and average cost effectiveness for various single interventions to control HIV/AIDS in Sear-D region, compared with no intervention†
| Intervention | Coverage level | Yearly costs ($Int, millions) | Yearly infections averted (millions)† | Average cost effectiveness ratio ($Int/infection averted) | Yearly DALYs averted (millions)† | Average cost effectiveness ratio ($Int/DALY averted) |
|---|---|---|---|---|---|---|
| Mass media | 100% | 33 | 0.11 | 309 | 1.8 | 18 |
| Peer education for sex workers | 50%
|
78
|
1.49
|
53
|
24.6
|
3
|
| 80%
|
115
|
2.20
|
52
|
36.1
|
3
|
|
| 95% | 133 | 2.49 | 53 | 40.9 | 3 | |
| Peer education and treatment of sexually transmitted infections for sex workers
|
50%
|
83
|
1.82
|
45
|
29.9
|
3
|
| 80%
|
122
|
2.57
|
47
|
42.1
|
3
|
|
| 95%
|
141
|
2.85
|
50
|
46.6
|
3
|
|
| School based education | 50%
|
174
|
0.01
|
13 326
|
0.2
|
790
|
| 80%
|
175
|
0.02
|
8544
|
0.3
|
506
|
|
| 95% | 176 | 0.02 | 7288 | 0.4 | 432 | |
| Voluntary counselling and testing | 95% | 207 | 0.32 | 642 | 5.2 | 40 |
| Prevention of mother to child transmission | ANC | 268 | 0.04 | 7191 | 0.9 | 310 |
| Treatment of sexually transmitted infections | Current | 177 | 0.34 | 522 | 5.6 | 32 |
| ANC
|
297
|
0.66
|
448
|
10.9
|
27
|
|
| 95% | 356 | 1.08 | 330 | 17.7 | 20 | |
| Antiretroviral therapy: | ||||||
| No intensive monitoring, first line drugs only | ANC | 550 | 0.03 | 18 884 | 1.0 | 542 |
| Intensive monitoring, first line drugs only | ANC | 588 | 0.03 | 21 788 | 1.0 | 570 |
| No intensive monitoring, first and second line drugs | ANC | 1671 | 0.03 | 56 718 | 1.3 | 1319 |
| Intensive monitoring, first and second line drugs | ANC | 1774 | 0.03 | 55 188 | 1.4 | 1280 |
ANC=antenatal care coverage level.
International dollars are a hypothetical unit of currency that has the same purchasing power that the US$ has in the United States at a given point in time. Details of this approach are discussed elsewhere.12
Intervention benefits are not comparable with current epidemiological estimates because results in this analysis are computed in relation to a “no intervention” comparator, which subtracts current levels of condom use, treatment of sexually transmitted infections, and antiretroviral treatment.
Table 3.
Annual costs in international dollars ($Int),* DALYs averted, and incremental cost effectiveness of non-dominated† intervention combinations to control HIV/AIDS in Afr-E region
|
Intervention package
|
Yearly costs ($Int, millions)
|
Incremental cost effectiveness ratio ($Int/DALYs averted)
|
||
|---|---|---|---|---|
| No | Details | Yearly DALYs averted (millions)‡ | ||
| D1 | Mass media | 16 | 4.5 | 3 |
| D2 | D1 + peer education and treatment of sexually transmitted infections for sex workers, 50% coverage | 57 | 15.6 | 4 |
| D3 | D1 + peer education and treatment of sexually transmitted infections for sex workers, 80% coverage | 79 | 21.3 | 4 |
| D4 | D1 + peer education and treatment of sexually transmitted infections for sex workers, 95% coverage | 89 | 23.8 | 4 |
| D5 | D4 + prevention of mother to child transmission, antenatal care coverage level | 249 | 27.3 | 46 |
| D6 | D5 + treatment of sexually transmitted infections, current coverage | 290 | 27.9 | 68 |
| D7 | D5 + treatment of sexually transmitted infections, antenatal care coverage | 357 | 28.7 | 80 |
| D8 | D7 + voluntary counselling and testing, 95% coverage | 742 | 30.5 | 220 |
| D9 | D8 + treatment of sexually transmitted infections, expanded to 95% coverage | 859 | 30.9 | 290 |
| D10 | D9 + antiretroviral therapy, no intensive monitoring, first line drugs only | 2125 | 33.2 | 547 |
| D11 | D10 + school based education, 95% coverage | 2202 | 33.3 | 631 |
| D12 | D11 + antiretroviral therapy, intensive monitoring, first line drugs only | 2350 | 33.4 | 1144 |
| D13 | D11 + antiretroviral therapy, intensive monitoring, first and second line drugs | 7483 | 34.4 | 5175 |
International dollars are a hypothetical unit of currency that has the same purchasing power that the US$ has in the United States at a given point in time. Details of this approach are discussed elsewhere.12
Excludes combinations that were more costly but less effective than others (dominated interventions) and those with higher incremental cost effectiveness ratios than more effective options (weakly dominated interventions).
Intervention benefits are not comparable with current epidemiological estimates because results in this analysis are computed in relation to a “no intervention” comparator, which subtracts current levels of condom use, treatment of sexually transmitted infections, and antiretroviral treatment.
Table 4.
Annual costs in international dollars ($Int),* DALYs averted, and incremental cost effectiveness of non-dominated† intervention combinations to control HIV/AIDS in Sear-D region
|
Intervention package
|
Yearly costs ($Int, millions)
|
Incremental cost effectiveness ratio ($Int/DALYs averted)
|
||
|---|---|---|---|---|
| No | Details | Yearly DALYs averted (millions)‡ | ||
| D1 | Peer education and treatment of sexually transmitted infections for sex workers, 50% coverage | 83 | 29.9 | 3 |
| D2 | Peer education and treatment of sexually transmitted infections for sex workers, 80% coverage | 122 | 42.1 | 3 |
| D3 | Peer education and treatment of sexually transmitted infections for sex workers, 95% coverage | 141 | 46.6 | 4 |
| D4 | D3 + mass media, 100% coverage | 175 | 47.3 | 51 |
| D5 | D4 + treatment of sexually transmitted infections, 95% coverage | 511 | 49.8 | 133 |
| D6 | D5 + voluntary counselling and testing, 95% coverage | 693 | 50.4 | 317 |
| D7 | D6 + antiretroviral therapy, no intensive monitoring, first line drugs only | 1149 | 51.0 | 760 |
| D8 | D7 + prevention of mother to child transmission, antenatal care coverage level | 1416 | 51.3 | 850 |
| D9 | D8 + antiretroviral therapy, intensive monitoring, first line drugs only | 1443 | 51.3 | 1295 |
| D10 | D9 + school based education, 95% coverage | 1620 | 51.4 | 2192 |
| D11 | D10 + antiretroviral therapy, intensive monitoring, first and second line drugs | 2481 | 51.6 | 4406 |
International dollars are a hypothetical unit of currency that has the same purchasing power that the US$ has in the United States at a given point in time. Details of this approach are discussed elsewhere.12
Excludes combinations that were more costly but less effective than others (dominated interventions) and those with higher incremental cost effectiveness ratios than more effective options (weakly dominated interventions).
Intervention benefits are not comparable with current epidemiological estimates because results in this analysis are computed in relation to a “no intervention” comparator, which subtracts current levels of condom use, treatment of sexually transmitted infections, and antiretroviral treatment.
Figure 1.
Expansion path for incremental addition of cost effective interventions for HIV/AIDS in regions Afr-E (top) and Sear-D (bottom). (See tables 3 and 4 for description of combinations D1-D13 and D1-D11)
The expansion paths for the two regions are similar. In settings of extreme resource constraints, interventions focused on mass media and peer education and treatment of sexually transmitted infections for sex workers would be adopted first if cost effectiveness were the only criterion for prioritising interventions. In Afr-E health gains, measured in DALYs, would be maximised by adding prevention of mother to child HIV transmission and treatment of sexually transmitted infections in the community next, followed by voluntary counselling and testing, antiretroviral therapy, and school based education. All of these interventions would be regarded as highly cost effective based on standard benchmarks.12 The inclusion of second line drugs in antiretroviral regimens would be the last addition to the package of interventions in Afr-E. In Sear-D decision makers considering only the maximisation of population health would add treatment of sexually transmitted infections in the community, voluntary counselling and testing, antiretroviral therapy (with first line drugs), and prevention of mother to child transmission—all meeting the benchmark for highly cost effective interventions12—before adding school based education, which would be categorised as “cost effective” but not “highly cost effective,” or second line antiretrovirals, which fall just beyond the threshold defining “cost effective” interventions.
Sensitivity and uncertainty analyses
The expansion path provides useful information on cost effectiveness along with information on total costs and total effects, although the use of point estimates suggests a higher level of precision than is justified by the data. Many sources of uncertainty cannot be captured in the usual statistical confidence intervals, including uncertainty about the quantity of inputs required to run a programme, the actual use of services by patients, and unit costs. We tested the sensitivity of the rankings to variation in the assumptions around key parameters, and the ranking of interventions remained stable (see appendix for details).
In Afr-E a reduction in programme costs relative to patient costs would make treatment of sexually transmitted infections at high coverage and school based education more cost effective. In Sear-D an increase in programme relative to patient costs would make preventing mother to child transmission more attractive relative to other interventions. Uncertainty analyses also reveal stable outcomes across a range of plausible behavioural and biological assumptions in the 10 best fitting parameter sets (calibrated to match baseline projections). In Sear-D the expansion path was unchanged across the 10 parameter sets, except that preventing mother to child transmission could enter before the most basic antiretroviral therapy, and school based education could move between various antiretroviral treatment strategies. In Afr-E there was modest variation across the 10 parameter sets in the ordering of interventions, with ambiguity in the rankings of mass media and sex worker interventions, of preventing mother to child transmission and treating sexually transmitted infections in the community, and of school based education relative to voluntary counselling and testing and first line antiretroviral treatment.
For both regions, incremental cost effectiveness ratios all varied within a range of 60% above or below the point estimate, with the exceptions of mass media (in Sear-D), which could have a ratio up to 70% lower; school based education, which could have a ratio up to 2.5 times as high as the point estimate in Sear-D and was nearly 90% lower in one parameter set for Afr-E; and treating sexually transmitted infections in the community, which could be up to twice as costly in Afr-E as the point estimate.
Discussion
This study re-examines HIV/AIDS intervention strategies in a way that allows critical assessment of the cost effectiveness of current strategies and plans for the future use of extra resources that may become available. We have evaluated interventions singly and in combination, taking into account synergies in both costs and effects when interventions are implemented concurrently.
Because of the substantial uncertainties in many of our assumptions, we suggest that our results be viewed by broad bands of incremental cost effectiveness ratios. For example, in sub-Saharan Africa mass media and providing education and treatment of sexually transmitted infections for sex workers are virtually indistinguishable in terms of incremental cost effectiveness, but we can be more confident that school based education, at around $Int600 per DALY averted—even subject to a relatively wide range of uncertainty—requires greater resources to produce a given health benefit than peer education of sex workers, at less than $Int5 per DALY; or that use of second line antiretrovirals, at around $Int5000 per DALY averted, is substantially more costly per healthy life-year gained than the initial introduction of first line antiretrovirals, at about $Int500 per DALY.
Implications of results
Our results indicate that syndromic management of sexually transmitted infections can substantially reduce the health burden of HIV/AIDS in the population. There has been extensive debate over the role of treating sexually transmitted infections in the prevention of HIV infection because of apparently discrepant findings in three large, community based trials.16-20 Our results are consistent with recent syntheses of the findings from these trials,21,22 which conclude that such treatment has substantial potential to reduce HIV transmission, particularly in HIV epidemics at less advanced stages, as in both of the regions examined here (compared with the epidemic in Uganda). Our conclusion that treating sexually transmitted infections would be among the most cost effective interventions against HIV transmission should, however, be revisited as new information emerges.
Another important finding is that antiretroviral therapy would be included in a package of interventions for HIV/AIDS in both regions on the basis of cost effectiveness. A strict literal interpretation of the stated targets in the millennium development goals would limit the focus to interventions that reduce transmission, and evidence on the impact of treatment on transmission remains limited. However, treatment offers relatively good value for money in both regions in terms of broad measures of population health outcomes. Cost effectiveness ratios for first line HAART are lower than those for school based education, and some variant of HAART falls well below the threshold for very cost effective interventions in both regions. Although we found the addition of second line antiretrovirals to be relatively costly per added year of healthy life, their prices could well fall, as did the costs of first line treatment, which would lower these cost effectiveness ratios accordingly.
In addition, the direct impacts of antiretroviral therapy reported here might understate the overall social benefits of treatment. For example, the availability of treatment may encourage people to present voluntarily for counselling and testing, which is critical to overcoming denial, stigma, and discrimination—among the main barriers to effective prevention. It would also allow key workers such as those in the medical and education sectors to report more regularly for work, thereby relieving staff shortages in those sectors in many countries. These issues reinforce the finding that antiretrovirals should be offered in combination with preventive strategies.
Limitations of study
Several limitations in this study deserve mention. Some interventions that were not included in this analysis may be effective strategies. In addition, the interventions that we did include have been formulated in a small number of ways among the many possibilities. For example, we considered a basic variant of preventing mother to child transmission that falls short of the most recently published official recommendations.23 Although a regional analysis is intended to provide broad guidance to decision makers, many factors can cause variability in both costs and effects of interventions across settings. Although they are unlikely to affect our overall conclusions, continuing efforts are required to expand the scope of strategies that are analysed and consider additional alternatives for feasible implementation.
Many important uncertainties remain about the trajectory of HIV/AIDS epidemics and the potential effectiveness of interventions when expanded to full scale. Developing a better understanding of sexual behaviours in different settings will be critical, as will strengthening the empirical link between behavioural and epidemiological models. In considering the likely impact of interventions, we extrapolated most assumptions from a limited number of relatively small scale studies, so precise and reliable estimates of the effectiveness of large scale prevention programmes are still needed.
Conclusions
We emphasise that decisions are never made only on cost effectiveness criteria. Many other factors influence priority setting. For HIV/AIDS in particular, arguments have been made in support of general or specific intervention strategies based on ethical criteria and human rights, so policy makers should interpret our results in the context of these other important considerations.
A previous analysis indicated that the millennium development goal for HIV/AIDS could be achieved by application of a comprehensive response to prevention and treatment.7 Our analysis suggests that the financial constraints to implementing such a comprehensive approach to combating HIV/AIDS should not be regarded as the principal obstacle. A critical policy question that remains, however, is how to ensure that the massive undertaking required to respond effectively to the HIV pandemic can be sustained. Our findings that a combination of prevention and treatment can be highly cost effective brings into sharper focus the importance of overcoming other constraints such as managerial needs, political commitment, infrastructure, and human resource requirements.
What is already known on this topic
Previous studies of intervention priorities for HIV/AIDS in resource poor settings have either focused on comprehensive intervention packages or assembled cost effectiveness outcomes from independent studies of individual interventions
Recent reductions in costs of antiretroviral drugs make re-evaluation of the cost effectiveness of treatment essential
What this study adds
A comprehensive and standardised analysis of available interventions singly and in different combinations shows that “best buys” in HIV prevention include mass media campaigns, interventions focused on female sex workers, and treatment of other sexually transmitted infections
Cost effectiveness criteria would support the inclusion of antiretroviral therapy in a package of high value interventions, and treatment is expected to produce other benefits not captured in a cost effectiveness framework
Amendment
This is Version 2 of the paper. In this version, minor changes have been made to the text to clarify that the two regions studied are based on WHO grouping according to overall mortality, not HIV/AIDS epidemiology, and that cost effectiveness was the sole criterion used in prioritising interventions. A small correction in the discussion (p 5) means it now states: “Cost effectiveness ratios for first line HAART are lower than [not `similar to'] those for school based education.”
Supplementary Material
 Further details of the methods used appear on bmj.com
Further details of the methods used appear on bmj.com
We acknowledge valuable comments from David Evans, Tessa Tan-Torres, Stephen Lim, Jim Yong Kim, and Yves Souteyrand. We thank Peter Ghys, Karen Stanecki, and Neff Walker for providing baseline estimates and forecasts of HIV prevalence, John Stover for much useful input and guidance in development of the model, Kevin O'Reilly and George Schmid for helpful discussions on interventions, and Mark Sculpher and Virginia Wiseman for their constructive reviews of this and other papers in the cluster.
Contributors: All authors contributed to the conception, design and interpretation of data, and drafting of the manuscript. DRH, CH, and JAS developed the model. RB and JAL produced costing inputs. DRH and JAS completed the technical analysis. All authors approved the submitted version of the manuscript. DRH is guarantor for the study.
Funding: WHO provided financial support for the study in the form of salary support for staff members (JAL and CH) and funding for RB and DRH. WHO staff members received no external funding. Members of the WHO-CHOICE team helped frame the questions for the analysis and provided guidance on standardised methods. DRH and JAS were supported by a grant (P01 AG17625) from the National Institute on Aging, which had no role in the study design, execution, or reporting.
Competing interests: None declared.
References
- 1.UNAIDS, World Health Organization. AIDS epidemic update: December 2004. Geneva: UNAIDS, WHO, 2004.
- 2.Dixon S, McDonald S, Roberts J. The impact of HIV and AIDS on Africa's economic development. BMJ 2002;324: 232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherjee JS, Farmer PE, Niyizonkiza D, McCorkle L, Vanderwarker C, Teixeira P, et al. Tackling HIV in resource poor countries. BMJ 2003;327: 1104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez JP, Bertozzi S. Resource availability for HIV/AIDS and the funding gap. XV International AIDS Conference Satellite Session: resource tracking and priority setting, July 2004. Kaiser Family Foundation. www.kff.org/hivaids/upload/Resource-Availability-for-HIV-AIDS-the-Funding-Gap.pdf (accessed 28 Oct 2005).
- 5.Schwartlander B, Stover J, Walker N, Bollinger L, Gutierrez JP, McGreevey W, et al. AIDS. Resource needs for HIV/AIDS. Science 2001;292: 2434-6. [DOI] [PubMed] [Google Scholar]
- 6.Stover J, Walker N, Garnett GP, Salomon JA, Stanecki KA, Ghys PD, et al. Can we reverse the HIV/AIDS pandemic with an expanded response? Lancet 2002;360: 73-7. [DOI] [PubMed] [Google Scholar]
- 7.Salomon JA, Hogan DR, Stover J, Stanecki KA, Walker N, Ghys PD, et al. Integrating HIV prevention and treatment: from slogans to impact. PLoS Med 2005;2(1): e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creese A, Floyd K, Alban A, Guinness L. Cost-effectiveness of HIV/AIDS interventions in Africa: a systematic review of the evidence. Lancet 2002;359: 1635-43. [DOI] [PubMed] [Google Scholar]
- 9.Marseille E, Hofmann PB, Kahn JG. HIV prevention before HAART in sub-Saharan Africa. Lancet 2002;359: 1851-6. [DOI] [PubMed] [Google Scholar]
- 10.Walker D. Cost and cost-effectiveness of HIV/AIDS prevention strategies in developing countries: is there an evidence base? Health Policy Plan 2003;18: 4-17. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez JP, Johns B, Adam T, Bertozzi SM, Edejer TT, Greener R, et al. Achieving the WHO/UNAIDS antiretroviral treatment 3 by 5 goal: what will it cost? Lancet 2004;364: 63-4. [DOI] [PubMed] [Google Scholar]
- 12.Evans DB, Tan-Torres Edejer T, Adam T, Lim SS, for the WHO-CHOICE MDG Team. Achieving the millennium development goals for health: Methods to assess the costs and health effects of interventions for improving health in developing countries. BMJ 2005;331: 1137-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollinger L, Cooper-Arnold K, Stover J. Where are the gaps? The effects of HIV-prevention interventions on behavioral change. Stud Fam Plann 2004;35: 27-38. [DOI] [PubMed] [Google Scholar]
- 14.Koopman JS, Jacquez JA, Welch GW, Simon CP, Foxman B, Pollock SM, et al. The role of early HIV infection in the spread of HIV through populations. J Acquir Immune Defic Syndr Hum Retrovirol 1997;14: 249-58. [DOI] [PubMed] [Google Scholar]
- 15.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000;342: 921-9. [DOI] [PubMed] [Google Scholar]
- 16.Grosskurth H, Mosha F, Todd J, Mwijarubi E, Klokke A, Senkoro K, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet 1995;346: 530-6. [DOI] [PubMed] [Google Scholar]
- 17.Wawer MJ, Sewankambo NK, Serwadda D, Quinn TC, Paxton LA, Kiwanuka N, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet 1999;353: 525-35. [DOI] [PubMed] [Google Scholar]
- 18.Kamali A, Quigley M, Nakiyingi J, Kinsman J, Kengeya-Kayondo J, Gopal R, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet 2003;361: 645-52. [DOI] [PubMed] [Google Scholar]
- 19.Orroth KK, Korenromp EL, White RG, Changalucha J, de Vlas SJ, Gray RH, et al. Comparison of STD prevalences in the Mwanza, Rakai, and Masaka trial populations: the role of selection bias and diagnostic errors. Sex Transm Infect 2003;79: 98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet 2000;355: 1981-7. [DOI] [PubMed] [Google Scholar]
- 21.Korenromp EL, Bakker R, de Vlas SJ, Gray RH, Wawer MJ, Serwadda D, et al. HIV dynamics and behaviour change as determinants of the impact of sexually transmitted disease treatment on HIV transmission in the context of the Rakai trial. AIDS 2002;16: 2209-18. [DOI] [PubMed] [Google Scholar]
- 22.White RG, Orroth KK, Korenromp EL, Bakker R, Wambura M, Sewankambo NK, et al. Can population differences explain the contrasting results of the Mwanza, Rakai, and Masaka HIV/sexually transmitted disease intervention trials?: A modeling study. J Acquir Immune Defic Syndr 2004;37: 1500-13. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Antiretroviral drugs and the prevention of mother-to-child transmission of HIV infection in resource-limited settings. Geneva: WHO, 2005.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.