Abstract
Insects are major vectors of plant and animal disease, and bacterial phytopathogens are often disseminated by flies. We have previously reported that some isolates of the phytopathogenic bacterial species Erwinia carotovora infect Drosophila and activate an immune response. Using a genetic screen, we have now identified two genes that are required by E. carotovora to infect Drosophila. One of these genes has a regulatory role whereas the other, evf, confers an infectious phenotype: its transfer to non-infectious Erwinia strains or to several enterobacteria improves survival in the gut and triggers the immune response. Overexpression of Erwinia virulence factor (evf) allowed bacteria to colonize the apical side of the gut epithelium and in some cases to spread to the body cavity. Our results demonstrate a specific interaction between plant pathogens and flies that promote their dissemination.
Introduction
Flies have often been thought to be involved in the transmission of animal and plant diseases. They live on decaying media, enriched in microorganisms, and are ideal vectors for microbial dissemination through food contamination because of their close association with animal or plant communities. Although the potential hazard of flies towards humans is generally accepted, little is known about the interactions between bacteria and potential fly vectors (Grubel et al., 1997; Kobayashi et al., 1999). In contrast with the situation observed for plague bacteria and fleas (Hinnebusch et al., 2002) or for many protozoan parasites that infect humans and mosquitoes (Beerntsen et al., 2000) in which the insect is an obligate host, there is no molecular report describing a specific interaction of a bacterial pathogen with a fly that allows its persistence. As a consequence of our ignorance, it is generally assumed that flies are passive vectors and transmit microbes by three potential means that might not involve specific interactions between the two partners: carriage on the body, regurgitation and defecation.
The fruitfly Drosophila melanogaster lives in decaying fruit and has been occasionally implicated in the transmission of phytopathogens such as the enteric bacteria Erwinia carotovora (Molina et al., 1974; Kloepper et al., 1981). E. carotovora species induce the soft rotting of many fruits and potatoes through the production of a battery of pectinolytic enzymes (Barras et al., 1994). Recently, we identified a strain of Erwinia carotovora carotovora, Ecc15, that—unlike most bacterial species—induced a systemic immune response in Drosophila larvae after natural ingestion (Basset et al., 2000). Feeding of larvae with living Ecc15 induced a strong expression of the antimicrobial peptide genes in the larval fat body, a functional equivalent of mammalian liver. This bacterial strain is not pathogenic for its host, but its capacity to induce a systemic immune response suggests that it has infectious properties and can be recognized by the Drosophila innate immune system. Interestingly, out of 16 Ecc strains originally tested, only 3 had the capacity to infect Drosophila larvae by natural infection, suggesting the existence of specific genes that allowed Ecc15 to interact with its insect host (Basset et al., 2000).
Here, using a genetic screen, we have identified two genes that are required by E. carotovora to infect Drosophila. One gene has a regulatory role whereas the second, evf, confers an infectious phenotype not only on non-infectious Ecc strains, but also on various enterobacteria. The presence of evf increases bacterial persistence in the host, favouring the efficient dissemination of Erwinia by Drosophila even if it triggers the immune response. Bacteria overexpressing evf were able to colonize the entire midgut and to spread to the body cavity.
Results
Two genes required by Ecc15 to infect Drosophila
A library of 5,500 Ecc15 derivatives carrying a randomly inserted NKBOR mini-transposon (Rossignol et al., 2001) was screened for mutants that had lost the capacity to induce the expression of the antibacterial peptide encoding the gene Diptericin in the larval fat body. This screen was facilitated by the use of flies carrying a Diptericin–green fluorescent protein (GFP) reporter gene fusion that mimics the expression of the endogenous gene (Tzou et al., 2000). Using this assay (Fig. 1A), we selected four mutants carrying NKBOR insertions in two different genes (see below) that prevented Ecc15 from inducing Diptericin–GFP expression in the fat body. None of these mutants were impaired in growth (data not shown). Northern blot analysis demonstrated that all mutations had a strong effect on Diptericin expression (Fig. 1B). In addition to the fat body, Ecc15 also induces Diptericin expression in the cells of the anterior midgut (Tzou et al., 2000). Figure 1A shows that the two Ecc15 mutants have also lost the capacity to induce a Diptericin–lacZ reporter gene in this tissue. The cloning by plasmid rescue and characterization of the regions flanking the NKBOR mini-transposon in the four mutants allowed the identification of two genes (Fig. 1C): homologue of Rap (hor), which has previously been characterized (Thomson et al., 1997), and a new gene, evf; three independent insertions were found in this gene). In E. carotovora, Hor has previously been identified as a regulator of phytopathogenicity (Thomson et al., 1997); as expected, the hor mutant that we isolated was affected in its ability to infect plants in a potato assay (Fig. 1A). In contrast, the evf mutant retained the capacity to infect plants. We cannot predict an activity for Evf because no similarities with known proteins or domains have been found. Interestingly, Southern blot analyses showed that the hor gene was found in all the Ecc isolates we tested, whereas evf was present only in strains Ecc15 and Ecc1488, two isolates that naturally infect Drosophila (Fig. 1D).
Figure 1.
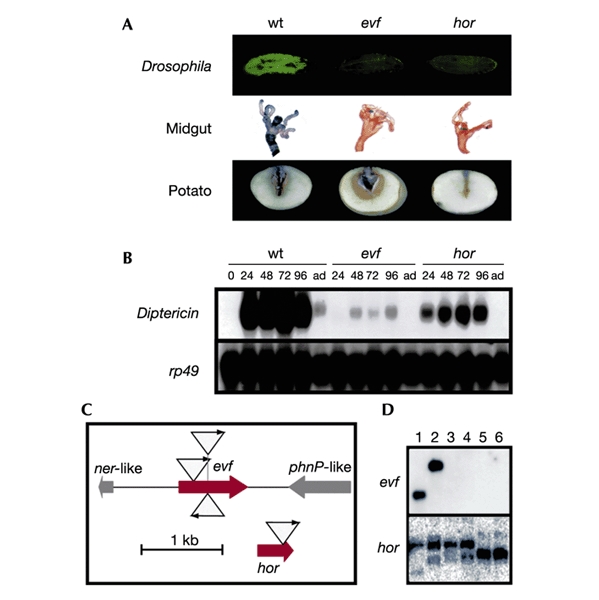
Identification of two genes of Erwinia required to infect Drosophila larvae. (A) GFP expression in larvae carrying a Diptericin–GFP reporter gene after infection by wild-type (wt) Ecc15, evf and hor mutants. Top, Local expression of a Diptericin–lacZ reporter gene by the same strains. Middle, LacZ staining (Tzou et al., 2000) was performed on gut collected 1 d after infection. Bottom, Infection of potatoes by the same bacterial strains. Potatoes were infected as described previously (Jones et al., 1993). (B) Northern blot analysis of Diptericin gene expression after natural infection of wild-type Drosophila with wild-type Ecc15, evf and hor mutants. RNA samples were extracted from Drosophila larvae collected at different time points after ingestion (24, 48, 72 and 96 h, and 'ad' for adults) and processed as described previously (Basset et al., 2000). rp49 hybridization was performed to normalize RNA samples. (C) Schematic representation of NKBOR insertion in regions containing evf and hor genes. Insertions of transposon NKBOR are represented by triangles with arrows. Genes located around evf are indicated. (D) Southern blot hybridizations of 32P-labelled evf and hor genes with EcoRI- and PvuII-cleaved total DNA from Ecc15 (lane 1), Ecc1488 (lane 2), Ecc1401 (lane 3), Ecc2140 (lane 4), Ecc2145 (lane 5) and Ecc2046 (lane 6).
Hor regulates evf expression
To analyse the relationships between hor and evf, we inserted hor and evf under the control of a constitutive promoter on a pSC101 derivative (pOM1; Espeli et al., 2001). The resulting plasmids, called pOM1-hor and pOM1-evf, were able to rescue their respective mutations as monitored by measurement of the expression of a Diptericin–lacZ reporter gene (Fig. 2A). Constitutive expression of evf conferred the capacity to infect Drosophila on the hor mutant, indicating that Evf acts downstream of Hor. In contrast, the constitutive expression of hor was not able to rescue the lack of Evf. Strikingly, we observed that Ecc15 derivatives carrying pOM1-evf induced significant lethality in larvae (see below). This lethality was probably responsible for the reduced Diptericin–lacZ expression compared with that obtained with wild-type Ecc15. The regulation of evf expression by Hor was confirmed by reverse transcription–polymerase chain reaction (RT–PCR) experiments done on total RNA extracted from dissected gut, obtained from larvae infected by the wild type, and by the evf and hor mutants (Fig. 2B): neither evf expression nor hor expression was detected in hor mutants, and only evf expression was affected in evf mutants. These results indicated that the expression of evf is under the control of Hor. Regulation of evf expression by Hor is reminiscent of the situation encountered in other bacteria in which Hor homologues directly regulate the expression of virulence factors (see Discussion) and suggested a critical role for Evf in the interaction with Drosophila.
Figure 2.
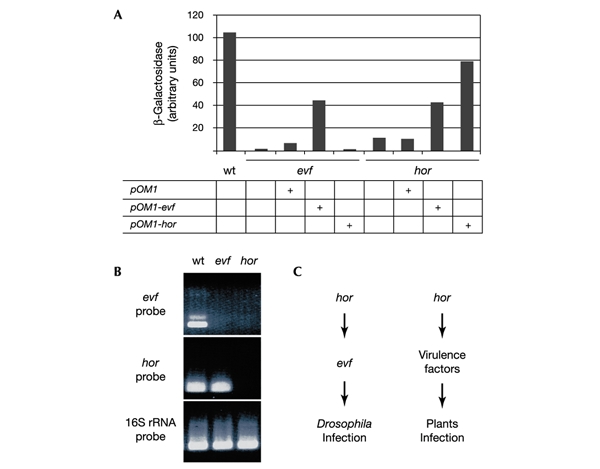
Epistatic relationship between evf and hor. (A) Quantitative measurements of β-galactosidase activity were performed with Drosophila larvae carrying the Diptericin–lacZ reporter gene collected 24 h after natural infection (Basset et al., 2000) by Ecc15 derivatives. Each mutant was transformed by pOM1, pOM1-evf and pOM1-hor. Bars represent mean results of three samples of five Drosophila larvae. wt, wild type. (B) RNA expression was monitored by RT–PCR with RNA isolated from the gut of 30 Drosophila larvae 6 h after infection by wild-type (wt) Ecc15, evf mutant and hor mutant. evf mRNA, hor mRNA and 16S rRNA were analysed as indicated; 16S rRNA was used as a positive control. (C) Genetic networks required for Drosophila and plants infection by Ecc15.
Bacteria expressing evf trigger the immune response
The importance of Evf in Drosophila infection was further evaluated by transferring the evf gene to a non-infectious Ecc strain, Ecc2046. Larvae carrying a Diptericin–lacZ fusion infected by Ecc2046 carrying pOM1-evf strongly expressed the reporter gene, whereas the strain containing the vector alone did not induce any immune response (Fig. 3A). In contrast, larvae infected by Ecc2046 carrying pOM1-hor did not express Diptericin (data not shown). This experiment indicated that the presence of evf was sufficient to explain the difference in infectious properties observed between the Ecc2046 and Ecc15 strains. This clearcut result prompted us to test the effect of evf expression in other enteric bacteria. Escherichia coli, Salmonella typhimurium and Serratia marcescens are three Gram-negative bacterial species that do not trigger any immune response in Drosophila after natural infection. The presence of pOM1-evf in these three bacteria induced a strong antibacterial response, whereas the presence of the vector alone had no effect (Fig. 3A). Taken together, our results indicated that the presence of Evf was sufficient by itself to promote a specific interaction between enteric bacteria and Drosophila larvae, resulting in the synthesis of antibacterial peptides.
Figure 3.

Immune response and persistence of bacteria expressing evf. (A) β-Galactosidase assay revealing the immune response in Ecc15, Ecc2046, E. coli MG1655, Salmonella typhimurium LT2 and Serratia marcescens expressing evf constitutively. Wild-type Ecc15 and Ecc15 carrying pOM1 were used as positive controls; Ecc2046, E. coli MG1655, Salmonella typhimurium LT2 and Serratia marcescens carrying pOM1 plasmid were used as negative controls. Lane C, banana alone. (B) Bacterial persistence was measured in wild-type CantonS Drosophila larvae (Basset et al., 2000). The number of colony-forming units per larva obtained at each time point after infection is the mean of 120 larvae infected. (C) Visualization of Ecc15 derivatives carrying a GFP marker gene in Drosophila larvae. Drosophila larvae were infected with Ecc15 carrying pOM1–GFP (a, d), with evf mutant carrying pOM1–GFP (b, e), and with evf mutant carrying pOM1-evf–GFP (c, f). Pictures were taken 2 h (a–c) and 8 h (d–f) after infection. Exposure times were shorter in (a–c) than in (d–f). Magnification × 10.
Expression of evf enhances colonization in the host
The selective advantage that evf might confer on Ecc15 was assessed by analysing its effects on bacterial persistence and pathogenicity in larvae. By using wild-type Ecc15, the evf mutant or the evf mutant carrying pOM1-evf, we observed that the persistence of Ecc15 derivatives 8 h after ingestion was correlated with the level of expression of evf (Fig. 3B); for the evf mutant the number of bacteria was very much reduced in the gut compared with that of wild-type Ecc15. In contrast, the constitutive expression of evf resulted in a more than tenfold increase in the number of bacteria present in the larvae. This effect can be detected directly by using the same strains expressing GFP constitutively (Fig. 3C). For all strains, bacteria were detected in the anterior midgut only during the first hours after ingestion. By 8 h after ingestion, the bacteria were visible only in the midgut of larvae infected by strains expressing evf constitutively, and these bacteria were often able to colonize the entire midgut. We used immunostaining to analyse more precisely the localization of the evf mutant, or the evf mutant carrying pOM1-evf, in larvae 6 h after ingestion. We observed that the evf mutant did not persist, and rare bacteria remained associated with food in the lumen of the gut (Fig. 4A). In contrast, the same mutant overexpressing evf often colonized the gut edge (Fig. 4B). In 30% of the larvae tested, bacteria were also found in the haemolymph (Fig. 4C), indicating that overexpression of evf allowed the spreading of bacteria across the gut barrier.
Figure 4.
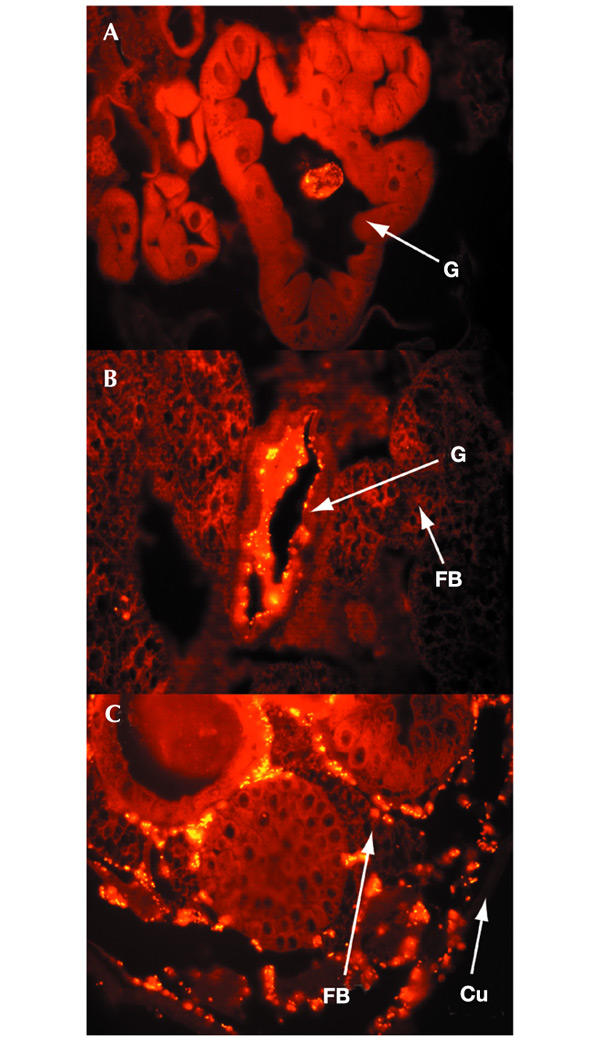
Representative immunostained sections of Drosophila larvae infected with evf mutant carrying pOM1–GFP (A) and with evf mutant carrying pOM1-evf–GFP (B, C). Thirty larvae infected with evf mutant carrying pOM1–GFP or with evf mutant carrying pOM1-evf–GFP were sectioned and bacteria were detected by anti-GFP antibodies. The evf mutant did not persist and rare bacteria can be detected with food in the lumen of the anterior midgut (A). Similar results were obtained with wild-type Ecc15 (data not shown). The evf mutant overexpressing evf colonized the epithelium of the entire midgut (B). In 30% of the larvae, bacteria carrying pOM1-evf were detected in the haemolymph (C, a case of major systemic infection). FB, fat body; G, gut; Cu, external cuticle. Magnification × 40.
As described above, we observed a strong lethality (up to 60%) in larvae infected by Ecc15 overexpressing evf that was apparent after 3–12 h (see Supplementary Information). It is important to note that all Ecc15 derivatives carrying pOM1-evf behaved identically to Ecc15 after direct injection into the body cavity of Drosophila adults (data not shown); they were not pathogenic for wild-type Drosophila, whereas all these derivatives kill imd mutants that do not synthesize antibacterial peptides (Lemaitre et al., 1995). Taken together, these results showed that evf expression was specifically required to establish the interaction between Ecc15 and Drosophila larval gut.
Discussion
The strain Ecc15 was initially identified for its capacity to induce an immune response in Drosophila larvae after natural infection. The use of this strain has been a determinant in revealing the ability of Drosophila to activate a systemic immune response adapted to its aggressors (Vidal et al., 2001) and to induce local immune responses (Tzou et al., 2000). The high specificity of the Drosophila–Ecc15 interaction suggested that this strain possesses a unique mechanism to infect Drosophila and that this infection can induce the expression of antimicrobial peptide-encoding genes.
Here, we have identified a single gene, evf, which is sufficient to specify an interaction between Erwinia species and the fruitfly Drosophila and to trigger the immune response. Our study demonstrated that flies are not passive vectors for bacteria, and that specific interactions allow persistence in larvae that might ensure effective dissemination of the bacteria. The observation that evf was found in only a limited number of Erwinia isolates suggests that this gene has been acquired recently by horizontal transfer. Interestingly, one Ecc strain, Ecc1401, does not possess the evf gene but still induces an immune response in Drosophila, suggesting that Ecc species might have selected other strategies to persist in Drosophila.
Our data showing that evf is controlled by the hor gene indicate that, in Ecc15, Hor is a key regulator, able to transmit signals from different environments to at least two types of effector: those involved in plant pathogenesis and those involved in Drosophila infection (Fig. 2C). Proteins strongly related to Hor (a homologue of RovA, also called Rap and SlyA) have been identified in several Gram-negative pathogens (Yersinia pseudotuberculosis, Y. enterolitica, Serratia marcescens, Salmonella typhimurium and E. coli) and control the expression of virulence genes such as tissuespecific adhesin, invasin, haemolysin and protease (Nagel et al. (2001) and references therein). Our data now extend the function of this class of regulators to the regulation of genes involved in Drosophila infection.
We observed that Evf promoted the adhesion of bacteria to the gut cells and was associated with crossing of the intestinal barrier. The change in properties induced by Evf results in the activation of the immune response either by a signal sent by gut cells or by the presence of bacteria in the haemolymph. It is possible that a function of Evf is to disrupt the peritrophic membrane, a chitinous membrane that lines the insect gut and prevents bacteria from entering the gut cells. Alternatively, Evf might permit the proliferation of bacteria in this environment, or it might act as a toxin that disturbs the physiology of the gut cells. Further studies are required to analyse the molecular activity of Evf. Interestingly, the presence of evf conferred infectious properties on the three enterobacteria tested, indicating that the way in which Evf ensures their survival in Drosophila can be effective in enterobacteria in general. This opens up the possibility of using Drosophila as a host model for Gram-negative bacterial pathogens.
Speculation
The exact nature of the Drosophila–Ecc15 interaction in nature requires further investigation. However, it is striking to observe that the level of evf expression modulates the outcome of the Drosophila–Erwinia interaction: in the absence of evf, the bacterium does not persist in larvae, whereas when evf is overexpressed, the bacterium becomes highly pathogenic. It is tempting to speculate that the level of evf expression in Ecc15 results from a beneficial co-evolution between the two partners: Ecc15 uses Drosophila as a vector for spreading, whereas Drosophila might benefit from transmitting bacteria that induce rotting of fruit and facilitate the life cycle of the larvae. In contrast with many other phytopathogenic bacteria, Erwinia species do not persist in the soil (Agrios, 1997), and the acquisition of a gene increasing survival in an insect that can be used as a vector might be important in spreading the bacteria from one plant to another. In agreement with this hypothesis, we have indeed observed that bacteria expressing evf were preferentially disseminated by Drosophila larvae; such larvae were still able, 24 h after infection, to propagate soft rot development in carrots (data not shown). Finally, we speculate that such interactions are not restricted to Erwinia and Drosophila but that other bacteria, including human pathogens, have developed ways of persisting in housefly vectors, as suggested previously (Douglas & Beard, 1996; Kaslow & Welburn, 1996; Kobayashi et al., 1999).
Methods
Isolation and characterization of Ecc15 mutants.
Random mutagenesis of Ecc15 was performed as described by Rossignol et al. (2001). A total of 5,500 KanR colonies were screened: pellets obtained from 10-ml cultures were used to feed Diptericin–GFP Drosophila larvae (Tzou et al., 2002). Candidates that were unable to induce the expression of the Diptericin–GFP fusion after 48 h of infection were tested again by the same method and subsequently tested for the lack of induction of a Diptericin–lacZ reporter fusion. DNA from each mutant was extracted and digested by BglII, ligated and transformed into a DH5α pir strain. This allowed us to select a plasmid containing NKBOR with the flanking regions. Sequencing of one flanking region was performed by using the oligonucleotide 5′-ATTTTGAGTGACACAGGAAC-3′. The rescued plasmid was digested with BglII and BamHI to delete the IS10 inverted repeat close to R6K ori (see Rossignol et al. 2001), ligated and transformed in DH5α pir. The sequence of the second flanking region was determined by using the oligonucleotide 5′-GGATCATATGACAAGATGTG-3′.
Construction of plasmids.
pOM1-evf was obtained by cloning a 967-bp BamHI–HindIII PCR fragment containing evf, obtained by using oligonucleotides 5′-AGTGGATCCTGTAACCCCCCCAATAGG-3′ and 5′-AAGCCCAAGCTTAAAATCGAATGATTTAGA-3′. pOM1-hor was obtained by cloning a 479-bp fragment containing hor by using oligonucleotides 5′-AGTGGATCCTAACAATAAGGAGAGGTG-3′ and 5′-AGTAAGCTTCCTCTGCGTAACCCAAAT-3′. The pOM1–GFP and pOM1-evf–GFP plasmids were obtained by cloning a 1.5-kbp EcoRI fragment amplified from pFVP25.1 into pOM1 and pOM1-evf, respectively, linearized by EcoRI (Valdivia & Falkow, 1996). The PCR fragment was obtained by using the oligonucleotides 5′-ATTGTCTCATGAGCGGAT-3′ and 5′-ATCGACGAATTCCGCAGTTATTTGTACAA-3′.
RNA extraction and RT–PCR analysis.
Total RNA from dissected guts were extracted with the kit Gram-cracker Reagents and RNAwiz (Ambion). RT–PCRs were performed on 1 μg of total RNA with the Titan-one RT–PCR system (Roche): evf mRNA was detected by using oligonucleotides 5′-ATTCAAGATGTGGATCTG-3′ and 5′-AGTAAGCTTGGTAATTGAATTTGCTTGG-3′, whereas hor mRNA was detected by using oligonucleotides 5′-GGAATTGCCATTAGGATC-3′ and 5′-GCCAATATATTTTTCTCAAGACGCG-3′. The positive control 16s rRNA was detected by the same method using oligonucleotides 5′-TAGCGATTCCGACTTCA-3′ and 5′-AACGCGAAGAACCTTAC-3′.
Immunostaining.
Third-instar larvae were infected with Ecc15 derivatives carrying a GFP-expressing plasmid. Larvae were fixed for 2 h in Carnoy (ethanol:chloroform:acetic acid, 6:3:1 by vol.), washed three times (30 min each) in ethanol, incubated overnight in methyl benzoate and embedded in paraffin blocks. Animals were cut into 0.6-μm serial transverse sections that were deposited on slides and rehydrated by standard procedures. Samples were blocked for 20 min with 10% normal goat serum in Tris-buffered saline plus 0.3% Triton (TBT), hybridized with a 1:50 dilution of mouse anti-GFP antibody (Roche) for 2 h and then washed three times (5 min each) in TBT. Samples were incubated for 1 h with a 1:250 dilution of Alexa-594-conjugated anti-mouse antibody, washed again and mounted with aqueous mounting medium (DAKO Paramount). All antibodies were diluted in TBT plus 10% normal goat serum.
Supplementary data are available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/4-embor730-s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
We thank R. Khush and N. Vodovar for critical reading of the manuscript, V. Méjean for helpful discussions, and A. Guenon des Mesnards, B. Limbourg-Bouchon, A.-L. Finoux, B. Maroni, M.-A. Michellod and E. Nicolas for technical assistance. This work was supported by the Centre National de la Recherche Scientifique, Association pour la Recherche sur le Cancer, and Program Microbiologie PRFMMIP. A.B. was supported by the Fondation pour la Recherche Médicale during part of this work.
Footnotes
The nucleotide sequence of evf has been submitted to GenBank under accession number AY167732.
References
- Agrios G.A. (1997) Plant Pathology. Academic Press, New York. [Google Scholar]
- Barras F., Van Gijsegem F. & Chatterjee A.K. (1994) Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu. Rev. Phytopathol., 32, 201–234. [Google Scholar]
- Basset A. et al. (2000) The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl Acad. Sci. USA, 97, 3376–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerntsen B.T., James A.A. & Christensen B.M. (2000) Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev., 64, 115–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A.E. & Beard C.B. (1996) in Biology of the Insect Midgut (eds Lehane, M.J. & Billingsley, P.F.) 419–431. Chapman & Hall, London. [Google Scholar]
- Espeli O., Moulin L. & Boccard F. (2001) Transcription attenuation associated with bacterial repetitive extragenic BIME elements. J. Mol. Biol., 314, 375–386. [DOI] [PubMed] [Google Scholar]
- Grubel P. et al. (1997) Vector potential of houseflies (Musca domestica) for Helicobacter pylori. J. Clin. Microbiol., 35, 1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch B.J. et al. (2002) Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science, 296, 733–735. [DOI] [PubMed] [Google Scholar]
- Jones S. et al. (1993) The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J., 12, 2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow D.C. & Welburn S. (1996) in Biology of the Insect Midgut (eds Lehane, M.J. & Billingsley, P.F.) 432–462. Chapman & Hall, London. [Google Scholar]
- Kloepper J.W., Brewer J.W. & Harrison M.D. (1981) Insect transmission of Erwinia carotovora var. carotovora and Erwinia carotovora var. atroseptica to potato plants in the field. Am. Potato J., 58, 165–175. [Google Scholar]
- Kobayashi M. et al. (1999) Houseflies: not simple mechanical vectors of enterohemorrhagic Escherichia coli O157:H7. Am. J. Trop. Med. Hyg., 61, 625–629. [DOI] [PubMed] [Google Scholar]
- Lemaitre B. et al. (1995) A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc. Natl Acad. Sci. USA, 92, 9465–9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J.J., Harisson M.D. & Brewer J.W. (1974) Transmission of Erwinia carotovora var. atropeptica by Drosophila melanogaster meig. I. Acquisition and transmission of the bacterium. Am. Potato J., 51, 245–250. [Google Scholar]
- Nagel G., Lahrz A. & Dersch P. (2001) Environmental control of invasin expression in Yersinia pseudotuberculosis is mediated by regulation of RovA, a transcriptional activator of the SlyA/Hor family. Mol. Microbiol., 41, 1249–1269. [DOI] [PubMed] [Google Scholar]
- Rossignol M., Basset A., Espeli O. & Boccard F. (2001) NKBOR, a mini-Tn10-based transposon for random insertion in the chromosome of Gram-negative bacteria and the rapid recovery of sequences flanking the insertion sites in Escherichia coli. Res. Microbiol., 152, 481–485. [DOI] [PubMed] [Google Scholar]
- Thomson N.R. et al. (1997) The rap and hor proteins of Erwinia, Serratia and Yersinia: a novel subgroup in a growing superfamily of proteins regulating diverse physiological processes in bacterial pathogens. Mol. Microbiol., 26, 531–544. [DOI] [PubMed] [Google Scholar]
- Tzou P. et al. (2000) Tissuespecific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity, 13, 737–748. [DOI] [PubMed] [Google Scholar]
- Tzou P., Meister M. & Lemaitre B. (2002) Methods for studying infection and immunity in Drosophila. Methods Microbiol., 31, 507–529. [Google Scholar]
- Valdivia R.H. & Falkow S. (1996) Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol., 22, 367–378. [DOI] [PubMed] [Google Scholar]
- Vidal S. et al. (2001) Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-κB-dependent innate immune responses. Genes Dev., 15, 1900–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


