Summary
Conference on the Hsp90 Chaperone Machine
Introduction
Hsp90 (heat-shock protein 90) is an abundant molecular chaperone, but its function seems to be restricted to the folding of proteins involved in cell signalling, such as transcription factors and protein kinases. This restricted set of 'clients' (see Fig. 1) makes Hsp90 an attractive target for cancer therapeutics. As an anti-Hsp90 drug is now in clinical trials, the meeting was relevant to a broad range of scientists interested in its chaperone activity. However, even with this clinical relevance, a meeting devoted to just one chaperone might seem like too much of a good thing, unless one takes into account the myriad of co-chaperones that regulate Hsp90. These co-chaperones modulate the ATPase activity of Hsp90 and many of them have intrinsic chaperone activity of their own, providing some measure of specificity for different Hsp90 clients. There is also some crossover between different chaperone machineries, as some co-chaperones interact with Hsp70 as well as Hsp90.
Figure 1.
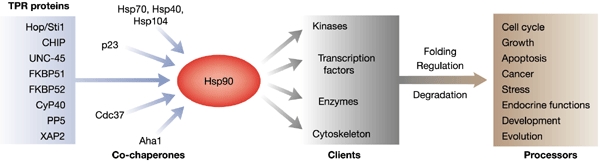
Examples of roles of Hsp90 and its co-chaperones in different cellular processes through their interactions with different client proteins.

The first international meeting on the Hsp90 (heat-shock protein 90) chaperone machine was held in Arolla, Switzerland, from 24 to 28 August 2002. Located in a beautiful isolated chalet, high in the Swiss Alps, this was a highly interactive and productive gathering, whose success owed much to its organizers, Didier Picard and Johannes Buchner.
The conference was opened by D. Smith (Scottsdale, AZ, USA), who gave a historical perspective on the interactions of Hsp90 and its co-chaperones with steroid receptors (reviewed by Pratt & Toft, 1997). His presentation began with a review of the work of Toft and Gorksi from the late 1960s, in which an oestrogen receptor was identified and found to exist in a large complex of proteins. Smith followed the identification and characterization of Hsp90 and other receptor-associated chaperones in this complex through to his recent studies on the regulation of the hormone-binding affinity of Hsp90-bound immunophilins. The theme of Hsp90 regulation, in particular by Hsp90-binding co-chaperones, and the variety of protein clients and physiological processes that are affected by the Hsp90 machinery was continued throughout the meeting.
Hsp90 structure and function
Structural studies have provided new insights into the mechanism of action of Hsp90. The Hartl and Pearl groups have published crystal structures for the amino-terminal ATPase domain (Stebbins et al., 1997; Prodromou et al., 1997). In Arolla, P. Meyer (London, UK) from the Pearl group presented the crystal structure (2.5-Å resolution) of the middle domain of Hsp90 (amino acids 273–560), which itself contains three domains—two αβ5α-domains and a linker consisting of three helices. One of the αβ5α-domains is structurally homologous to domains in the ATP-binding proteins mutL and DNA gyrase B, although there are some differences between them: Hsp90 lacks a helix that is present in the corresponding αβ5α-domain of mutL, and DNA gyrase B has a hydrophobic protruding loop that is absent from the other two proteins. The presence of this unusual exposed hydrophobic loop suggests that this is a possible client-binding site. The structural homology between the middle domain of Hsp90 with those of mutL and DNA gyrase B has led to the identification of residues that have a role in the binding and hydrolysis of ATP that is performed by the N-terminal domain. The Pearl group also presented studies of the solution structure of the N-terminal domain based on nuclear magnetic resonance (NMR) techniques. In particular, R. Selak (London, UK) spoke about preliminary work on the assignment of NMR peaks and structural analysis. Changes in chemical shifts caused by ATP, AMPpNp and ADP binding were observed for many protons in the spectrum of the N-terminal domain, suggesting that the conformational changes that occur on nucleotide binding are not just localized to the ATP-binding site. The next challenge is to assign all these peaks and to quantify the structural changes.
The role of dimerization of the N-terminal domain in the ATPase cycle was presented by K. Richter from the Buchner lab (Garching, Germany), who has used heterodimers of truncated and full-length Hsp90 to establish the effects of dimerization specifically on ATP binding and hydrolysis. The first 16 amino acids of Hsp90 were shown to be important for the dimerization-stimulated increase in ATPase activity that occurs once ATP is bound.
Several key questions about the relationship between the structure and function of Hsp90 remain unanswered at this time. First, what forms the binding site or sites for client proteins? Despite the attention given to Hsp90 in recent years, surprisingly little is known about how Hsp90 interacts with client proteins. Second, what is the mechanism by which Hsp90 alters client conformation? There could be an active reshaping of the client protein that involves several domains in the Hsp90 dimer; alternatively, Hsp90 binding may stabilize an otherwise transient client conformation. Another issue to be resolved is the existence of a second ATP-binding site at the carboxy terminus of Hsp90. Several recently published reports (for example, Marcu et al., 2000; Garnier et al., 2002; Soti et al., 2002) provide evidence for the existence of such a site, but what forms the second site and how it influences Hsp90 function remain unclear. Furthermore, recent structural analyses do not support the existence of a second ATP-binding site (C. Prodromou and F. Meyer, personal communication).
Hsp90 co-chaperones
Hsp90 does not usually function alone but interacts with a host of other factors, or co-chaperones, that influence its interaction with nucleotides and client proteins. The best-characterized model for the function of co-chaperones comes from studies of the interaction of Hsp90 with nuclear receptors such as the glucocorticoid receptor (GR). In this model (Fig. 2), the co-chaperones appear to enter and exit Hsp90–GR complexes in a defined order. The co-chaperones can be broadly divided on a structural basis into those containing tetratricopeptide repeat (TPR) domains and those that do not; among the latter there is little structural similarity. Functional characterization of co-chaperones is still at an early stage, although recent studies have established that some co-chaperones (such as Cdc37 and p23) have the ability to bind independently to Hsp90 clients whereas others do not (Hop/Sti1).
Figure 2.
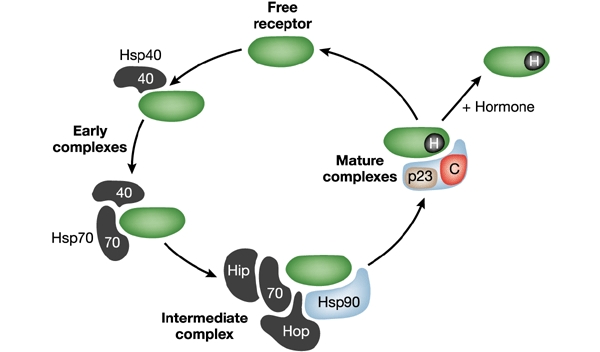
Co-chaperones of Hsp70 and Hsp90 enter and exit chaperone–GR (glucocorticoid receptor) protein complexes in a defined order. This cycling reflects the co-operation between Hsp70 and Hsp90, and the role of different co-chaperones, for example p23, Hsp40, Hip, Hop and others, such as immunophilins, in the folding process. The GR protein is shown in green. H, hormone; C, other co-chaperones, for example immunophilin, FKBP51, FKBP52, CyP40 and PP5.
Several talks focused on the co-chaperones that regulate Hsp90 ATPase activity. In one of these talks, C. Prodromou (London, UK) introduced the audience to Aha1, a co-chaperone of yeast Hsp90 and homologue of Hch1, which was originally identified as a multicopy suppressor of the growth phenotype of an Hsp90-mutant yeast strain (Nathan et al., 1999). Aha1 was shown to be a novel Hsp90 co-chaperone that stimulates the basal ATPase activity of yeast Hsp90 up to tenfold. In addition, W. Obermann (Bonn, Germany) showed that C143, the human homologue of Aha1 has a similar function. Another protein shown to upregulate the ATPase activity of Hsp90 is GR itself. Members of S. Jackson's group (Cambridge, UK) described how an in vitro refolded form of the GR ligand-binding domain specifically stimulates the basal ATPase activity of human Hsp90. Interestingly, this response is not observed with unfolded or partially folded protein substrates that are also known to bind to Hsp90.
Rather than stimulating ATPase activity, some co-chaperones are inhibitory, and among these is Hop/Sti1, which forms a stable complex with Hsp90 and Hsp70 through distinct TPR domains. Evidence for a model in which Hop/Sti1 is able to stimulate the basal ATPase activity of Hsp70 but can also inhibit that of Hsp90 was presented by J. Buchner (Garching, Germany). A key recognition motif for the binding of Hop/Sti1 to both Hsp90 and Hsp70 is the EEVD sequence, that is present at the C termini of both chaperones (Scheufler et al., 2000). However, as these are bound by distinct TPR domains, Hop/Sti1 must be able to discriminate between motifs in Hsp90 and those in Hsp70. In one talk, O. Odunuga from G. Blatch's lab (Cape Town, South Africa) presented a mutagenic analysis of the Hop/Sti1 TPRs in which he identified amino acids that confer specificity of binding. For example, the dual substitution A49F plus K50E of Hop TPR1 reduces binding to Hsp70 and confers the ability to bind Hsp90. These substitutions mimic corresponding residues in the Hsp90-binding TPR2a domain of Hop/Sti1. J. Young of the Hartl group (Martinsried, Germany) presented studies on Tom70, a TPR protein that mediates the import of proteins into mitochondria. As with Hop/Sti1, Tom70 binds both Hsp70 and Hsp90, but in contrast, binding of Tom70 to these chaperones is mediated through a single, dualspecificity TPR domain. Interestingly, inhibition of either Hsp90 (with geldanamycin) or Hsp70 (by overexpressing a mutant form of the co-chaperone Bag1) impeded mitochondrial import by Tom70, suggesting that there is a requirement for sequential Hsp interactions.
Other co-chaperones include several large immunophilins of the FK506-binding and cyclosporin-binding families (FKBPs and cyclophilins (CyPs), respectively), which contain TPR domains that mediate their binding to Hsp90. X-ray crystallographic structures for two of these, FKBP51 and CyP40, were presented in talks by D. Smith (in collaboration with J. Clardy, Ithaca, NY, USA) and M. Walkinshaw (Edinburgh, UK; in collaboration with T. Ratajczak, Nedlands, Australia), respectively. Both CyP40 and FKBP51 contain a C-terminal α-helix that extends beyond the TPR. The functional importance of this extended helix remains unclear, although it contains a putative calmodulin (CaM)-binding site. As presented by A. Breiman (Tel Aviv, Israel), high-molecular-weight plant FKBPs, which have a role in plant development and morphogenesis, also have FKBP and TPR domains, as well as a putative CaM-binding site. For FKBP73, which functions as a homodimer, CaM was shown to inhibit dimerization, suggesting that CaM may regulate these complexes. FKBP73 was also shown to associate with dynein, as has been observed with several of the mammalian receptor-associated immunophilins.
How the TPR domains of these immunophilins bind to Hsp90 was discussed in detail; T. Ratajzak mapped out regions in the C-terminal domain of Hsp90 that are important for dimerization, immunophilin binding and novobiocin binding. The region between Hsp90 residues 653 and 673, which is leucine rich, was found to be the primary dimerization and immunophilin-binding site.
Cdc37 was first described as an Hsp90 co-chaperone that interacts only with protein kinases. This view has changed due to recent findings that it interacts both with the human androgen receptor (Rao et al., 2001) and with hepadnavirus reverse transcriptase, as described by J. Hu (Boston, MA, USA). The role of Cdc37 in protein kinase folding has yet to be fully characterized, although it is known to function in association with Hsp90 and to have a chaperone function of its own. Studies presented in Arolla suggest that Cdc37 may regulate protein kinase activity in addition to promoting folding of the nascent kinase polypeptide. A. Basso and N. Rosen (New York, NY, USA) presented evidence that Akt (a protein kinase also known as protein kinase B) is biologically active when in a complex with Cdc37. In another presentation, C. Gonzalez (Heidelberg, Germany) described how the Aurora B protein kinase and Cdc37 migrate together to spindle microtubules where the kinase functions. He also showed that Aurora B is not always dependent on Cdc37 or Hsp90 for activity; in some cancer cells it seems to be an active kinase that does not require either of these chaperones.
Evidence for these putative regulatory functions of Cdc37 was augmented by new studies showing that Cdc37 can protect protein kinases from the inhibitory actions of other co-chaperones. A. Citri (Rehovot, Israel), for example, showed that Cdc37 overexpression protects the oncogenic tyrosine kinase ErbB2 from degradation induced by the co-chaperone Chip, which interacts with Hsp90 and functions as an E3 ubiquitin ligase (Cyr et al., 2002). Because Cdc37 is upregulated in transformed cells and tissues, these data are important for understanding how chaperones contribute to cellular decisions regarding protein turnover. Similar conclusions about the role of Cdc37 in protecting protein activity were presented by J. Jimenez (Sevilla, Spain). In the yeast Schizosaccharomyces pombe, p23 overexpression downregulates the cyclin-dependent protein kinase Cdc2, and is lethal. Cdc37 overexpression suppresses this downregulation, however, suggesting that Cdc37 and p23 have antagonistic functions in this system. Together, these studies reinforce the view that competition between co-chaperones for both Hsp90 and clients leads to distinct functional outcomes depending on the combination of co-chaperones expressed.
Evidence that p23 may function outside the Hsp90 chaperone machinery was presented by B. Freeman (Champaign-Urbana, IL, USA). His studies showed that p23 can regulate the association of hormone-bound nuclear receptors with their response elements in chromatin. This activity may facilitate the rapid decrease in activity by nuclear receptors in response to hormone withdrawal.
Hsp90 at the interface of multiple cellular pathways
Hsp90 influences the activity and stability of a wide range of client proteins that function as key regulators in cellular growth, differentiation and death pathways. Among the more than 100 known Hsp90 clients (summarized on the Picard lab home page: http://www.picard.ch/DP/DPhome.html) are the steroid receptors, various PAS (Per–Arnt–Sim) family transcription factors, tyrosine kinases, serine/threonine kinases, G-protein subunits, nitric oxide synthase and telomerase. J. Hu updated the audience on his recent successful efforts to reconstitute all of the factors required for reverse transcriptase activation in vitro. Interestingly, Hu settled on the same five chaperone components—Hsp90, Hsp70, Hsp40, Hop/Sti1 and p23—that Pratt and Toft identified as being the minimal requirements for the efficient maturation of steroid receptor complexes. However, these five proteins are not sufficient for all Hsp90 client proteins; L. Poellinger (Stockholm, Sweden) presented his findings that XAP2, an FKBP-class TPR immunophilin, regulates nuclear localization of the aryl hydrocarbon receptor (AhR), thus influencing cellular responses to dioxins and other xenobiotic compounds that activate AhR. Additionally, Poellinger introduced HIF1-α, which belongs to the same family of PAS transcription factors as AhR and regulates cellular responses to hypoxia, as an Hsp90 client protein whose stability is regulated by the tumour suppressor protein complex VHL (von Hippel–Lindau complex). In another twist on Hsp90 function, J. Frydman (Stanford, CA, USA) presented her findings that Hsp90 is required for VHL degradation but not for its folding or assembly. T. Rein (Munich, Germany), whose lab is interested in glucocorticoid resistance, showed that overexpression of the Hsp90 co-chaperone and immunophilin FKBP51 decreases cellular responses to glucocorticoids.
Pharmacology of Hsp90
Hsp90 has several protein interaction and nucleotide-binding sites that are involved in its functions, and there are many natural products that specifically bind Hsp90, providing candidate compounds for therapeutic use. Since the key discovery by Whitesell et al. (1994) that geldanamycin (GA) binds Hsp90 and promotes dissociation of Hsp90 complexes and proteolytic degradation of the vsrc client protein (an oncogenic product associated with certain cancers), several naturally occurring and man-made inhibitors of Hsp90 have been identified. GA, the related ansamycin compounds and radicicol bind the N-terminal ATPase domain, whereas novobiocin and cisplatin seem to bind in the C-terminal half of Hsp90. In Arolla, we heard about some exciting progress on the potential therapeutic use of these inhibitors for cancer treatment.
As Hsp90 is involved in many growth-regulatory pathways, targeting Hsp90 could provide a powerful means of combating cancer. One might also expect that Hsp90 inhibitors would be highly toxic to normal cells. In fact, like many chemotherapeutic agents, GA has a dose-dependent selectivity for tumour cells. P. Ivy (Rockville, MD, USA) and C. Erlichman (Rochester, MN, USA) presented a poster that summarized results from four ongoing phase I clinical trials in advanced cancer patients in which the toxicity of 17-allylamino-1-deoxygeldanamycin is being assessed. Although several dose-limiting and non-dose-limiting toxicities have been observed, these are less problematic in certain dosing regimens. No objective clinical responses have yet been reported, but there is guarded optimism for planned phase I combination drug trials and for phase II single-agent trials. In separate talks by L. Neckers (Rockville, MD, USA) and N. Rosen, we learned that, in addition to enhancing degradation of ErbB2/Neu and other growth-promoting clients, GA has properties that could potentiate the effectiveness of other anti-cancer regimens. For example, GA can reverse adriamycin (also known as doxorubicin) resistance in cultured cells by promoting degradation of Hsp90 client proteins including the tumour necrosis factor receptor interacting protein (RIP) and IκB kinase—factors that promote activation of NFκB and resistance to apoptosis. In addition, GA-stimulated inactivation of Akt sensitizes cells to chemotherapeutic agents such as taxanes and adriamycin. The GA-stimulated degradation of the transcription factor HIF1-α promotes hypoxia and renders cells more sensitive to ionizing radiation. In relation to the selective toxicity of GA in tumour cells, Rosen speculated that GA and other natural products that bind Hsp90 might function as growth regulators by inhibiting or reducing, in some other way, the activity of Hsp90 in the host organism. This was proposed as an alternative to them functioning as toxins intended to inhibit competitors or predators. This is an interesting hypothesis, which merits formal testing. Last, L. Whitesell (Tucson, AZ, USA) gave a progress report on his efforts to identify novel small-molecule inhibitors of chaperones. He discussed one particularly interesting candidate molecule that can inhibit luciferase folding in cells and revert v-Src-transformed cells; the target for this inhibitor, which is presumed to be a chaperone protein, has not been identified.
Hsp90 in development and evolution
A variety of studies have shown that Hsp90 influences animal and plant development, and some fascinating examples were presented at Arolla. Y. Argon (Chicago, IL, USA) has generated a mouse line with a disrupted gene for Grp94, an Hsp90 homologue that is localized to the endoplasmic reticulum. Homozygous knockouts of the gene encoding Grp94 are embryonic lethal due to a failure of mesoderm to form; this phenotype is thought to result from the inactivation of an essential cell surface receptor (activin receptor 1A was suggested) or a secreted ligand that requires GRP94 for proper folding and maturation.
As an example of biological adaptation in host–parasite relationships, J. Clos (Hamburg, Germany) discussed the relationship between Hsp90 and life-stage differentiation in the protozoan Leishmania donovani. A temperature shift from 25 °C to 37 °C, and acidification, which mimics the transition the parasite experiences as it passes from the insect vector to the lysosomal compartment of a mammalian host, results in growth arrest, a corresponding stress response, and differentiation from the promastigote to amastigote life stage. Clos presented evidence suggesting that the life cycle of L. donovani is tightly regulated by Hsp90 homeostasis. In one set of experiments, he selected for continued cell growth in the presence of GA; the resulting cells showed episomal amplification of Hsp90 genes to a point at which, remarkably, Hsp90 comprised 30% of the total cellular protein.
Describing one of the most biologically significant discoveries related to Hsp90, C. Queitsch presented her work from the Lindquist lab (Cambridge, MA, USA) on the role of Hsp90 as a facilitator of evolution. Using the plant Arabidopsis thaliana to confirm and extend earlier studies in Drosophila (Rutherford & Lindquist, 1998), Queitsch observed that an assortment of morphogenetic responses occur in a single Arabidopsis genetic background when the function of Hsp90 is compromised by exposing seedlings to mild heatstress, by reducing Hsp90 levels by specific RNAi (RNA-mediated interference), or by treating seedlings with GA. This variation in response makes an elegant case for Hsp90 being able to buffer the effects of genetic variation on development under normal conditions, while allowing this variation to be expressed when organisms confront stressful environmental challenges. Extrapolating freely, we are indebted to Hsp90, not only for bringing us together in Arolla, but also perhaps for bringing us to where we are as a species.
Perspectives
In conclusion, the Arolla meeting gave those involved in Hsp90-related research an opportunity to review their progress and to see how this field has expanded into areas as diverse as cancer therapeutics and evolutionary theory. Conversely, many questions remain and the road ahead is a long one. A complete structural analysis will be especially important for understanding Hsp90's mechanism of action, for the characterization of the client-binding site and in determining whether a second ATP-binding site exists at the C terminus. In addition, it remains unclear whether the rather restricted Hsp90 'client base' is expanded after the heat shock that induces Hsp90 upregulation (after which the protein was named). Analysis of co-chaperones has also emerged as having importance in a wide range of research areas, and genomic and proteomic approaches will hopefully reveal the true extent of the role of the Hsp90 chaperone machinery in mammalian systems.

References
- Cyr D.M., Hohfeld J. & Patterson C. (2002) Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem. Sci., 27, 368–375. [DOI] [PubMed] [Google Scholar]
- Garnier C. et al. (2002) Binding of ATP to heat shock protein 90: evidence for an ATP-binding site in the C-terminal domain. J. Biol. Chem., 277, 12208–12214. [DOI] [PubMed] [Google Scholar]
- Marcu M.G., Chadli A., Bouhouche I., Catelli M. & Neckers L.M. (2000) The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem., 275, 37181–37186. [DOI] [PubMed] [Google Scholar]
- Nathan D.F., Vos M.H. & Lindquist S. (1999) Identification of SSF1, CNS1, and HCH1 as multicopy suppressors of a Saccharomyces cerevisiae Hsp90 loss-of-function mutation. Proc. Natl Acad. Sci. USA, 96, 1409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt W.B. & Toft D.O. (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev., 18, 306–360. [DOI] [PubMed] [Google Scholar]
- Prodromou C. et al. (1997) Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell, 90, 65–75. [DOI] [PubMed] [Google Scholar]
- Rao J. et al. (2001) Functional interaction of human Cdc37 with the androgen receptor but not with the glucocorticoid receptor. J. Biol. Chem., 276, 5814–5820. [DOI] [PubMed] [Google Scholar]
- Rutherford S.L. & Lindquist S. (1998) Hsp90 as a capacitor for morphological evolution. Nature, 396, 336–342. [DOI] [PubMed] [Google Scholar]
- Scheufler C. et al. (2000) Structure of TPR domain–peptide complexes: critical elements in the assembly of the Hsp70–Hsp90 multichaperone machine. Cell, 101, 199–210. [DOI] [PubMed] [Google Scholar]
- Soti C., Racz A. & Csermely P. (2002) A nucleotide-dependent molecular switch controls ATP binding at the C-terminal domain of Hsp90. N-terminal nucleotide binding unmasks a C-terminal binding pocket. J. Biol. Chem., 277, 7066–7075. [DOI] [PubMed] [Google Scholar]
- Stebbins C.E. et al. (1997) Crystal structure of an Hsp90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell, 89, 239–250. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Mimnaugh E., De Costa B., Myers C. & Neckers L. (1994) Inhibition of heat shock protein HSP90–pp60v–src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl Acad. Sci. USA, 91, 8324–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]


