Summary
Regulators of endocytosis and the actin cytoskeleton
Abstract
The Ark/Prk serine/threonine kinases initiate phosphorylation cycles that control the endocytic machinery in mammalian cells and in yeast, and the actin cytoskeleton in yeast. The members of this protein family are unified by homologies in their kinase domain, but are generally diverse in their other domains. The evolution of Ark/Prk family members in different organisms may have allowed the conserved role of the kinase domain, which is required for the phosphorylation of both endocytic and cytoskeletal components, to be coupled to other functional domains.
Introduction
The Ark/Prk serine/threonine protein kinase family has recently been shown to be important in controlling endocytosis and the actin cytoskeleton. The members of this family (Fig. 1A) are defined by their homology in an amino-terminal kinase domain and by a consensus phosphorylation target site (L(I)XXQXTG) that is found in all substrates analysed so far. Strikingly, functional studies indicate that all of the characterized Ark/Prk kinases have related roles in endocytosis in mammalian cells, and in both endocytosis and actin dynamics in yeast. Interestingly, little conservation exists outside the kinase domain. This is undoubtedly important for the substrate specificity of individual family members and may be responsible for other functions that are unique to specific Ark/Prk proteins (Fig. 1B). In this respect, it is important to note that increasing evidence of the actin cytoskeleton being required for endocytosis in mammalian cells, as it is in yeast, suggests that this family has evolved to regulate several steps in a common process.
Figure 1.
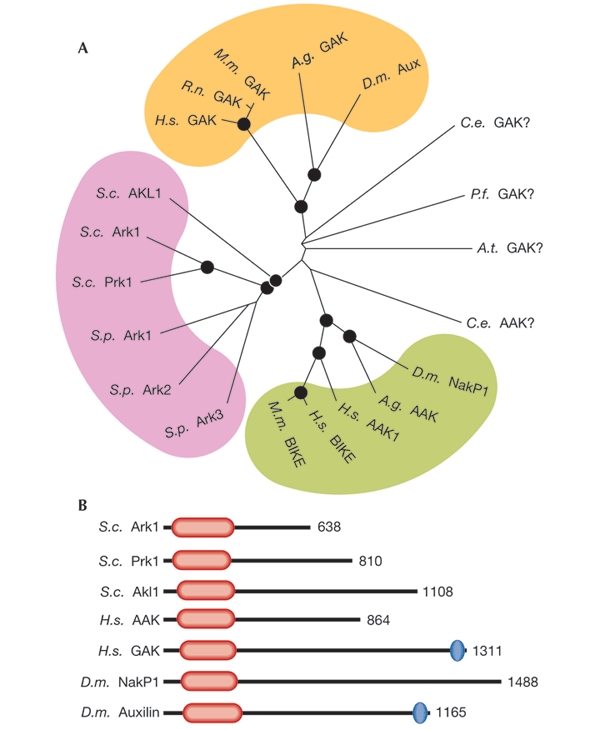
(A) Unrooted phylogenetic tree of the kinase domains of the Ark1/Prk1 family produced from alignments generated using ClustalX software. The black circles indicate nodes that scored a significance of greater than 90% probability. Coloured areas denote a possible subfamily. The yeast kinase members all fall into a distinct group (pink) as do the AAK1-related (green) and GAK-related (yellow) members. Kinases that fall outside these groups are denoted as in the database (GAK for Plasmodium falciparum (NP_701816) and Arabidopsis thaliana (T01122)) or according to the group to which they lie nearest (Caenorhabditis elegans AAK (NP_497929) and GAK (NP_508971)). Schizosaccharomyces pombe family members have been given the names Ark1, Ark2 and Ark3 to indicate their position in the yeast group, although there is no functional evidence at present to show that these kinases associate with actin. (B) Diagram of Ark/Prk kinase domain structures. In all cases, the kinase domain is near the amino terminus (red lozenge). The length of the region downstream of the kinase domain is extremely variable. Only two of the proteins depicted have other recognizable homology domains. These are the DNA J domains (blue ovals), which in both cases are situated at the extreme carboxyl terminus of the protein. In the kinase domains, Ark1 is 73% identical to Prk1, 48% to Akl1, and 38% to both NakP1 and AAK1. The kinase domain of AAK1 is 66% identical to NakP1 and 39% to GAK. AAK, adaptor-associated kinase; BIKE, BMP2-inducible kinase; GAK, cyclin-G-associated kinase; NakP1, Numb-associated kinase P1; Aux, Auxilin; A.g., Anopheles gambiae; A.t., Arabidopsis thaliana; C.e., Caenorhabditis elegans; D.m., Drosophila melanogaster; H.s., Homo sapiens; M.m., Mus musculus; P.f., Plasmodium falciparum; R.n., Rattus norvegicus; S.c., Saccharomyces cerevisiae; S.p., Schizosaccharomyces pombe.
This review focuses on our knowledge of the roles of the Ark/Prk kinases and highlights the idea that some of the target substrates of these kinases may be key links between the endocytic pathway and the actin cytoskeleton.
Endocytosis in mammals
Endocytosis is the fundamental cellular process by which all eukaryotic cells internalize material from the extracellular medium through vesicles. These vesicles bud from the plasma membrane and are delivered to an endosomal compartment where their content is sorted, and recycled to the cell surface, transported across the cell or targeted for degradation in lysosomes.
In mammalian cells, the initial internalization step can occur by different mechanisms, the best-characterized of which involves clathrin-coated pits (Brodsky et al., 2001). These structures form at the plasma membrane and have a striking lattice morphology that results from the polymerization of cytosolic clathrin onto the membrane. The clathrin-binding adaptor complex, AP2, links transmembrane receptors that are destined for internalization to the clathrin lattice by associating with internalization motifs in their cytoplasmic tails (Ohno et al., 1995). A high-molecular-weight GTPase, dynamin, is required for the budding of coated pits (McNiven et al., 2000a), and a host of other molecules, principally the binding partners for clathrin, AP2 and/or dynamin, are implicated in the formation of these vesicles. These other molecules include Eps15, epsin, amphiphysin, intersectin, syndapin and Rab5 (Brodsky et al., 2001).
The role of actin in endocytosis in mammals
A requirement for actin in clathrin-mediated uptake in mammalian cells is supported by the recent discovery of several molecular links between endocytic proteins and the actin cytoskeleton. Mammalian actin-binding protein 1 (Abp1) and cortactin, both activators of the Arp2–Arp3 complex, are now known to bind dynamin (McNiven et al., 2000b; Kessels et al., 2001). Also, the dynamin-binding partners, syndapin and intersectin, could indirectly regulate actin assembly through their interactions with N-WASP (a homologue of Wiskott-Aldrich syndrome protein)—a potent activator of the Arp2–Arp3 complex (Qualmann & Kessels, 2002)—which is crucial for driving actin nucleation (Welch & Mullins, 2002). Perhaps the strongest link between actin and clathrin-coated vesicles is the F-actin binding protein Huntingtin-interacting protein 1R (HIP1R), which can physically link clathrin-coated vesicles with actin in vitro and which co-localizes with coated pits in vivo (Engqvist-Goldstein et al., 2001). Recently, local actin rearrangements that are associated with the budding of clathrin-coated vesicles from the plasma membrane have been visualized using evanescent wave microscopy (Merrifield et al., 2002).
Endocytosis in yeast
Early studies on endocytosis mutants in yeast suggested that the initial steps of internalization might be different from those of the clathrin-mediated pathway in mammalian cells. Deletion, or mutations, of the yeast clathrin gene results in only a partial, albeit significant, endocytic block (Payne et al., 1987). Many mutations identified in endocytic screens affect proteins that associate either physically or functionally with actin (Geli & Riezman, 1998). In addition, an intact and dynamic actin cytoskeleton is necessary for endocytosis (Kubler & Riezman, 1993; Ayscough et al., 1997; Belmont et al., 1999; Ayscough, 2000). However, recent data suggest that the apparent differences between the endocytic machinery of lower and higher eukaryotes reflect the distinct approaches used to study them.
In yeast, two distinct actin structures can be visualized by using rhodamine–phalloidin staining. Actin cables, which are cytoplasmic bundles of relatively long filaments, are important for vesicle movement and for generating cell polarity. In contrast to this, endocytic proteins associate with cortical actin patches, which are plasma-membrane-associated complexes that comprise short filaments and a plethora of actin binding and regulatory proteins.
The association between cortical actin patches and endocytic proteins in yeast raises the question of whether endocytic machinery components are involved in actin-patch recruitment and of how the site of endocytosis is established. Sla2 is a yeast protein that interacts with the clathrin light-chain in a two-hybrid screen (Henry et al., 2002), the mammalian homologue of this protein being HIP1R (Engqvist-Goldstein et al., 2001). Similarly to HIP1R, Sla2 contains a carboxy-terminal domain able to interact with actin. The recruitment of Sla2 to endocytic sites seems to be an early endocytic event in yeast. Sla2 is able to interact with other components of the endocytic machinery including Sla1 (K.R.A. and colleagues, unpublished observations), which is itself part of a complex that contains Pan1 and End3, which is important for endocytosis in yeast (Tang & Cai, 1996; Tang et al., 1997; 2000). In this complex, Sla1 and Pan1 have been shown to interact with proteins that regulate actin dynamics. For example, Pan1 binds directly to and activates the Arp2–Arp3 complex, which is crucial for driving cortical actin polymerization (Duncan et al., 2001), and Sla1 binds to both Las17 (the yeast WASP homologue) and Abp1, which are activators of Arp2–Arp3 (Madania et al., 1999; Kessels et al., 2000). A loss of Sla1 leads to an increase in stabilized actin fibres (F-actin), suggesting that it may inhibit these actin regulators. Pan1 and Sla1 may be compartmentalized with components of the endocytic machinery, confining their activities to the appropriate regions of the plasma membrane.
Parallels between endocytosis in yeast and mammals
Pan1 is the yeast homologue of Eps15, which associates with AP2 in mammalian cells (Benmerah et al., 1995). Despite the considerable molar excess of AP2 over Eps15, dominant-negative mutants of Eps15 and anti-Eps15 antibodies block endocytosis (Benmerah et al., 1998; Carbone et al., 1997). Eps15 also binds epsin (Chen et al., 1998), and Pan1 interacts with the yeast epsins (Ent1 and Ent2) in a two-hybrid assay (Wendland & Emr, 1998), suggesting that at least some elements of Pan1 function have been conserved. Pan1 also binds to, and co-localizes with, yAP1801 and yAP1802 (Wendland & Emr, 1998). Because the mammalian homologue, AP180, of yAP1801 and yAP1802 binds clathrin and AP2 (Ahle & Ungewickell, 1986), this interaction may provide a further link between the Sla1–End3–Pan1 complex and clathrin (Fig. 2). Sla1 also acts as an endocytic adaptor, recognizing NPFX(1,2)D motifs in proteins such as the pheromone receptors Ste2 and Ste3, and facilitating their internalization (Howard et al., 2002). It thereby links cargo recruitment both to the endocytic machinery and to the actin cytoskeleton.
Figure 2.
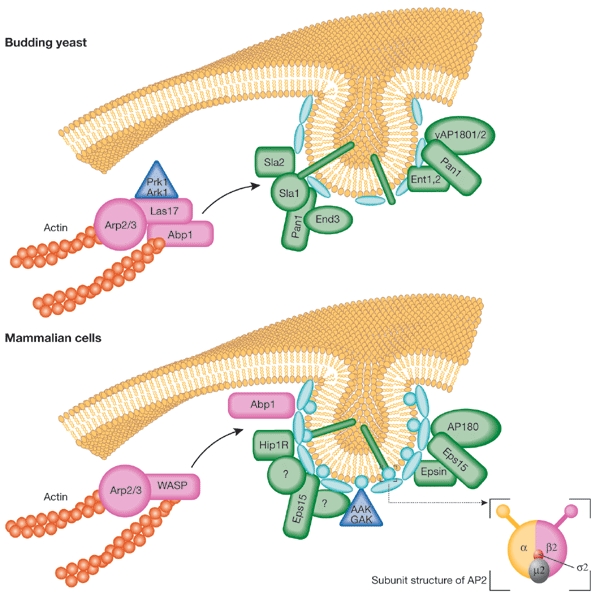
Comparison of early steps of endocytosis in yeast and mammalian cells. Some of the main components that are required for the early endocytic steps are shown, with emphasis on the interactions for which there is clear evidence and that might be affected directly or indirectly by the Ark/Prk kinases. Homologues are indicated where known, and functionality is reflected by colour (endocytic components in green, cytoskeletal regulators in pink, clathrin and AP2 in pale blue ellipses and circles, respectively, and cargo as green rods). The insert shows a schematic representation of the subunit composition of the AP2 adaptor complex. AAK, adaptor-associated kinase; Abp1, actin-binding protein 1; GAK, cyclin-G-associated kinase; WASP, Wiskott–Aldrich syndrome protein.
Thus, whether in yeast or in mammalian cells, endocytosis requires multivalent interactions and the formation of dynamic macromolecular protein complexes (Fig. 2) that may be coupled to actin assembly and disassembly. Because endocytosis is a constitutive process that involves continuous recycling of its machinery, these complexes must be capable of rapid and regulated assembly and disassembly. Reversible phosphorylation is therefore a plausible regulatory mechanism, and members of the Ark/Prk family of protein kinases seem to be key regulators of this process.
The Ark1/Prk1 family
The family of actin-regulating kinases in Saccharomyces cerevisiae comprises three members: Ark1, Prk1 and the lesser-studied Akl1. PRK1 interacts at a genetic level with PAN1 (Zeng & Cai, 1999), and Prk1 phosphorylates Pan1 at its N terminus on multiple repeats of the consensus target phosphorylation site L(I)xxQxTG (where x is any amino acid). Sla1 also contains multiple LxxQxTG repeats and is a substrate for Prk1 (Zeng et al., 2001). In both Sla1 and Prk1, these phosphorylation sites are in the regions required for their interaction, suggesting that modification may regulate assembly and/or disassembly of the Pan1–Sla1 complex. This idea is supported in vitro by the failure of phosphorylated Pan1 to bind to Sla1 and vice versa. Overexpression of Prk1 results in the dissociation of Sla1, but not Pan1, from cortical complexes (Zeng et al., 2001), possibly as a consequence of phosphorylation.
Evidence suggests that the role of Ark1 may overlap with that of Prk1. Both Sla1 and Pan1 are phosphorylated in prk1−/− cells, but not in cells that lack both kinases (Zeng et al., 2001). Whether Ark1 recognizes only the LxxQxTG motif or also variations thereof has not yet been explored, but it should be noted that in addition to five LxxQxTG motifs, Sla1 has seven QxTG motifs and ten L(I)(M)xxxxTG motifs. This raises the possibility that different subsets of target sites may be phosphorylated in response to different signals.
Further evidence for Ark1/Prk1 involvement in endocytosis comes from the Prk1-specific substrate Ent1. Although overexpression of mutants that mimic constitutively phosphorylated or dephosphorylated Ent1 has no significant effect on endocytosis in wild-type cells, it does inhibit endocytosis in a Pan1 temperature-sensitive mutant (pan1-20) background (Watson et al., 2001).
Ark1 was originally identified through a two-hybrid screen for proteins that bind to Sla2 (Cope et al., 1999). The interaction is through the Ark1 C-terminal region, which shows no homology with Prk1, indicating that the kinases may have distinct roles. This idea is supported by a series of genetic studies in which either ark1 or prk1 was deleted with mutations in other genes including abp1, sla2 and rvs167 (yeast amphiphysin). Different phenotypes resulted (Cope et al., 1999), suggesting that Ark1 and Prk1 may regulate actin and the endocytic machinery through parallel pathways.
If Ark1 and Prk1 phosphorylation of Pan1 and Sla1 changes the ability of these proteins to interact, the kinases may need to be spatially segregated from their substrate until required. Fluorescence resonance energy transfer (FRET) and fluorescence microscopy have shown that Sla1 and Abp1 are present individually in discrete cortical patches as well as together in a distinct subset of patches (Warren et al., 2002). Because Abp1 is known to bind to Prk1 (Fazi et al., 2002) and is necessary for the correct localization of both Ark1 and Prk1 (Cope et al., 1999), it seems likely that these kinases are normally associated with the actin cytoskeleton. Thus, discrete actin patches could segregate the kinases from the substrate, and the merging of these patches, presumably at sites of endocytosis, would then give them access to Pan1 and Sla1.
Although little is known about the details of the actin rearrangements that are required to drive endocytosis (Schafer, 2002), it seems likely that, once recruited to sites of endocytosis, an actin patch must change markedly to favour the invagination or scission process, relying on both actin polymerization and depolymerization. Sla1 may be an initiator of actin disassembly, as its deletion leads to a more stable actin cytoskeleton (Ayscough et al., 1997; Warren et al., 2002). Subsequent phosphorylation by Ark1 or Prk1 could cause Sla1 to dissociate from the endocytic complex, allowing other proteins such as Sla2 to interact with actin to drive the formation of new structures required for endocytosis.
Phosphorylation cycles in yeast
An understanding of the mechanisms by which Ark1 and Prk1 regulate endocytosis requires knowledge of the reversible nature of phosphorylation. Although Prk1 phosphorylation has been shown to inhibit Pan1 function, it may be the balance between phosphorylation and dephosphorylation that is important. Current data support a model in which, following the association of actin with the endocytic machinery, Ark1 and Prk1 can phosphorylate Sla1, Pan1 and Ent1 and, at least in the case of Sla1, cause its dissociation from a large complex. In the absence of both Ark1 and Prk1, all of the actin and endocytic machinery is found in one large patch that dissociates from the cell cortex. This actin may be insufficiently dynamic to facilitate endocytosis. Thus, the normal role of Ark1 and Prk1 could be the positive regulation of endocytosis.
If Pan1 and Sla1 are phosphorylated and then lost from the complex, dephosphorylation might allow their re-incorporation into cortical complexes. This remains to be shown, as the role of phosphatases in endocytosis has not been extensively studied. However, interactions between Sla1 and the yeast homologue of protein phosphatase 1, Glc7 (Tu et al., 1996; Venturi et al., 2000), as well as between Pan1 and Glc7 have been reported (Uetz et al., 2000). Furthermore, Scd5, a suppressor of clathrin heavy-chain deficiency, was recently shown to rely on its interaction with Glc7 to maintain endocytosis and actin dynamics (Chang et al., 2002). Because Scd5 is also required in conjunction with clathrin for the association of Sla2 with cortical actin (Henry et al., 2002), one scenario is that the Glc7 phosphatase recruits Sla2 to initiate new sites of endocytosis.
Mammalian family members
There is increasing evidence that reversible cycles of phosphorylation also control the coated-vesicle cycle in mammalian cells. In vitro dephosphorylation of dynamin, amphiphysin and epsin promotes their assembly into complexes with clathrin and AP2 (Slepnev et al., 1998). In neuronal cells, recycling of synaptic vesicles through clathrin-coated pits requires the concerted dephosphorylation of these and several other endocytic proteins, including Eps15 and AP180 (Cousin & Robinson, 2001). On neuronal stimulation, dephosphorylation occurs rapidly to promote vesicle uptake and/or recycling, whereas re-phosphorylation, which is also essential, occurs more slowly.
Members of the Ark/Prk family of kinases have recently been identified in mammalian cells. These include cyclin-G-associated kinase (GAK) and adaptor-associated kinase 1 (AAK1). Substrate analyses coupled to functional studies indicate that, like their counterparts in yeast, these kinases are important for endocytosis.
GAK was originally identified as a ubiquitous homologue of the neuronal protein auxilin, and shares its role as a cofactor to the heat shock cognate protein (Hsc70) in uncoating clathrin-coated vesicles (Greener et al., 2000; Umeda et al., 2000). GAK is a component of these vesicles (Korolchuk & Banting, 2002), and, in vitro, its kinase domain can phosphorylate the medium subunits of both the plasma membrane adaptor complex AP2 and the trans-Golgi network adaptor complex AP1, which are known as μ2 and μ1, respectively (Fig. 2, inset; Umeda et al., 2000). Both μsubunits are involved in cargo recruitment into clathrin-coated vesicles (Ohno et al., 1995).
AAK1 was identified as a binding partner for the appendage or ear domain of the α-adaptin component of AP2 complexes (Conner & Schmid, 2002). It co-purifies with AP2, and localizes to active sites of endocytosis in rat hippocampal neurons, and also with coated pits in HeLa cells. A recombinant form can phosphorylate μ1 and μ2. Phosphorylation of μ2 occurs in vivo on serine and threonine residues (Wilde & Brodsky, 1996), and Thr 156, which is located in a typical consensus site for the Ark/Prk kinase family, has been shown to be an in vitro phosphorylation site (Pauloin & Thurieau, 1993). μ2 phosphorylation has been shown to be important for endocytosis (Olusanya et al., 2001) as the sequestration of the cargo molecule transferrin into newly formed coated pits is inhibited if μ2 phosphorylation is specifically blocked. Other evidence for this is that transfection of HeLa cells with a mutant form of μ2, which cannot be phosphorylated, inhibits transferrin uptake.
Several complementary studies have shed light on the role of μ2 phosphorylation. The binding of endocytic cargoes, such as the transferrin receptor, by μ2 is known to depend on the recognition of sorting motifs in the cytoplasmic tails of transmembrane receptors (Ohno et al., 1995). Phosphorylated μ2 binds to peptides that contain such motifs with a significantly higher affinity than the unphosphorylated form (Ricotta et al., 2002). The crystal structure of the AP2 complex (comprising α- and β2-adaptin without their ear domains) suggests that unphosphorylated μ2 cannot bind cargo because the binding site is partially blocked by β2-adaptin (Collins et al., 2002). It also indicates that a substantial conformational change is required to make the binding site accessible. Evidence that a conformational change occurs when AP2 is incorporated into coated pits comes from experiments showing that incorporation of AP2 into clathrin coats alters its sensitivity to proteolysis (Matsui & Kirchhausen, 1990) and increases its affinity for cargo (Rapoport et al., 1997). Such a change could be effected by phosphorylation of μ2 on Thr 156, which is located on an exposed surface of μ2.
Understanding the crucial role of μ2 phosphorylation in the formation of coated vesicles requires the identification and characterization of the kinases responsible for Thr 156 phosphorylation in vivo. Given their ability to phosphorylate μ2 in vitro, both GAK and AAK1 are candidates. To determine which of these is relevant for the coated-vesicle cycle, adaptor complexes were subfractionated, and a fraction enriched in AAK1 but devoid of GAK was found to contain most of the μ2 kinase activity (Ricotta et al., 2002). Together with the localization of AAK1 to sites of endocytosis, compared with the perinuclear/cytoplasmic localization of GAK (Greener et al., 2000), this suggests that AAK1 is probably the in vivo μ2 kinase. Given the in vitro substrate specificities of AAK1 and GAK, and the enrichment of the latter in coated vesicles, it is possible that GAK preferentially phosphorylates μ1 in vivo, perhaps to promote cargo recruitment into coated vesicles at the trans-Golgi network. Similarly to AAK1, it can also bind to the ear domain of α-adaptin, suggesting that it may nevertheless have a role in coated vesicle formation at the cell surface, although this is likely to be independent of its kinase domain and may be related to the uncoating process. However, it is also important to consider the alternative possibilities that, by analogy with the suggested overlapping functions of Ark1 and Prk1, different signals may independently activate AAK1 or GAK to phosphorylate μ2, or that other substrates for GAK may be present at the plasma membrane. Given that AAK1 is probably the closest mammalian Prk1 homologue (Fig. 1A), it is intriguing to speculate that these substrates might regulate interactions between the endocytic machinery and the actin cytoskeleton.
Conclusions
We have focused on the best-characterized members of the Ark1/Prk1 family. However, other family members are emerging and, intriguingly, the paradigm of a common role in endocytosis is maintained. So, for example, the Numb-associated kinase (Nak) of Drosophila phosphorylates Numb, which functions in cell fate determination during cell division (Chien et al., 1998). Numb has been shown to be an endocytic protein (Santolini et al., 2000) and, furthermore, Numb-mediated asymmetrical cell division in Drosophila requires α-adaptin (Berdnik et al., 2002).
Major challenges for the future will be to analyse the regulation of these kinases and the counterbalance of their activities by phosphatases. Analysis of the in vivo substrate specificities of these molecules, coupled with improved microscopy techniques that allow local actin dynamics to be imaged (Merrifield et al., 2002), should provide important insights into these issues. Because family membership is now dictated largely by homologies in the kinase domains, it is crucial to determine how kinase activity is integrated with the function of the divergent C termini of these proteins (Fig. 1B), which probably regulate their in vivo specificities.
What is certain is that we have seen the emergence of a new kinase family that seems to have a central role in endocytosis from yeast through to man. At this early stage, many of the in vivo roles of these proteins remain undetermined, so their breadth of function is still unknown. However, the range of approaches that are being used to investigate these proteins should lead to answers to the many questions about the relationship between the actin cytoskeleton and endocytosis, and the role of phosphorylation and dephosphorylation.
Acknowledgments
We thank C. Smythe and S. Winder for helpful comments on the manuscript. E.S. is supported by the Medical Research Council (MRC), the British Heart Foundation and the Biotechnology and Biological Sciences Research Council. K.R.A. is an MRC Senior Research Fellow (G117/394).
References
- Ahle S. & Ungewickell E. (1986) Purification and properties of a new clathrin assembly protein. EMBO J., 5, 3143–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough K.R. (2000) Endocytosis and the development of cell polarity in yeast require a dynamic F-actin cytoskeleton. Curr. Biol., 10, 1587–1590. [DOI] [PubMed] [Google Scholar]
- Ayscough K.R., Stryker J., Pokala N., Sanders M., Crews P. & Drubin D.G. (1997) High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol., 137, 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont L.D., Patterson G.M. & Drubin D.G. (1999) New actin mutants allow further characterization of the nucleotide binding cleft and drug binding sites. J. Cell Sci., 112, 1325–1336. [DOI] [PubMed] [Google Scholar]
- Benmerah A., Gagnon J., Begue B., Megarbane B., Dautry V.A. & Cerf B.N. (1995) The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J. Cell Biol., 131, 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A., Lamaze C., Begue B., Schmid S.L., Dautry V.A. & Cerf B.N. (1998) AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol., 140, 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdnik D., Torok T., Gonzalez-Gaitan M. & Knoblich J.A. (2002) The endocytic protein α-adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell, 3, 221–231. [DOI] [PubMed] [Google Scholar]
- Brodsky F.M., Chen C.Y., Knuehl C., Towler M.C. & Wakeham D.E. (2001) Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol., 17, 517–568. [DOI] [PubMed] [Google Scholar]
- Carbone R., Fre S., Iannolo G., Belleudi F., Mancini P., Pelicci P.G., Torrisi M.R. & Di Fiore P.P. (1997) Eps15 and Eps15R are essential components of the endocytic pathway. Cancer Res., 57, 5498–5504. [PubMed] [Google Scholar]
- Chang J.S., Henry K., Wolf B.L., Geli M. & Lemmon S.K. (2002) Protein phosphatase-1 binding to Scd5p is important for regulation of actin organization and endocytosis in yeast. J. Biol. Chem., 277, 45880–45886. [DOI] [PubMed] [Google Scholar]
- Chen H., Fre S., Slepnev V.I., Capua M.R., Takei K., Butler M.H., Di F.P. & De C.P. (1998) Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature, 394, 793–797. [DOI] [PubMed] [Google Scholar]
- Chien C.T., Wang S., Rothenberg M., Jan L.Y. & Jan Y.N. (1998) Numb-associated kinase interacts with the phosphotyrosine binding domain of Numb and antagonizes the function of Numb in vivo. Mol. Cell. Biol., 18, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B.M., McCoy A.J., Kent H.M., Evans P.R. & Owen D.J. (2002) Molecular architecture and functional model of the endocytic AP2 complex. Cell, 109, 523–535. [DOI] [PubMed] [Google Scholar]
- Conner S.D. & Schmid S.L. (2002) Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol., 156, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope M.J., Yang S., Shang C. & Drubin D.G. (1999) Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J. Cell Biol., 144, 1203–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin M.A. & Robinson P.J. (2001) The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci., 24, 659–665. [DOI] [PubMed] [Google Scholar]
- Duncan M.C., Cope M.J., Goode B.L., Wendland B. & Drubin D.G. (2001) Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nature Cell Biol., 3, 687–690. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A.E., Warren R.A., Kessels M.M., Keen J.H., Heuser J. & Drubin D.G. (2001) The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J. Cell Biol., 154, 1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazi B. et al. (2002) Unusual binding properties of the SH3 domain of the yeast actin-binding protein Abp1: structural and functional analysis. J. Biol. Chem., 277, 5290–5298. [DOI] [PubMed] [Google Scholar]
- Geli M.I. & Riezman H. (1998) Endocytic internalization in yeast and animal cells: similar and different. J. Cell Sci., 111, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Greener T., Zhao X., Nojima H., Eisenberg E. & Greene L.E. (2000) Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J. Biol. Chem., 275, 1365–1370. [DOI] [PubMed] [Google Scholar]
- Henry K.R., D'Hondt K., Chang J., Newpher T., Huang K., Hudson R.T., Riezman H. & Lemmon S.K. (2002) Scd5p and clathrin function are important for cortical actin organization, endocytosis, and localization of sla2p in yeast. Mol. Biol. Cell, 13, 2607–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J.P., Hutton J.L., Olson J.M. & Payne G.S. (2002) Sla1p serves as the targeting signal recognition factor for NPFX(1,2)D- mediated endocytosis. J. Cell Biol., 157, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels M.M., Engqvist-Goldstein A.E. & Drubin D.G. (2000) Association of mouse actin-binding protein 1 (mAbp1/SH3P7), an Src kinase target, with dynamic regions of the cortical actin cytoskeleton in response to Rac1 activation. Mol. Biol. Cell, 11, 393–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels M.M., Engqvist-Goldstein A.E., Drubin D.G. & Qualmann B. (2001) Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J. Cell Biol., 153, 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk V.I. & Banting G. (2002) CK2 and GAK/auxilin2 are major protein kinases in clathrin-coated vesicles. Traffic, 3, 428–439. [DOI] [PubMed] [Google Scholar]
- Kubler E. & Riezman H. (1993) Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J., 12, 2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madania A., Dumoulin P., Grava S., Kitamoto H., Scharer-Brodbeck C., Soulard A., Moreau V. & Winsor B. (1999) The Saccharomyces cerevisiae homologue of human Wiskott–Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol. Biol. Cell, 10, 3521–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W. & Kirchhausen T. (1990) Stabilization of clathrin coats by the core of the clathrin- associated protein complex AP-2. Biochemistry, 29, 10791–10798. [DOI] [PubMed] [Google Scholar]
- McNiven M.A., Cao H., Pitts K.R. & Yoon Y. (2000a) The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem. Sci., 25, 115–120. [DOI] [PubMed] [Google Scholar]
- McNiven M.A., Kim L., Krueger E.W., Orth J.D., Cao H. & Wong T.W. (2000b) Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol., 151, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield C.J., Feldman M.E., Wan L. & Almers W. (2002) Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nature Cell Biol., 4, 691–698. [DOI] [PubMed] [Google Scholar]
- Ohno H., Stewart J., Fournier M.C., Bosshart H., Rhee I., Miyatake S., Saito T., Gallusser A., Kirchhausen T. & Bonifacino J.S. (1995) Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science, 269, 1872–1875. [DOI] [PubMed] [Google Scholar]
- Olusanya O., Andrews P.D., Swedlow J.R. & Smythe E. (2001) Phosphorylation of threonine156 of the μ2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr. Biol., 11, 896–900. [DOI] [PubMed] [Google Scholar]
- Pauloin A. & Thurieau C. (1993) The 50 kDa protein subunit of assembly polypeptide (AP) AP-2 adapter from clathrin-coated vesicles is phosphorylated on threonine-156 by AP-1 and a soluble AP50 kinase which copurifies with the assembly polypeptides. Biochem. J., 296, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G.S., Hasson T.B., Hasson M.S. & Schekman R. (1987) Genetic and biochemical characterization of clathrin-deficient Saccharomyces-cerevisiae. Mol. Cell. Biol., 7, 3888–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann B. & Kessels M.M. (2002) Endocytosis and the cytoskeleton. Int. Rev. Cytol., 220, 93–144. [DOI] [PubMed] [Google Scholar]
- Rapoport I., Miyazaki M., Boll W., Duckworth B., Cantley L.C., Shoelson S. & Kirchhausen T. (1997) Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J., 16, 2240–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta D., Conner S.D., Schmid S.L., von Figura K. & Honing S. (2002) Phosphorylation of the AP2 μ subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J. Cell Biol., 156, 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolini E., Puri C., Salcini A.E., Gagliani M.C., Pelicci P.G., Tacchetti C. & di Fiore P.P. (2000) Numb is an endocytic protein. J. Cell Biol., 151, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D.A. (2002) Coupling actin dynamics and membrane dynamics during endocytosis. Curr. Opin. Cell Biol., 14, 76–81. [DOI] [PubMed] [Google Scholar]
- Slepnev V.I., Ochoa G.C., Butler M.H., Grabs D. & Camilli P.D. (1998) Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science, 281, 821–824. [DOI] [PubMed] [Google Scholar]
- Tang H.Y. & Cai M. (1996) The EH-domain-containing protein Pan1 is required for normal organization of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Cell. Biol., 16, 4897–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H.Y., Munn A. & Cai M. (1997) EH domain proteins Pan1p and End3p are components of a complex that plays a dual role in organization of the cortical actin cytoskeleton and endocytosis in Saccharomyces cerevisiae. Mol. Cell. Biol., 17, 4294–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H.Y., Xu J. & Cai M. (2000) Pan1p, End3p, and S1a1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol. Cell. Biol., 20, 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J., Song W. & Carlson M. (1996) Protein phosphatase type 1 interacts with proteins required for meiosis and other cellular processes in Saccharomyces cerevisiae. Mol. Cell. Biol., 16, 4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P. et al. (2000) A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae. Nature, 403, 623–627. [DOI] [PubMed] [Google Scholar]
- Umeda A., Meyerholz A. & Ungewickell E. (2000) Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur. J. Cell Biol., 79, 336–342. [DOI] [PubMed] [Google Scholar]
- Venturi G.M., Bloecher A., Williams-Hart T. & Tatchell K. (2000) Genetic interactions between GLC7, PPZ1 and PPZ2 in Saccharomyces cerevisiae. Genetics, 155, 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren D.T., Andrews P.D., Gourlay C.W. & Ayscough K.R. (2002) Sla1p couples the yeast endocytic machinery to proteins regulating actin dynamics. J. Cell Sci., 115, 1703–1715. [DOI] [PubMed] [Google Scholar]
- Watson H.A., Cope M.J., Groen A.C., Drubin D.G. & Wendland B. (2001) In vivo role for actin-regulating kinases in endocytosis and yeast epsin phosphorylation. Mol. Biol. Cell, 12, 3668–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M.D. & Mullins R.D. (2002) Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol., 18, 247–288. [DOI] [PubMed] [Google Scholar]
- Wendland B. & Emr S.D. (1998) Pan1p, yeast eps15, functions as a multivalent adaptor that coordinates protein–protein interactions essential for endocytosis. J. Cell Biol., 141, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A. & Brodsky F.M. (1996) In vivo phosphorylation of adaptors regulates their interaction with clathrin. J. Cell Biol., 135, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G. & Cai M. (1999) Regulation of the actin cytoskeleton organization in yeast by a novel serine/threonine kinase Prk1p. J. Cell Biol., 144, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G., Yu X. & Cai M. (2001) Regulation of yeast actin cytoskeleton-regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p. Mol. Biol. Cell, 12, 3759–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]


