Abstract
We report in this study the in vivo efficacy of nine 2-substituted quinolines on the Leishmania amazonensis cutaneous infection murine model and on the Leishmania infantum and Leishmania donovani visceral infection murine models. In the case of the L. amazonensis model, quinolines were administered orally at 25 mg/kg twice daily for 15 days. Quinolines 1, 2, 3, and 7 reduced by 80 to 90% the parasite burdens in the lesion, whereas N-methylglucamine antimoniate (Glucantime), administered by subcutaneous injections at 100 mg [28 mg Sb(V)] per kg of body weight daily, reduced the parasite burdens by 98%. In visceral leishmaniasis due to L. infantum, mice treated orally at 25 mg/kg daily for 10 days with quinolines 1, 4, 5, and 6 showed a significant reduction of parasite burdens in the liver and spleen. These quinolines were significantly more effective than meglumine antimoniate to reduce the parasite burden in both the liver and spleen. Also, the oral in vivo activity of three quinolines (quinolines 4, 5, and 2-n-propylquinoline) were determined against L. donovani (LV 9) at 12.5 and 25 mg/kg for 10 days. Their activity was compared with that of miltefosine at 7.5 mg/kg. Miltefosine, 2-n-propylquinoline, and quinoline 5 at 12.5 mg/kg significantly reduced the parasite burdens in the liver by 72, 66, and 61%, respectively. From the present study, quinoline 5 is the most promising compound against both cutaneous and visceral leishmaniasis. The double antileishmanial and antiviral activities of these compounds suggest that this series could be a potential treatment for coinfection of Leishmania-human immunodeficiency virus.
For the last 50 years, the pentavalent antimony compounds sodium stilbogluconate (Pentostam; Glaxo Wellcome, United Kingdom) and meglumine antimoniate (Glucantime; Aventis, France) have been the first-line treatments for leishmaniasis. Amphotericin B and pentamidine, the parenteral alternatives to antimony, cause serious and irreversible toxic effects which preclude their use (7). New approaches have been proposed, including the use of other nonparenteral agents such as the aminoglycoside, aminosidine (topical application), or oral agents. Oral administration has the advantage of reducing socioeconomic difficulties that are present in areas of endemicity where health facilities are lacking. Over the last 4 years, hexadecylphosphocholine (HePC; miltefosine), an antineoplastic agent, has been identified as the first effective oral treatment for visceral infection (23).
With this in mind, we have searched for other alternative drugs based on empirical screening or ethnopharmacological studies and thereafter established an in vitro structure-activity relationship. Following this approach, we have previously reported the efficacy of 2-substituted quinolines in the experimental treatment of cutaneous and visceral leishmaniasis by oral and parenteral routes (10, 11).
VL is considered an important potential opportunistic disease among patients infected with human immunodeficiency virus type 1 (HIV-1), according to epidemiological data established by the World Health Organization reporting that HIV-1-Leishmania coinfection has spread to 33 countries throughout the world (8).
In a recent study, Fakhfakh et al. (9) described the efficacy of 2-substituted quinolines against both HIV and Leishmania, suggesting that oral therapy with these compounds could treat Leishmania-HIV coinfection.
From structure-activity relationships, especially those based on the nature of the alkyl chain branched at the 2-position of quinoline, we have selected the compounds with the best in vitro activity as promising drug candidates (9). Therefore, we are interested in establishing simple low-cost methods to prepare these quinolines and deduce in vivo structure-activity relationships.
Thus, in the present study, we report on the antileishmanial activity of nine compounds selected from in vitro studies with Leishmania amazonensis after oral treatment in an experimental cutaneous leishmaniasis model in BALB/c mice, Leishmania infantum in an experimental visceral leishmaniasis in BALB/C mice, or Leishmania donovani under the same conditions. The efficacy of quinolines was compared with the activity of meglumine antimoniate and with oral treatment with miltefosine in the experimental L. donovani infection.
MATERIALS AND METHODS
Chemicals.
The 2-substituted quinolines were synthesized in the Laboratory of Pharmacognosy, Faculty of Pharmacy, Chātenay-Malabry, France, by procedures described elsewhere (9). Each compound was shown to have in vitro antileishmanial activity against amastigote forms of L. amazonensis and L. donovani. Physical and spectral data (proton and carbon-13 nuclear magnetic resonance and mass spectrometry) were used to check the purity of 2-substituted quinolines.
Commercial preparations of meglumine antimoniate were obtained from Aventis, Paris, France, as a solution in a vial. Miltefosine (1-O-hexadecylphosphocholine; no. 000761) was provided by Zentaris (Frankfurt, Germany).
Parasites.
L. amazonensis (IFLA/BR/67/PH8) and L. infantum (MHOM/FR/91/LEM2259V, isolated from the bone marrow of HIV-infected patients with visceral leishmaniasis) (15) were kindly provided by the Laboratory of Parasitology, University Hospital of Montpellier, France. L. donovani (MHOM/ET/1967/L82-LV9) was also used in this study.
Female BALB/c mice and golden hamsters (Mesocricetus auratus) were supplied by La Plata, Argentina and bred at the Instituto de Investigaciones en Ciencias de la Salud, Asuncion, Paraguay. Golden hamsters were used to maintain the parasites.
In vivo evaluation against L. amazonensis.
L. amazonensis was used and maintained by passage every 8 weeks in hamsters. BALB/c mice (n = 9) were inoculated in the left hind footpad with 1 × 106 amastigotes obtained from donor hamsters. The parasites were delivered in 100 μl of phosphate-buffered saline (PBS). The growth of lesions was determined weekly by measuring the diameters of both rear feet with a direct reading vernier caliper (Kroelin 1ODI 00T6). The size of the lesion in millimeters was calculated by subtracting the measurement of the uninfected foot from that of the infected foot. Measurements commenced 1 day prior to the inoculation of amastigotes and were continued for 9 weeks. For each experiment, the mean and standard error of the mean were calculated. The treatment was initiated 5 weeks after inoculation when the infection was well established, and the lesions were obvious. Two days before administration of drug, the mice were randomly divided into groups of 10. N-Methylglucamine antimoniate was dissolved in 50 μl of PBS and administered to BALB/c mice in regimens of 28 mg of Sb(V) per kg of body weight daily for 15 days by the subcutaneous route. 2-Substituted quinolines were tested at a dose level of 25 mg/kg of body weight/day and were formulated in 50 μl PBS and 5 μl of polysorbate (Tween 80; OSI, France). Drugs were administered daily (twice) by the oral route for 15 days. The untreated group received 50 μl of PBS and 5 μl of Tween 80 daily. The animals were sacrificed 1 week after the end of treatment to assess the parasite loads in the infected footpad. Briefly, the mice were killed and the lesions of the infected footpad were excised, weighed, and homogenized in a glass with a Teflon pestle (Potter; OSI) in 5 ml of RPMI 1640 (Gibco, France) tissue culture medium supplemented with 10% fetal calf serum, 1 ml of glutamine (29.4 mg/liter; GIBCO, France), penicillin (100 U/ml), and streptomycin (100 μg/ml). Plates were examined, and the number of amastigotes per host lesion cell nucleus was counted. The number of amastigotes per nucleus × lesion weight in grams × 107 is approximately equal to the total number of amastigotes per organ (10). Parasite suppression was calculated from the ratio of the mean lesion amastigote counts of drug-treated mice and the mean lesion amastigote counts of untreated mice multiplied by 100 to obtain the percentage of parasite suppression.
In vivo evaluation against L. infantum.
L. infantum was maintained by serial passage in golden hamsters which were infected intravenously with 107 amastigotes derived from the livers of infected hamsters. BALB/c mice, randomly sorted into groups of 8 to 10 were infected intravenously by injection of 5 × 106 amastigotes in 100 μl medium derived from homogenates of infected hamster spleens. One day after the last drug administration, the mice were weighed and killed, and the livers and spleens were removed and weighed. Liver impressions were prepared and stained by Giemsa. The numbers of amastigotes per host liver cell nucleus were counted (500 liver nuclei from each animal were examined under oil immersion). The number of amastigotes per organ per nucleus × liver mass in mg × 4 × 105 is approximately the total number of amastigotes per liver (6). Parasite suppression was calculated from the ratio of the mean liver amastigote counts of drug-treated mice and the mean liver amastigotes counts of untreated mice multiplied by 100 to obtain the percentage of parasite suppression.
The treatments were initiated 1 week after parasite inoculation. The BALB/c mice were first weighed and then randomly divided into groups of eight. Quinolines were formulated in 100 μl of carboxymethyl cellulose and Tween 80 and administered daily at 25 mg/kilogram body weight/day for 10 days by the oral route. The reference drug (N-methylglucamine antimoniate) was dissolved in 100 μl of carboxymethyl cellulose-Tween and administered at 100 mg/kg or at a dose level of 28 mg of Sb(V)/kg/day by the subcutaneous route.
In vivo evaluation against L. donovani.
Six- to 8-week-old BALB/c mice (Elevages Janvier, France) were infected intravenously with 107 L. donovani amastigotes derived from spleen hamsters and randomly sorted into groups of 7 to 10. The treatment was started 1 week after infection and was continued for 10 days. One group received the reference drug HePC (miltefosine) orally at 7.5 mg/kg of body weight. In these experiments, synthetic quinolines (4 and 5) and 2-n-propylquinoline (10) were tested at two different doses administered orally at 25 or 12.5 mg/kg for 10 days. All doses were administered consecutively on days 7 to 17 after infection. At day 24, all groups were sacrificed and livers and spleens were weighed. Parasite numbers were determined by counting the number of amastigotes/500 liver cells in Giemsa-stained impression smears prepared from the liver and multiplying that value by the weight of the liver (in milligrams) (22).
Statistical analysis.
For cutaneous leishmaniasis, the means of the size and the parasite load of the infected footpad of the untreated and drug-treated groups were compared using the unpaired Student t test. For visceral infection, the mean number of parasites per gram of liver of treatment groups and controls were compared using Student's t test or the Kruskal-Wallis nonparametric analysis of variance test for comparing two groups. Significance was established for a P value of <0.05.
Toxicological data.
The behavior of mice was monitored daily throughout the experiment, and mice were weighed at the end of experiment to compare the weight of treated mice with that of untreated ones.
RESULTS
In all experimental leishmaniasis models, we observed no significant loss of weight for the total compounds in any treated mice, suggesting low toxicity at the doses used in the experiments.
Efficacy of oral treatment with quinolines on experimental infection with Leishmania amazonensis.
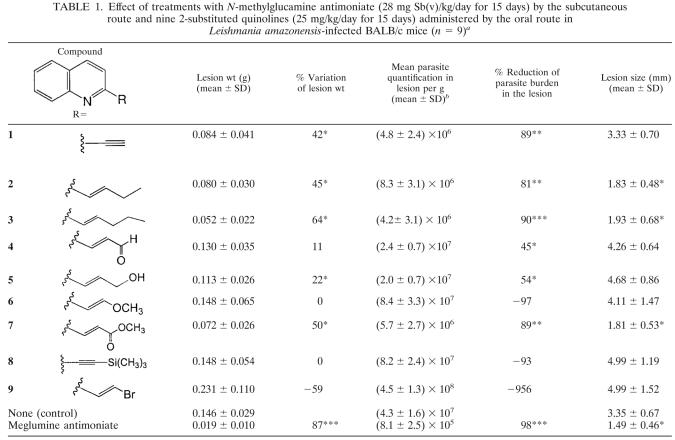
This study is the first one devoted to the in vivo evaluation of the most promising 2-alkylated quinolines compared on three experimental leishmaniasis models. The effects of subcutaneous and oral treatments with meglumine antimoniate or with 2-substituted quinolines by the subcutaneous or oral route during the course of infection of BALB/c mice infected with L. amazonensis are presented in Table 1. The subcutaneous treatment with the antimonial drug at 28 mg/kg of Sb(V) for 15 days significantly reduced the lesion weight by 87.3% (P < 0.0001) and the parasite loads by 98.4% (P < 0.0001) versus the untreated mice. At 8 weeks postinfection, footpad lesions of the untreated mice were larger than those of the treatment groups, except for mice treated with quinolines 6, 8 and 9, in which the lesion sizes were 4.11 ± 1.47, 4.99 ± 1.19, and 4.99 ± 1.52 mm, respectively. The inefficacy of these quinolines was confirmed by the large increase in the parasite burden in the lesions, by 97.1, 93, and 957%, respectively. The mice treated with quinolines 2, 3, and 7 had significantly (P < 0.05) smaller lesion sizes than untreated mice. Lesion sizes were 1.83 ± 0.48 mm for the group treated with quinoline 2, 1.93 ± 0.68 mm for the group treated with quinoline 3, and 1.81 ± 0.53 mm for the group treated with quinoline 7 compared with 3.35 ± 0.67 mm for untreated animals (Fig. 1). The parasite burdens in the infected footpads were significantly reduced (P < 0.0001) by quinoline 3 and by quinolines 2 and 7 (P < 0.001), by 81, 90, and 89%, respectively. We also observed that the mice treated with quinolines 1 and 5 showed a significant reduction of the lesion weight (P < 0.05) by 42 and 22%, respectively, compared with the untreated mice. Parasite burdens in the footpad lesions were significantly (P < 0.05) reduced in the groups treated with quinolines 1, 4, and 5. The mean reduction of parasite burden was 89% for the group treated with quinoline 1, 45% for the group treated with quinoline 4, and 54% for the group treated with quinoline 5. Compound 9 was responsible for a very large increase in the parasite burden, suggesting that it might affect the functioning of the immune system. Further studies to evaluate this phenomenon would be interesting.
TABLE 1.
Effect of treatments with N-methylglucamine antimoniate (28 mg Sb(v)/kg/day for 15 days) by the subcutaneous route and nine 2-substituted quinolines (25 mg/kg/day for 15 days) administered by the oral route in Leishmania amazonensis-infected BALB/c mice (n = 9)a
Statistical significance compared to untreated mice is indicated as follows: *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
Values are means±standard deviations of results for 9 mice.
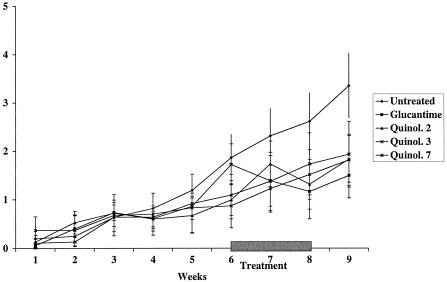
FIG. 1.
Efficacy of a 15-day treatment course with meglumine antimoniate [28 mg of Sb(V) per kg per day] and quinolines (Quinol.) 2, 3, and 7 administered orally at 25 mg/kg twice daily during the course of infection of BALB/c mice (n = 10) with L. amazonensis. Treatments were started on the sixth week postinfection and were continued for 15 days. Each point represents the mean difference in size ± standard deviation of the mean between infected and uninfected footpads.
Efficacy of oral treatments with quinolines in experimental Leishmania infantum infection.
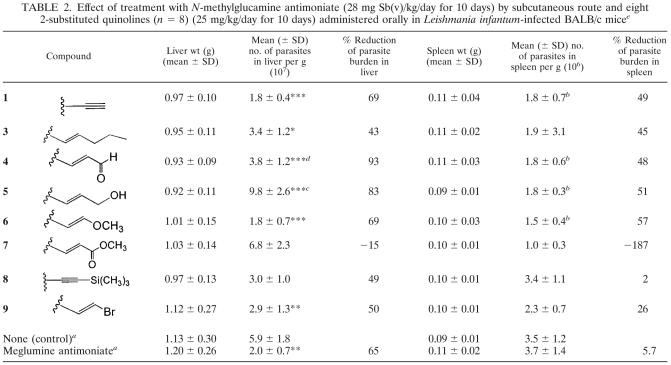
Table 2 presents the parasite burdens in the livers and spleens of untreated and treated mice. Treatment with quinolines 4, 5, 6, and 1 at 25 mg/kg significantly reduced the liver parasite burdens compared to the burdens in the untreated mice: for quinoline 1, by 69% (P < 0.0001); for quinoline 4, by 93% (P < 0.0001); for quinoline 5, by 83% (P < 0.0001); for quinoline 6, by 69% (P < 0.0001). Treatments with quinolines 4 and 5 caused a significant reduction in parasite burden in the liver and spleen compared to the burdens in the mice treated with meglumine antimoniate: for quinoline 4, liver, P < 0.0001; spleen, P < 0.001; and for quinoline 5, liver, P < 0.001; spleen, P < 0.05. Treatment with meglumine antimoniate had no effect on spleen parasite burdens. The treatments with quinolines 3 and 9 resulted in significant reductions in liver parasite burden, by 43% (P < 0.05) and 50% (P < 0.001), respectively, but had no significant effect on spleen parasite burdens. Only the treatment with quinoline 7 increased the parasite burdens in the liver and spleen compared to the untreated group. Treatment with quinoline 8 had a weak suppressive effect, reducing the parasite burdens in the liver by 49% (not significantly) and had no effect on the spleen parasite burdens.
TABLE 2.
Effect of treatment with N-methylglucamine antimoniate (28 mg Sb(v)/kg/day for 10 days) by subcutaneous route and eight 2-substituted quinolines (n = 8) (25 mg/kg/day for 10 days) administered orally in Leishmania infantum-infected BALB/c micee
n = 18.
Statistically significant (P < 0.05) compared with meglumine antimoniate-treated mice.
Statistically significant (P < 0.001) compared with meglumine antimoniate-treated mice.
Statistically significant (P < 0.0001) compared with meglumine antimoniate-treated mice.
Statistical significance compared to untreated mice is indicated as follows: *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
Efficacy of oral treatments with three quinolines in experimental Leishmania donovani infection.
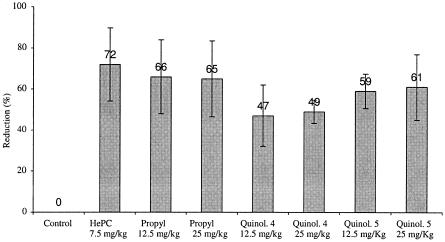
Oral treatment with miltefosine at 7.5 mg/kg for 10 days caused a significant reduction in liver parasite burden in mice infected with L. donovani (72%; P < 0.002). Treatment at 12.5 mg/kg with 2-n-propylquinoline resulted in a significant reduction in liver parasite burdens (66%; P < 0.002), but with the dose of 25 mg/kg, we observed a similar significant reduction (65%; P < 0.002) (Fig. 2).
FIG. 2.
Reduction of parasite burden of L. donovani in livers of mice treated with HePC or 2-substituted quinolines (Quinol.) by the oral route compared to untreated mice.
Treatments with quinolines 4 and 5 at 25 mg/kg resulted in a significant reduction in parasite burden in the liver: for quinoline 4, 49% (P < 0.002); for quinoline 5, 61% (P < 0.003). A decrease in the dose of these compounds to 12.5 mg/kg caused a similar significant reduction in the parasite burden in the liver, by 47% (P < 0.02) and 59% (P < 0.003), respectively.
DISCUSSION
Our results indicated that E-3-quinol-2-yl-prop-2-en-1-ol (quinoline 5 in this study) was one of most active compounds and displayed a significant in vitro activity against the intracellular amastigote forms of L. amazonensis (LV 79), with a 50% inhibitory concentration (IC50) of around 3 to 5 μM, and against L. infantum (MHOM/MA/BE/67), with an IC50 of around 2 μM and 3 μM against the promastigote forms of L. donovani (9). Furthermore, quinoline 5 was evaluated ex vivo for its antiviral activity against HIV-1 replication in CEM4fx cells. In this case, it exhibited submicromolar antiviral activity (IC50 of around 1 μM) and also showed some activity against human T-cell leukemia virus type 1-infected cells (12, 13).
Cutaneous and visceral leishmaniasis remain a therapeutic challenge and a serious public health problem. Pentavalent antimonials are the main drugs used in the treatment of all forms of leishmaniasis, but the description of cases of resistance to these drugs and the fact that they have to be administered parenterally represent the major limitations for the chemotherapy of leishmaniasis. Unfortunately, no ideal therapies for the leishmaniases have yet been identified (7). The first oral treatment for visceral leishmaniasis, miltefosine (23, 24), is now registered in India, Germany, and Colombia. The development of a second oral drug, sitamaquine (27), is in a phase 2 trial, and two drugs which can be given by the topical route, imiquimod (1) and paromomycin (2), are in the same stage of development. The main observation from this study is that oral treatment with 2-substituted quinolines was found to be effective in murine models of cutaneous and visceral leishmaniases. The in vitro and in vivo activity of 2-substituted quinoline series previously described justify the pharmacomodulations at position 2 of the quinoline ring (9-11). Thus, from the preceding in vitro studies, the choice of the moiety at position 2 of the quinoline ring was based on the following criteria. First, it is essential to have a nonsaturated structure allowing potential chemical reactions with putative targets. Thus, ethyne and ethylene groups have been selected. Second, we showed that the optimal length of the alkyl chain to obtain the maximal antileishmanial activity should not exceed five carbons (9). Third, the nature of the chemical groups at the C-2 extremity seemed to have a limited effect on in vitro antileishmanial activity. This study has shown that four quinolines (1, 3, 4, and 5) administered to mice orally exhibited a significant efficacy in both cutaneous and visceral leishmaniases (Table 3); these compounds are all 2-alkenylquinolines. In previous studies (9), we showed that quinolines 3, 4, and 5 were able to inhibit HIV-1 replication in CEM4fx cells. These data suggest a role for these compounds in the treatment of cases of AIDS-visceral leishmaniasis coinfection. We observed that these compounds possess an unsaturated side chain with three carbon atoms. Two quinolines (quinolines 3 and 7) were specifically active against cutaneous leishmaniasis, and two quinolines (quinolines 4 and 5) were only active against visceral leishmaniasis infection. We observed that 2-alkenylquinoline 3 (containing a pent-2-en-1-yl chain) had antileishmanial properties similar to those of prop-2en-1-yl quinoline previously described and isolated from the Bolivian plant Galipea longiflora (10, 11). With these new analogs, we can now confirm (i) their oral efficacy and (ii) the recent in vitro results obtained against the amastigote forms of L. amazonensis, L. infantum, and L. donovani (9). These compounds, with low molecular weight, have exhibited a variety of biological properties such as antiprotozoal activity against different species, in particular Plasmodium (14) and Trypanosoma cruzi (20), and were found to be potent inhibitors of the HIV-1 integrase (28). Furthermore, these compounds could be used in combination with classical antileishmanial drugs to prevent the emergence of drug resistance. In addition, since various data from clinical studies with AIDS patients living in zones of leishmaniasis endemicity have shown that visceral leishmaniasis promoted the clinical progression of AIDS (16), these quinolines seem to be promising for the treatment of HIV-visceral leishmaniasis-coinfected patients.
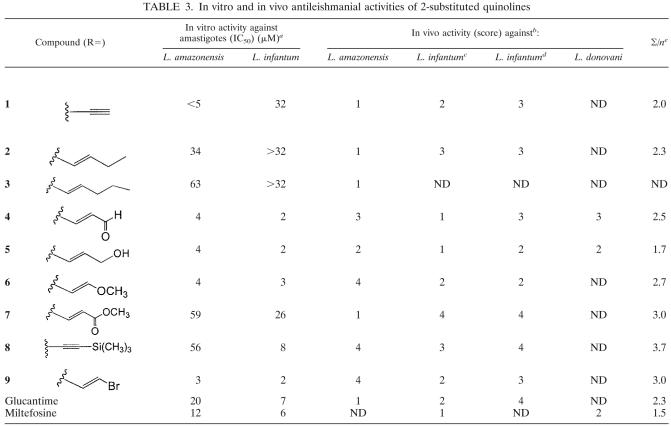
TABLE 3.
In vitro and in vivo antileishmanial activities of 2-substituted quinolines
Data are from Fakhfakkh et al. (9).
Score: 1, % reduction > 80%; 2, 50% < % reduction < 80%; 3, 25% < % reduction < 50%; 4, 0 < % reduction < 25%. ND, not determined.
Reduction of parasite burden in the liver.
Reduction of parasite burden in the spleen.
∑/n, ratio of ∑ score to number of in vivo models.
The mechanism of action of 2-substituted quinolines in Leishmania has not been investigated but will be developed in a future study. However, some data concerning the mode of action of quinolines on Leishmania are available. Thus, sitamaquine, a 8-amino-quinoline, induces fine structural alterations in Leishmania tropica within human macrophages (18); sitamaquine induces also a rapid collapse of the mitochondrial inner membrane potential of L. donovani promastigotes (25) and promotes a fast and extensive alkalinization of the L. donovani acidocalcisomes (26). Only a few quinolines (imidazoquinolinamines) such as imiquimod have also been demonstrated to be potent inducers of alpha interferon and cytokines in both in vitro and in vivo experiments (5).
To date, chemical leads have been identified for this group of compounds, and optimization by activities both in culture and on animal models has been carried out. In previous studies, the potential importance of metabolites was highlighted, since some analogs exhibited strong in vivo activity, whereas in vitro action was weak, and in this way, some active metabolites of 2-propylquinoline have been identified (3, 17).
It is noteworthy that an inhibitory effect of 2-n-propylquinoline on the P-glycoprotein activity of intestine cells has been recently described (4). It would be interesting to determine whether this effect is also observed in Leishmania, since antimonial resistance is linked to Leishmania P-glycoprotein overexpression (19, 21).
We demonstrated in this study that quinolines 4, 5, and 6 exhibited the best activities with the L. infantum mouse model, whereas quinolines 1, 3, and 7 were the best with the L. amazonensis model. When comparing all the molecules on these infection models, quinoline 5 clearly emerged as the single compound with satisfactory activity in the three in vivo models (Table 3). Furthermore, it presents several advantages, it has good physicochemical properties (chemically stable, solid state), low toxicity, and excellent activity and it is easily prepared from quinaldine in three-step synthesis. The preparation of a quinoline salt could improve its physicochemical properties further and allow its use by the oral or topical route as a treatment or in association with classical treatments of leishmaniasis. Finally, it may also be seen as a possible natural metabolite of the natural active compound prop-2-en-1-yl quinoline. We therefore propose to focus on quinoline 5 for industrial development with companies from areas of endemicity. The in vitro antiretroviral properties of the compounds tested in this study have been recently demonstrated against HIV-1 (9) and against human T-cell leukemia virus type 1 (12, 13), suggesting that the most promising compounds could be used for HIV-leishmaniasis coinfection. Their preparation by chemical synthesis is easily transposable in emerging countries with an industrial partner which could produce them on a large scale at low cost.
In conclusion, further studies of pharmacokinetics, dosage optimization, and mechanism of action will be carried out in relation to antileishmanial activity to determine an adequate dosing regimen for therapeutic use.
REFERENCES
- 1.Arevalo, I., B. Ward, R. Miller, T. C. Meng, E. Najar, E. Alvarez, G. Matlashewski, and A. Llanos-Cuentas. 2001. Successful treatment of drug-resistant cutaneous leishmaniasis in human by use of imiquimod, an immunomodulator. Clin. Infect. Dis. 33:1847-1851. [DOI] [PubMed] [Google Scholar]
- 2.Armijos, R. X., M. M. Weigel, M. Calvopina, M. Mancheno, and R. Rodriguez. 2004. Comparison of the effectiveness of two topical paromomycin treatments versus meglumine antimoniate for New World cutaneous leishmaniasis. Acta Trop. 91:153-160. [DOI] [PubMed] [Google Scholar]
- 3.Belliard, A. M., B. Baune, M. Fakhfakh, R. Hocquemiller, and R. Farinotti. 2003. Determination of the human cytochrome P 450 involved in the metabolism of 2-n-propylquinoline. Xenobiotica 33:341-355. [DOI] [PubMed] [Google Scholar]
- 4.Belliard, A. M., C. Leroy, H. Banide, R. Farinotti, and B. Lacour. 2003. Decrease of intestinal P-glycoprotein activity by 2n-propylquinoline, a new oral treatment for visceral leishmaniasis. Exp. Parasitol. 103:51-56. [DOI] [PubMed] [Google Scholar]
- 5.Buates, S., and G. Matlashewski. 2001. Identification of genes induced by a macrophage activator, S-28463, using gene expression array analysis. Antimicrob. Agents Chemother. 45:1137-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buffet, P. A., A. Sulahan, Y. J. F. Garin, N. Nassar, and F. Derouin. 1995. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissue of infected mice. Antimicrob. Agents Chemother. 39:2167-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis-current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19:502-508. [DOI] [PubMed] [Google Scholar]
- 8.Desjeux, P. 1998. UNAIDS. Leishmania and HIV in gridlock. WHO and UN programme on HIV/AIDS; WHO/CTD/LEISH/98.9. World Health Organization, Geneva, Switzerland.
- 9.Fakhfakh, M., A. Fournet, E. Prina, J. F. Mouscadet, X. Franck, R. Hocque-miller, and B. Figadère. 2003. Synthesis and biological evaluation of substituted quinolines: potential treatment of protozoal and retroviral coinfections. Bioorg. Med. Chem. 11:5013-5023. [DOI] [PubMed] [Google Scholar]
- 10.Fournet, A., M. E. Ferreira, S. Torres de Ortiz, S. Fuentes, H. Nakayama, A. Rojas de Arias, A. Schinini, and R. Hocquemiller. 1996. In vivo efficacy of 2-substituted quinolines in experimental treatment of New World cutaneous leishmaniasis caused by Leishmania amazonensis. Antimicrob. Agents Chemother. 40:2447-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournet, A., J. C. Gantier, A. Gautheret, L. Leysalles, M. H. Munos, J. Mayrargue, H. Moskowitz, A. Cavé, and R. Hocquemiller. 1994. The activity of 2-substituted quinoline alkaloids in BALB/c mice infected with Leishmania donovani. J. Antimicrob. Chemother. 33:537-544. [DOI] [PubMed] [Google Scholar]
- 12.Fournet, A., M. Mahieux, M. A. Fakhfakh, X. Franck, R. Hocquemiller, and B. Figadère. 2003. Substituted quinolines induce inhibition of proliferation of HTLV-1 infected cells. Bioorg. Med. Chem. Lett. 13:891-894. [DOI] [PubMed] [Google Scholar]
- 13.Franck, X., A. Fournet, E. Prina, R. Mahieux, R. Hocquemiller, and B. Figadère. 2004. Biological evaluation of substituted quinolines. Bioorg. Med. Chem. Lett. 14:3635-3638. [DOI] [PubMed] [Google Scholar]
- 14.Gantier, J. C., A. Fournet, M. H. Munos, and R. Hocquemiller. 1996. The effect of some 2-substituted quinolines isolated from Galipea longiflora on Plasmodium vinckei petteri infected mice. Planta Med. 62:285-286. [DOI] [PubMed] [Google Scholar]
- 15.Garin, Y. J. F., A., Sulahian, F. Pratlong, P. Méneceur, J. P. Gangneux, E. Prina, J. P. Dedet, and F. Derouin. 2001. Virulence of Leishmania infantum is expressed as a clonal and dominant phenotype in experimental infections. Infect. Immun. 69:7365-7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerin, P. J., P. Olliaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, M. K. Wasunna, and A. D. Bryceson. 2002. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 17.Iglarz, M., B. Baune, J. C. Gantier, R. Hocquemiller, and R. Farinotti. 1998. Determination of 2-n-propylquinoline in mouse plasma and liver by HPLC. J. Chromatogr. B 714:335-340. [DOI] [PubMed] [Google Scholar]
- 18.Langreth, S. G., J. D. Berman, G. P. Riordan, and L. S. Lee. 1983. Fine-structural alterations in Leishmania tropica within human macrophages exposed to antileishmanial drugs in vitro. J. Protozool. 30:555-561. [DOI] [PubMed] [Google Scholar]
- 19.Loiseau, P. M., L. Imbertie, C. Bories, D. Beteder, and I. De Miguel. 2002. Design and antileishmanial activity of amphotericin B-loaded stable ionic amphiphile biovector fomulations. Antimicrob. Agents Chemother. 46:1597-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama, H., M. E. Ferreira, A. Rojas de Arias, N. V. de Bilbao, A. Schinini, and A. Fournet. 2001. Experimental treatment of chronic Trypanosoma cruzi infection in mice with 2-n-propylquinoline. Phytother. Res. 15:630-632. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Victoria, J. M., A. Di Pietro, D. Barron, A. G. Ravelo, S. Castanys, and F. Gamarro. 2002. Multidrug resistance phenotype mediated by the P-glycoprotein-like transporter in Leishmania: a search for reversal agents. Curr. Drug Targets 3:311-333. [DOI] [PubMed] [Google Scholar]
- 22.Stauber, L. A., E. M. Franchino, and J. Grun. 1958. An eight-day method for screening compounds against Leishmania donovani in the golden hamster. J. Protozool. 5:269-273. [Google Scholar]
- 23.Sundar, S., and H. W. Murray. 2005. Availability of miltefosine for the treatment of kala-azar in India. Bull. W. H. O. 83:394-395. [PMC free article] [PubMed] [Google Scholar]
- 24.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fisher, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 25.Vercesi, A. E., and R. Docampo. 1992. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem. J. 284:463-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vercesi, A. E., C. O. Rodrigues, R. Catisti, and R. Docampo. 2000. Presence of a Na(+)/H(+) exchanger in acidocalcisomes of Leishmania donovani and their alkalization by anti-leishmanial drugs. FEBS Lett. 473:203-206. [DOI] [PubMed] [Google Scholar]
- 27.Yeates, C. 2002. Sitamaquine (GlaxoSmithKline/Walter Reed Army Institute). Curr. Opin. Investig. Drugs 3:1446-1452. [PubMed] [Google Scholar]
- 28.Zouhiri, F., J. F. Mouscadet, K. Mekouar, D. Desmaële, D. Savouré, H. Leh, F. Subra, M. Le Bret, C. Auclair, and J. d'Angelo. 2000. Structure-activity relationships and binding mode of styrylquinolines as potent inhibitors of HIV-1 integrase and replication of HIV-1 in cell culture. J. Med. Chem. 43:1533-1540. [DOI] [PubMed] [Google Scholar]